Abstract
The placenta adapts to maternal environment and its alterations may have a lasting impact on child's temperament development. Prenatal stress has been linked to both a downregulation of monoamine oxidase A (MAOA) gene expression in the placenta and to difficult temperament. Capitalizing on an ongoing longitudinal study, we analysed data from 95 mother–child dyads to investigate whether MAOA mediates the association between prenatal stress and infant temperament. Prenatal stress was defined as exposure to Superstorm Sandy (Sandy) during pregnancy. Infant temperament was measured by Infant Behaviour Questionnaire-Revised. MAOA gene expression was quantified in placenta tissue. The Smiling and Laughter subscale score was independently associated with Sandy exposure and MAOA placental gene expression. Mediation analysis confirmed that MAOA expression partially mediated the relationship between Sandy and Smiling and Laughter subscale, suggesting that in utero exposure to Sandy could induce lower frequency of smiling and laughter via downregulation of placental MAOA gene expression. These effects could compromise optimal temperamental trajectory and contribute to risk for psychological problems. Placental epigenetic markers can contribute to a multidimensional model of early intervention for high-risk children.
Keywords: epigenetic impact, MAOA, prenatal stress, temperament
1 | INTRODUCTION
Temperament is defined as an early appearing pattern of individual differences in emotional, motor, and attentional reactivity and regulation in response to stimulation (Rothbart & Ahadi, 1994). Aspects of difficult temperament including high reactivity, negative emotionality, and withdrawal have been associated with a range of psychological disorders, including mood and anxiety disorders, disruptive behaviours, and attention deficit hyperactivity disorder later in childhood (for review, see Muris & Ollendick, 2005). Because temperament is embedded in the biological makeup, but also somewhat malleable to environmental influences (Saudino, 2005), research with an eye for such influences can facilitate intervention for children at risk for psychopathology.
Stress is a potent environmental insult, particularly during critical in utero development. Not surprisingly, studies have documented the link between prenatal stress and problematic temperament in infants and psychological problems in later childhood. Infants of mothers who experienced stress during pregnancy were found to have greater negative reactivity, problems adapting to new situations, and difficulties regulating their attention (Buitelaar, Huizink, Mulder, Robles De Medina, & Visser, 2003; Davis et al., 2007), and children who were exposed to stress in utero were more likely to exhibit mood and behavioural problems during childhood (Davis & Sandman, 2012; Van Den Bergh & Marcoen, 2004).
Exposure to stress in utero is mediated by the placenta (Bronson & Bale, 2015), an organ labelled as the “third brain,” which links the developed brain of the mother with that of the developing foetus (Yen, 1994). The placenta is primarily a foetus organ, shaped by the maternal milieu by tailoring its functions in response to environmental cues. It creates an internal ecosystem charged with preparing the growing foetus for the demands of the world outside the utero. If there is a mismatch between in utero and external environment, the child may be embarking upon a maladaptive developmental trajectory (Glover, 2011). The placenta can therefore provide vital information about early environmental influence on the early childhood characteristics. The placental adaptations to stress during foetal development may employ an epigenetic mechanism, which influences placental gene expression without changing the gene sequence (Lesseur, Paquette, & Marsit, 2014).
Such placental alteration has been noted for the expression of placental monoamine oxidase A (MAOA) gene in association with prenatal stress; however, child outcomes were not assessed. Blakeley, Capron, Jensen, O'Donnell, and Glover (2013) reported that prenatal depression and anxiety were associated with downregulation of MAOA in human placentas. MAOA is an enzyme responsible for the breakdown of three major neurotransmitters: serotonin, dopamine, and norepinephrine, and MAOA gene has been implicated in a range of disorders including drug abuse (Vanyukov et al., 2004), anxiety (Tadic et al., 2003), attention deficit hyperactivity disorder (Jiang et al., 2001), anorexia nervosa (Urwin & Nunn, 2005), and antisocial behaviour (Samochowiec et al., 1999). Moreover, a deficiency of MAOA enzyme activity due to a point mutation of the MAOA gene has been correlated with heightened levels of aggression (Brunner, Nelen, Breakefield, Ropers, & van Oost, 1993).
The fact that MAOA gene (along with neurotransmitter transporter genes) is expressed in the placenta indicates its involvement in the regulation of these key neurotransmitters in foetal circulation. Aberrations of its expression in the placenta may lead to altered foetal neurodevelopment (Zhang, Smith, Liu, & Holden, 2010). To date, however, no study has examined the association between prenatal stress and child outcomes, such as temperament, as mediated by expression of the MAOA gene in the placenta.
To achieve this aim, this study evaluates the interrelationship between prenatal stress, placental MAOA gene expression, and child temperament at 12 months of age in a sample drawn from an ongoing longitudinal study of mothers and their children, utilizing stored placenta tissues. Prenatal stress is defined as exposure to a natural disaster, Superstorm Sandy (Sandy), which devastated the New York City area in late 2012, and affected participants from an ongoing cohort study. As dysregulation of placental gene functioning may compromise the optimal neurodevelopment of children, a greater understanding of this biological mechanism is of critical importance for early identification of risk and intervention.
2 | METHOD
2.1 | Participants
The sample consisted of participants (N = 95) from a large ongoing longitudinal study for whom placental gene expression and infant temperament data were available. Participants were recruited from prenatal obstetrics and gynaecological clinics in metropolitan New York during the second trimester of pregnancy and at that time their demographic information (shown in Table 1) was collected. In short, approximately 7% of mothers identified as Asian, 27% as Black or African American, 52% as Hispanic, 8% as White, and 5% selected “Other.” Fifty-five per cent of the children are female; 56% of mothers identified as single. The age of mothers ranged from 17 to 44 (M = 26.91, SD = 5.87). The complete study sample, from which current participants were drawn, is described elsewhere (Finik & Nomura, 2017).
TABLE 1.
Demographic characteristics of mothers and children in the current study (N = 95)
| Variable | Total (N = 95) M (SD) | Sandy (N = 43) M (SD) | No Sandy (N = 52) M (SD) | p value |
|---|---|---|---|---|
| Age of mother | 26.89 (5.86) | 28.07 (6.01) | 25.95 (5.62) | .08 |
|
| ||||
| Parity | 2.89 (2.02) | 2.72 (1.83) | 3.04 (2.17) | .90 |
| EPDSa | 4.27 (5.46) | 4.46 (6.35) | 4.11 (4.66) | .77 |
|
| ||||
| N (%) | N (%) | N (%) | ||
|
| ||||
| Race | .14 | |||
| Asian | 7 (7.4) | 6 (14) | 1 (1.9) | |
| Black | 26 (27.4) | 9 (20.9) | 17 (32.7) | |
| Hispanic | 49 (51.6) | 21 (48.8) | 28 (53.8) | |
| White | 8 (8.4) | 5 (11.6) | 3 (5.8) | |
| Other | 5 (5.3) | 2 (4.7) | 3 (5.8) | |
|
| ||||
| Marital status | <.001 | |||
| Married | 36 (37.9) | 26 (60.5) | 10 (19.2) | |
| Common law | 6 (6.3) | 5 (11.6) | 1 (1.9) | |
| Single | 53 (55.8) | 12 (27.9) | 41 (78.8) | |
|
| ||||
| Education | <.001 | |||
| Some high school | 17 (17.9) | 3 (7) | 14 (26.9) | |
| High school /GED diploma | 20 (21.1) | 6 (14) | 14 (26.9) | |
| Some college | 29 (30.5) | 11 (25.6) | 18 (34.6) | |
| Associate's | 11 (11.6) | 6 (14) | 5 (9.6) | |
| Bachelor | 10 (10.5) | 9 (20.9) | 1 (1.9) | |
| Graduate | 8 (8.4) | 8 (18.6) | 0 | |
|
| ||||
| Sex of child | .15 | |||
| Female | 52 (54.7) | 20 (46.5) | 32 (61.5) | |
| Male | 43 (45.3) | 23 (53.5) | 20 (38.5) | |
Note. EPDS = Edinburgh Postnatal Depression Scale; GED = General Educational Development.
Assessed at 6 months postpartum.
Participants were excluded if they were positive for human immunodeficiency virus infection, maternal psychosis, maternal age < 15 years, life-threatening medical complications related to the mother, and congenital or chromosomal abnormalities in the foetus. They were also excluded if they indicated plans to relocate out of the geographic area during the study. All participants were consented as per protocol approved by the Institutional Review Boards at the City University of New York, New York Presbyterian-Queens, and Icahn School of Medicine at Mount Sinai.
3 | PROCEDURE
3.1 | Placenta collection and RNA extraction
Placenta was collected at the time of delivery. A detailed description of the placenta collection can be found elsewhere (Lambertini, Chen, & Nomura, 2015). In short, biopsies, free of maternal decidua, were collected from each placenta quadrant midway between the cord insertion and the placenta rim within an hour of delivery to maintain the optimal integrity of RNA. The collected tissues were first placed into a liquid nitrogen tank for 24 hr for “snap freezing” and then transferred to a −80 °C freezer. RNA was extracted from placenta samples using the Maxwell simplyRNA Tissue Kit (Promega, #AS1280), following the manufacturer's protocol. Extracted RNA was quantified using a Nanodrop spectrophotometer (Thermo Fisher Scientific Inc., #ND-2000) and stored at a −80 °C freezer until use.
3.2 | Gene expression profiling
Placental RNA was profiled using a custom-designed code set (NanoString Technologies, Seattle, WA). Briefly, 100 ng RNA was incubated in the presence of reporter and capture probes overnight at 65 °C. Following hybridization, unbound probes were removed, and the purified complexes were aligned and immobilized on imaging cartridges using an nCounter Prep Station II. Cartridges were then sealed and scanned in an nCounter Digital Analyser for code count detection. The NanoStringNorm package (Waggott et al., 2012) was used to normalize nCounter data. Code count data were first normalized against the geometric mean of spike-in controls to account for differences in hybridization and recovery. Differences in sample content were accounted for by normalizing the data against the geometric mean of the housekeeping genes GAPDH, RPL19, and RPLP0. The background threshold of detection was set at 2 standard deviations above the mean of the included negative controls. Expression below background threshold was set to the value of the limit of detection divided by the square root of 2 to maintain sample variability.
4 | MEASURES
4.1 | Superstorm Sandy exposure
Forty-three out of 95 (45.3%) mothers were exposed to Sandy while they were pregnant: 33 in the first trimester, 7 in the second trimester, and 3 in the third trimester. In the control group, eight mothers became pregnant after Sandy, and 44 mothers gave birth before Sandy. Sandy exposure status (i.e., in utero exposure) was coded as a dichotomous variable (Yes or No) based on the date of delivery.
4.2 | Infant temperament
Mothers completed the Infant Behaviour Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003) at 12 months (M = 12.93 months of age, SD = 1.79) post-partum. IBQ-R is composed of 91 questions about the frequency of specific child behaviours (e.g., How often did your baby smile or laugh when given a toy this week?), which comprise 14 subscales of temperament. They are Activity, Cuddliness, Fear, Sadness, High Intensity Pleasure (enjoyment from high stimulus intensity), Low Intensity Pleasure (enjoyment from low stimulus intensity), Approach (positive anticipation), Smiling and Laughter (during caretaking and play), Falling Reactivity (rate of recovery from distress), Duration of Orientation (attentional focusing), Perceptual Sensitivity, Distress to Limitations (frustration), Vocal Reactivity, and Soothability (Gartstein & Rothbart, 2003). The mothers reported weekly behaviour frequency on a scale ranging from 1 (never) to 7 (always), with an option to indicate the behaviour was not observed. The IBQ-R is a widely used measure with high internal consistency and good interrater reliability among relatives of the infant (Gartstein & Rothbart, 2003).
4.3 | Maternal depression
Maternal depression symptomatology at 6 months post-partum was measured by the Edinburgh Postnatal Depression Scale (EPDS; Murray & Carothers, 1990). The mothers were asked to report how they had been feeling in the past 7 days on a 4-point Likert scale with a range from “yes, all the time” to “no, not at all.” Some questions were reverse coded, and the sum score constituted the “maternal depression” score. The inventory is well-validated in different languages and has an acceptable reliability ranging from .79 to .86 with satisfactory sensitivity (79%) and specificity (85%) (Kheirabadi, Maracy, Akbaripour, & Masaeli, 2012).
4.4 | Statistics
First, a series of Pearson correlations were performed among 14 IBQ-R subscales of temperament and MAOA gene expression levels. Data were tested for normality, and all variables approximated the normal distribution.
Second, an independent samples t-test was conducted to compare 14 subscales of temperament and MAOA gene expression between children whose mothers were and were not exposed to Sandy during pregnancy.
Third, the demographic characteristics between the two groups were compared using independent samples t-tests and Fisher's exact tests (see Table 1). If the difference was significant, the relationship of the demographic characteristic with MAOA gene expression and notable temperament subscale scores was tested by an independent samples t-test or a one-way analysis of variance (ANOVA); covariation was assessed if either relationship was significant. Marital status was recoded into a dichotomous variable (single vs. married or common law), and education was recoded into a trichotomous variable (some high school education; high school/General Educational Development, some college or associate's degree; and college or graduate degree).
Fourth, an independent samples t-test was conducted to determine whether post-partum maternal depression scores (as measured by EPDS) differed between the two comparison groups. It was followed by a simple regression to determine whether post-partum depression predicted MAOA expression and notable IBQ-R subscale scores.
Additionally, gender differences in MAOA expression and notable temperament subscales were explored between comparison groups.
Fifth, the initial univariate analyses were followed by a mediation analyses to test whether MAOA gene expression mediated the association between Sandy exposure during pregnancy (i.e., in utero exposure) and infant temperament at 12 months of age. The PROCESS macro for SPSS with 10,000 bootstrapping resampling (Hayes, 2015) was used to evaluate the potential mediation effects of MAOA in the relationship between Sandy exposure as predictor variable and subscales of infant temperament of IBQ-R as outcome variables.
5 | RESULTS
5.1 | Pearson correlations
MAOA gene expression had the largest and strongest association with Smiling and Laughter subscale scores (r = .29, p = .004). MAOA expression was also positively associated with Low Intensity Pleasure (r = .23, p = .02) and High Intensity Pleasure (r = .24, p = .02) subscale scores and negatively associated with Activity (r = −.21, p = .04) subscales scores. The complete correlation matrix is shown in Table 2.
TABLE 2.
Correlations among the 14 IBQ-R subscales with MAOA
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MAOA | 1 | ||||||||||||||
| 2. Activity | −.21* | 1 | |||||||||||||
| 3. Distress to Limitations | −.06 | .41** | 1 | ||||||||||||
| 4. Fear | .06 | .33** | .45** | 1 | |||||||||||
| 5. Duration of Orienting | .11 | .28** | .18 | .33** | 1 | ||||||||||
| 6. Low Pleasure | .23* | .14 | −.17 | −.008 | .45** | 1 | |||||||||
| 7. Soothability | −.02 | −.12 | −.10 | −.12 | .07 | .05 | 1 | ||||||||
| 8. Falling Reactivity | .08 | −.01 | −.24* | −.14 | .30** | .42** | .39** | 1 | |||||||
| 9. Cuddliness | .14 | −.39** | −.24* | −.16 | .06 | .13 | .37** | .12 | 1 | ||||||
| 10. Perceptual Sensitivity | .20 | .21* | −.03 | .11 | .46** | .42** | .19 | .31** | .09 | 1 | |||||
| 11. Sadness | −.14 | .48** | .66** | .43** | .31** | −.02 | −.27** | −.16 | −.40** | .06 | 1 | ||||
| 12. Approach | .17 | .13 | .09 | .02 | .28** | .29** | .45** | .41** | .14 | .50** | .04 | 1 | |||
| 13. Vocal Reactivity | .10 | .32** | .06 | .22* | .52** | .48** | .28** | .50** | .08 | .45** | .12 | .56** | 1 | ||
| 14. Smile/Laughter | .29** | .06 | −.07 | .19 | .45** | .62** | .16 | .46** | .14 | .40** | −.08 | .45** | .64** | 1 | |
| 15. High Pleasure | .24* | .04 | −.11 | .007 | .30** | .53** | .25* | .43** | .23* | .41** | −.20 | .46** | .58** | .70** | 1 |
| M | 11.35 | 4.21 | 4.29 | 3.95 | 4.79 | 5.58 | 5.24 | 4.96 | 5.21 | 5.17 | 3.53 | 5.92 | 5.68 | 5.88 | 6.46 |
| SD | 1.04 | 1.07 | 1.19 | 1.26 | 1.27 | 1.01 | 0.98 | 1.04 | 1.12 | 1.30 | 1.24 | 0.88 | 0.95 | 0.92 | 0.62 |
Note. IBQ-R = Infant Behaviour Questionnaire-Revised; MAOA = monoamine oxidase A.
p < .05 level.
p < .01 level.
5.2 | Independent samples t-tests and one-way ANOVAs
Children whose mothers were exposed to Sandy (M = 5.59, SD = 1.06) compared with those who were not (M = 6.12, SD = 0.70) had lower Smiling and Laughter subscale scores, t(93) = 2.96, p = .004. There were no notable differences in scores on other temperament subscales by Sandy exposure status (see Table 3). Placental MAOA expression of mothers exposed to Sandy was lower (M = 11.06, SD = 1.11), compared with those who were not exposed (M = 11.59, SD = 0.93), t(93) = −2.55, p = .01.
TABLE 3.
Comparisons of the IBQ-R subscales between the two exposure groups
| IBQ-R subscale | Sandy (N = 43) M (SD) | No Sandy (N = 52) M (SD) | p value |
|---|---|---|---|
| Activity | 4.39 (0.90) | 4.07 (1.19) | .14 |
| Distress to Limitations | 4.49 (0.92) | 4.13 (1.36) | .13 |
| Fear | 3.73 (1.10) | 4.13 (1.35) | .12 |
| Duration of Orienting | 4.52 (1.28) | 5.01 (1.23) | .06 |
| Low Pleasure | 5.50 (1.00) | 5.64 (1.02) | .50 |
| Soothability | 5.26 (0.86) | 5.22 (1.07) | .85 |
| Falling Reactivity | 4.94 (0.99) | 4.97 (1.09) | .89 |
| Cuddliness | 5.12 (0.99) | 5.29 (1.23) | .49 |
| Perceptual Sensitivity | 5.00 (1.32) | 5.30 (1.29) | .26 |
| Sadness | 3.60 (1.17) | 3.47 (1.30) | .59 |
| Approach | 5.91 (0.84) | 5.93 (0.92) | .93 |
| Vocal Reactivity | 5.51 (1.00) | 5.82 (0.90) | .12 |
| Smile/Laughter | 5.59 (1.06) | 6.12 (0.70) | .004 |
| High Pleasure | 6.39 (0.61) | 6.52 (0.63) | .29 |
Note. Significant differences are in bold.
IBQ-R = Infant Behaviour Questionnaire-Revised.
The single mothers (M = 6.11, SD = 0.73) reported higher Smiling and Laughter scores than common law or married mothers (M = 5.60, SD = 1.05), t(93) = 2.79, p = .006. There were no significant differences in the MAOA expression, t(93) = 1.35, p = .18, between marital status groups. One-way ANOVA with Welch correction for unequal variances revealed that the three education groups differed in Smiling and Laughter scores, F(2, 92) = 8.56, p < .001. Games–Howell post hoc comparisons showed that children of mothers with highest education had significantly lower Smiling and Laughter scores (M = 5.15, SD = 1.32) than children of mothers with less than high school degree (M = 6.18, SD = 0.67, p = .02) and children of mothers with high school or associate's degree, or some college education (M = 6.02, SD = 0.71, p = .04). There were no significant differences in the MAOA expression among the three education groups, F(2, 92) = 1.35, p = .26.
The maternal postnatal depression symptomatology, as measured by the EPDS, was associated with neither MAOA expression (p = .50) nor Sandy exposure status (p = .77), nor Smiling and Laughter subscale score (p = .82). Therefore, it was not evaluated as a covariate in further analyses.
There were no gender differences in scores on Smiling and Laughter, t(93) = .24, p = .81, and in MAOA expression, t(93) = −1.29, p = .20.
5.3 | Mediation analyses
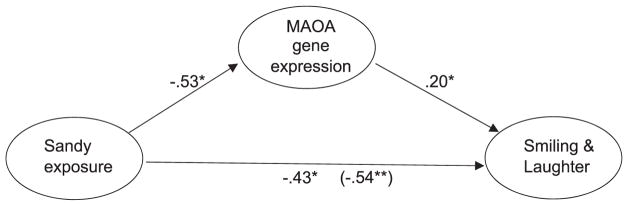
Evidence for mediation between in utero Sandy exposure and scores on temperament subscales via MAOA gene expression was tested. First, Sandy exposure in utero was a significant predictor for scores on Smiling and Laughter subscale scores (β = −.54, p = .004). Second, Sandy exposure significantly predicted MAOA gene expression (β = −.53, p = .01). Third, MAOA gene expression was significantly linked to scores on Smiling and Laughter subscale scores (β = .20, p = .02). The effect of Sandy exposure on Smiling and Laughter scores remained significant after controlling for MAOA expression (β = −.43, p = .02). Together, these findings show that the relationship between Sandy and Smiling and Laughter subscale scores was reduced when MAOA gene expression was introduced as the mediator in the model. Mediation analysis using bootstrapping procedure confirmed that MAOA expression mediated the relationship between Sandy exposure and reduced scores on Smiling and Laughter subscale scores (β = −.11, 95% confidence interval [CI; – .37, – .003], p < .05, 20.4%). This mediation model is illustrated in Figure 1. MAOA gene expression was not found to be mediating the relationship between Sandy and other significantly correlated temperament subscales (Activity, Low Pleasure, and High Pleasure Intensity).
FIGURE 1.
Effects of Superstorm Sandy and monoamine oxidase A (MAOA) gene expression on Smiling and Laughter temperament subscale score with total effects shown in parentheses (N = 95). Note. *p < .05. **p < .01
Because the comparison groups significantly differed on marital status (p < .001) and education (p < .001), and the marital status and education groups differed on Smiling and Laughter scores (ps < .04), they were included in the mediation model as covariates, and their effects were adjusted for statistically.
When mediation effects were tested in three separate adjusted mediation models with (a) marital status as a covariate, (b) education as a covariate, and (c) both education and marital status as covariates, we found that MAOA expression partially mediated the relationship between Sandy exposure and reduced Smiling and Laughter subscale scores after adjusting for the effect of marital status (β = −.11, 95% CI [−.36, −.0008], p = .05), but the model became only marginally significant after adjusting for education (β = −.09, 95% CI [−.34, +.002], p = .06) and after adjusting for both marital status and education (β = −.09, 95% CI [−.35, +.003], p = .06).
6 | DISCUSSION
The current study has three major findings. First, out of 14 subscales of infant temperament at 12 months, Smiling and Laughter scores were independently associated with both in utero Sandy exposure status and the MAOA gene expression. Second, when Sandy exposure and MAOA expression status were examined simultaneously, both Sandy exposure status and the MAOA expression were associated with Smiling and Laughter scores. Finally, the effect of Sandy exposure on decreased Smiling and Laughter scores was reduced (20.4%) when analysed together with the MAOA expression; however, the initial association remained significant, suggesting a partial mediation. Overall, our results suggest that in utero exposure to Sandy could induce lower rate of smiling and laughter via a biological mechanism of downregulation of placental MAOA gene expression.
Research has consistently documented links between prenatal stress and difficulties in child temperament, including greater distress to novelty, higher negative emotional reactivity, and more behaviour problems (Blair, Glynn, Sandman, & Davis, 2011; Davis et al., 2004; Gutteling et al., 2005; Nolvi et al., 2016). The placenta, as the interface between the mother and the foetus, may be responsible for mediating offspring's neurodevelopment in reaction to the environment through epigenetic mechanisms such as DNA methylation, histone modification, genomic imprinting, and non-coding RNA expression without affecting the gene sequence (Lesseur et al., 2014). The finding from our study suggests that prenatal stress could shape temperamental development epigenetically through alteration of placental MAOA gene expression.
The dysregulation of neurotransmitters serotonin, norepinephrine, and dopamine is frequently linked with mood, anxiety, neurodevelopmental disorders, and stress exposure. In the placenta, they are largely regulated by MAO enzymes (Bronson & Bale, 2015); therefore, lower MAOA expression in the placenta may increase foetal exposure to these neurotransmitters and disrupt finely tuned typical brain development, signalling the beginning of a maladaptive trajectory. For example, prenatal exposure to selective serotonin reuptake inhibitors, pharmacological treatment for mood disorders aimed at increasing serotonin levels, has been associated with internalizing and externalizing behaviours in childhood (see review in Oberlander, Gingrich, & Ansorge, 2009).
Smiling and laughter have been recognized as significant emotional and cognitive markers of infant development (Sroufe & Wunsch, 1972). Infants communicate through smiling and laughing before acquiring language skills (Harris, 2004; Mireault et al., 2015), to express their comfort to a caretaker (Rothbart, 1973), with great implications for social development. Additionally, smiling and laughing have been recognized as important identifiers in temperament studies. A study of temperament examined by IBQ-R of infants at low and high risk for autism found lower frequencies of smiling and laughing reported for high-risk infants, as compared with low-risk infants (Clifford, Hudry, Elsabbagh, Charman, & Johnson, 2013). Kagan (1997) grouped infants in his study sample according to their temperamental activity and distress level (either low or high reactivity) in response to a demonstration of a novel stimuli. At the 4-year follow-up assessment, smiling successfully identified children who were high and low in reactivity as infants, with low reactive children smiling more than high reactive children. Furthermore, smiling has been used as an indicator of developmental risk and disability and as a predictor of social competence (Benson & Haith, 2009). Therefore, lower reported smiling and laughter in our study may signify important temperamental difficulties.
There are several notable limitations in our study. First, infant temperament was assessed via maternal report. Although, substantial evidence shows parent report to be predictive of child behaviour in the laboratory (Rothbart & Bates, 2006), multiple informants or an independent observation of child's temperament during a laboratory visit could have strengthened our findings. Second, we selected the present sample from participants who had reported their child's temperament at 12 months post-partum and for whom placental tissues were available. Although our findings suggest new underlying biological mechanism, a larger sample could have further corroborated our results. Third, the mothers with lowest education attainment and single mothers reported higher smiling and laughing of their children. In our sample, the mothers exposed to Sandy had higher levels of education as compared with mothers without in utero exposure, and it is not possible to disentangle Sandy exposure and education effects on temperament in the current study. Further, when we examined the model with maternal education as a covariate, the association became only marginally significant (p = .06). Thus, when interpreting the results, the readers should consider a possibility that unmeasured factors associated with maternal education could have confounded the observed outcomes. Lastly, we can only speculate the stability of the MAOA gene expression.
Sex-specific stress pathways have been reported in placental epigenetic studies (Bale, 2011), as have interaction between MAOA gene and environment by gender (Enoch, Steer, Newman, Gibson, & Goldman, 2010). Though we did not find gender differences in the current study of 12-month-olds, we may detect them later in neurodevelopment as our participants mature.
The effect of prenatal stress is a multifaceted interaction of foetal genetic background, sex, maternal hormonal levels, and gestational age at the time of exposure (Bale, 2011), which our study was able to partially capture. Research has shown links between trimester-specific stress exposure and psychopathology in offspring (Beversdorf et al., 2005; Khashan et al., 2008); however, little is still known about trimester-specific stress effects on gene expression. The relatively small sample size did not allow for a systematic investigation of these differences in the current study. The majority of the Sandy-exposed mothers (77%) were in the first trimester; therefore, our results may be skewed towards the first trimester exposure.
As prenatal stress may be subjective and is rather intangible, we defined it as an objective exposure to Sandy (Yes or No). Including an account of a degree of hardship experienced by the mothers could delineate dose effects of disaster exposure and is a promising aim for future research.
Nonetheless, our findings suggest that stress experienced in utero could shape the temperamental development epigenetically through alteration of MAOA gene expression. As such, placental epigenetic markers are hopeful future screeners to identify children at risk for a suboptimal development that may be aided by early intervention services.
Highlights.
The study examined whether placental MAOA gene expression operates as a biological mediator in the relationship between pre-natal stress and infant temperament.
Mediation analyses revealed a partial mediation between prenatal stress and Smiling and Laughter subscale.
Stress in utero could change MAOA gene expression epigenetically, which could shape temperament development. Such placental gene expressions may become useful biomarkers for screening and intervention.
Acknowledgments
FUNDING
This work is supported by grants K01-080062, K01-080062S and R01-102729 from the National Institutes of Mental Health (NIMH), and PSC-CUNY, Queens College Research Enhancement Grant to the PI, Nomura.
We thank all the parents and children who consented to participate in this study. We also thank current and former research staff and assistants at Queens College, City University of New York for their contributions to this study. Gene expression assays were conducted in Chen's Laboratory at Icahn School of Medicine at Mount Sinai. We thank Qian Li, PhD, Yula Ma, MD, and Jia Chen, ScD for gene expression assays and analysis.
Funding information
National Institute of Mental Health, Grant/Award Number: K01 MH080062K01 MH080062SR01 MH102729; Queens College, CUNY, Grant/Award Number: Research Enhancement Grant
References
- Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14(4):348–356. doi: 10.3109/10253890.2011.586447. https://doi.org/10.3109/10253890.2011.586447 [DOI] [PubMed] [Google Scholar]
- Benson JB, Haith MM, editors. Social and emotional development in infancy and early childhood. Academic Press; 2009. pp. 418–419. [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, … Bauman ML. Timing of prenatal stressors and autism. Journal of Autism and Developmental Disorders. 2005;35(4):471–478. doi: 10.1007/s10803-005-5037-8. https://doi.org/10.1007/s10803-005-5037-8 [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14(6):644–651. doi: 10.3109/10253890.2011.594121. https://doi.org/10.3109/10253890.2011.594121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley PM, Capron LE, Jensen AB, O'Donnell KJ, Glover V. Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. Journal of Psychosomatic Research. 2013;75(4):341–345. doi: 10.1016/j.jpsychores.2013.07.002. https://doi.org/10.1016/j.jpsychores.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology Reviews. 2015;41:207–218. doi: 10.1038/npp.2015.231. https://doi.org/10.1038/npp.2015.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield X, Ropers HH, van Oost B. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262(5133):578–580. doi: 10.1126/science.8211186. https://doi.org/10.1126/science.8211186 [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, Robles De Medina PG, Visser GHA. Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging. 2003;24:S53–S60. doi: 10.1016/s0197-4580(03)00050-2. https://doi.org/10.1016/S0197-4580(03)00050-2 [DOI] [PubMed] [Google Scholar]
- Clifford SM, Hudry K, Elsabbagh M, Charman T, Johnson MH. Temperament in the first 2 years of life in infants at high-risk for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(3):673–686. doi: 10.1007/s10803-012-1612-y. https://doi.org/10.1007/s10803-012-1612-y [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. https://doi.org/10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. https://doi.org/10.1016/j.psyneuen.2011.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6(3):319–331. https://doi.org/10.1207/s15327078in0603_1 [Google Scholar]
- Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes, Brain and Behavior. 2010;9(1):65–74. doi: 10.1111/j.1601-183X.2009.00535.x. https://doi.org/10.1111/j.1601-183X.2009.00535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finik J, Nomura Y. Cohort profile: Stress in Pregnancy (SIP) study. International Journal of Epidemiology. 2017;46(5):1388–1388k. doi: 10.1093/ije/dyw264. https://doi.org/10.1093/ije/dyw264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior & Development. 2003;26(1):64–86. https://doi.org/10.1016/S0163-6383(02)00169-8 [Google Scholar]
- Glover V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(4):356–367. doi: 10.1111/j.1469-7610.2011.02371.x. https://doi.org/10.1111/j.1469-7610.2011.02371.x [DOI] [PubMed] [Google Scholar]
- Gutteling BM, De Weerth C, Willemsen-Swinkels SHN, Huizink AC, Mulder EJH, Visser GHA, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. European Child and Adolescent Psychiatry. 2005;14(1):41–51. doi: 10.1007/s00787-005-0435-1. https://doi.org/10.1007/s00787-005-0435-1 [DOI] [PubMed] [Google Scholar]
- Harris M. First words. In: Oats J, Grayson A, editors. Cognitive and language development in children. Malden, UK: Blackwell; 2004. pp. 61–112. [Google Scholar]
- Hayes AF. An index and test of linear moderated mediation. Multivariate Behavioral Research. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. https://doi.org/10.1080/00273171.2014.962683 [DOI] [PubMed] [Google Scholar]
- Jiang S, Xin R, Lin S, Qian Y, Tang G, Wang D, Wu X. Linkage studies between attention-deficit hyperactivity disorder and the monoamine oxidase genes. American Journal of Medical Genetics. 2001;105(8):783–788. doi: 10.1002/ajmg.10098. https://doi.org/10.1002/ajmg.10098 [DOI] [PubMed] [Google Scholar]
- Kagan J. Temperament and the reactions to unfamiliarity. Child Development. 1997;68(1):139–143. [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, … Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry. 2008;65(2):146–152. doi: 10.1001/archgenpsychiatry.2007.20. https://doi.org/10.1001/archgenpsychiatry.2007.20 [DOI] [PubMed] [Google Scholar]
- Kheirabadi GR, Maracy MR, Akbaripour S, Masaeli N. Psychometric properties and diagnostic accuracy of the Edinburgh Postnatal Depression Scale in a sample of Iranian women. Iranian Journal of Medical Sciences. 2012;37(1):32–38. [PMC free article] [PubMed] [Google Scholar]
- Lambertini L, Chen J, Nomura Y. Mitochondrial gene expression profiles are associated with maternal psychosocial stress in pregnancy and infant temperament. PLoS One. 2015;10(9):1–21. doi: 10.1371/journal.pone.0138929. https://doi.org/10.1371/journal.pone.0138929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesseur C, Paquette AG, Marsit CJ. Epigenetic regulation of infant neurobehavioral outcomes. Medical Epigenetics. 2014;2:71–79. doi: 10.1159/000361026. https://doi.org/10.1159/000361026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireault GC, Crockenberg SC, Sparrow JE, Cousineau K, Pettinato C, Woodard K. Laughing matters: Infant humor in the context of parental affect. Journal of Experimental Child Psychology. 2015;136:30–41. doi: 10.1016/j.jecp.2015.03.012. https://doi.org/10.1016/j.jecp.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Ollendick TH. The role of temperament in the etiology of child psychopathology. Clinical Child and Family Psychology Review. 2005;8:271–289. doi: 10.1007/s10567-005-8809-y. https://doi.org/10.1007/s10567-005-8809-y [DOI] [PubMed] [Google Scholar]
- Murray L, Carothers AD. The validation of the Edinburgh Post-Natal Depression Scale on a community sample. The British Journal of Psychiatry. 1990;157(2):288–290. doi: 10.1192/bjp.157.2.288. [DOI] [PubMed] [Google Scholar]
- Nolvi S, Karlsson L, Bridgett DJ, Korja R, Huizink AC, Kataja E-L, Karlsson H. Maternal prenatal stress and infant emotional reactivity six months postpartum. Journal of Affective Disorders. 2016;199:163–170. doi: 10.1016/j.jad.2016.04.020. https://doi.org/10.1016/j.jad.2016.04.020 [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: Molecular to clinical evidence. Clinical Pharmacology and Therapeutics. 2009;86(6):672–677. doi: 10.1038/clpt.2009.201. https://doi.org/10.1038/clpt.2009.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK. Laughter in young children. Psychological Bulletin. 1973;80(3):247–256. doi: 10.1037/h0034846. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA. Temperament and the development of personality. Journal of Abnormal Psychology. 1994;103(1):55–66. doi: 10.1037//0021-843x.103.1.55. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament in children's development. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology, Vol. III, Social, emotional and personality development. Hoboken, NJ: Wiley; 2006. pp. 96–166. [Google Scholar]
- Samochowiec J, Lesch KP, Rottmann M, Smolka M, Syagailo YV, Okladnova O, … Sander T. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Research. 1999;86(1):67–72. doi: 10.1016/s0165-1781(99)00020-7. https://doi.org/10.1016/S0165-1781(99)00020-7 [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. Journal of Developmental and Behavioral Pediatrics. 2005;26(3):214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA, Wunsch J. The development of laughter in the first year of life. Child Development. 1972;43(4):1326–1344. [PubMed] [Google Scholar]
- Tadic A, Rujescu D, Szegedi A, Giegling I, Singer P, Möller H-J, Dahmen N. Association of a MAOA gene variant with generalized anxiety disorder, but not with panic disorder or major depression. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2003;117B:1–6. doi: 10.1002/ajmg.b.10013. https://doi.org/10.1002/ajmg.b.10013 [DOI] [PubMed] [Google Scholar]
- Urwin RE, Nunn KP. Epistatic interaction between the monoamine oxidase A and serotonin transporter genes in anorexia nervosa. European Journal of Human Genetics. 2005;13:370–375. doi: 10.1038/sj.ejhg.5201328. https://doi.org/10.1038/sj.ejhg.5201328 [DOI] [PubMed] [Google Scholar]
- Van Den Bergh BRH, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8-and 9-year-olds. Child Development. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Maher BS, Devlin B, Tarter RE, Kirillova GP, Yu LM, Ferrell RE. Haplotypes of the monoamine oxidase genes and the risk for substance use disorders. American Journal of Medical Genetics. 2004;125B:120–125. doi: 10.1002/ajmg.b.20105. [DOI] [PubMed] [Google Scholar]
- Waggott D, Chu K, Yin S, Wouters BG, Liu F-F, Boutros PC. NanoStringNorm: An extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics. 2012;28(11):1546–1548. doi: 10.1093/bioinformatics/bts188. https://doi.org/10.1093/bioinformatics/bts188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SS. The placenta as the third brain. The Journal of Reproductive Medicine. 1994;39(4):277–280. [PubMed] [Google Scholar]
- Zhang H, Smith GN, Liu X, Holden JJa. Association of MAOA, 5-HTT, and NET promoter polymorphisms with gene expression and protein activity in human placentas. Physiological Genomics. 2010;42(1):85–92. doi: 10.1152/physiolgenomics.00220.2009. https://doi.org/10.1152/physiolgenomics.00220.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]