Abstract
Herein, we present on two 2,6-bis(4-ethynylpyridinyl)-4-fluoroaniline receptors that display solvatochromic absorption and emission. Neutral derivatives displayed opposite solvatochromic behavior as compared to the alkylated receptors. Adding anions induced changes in the absorption and emission spectra. In general, the fluorescence of the halogen bonding receptor was quenched less efficiently when compared to the hydrogen bonding receptor.
A halogen bond (XB) is an attractive noncovalent interaction between an electron-deficient halogen atom and a Lewis base. XBs are more directional and display different solvent dependencies1 compared to hydrogen bonds (HB), and can be applied in anion recognition and sensing.2 Recently, Beer’s macrocycles, rotaxanes and catenanes; Molina and Alkorta’s halotriazolium; and Taylor’s urea were reported as leading examples of fluorescent and colorimetric anion sensors that employ XBs.3 However, there are still relatively few spectroscopic studies devoted to XBing anion receptors. Herein we report a new UV-Vis/fluorescence responsive XB receptor that explores how XBing influences spectrophotometric properties in this system.
Building on previous studies conducted in our laboratory,4 we designed and synthesized a pair of 2,6-bis(4-ethynylpyridinyl)-4-fluoroaniline XB receptors (2a and 2b). Solution studies, crystal structures and computations supported our hypothesis that the intramolecular HB between the electron-deficient aniline and the two XB donor iodine atoms enhance the electrophilicity of the XB donors and preorganizes the bidentate XBing conformation.5 We refer to this new preorganization strategy as intramolecular HB-XB. Receptor 2b, lacks XB donors and was prepared to quantify C–H HBing and serve as a control. While synthesizing and characterizing the receptors, solvent dependent color changes were observed, especially under ultraviolet light. To better understand this solvatochromism, UV-Visible absorption and fluorescence emission studies were conducted for 1a, 1b and octyl derivatives 2a and 2b (Fig. 1).
Fig 1.
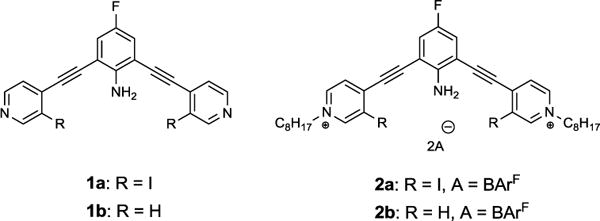
Structures of 2,6-bis(4-ethynylpyridinyl)-4-fluoroaniline XB donor 1a, HB donor 1b and octyl derivatives 2a, 2b.
Solvent dependence of both the absorption and emission spectra was observed for 1a and 1b (Fig. 2). Receptors 1a and 1b exhibited a major absorbance band in the range of 300-600 nm (Fig. S1 and S3). The absorption band of 1a is red shifted from 405 nm to 416 nm as the polarity of the solvent (with the exception of MeCN) is increased (Fig. S1). A similar direct correlation of bathochromic shift with increasing solvent polarity was obtained for 1b (Fig. S3). Soret bands from π–π* transitions exhibit significant charge-transfer character resulting from the electron-deficient pyridine and electron donating aniline.6 Polar solvents stabilize the excited state more than the ground state of the receptor. As a result, the energy for the HOMO to LUMO electron transition is lowered producing a bathochromic shift of the spectra with increasing solvent polarity. The λmax of 1a is always red shifted when compared to 1b in the same solvent, a result of the auxochrome iodine groups in 1a.7
Fig 2.

Solvatochromism of 1mM 1a (top left: under sunlight; bottom left: under 365nm UV light) and 1b (top right: under sunlight; bottom right: under 365nm UV light) in a set of solvents.
The solvatochromic effect on fluorescence was also investigated for 1a and 1b. The iodine atoms of 1a produce a “heavy atom effect” that enhances the probability of intersystem crossing leading to reduced fluorescence of 1a compared to 1b (Fig. 3).8 The emission spectra of 1a and 1b obey the same direct correlation between solvent polarity and λmax shift. However, methanol deviates from this trend for both 1a and 1b (Fig. S2 and S4). For instance, the emission band of 1b in methanol has a λmax at 475 nm which is 15 nm and 14 nm longer than in acetone and acetonitrile, respectively, and is even close to dimethylformamide (DMF, λmax = 476 nm) (Fig. S4). This deviation could result from the HB ability of the protic methanol. HBing to the amine of the fluoroaniline through an NH•••O type HB or to a pyridine nitrogen through an OH•••N HB could stabilize the excited state and shift the emission. Additionally, a drop in emission is observed for 1b, perhaps due to HB enhanced internal conversion and intersystem crossing.9
Fig 3.

Normalized fluorescence emission spectra of 1a (20 μM, solid line) and 1b (20 μM, dashed line) in various solvents.
To investigate the XB interaction and how it influences solvatochromism and fluorescence, the receptors were alkylated to increase their electron-deficiency and their binding affinity for anionic guests. Alkylation of the pyridines with octyl chains activated the XB and HB donors of 2a and 2b, respectively, while also enabling solubility in organic solvents.
The UV-Vis absorption and fluorescence emission spectra of 2a and 2b in various solvents are reported in Fig. 4. A negative solvatochromism was observed in DCM, acetone, MeCN and DMF for the absorption of both 2a and 2b which has also been observed in other pyridinium systems.10 This phenomenon has been explained by the ground state being more polar than the excited state11 and intramolecular charge transfer being favored by polar solvents.10 This produces a larger energy difference between the ground and excited states as the polarity of the solvent increases. Chloroform, MeOH and DMSO deviate from this trend, perhaps, due to the binding between the receptors and solvents or environment effect which give rise to conformational changes in the receptor molecule.12 Additionally, an obvious difference between 2a and 2b is the large blue shifting of 2a in DMF. We have solution and crystallographic evidence that derivatives of 2a can XB to the DMF carbonyl oxygen.13 This binding interaction may further stabilize the ground state. Ultimately, an 80 nm blue shift of absorbance in DMF is observed compared to dichloromethane (DCM, Fig. 4a). The HB in 2b has a similar but weaker effect, shifting the absorption peak from 472 nm in DCM to 466 nm in DMF (Fig. 4b).
Fig 4.

Absorption spectra of 2a (a) and 2b (b) in various solvents. Fluorescence emission spectra of 2a (c) and 2b (d) in various solvents (for excitation wavelengths and details see SI). All spectra were recorded at 20 μM of receptor.
2a was only fluorescent in nonpolar solvents (DCM and chloroform) and was nearly quenched in all other solvents (Fig. 4c). In contrast, 2b had stronger fluorescence than 2a due to lack of the heavy atom effect, but was also quenched in polar solvents (Fig. 4d). The fluorescence quenching is consistent with other probes that are weakly fluorescent in hydrophilic environments but strongly fluorescent in hydrophobic environments.14
Qualitative evaluation of the anion sensing capability of 2a and 2b in DCM was performed with a series of anions as their tetrabutylammonium salts (Cl−, Br−, I−, SCN−, NO3−, HSO4−, H2PO4− and ReO4−). Considering both solubility and polarity which may affect the noncovalent interaction between receptor and anion, we chose DCM as the solvent for these studies. The results are illustrated in Fig. 5 (see SI for full details). In general, the absorption band of XB receptor 2a at λmax 507 nm is red shifted from 509 to 535 nm when one molar equivalent of anion is added, and hypochromically blue shifted after the addition of excess anion (50 molar equivalents). Additionally, the absorption band of HB receptor 2b at 472 nm behaves similarly to 2a which is red shifted from 479 to 513 nm in the presence of one equivalent of anion and hypochromically blue shifted upon addition of excess anion (50 molar equivalents).
Fig 5.

Absorption spectra of 2a with TBA+I− (top left) and with TBA+Cl− (top middle-left), 2b with TBA+I− (top middle-right) and with TBA+Cl− (top right); followed by fluorescence emission spectra of 2a with TBA+I− (bottom left) and with TBA+Cl− (bottom middle-left), 2b with TBA+I− (bottom middle-right) and with TBA+Cl− (bottom right). All spectra were recorded at 20 μM of receptor in DCM solution (for excitation wavelengths and details see SI).
In the emission spectra, halides quenched the fluorescence of both 2a and 2b from 0 equivalent to 50 equivalents. For instance, fluorescence of 2a decreased by 20% after adding one equivalent of TBA+Cl− and declined to 50% of the initial value after 50 equivalents (Fig. 5, bottom middle-left). However, the receptor is more sensitive to iodide (Fig. 5, bottom left). The intensity significantly dropped to 2% of the original level at just one equivalent of iodide. Inter and intramolecular HBing have been shown to facilitate fluorescence quenching.15 The efficient fluorescence “turn-off” in 2a correlates with previous NMR studies that illustrate stronger binding between 2a and iodide compared to other anions.5 In the current system, the formation of strong XBs (C-I•••I−) in the 2a•I− complex could allow the necessary spin-orbital coupling for fluorescence quenching to occur.16 Moreover, the iodine17 and iodide18 present can act as heavy-atom quenchers. Considering these effects, intersystem crossing could be favored which leads to fluorescence quenching. Compared to 2a, fluorescence quenching of 2b is more efficient with chloride and bromide, and similar with iodide. In addition, thiocyanate (SCN−) quenched the fluorescence of both 2a and 2b. SCN– decreased fluorescence intensity of 2a to less than 35% of the original level at one equivalent but changed very little at 50 equivalents. However, the fluorescence of 2b was almost totally quenched after 50 equivalents.
Some of the oxoanions studied elicited different fluorescence responses compared to halides and SCN–. NO3– and ReO4– induced a similar fluorescence response in 2a and 2b as SCN– did. However, H2PO4– produced a 51% and 60% decrease in 2a and 2b, respectively, at one equivalent and totally quenched fluorescence when in excess. HSO4− affected the fluorescence of 2b in a similar way to SCN−, NO3− and ReO4−. However, in 2a, the solution turned cloudy and floccule formed, precipitating out the receptor in a couple minutes upon the addition of 50 equivalents of HSO4−. In general, 2b is quenched more than 2a with all anions, except for iodide. One hypothesis is that XB between 2a and the anions rigidifies the structure of the 2a•anion complex. The more planar/rigid structure which has less vibrational modes to absorb the excess energy leads to less efficient internal conversion. Thus, the more efficient internal conversion of 2b causes the lower quantum yield, and correspondingly lower fluorescence intensity.19
In summary, we have demonstrated that XB receptor 1a and HB receptor 1b exhibited similar solvatochromism in their UV-Vis and fluorescence spectra. As compared to 1a and 1b, octyl derivatives 2a and 2b exhibited opposite solvatochromism corresponding to their charged states and differences in binding ability. Further theoretical estimations of the electron density distributions of the HOMO and LUMO are ongoing in our lab which will help to explain how XBing or HBing influences solvatochromism. The anion induced fluorescence quenching of XB derivative 2a is less efficient than 2b with most anions, possibly due to the loose bolt effect. Additionally, 2a can selectively sense iodide over other anions by a significant fluorescence quenching after the addition of one equivalent. Such effects have been explored to better understand the nature of XB and may provide the opportunity to exploit XB in fluorescent/colorimetric anion sensors.
Supplementary Material
Acknowledgments
This work was funded by National Science Foundation (NSF) CAREER CHE-1555324, the Center for Biomolecular Structure and Dynamics CoBRE (NIH NIGMS grant P20GM103546) and the University of Montana (UM). We would like to thank the anonymous reviewers for their insightful comments and suggestions.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.(a) Robertson CC, Perutz RN, Brammer L, Hunter CA. Chemical Science. 2014;5(11):4179–4183. [Google Scholar]; (b) Laurence C, Graton J, Berthelot M, El Ghomari MJ. Chemistry A European Journal. 2011;17(37):10431–10444. doi: 10.1002/chem.201101071. [DOI] [PubMed] [Google Scholar]
- 2.(a) Beale TM, Chudzinski MG, Sarwar MG, Taylor MS. Chemical Society Reviews. 2013;42(4):1667–1680. doi: 10.1039/c2cs35213c. [DOI] [PubMed] [Google Scholar]; (b) Metrangolo P, Meyer F, Pilati T, Resnati G, Terraneo G. Angewandte Chemie International Edition. 2008;47(33):6114–6127. doi: 10.1002/anie.200800128. [DOI] [PubMed] [Google Scholar]; (c) Erdelyi M. Chemical Society Reviews. 2012;41(9):3547–3557. doi: 10.1039/c2cs15292d. [DOI] [PubMed] [Google Scholar]
- 3.(a) Zapata F, Caballero A, White NG, Claridge TD, Costa PJ, Félix V, Beer PD. Journal of the American Chemical Society. 2012;134(28):11533–11541. doi: 10.1021/ja302213r. [DOI] [PubMed] [Google Scholar]; (b) Mullaney BR, Thompson AL, Beer PD. Angewandte Chemie International Edition. 2014;53(43):11458–11462. doi: 10.1002/anie.201403659. [DOI] [PubMed] [Google Scholar]; (c) Caballero A, Zapata F, White NG, Costa PJ, Félix V, Beer PD. Angewandte Chemie. 2012;124(8):1912–1916. doi: 10.1002/anie.201108404. [DOI] [PubMed] [Google Scholar]; (d) Zapata F, Caballero A, Molina P, Alkorta I, Elguero J. The Journal of Organic Chemistry. 2014;79(15):6959–6969. doi: 10.1021/jo501061z. [DOI] [PubMed] [Google Scholar]; (e) Chudzinski MG, McClary CA, Taylor MS. Journal of the American Chemical Society. 2011;133(27):10559–10567. doi: 10.1021/ja202096f. [DOI] [PubMed] [Google Scholar]; (f) Saccone M, Palacio FF, Cavallo G, Dichiarante V, Virkki M, Terraneo G, Priimagi A, Metrangolo P. Faraday Discussions. 2017;203:407–422. doi: 10.1039/c7fd00120g. [DOI] [PubMed] [Google Scholar]
- 4.(a) Massena CJ, Riel AMS, Neuhaus GF, Decato DA, Berryman OB. Chemical Communications. 2015;51(8):1417–1420. doi: 10.1039/c4cc09242b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Riel AMS, Jessop MJ, Decato DA, Massena CJ, Nascimento VR, Berryman OB. Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials. 2017;73(2):203–209. doi: 10.1107/S2052520617001809. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Riel AMS, Decato DA, Berryman OB. Crystal Growth & Design. 2016;16(2):974–980. doi: 10.1021/acs.cgd.5b01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manuscript submitted. See SI for synthesis details.
- 6.(a) Kaletaş BK, Mandl C, van der Zwan G, Fanti M, Zerbetto F, De Cola L, König B, Williams RM. The Journal of Physical Chemistry A. 2005;109(29):6440–6449. doi: 10.1021/jp051035u. [DOI] [PubMed] [Google Scholar]; (b) Kaletaş BK, Joshi HC, van der Zwan G, Fanti M, Zerbetto F, De Cola L, König B, Williams RM. The Journal of Physical Chemistry A. 2005;109(42):9443–9455. doi: 10.1021/jp054651z. [DOI] [PubMed] [Google Scholar]
- 7.Dash DC. ANALYTICAL CHEMISTRY. Second. PHI Learning Pvt. Ltd; 2017. pp. 384–385. [Google Scholar]
- 8.Xiang Y, Zhao Y, Xu N, Gong S, Ni F, Wu K, Luo J, Xie G, Lu Z, Yang C. Journal of Materials Chemistry C. 2017;5(46):12204–12210. [Google Scholar]
- 9.Lin T, Liu X, Lou Z, Hou Y, Teng F. Journal of Molecular Structure. 2016;1123:49–54. [Google Scholar]
- 10.(a) Mazzoli A, Carlotti B, Bonaccorso C, Fortuna CG, Mazzucato U, Miolo G, Spalletti A. Photochemical & Photobiological Sciences. 2011;10(11):1830–1836. doi: 10.1039/c1pp05214d. [DOI] [PubMed] [Google Scholar]; (b) Carlotti B, Cesaretti A, Fortuna CG, Spalletti A, Elisei F. Physical Chemistry Chemical Physics. 2015;17(3):1877–1882. doi: 10.1039/c4cp04963b. [DOI] [PubMed] [Google Scholar]
- 11.Carlotti B, Consiglio G, Elisei F, Fortuna CG, Mazzucato U, Spalletti A. The Journal of Physical Chemistry A. 2014;118(20):3580–3592. doi: 10.1021/jp407342q. [DOI] [PubMed] [Google Scholar]
- 12.Marini A, Munoz-Losa A, Biancardi A, Mennucci B. The Journal of Physical Chemistry B. 2010;114(51):17128–17135. doi: 10.1021/jp1097487. [DOI] [PubMed] [Google Scholar]
- 13.Riel AMS, Jessop MJ, Decato DA, Massena CJ, Nascimento VR, Berryman OB. Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials. 2017;73(2):203–209. doi: 10.1107/S2052520617001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homocianu M, Airinei A, Dorohoi DO. Journal of Advanced Research in Physics. 2011;2(1):1–9. [Google Scholar]
- 15.(a) Zhao GJ, Han KL. The Journal of Physical Chemistry A. 2009;113(52):14329–14335. doi: 10.1021/jp903200x. [DOI] [PubMed] [Google Scholar]; (b) Shimada H, Nakamura A, Yoshihara T, Tobita S. Photochemical & Photobiological Sciences. 2005;4(4):367–375. doi: 10.1039/b416284f. [DOI] [PubMed] [Google Scholar]
- 16.Ottolenghi M, Goldschmidt CR, Potashnik R. The Journal of Physical Chemistry. 1971;75(8):1025–1031. [Google Scholar]
- 17.Davidson RS, Bonneau R, Joussot-Dubien J, Trethewey KR. Chemical Physics Letters. 1980;74(2):318–320. [Google Scholar]
- 18.Chmyrov A, Sandén T, Widengren J. The Journal of Physical Chemistry B. 2010;114(34):11282–11291. doi: 10.1021/jp103837f. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Mottaleb MSA. Laser Chemistry. 1984;4(1–6):305–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


