Abstract
Rationale:
While the health effects of beta-carotene have been studied extensively, a systematic examination of serum concentrations and long-term mortality, including cardiovascular disease (CVD) mortality, has not been reported.
Objective:
Explore whether serum beta-carotene is associated with overall and cause-specific mortality, and to elucidate the strength and dose-response of the association.
Methods and Results:
We conducted a prospective serologic analysis of 29,103 men in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. During 31 years of follow-up, 23,796 deaths occurred, including deaths due to CVD (9,869), cancer (7,692), respiratory disease (2,161), diabetes (119), injuries and accidents (1,255), and other causes (2,700). Serum beta-carotene was assayed using high-performance liquid chromatography. Adjusting for major risk factors measured, men with higher serum beta-carotene had significantly lower all-cause mortality (hazard ratios (HR)=0.81, 0.71, 0.69 and 0.64 for quintile 2 (Q2)-Q5 versus Q1, respectively; Ptrend<0.0001). Serum beta-carotene was significantly associated with risk of death from CVD, heart disease, stroke, cancer, respiratory disease, diabetes, injuries and accidents, and other causes (Q5 versus Q1, HR=0.21–0.73, all Ptrend<0.0001). The all-cause mortality association was not materially impacted by adjustment for fruit and vegetable consumption (albeit, estimated with some measurement error), and was generally similar across subgroups of smoking intensity, alcohol consumption, trial supplementation, and duration of follow-up, but was significantly modified by age, years of smoking, and body mass index (BMI), with stronger inverse associations among men who were younger, smoked fewer years, and had lower BMI (all Pinteraction≤0.0025).
Conclusions:
This study provides evidence that higher beta-carotene biochemical status is associated with lower overall, CVD, heart disease, stroke, cancer, and other causes of mortality. The dose-response associations over a 30-year period were not attenuated by adjustment for other important risk factors, and support greater fruit and vegetable consumption as a means to increase beta-carotene status and promote longevity.
Keywords: Beta-carotene, all-cause mortality, cause-specific mortality, multivariate analysis, epidemiology, biomarker, primary prevention, cardiovascular disease
INTRODUCTION
Despite substantial attention focused on the human health effects of beta-carotene, a systematic examination of the association between serum concentrations and long-term mortality has not been reported. This is particularly true with respect to cause-specific mortality (e.g., cardiovascular disease (CVD)) and the examination of dose-response relationships. The phytochemical beta-carotene is found naturally in green leafy vegetables, fruits and other yellow/orange plants, and it can be synthesized by microorganisms.1 As the major pro-vitamin A carotenoid, beta-carotene can be metabolized into bioactive retinol and other vitamin A compounds, which are critical for maintaining normal human physiology and homeostasis.2 In addition, previous in vitro and in vivo studies demonstrate that beta-carotene is a powerful antioxidant capable of neutralizing intracellular free radicals involved in the development of chronic illnesses, including CVD and cancer. Serum beta-carotene has also been inversely associated with systemic markers of inflammation and insulin resistance.3, 4
Several studies, including a recent meta-analysis, suggest an inverse association between circulating beta-carotene and total mortality,5–9 although there have been mixed results for risk of death from cancer and CVD, and data are sparse for heart disease, stroke, respiratory disease, and diabetes mortality.6–14 Most of these studies have been based on relatively small numbers of deaths, and had limited power to examine cause-specific mortality, dose-response associations, and risk among population subgroups. At the same time, large controlled trials reported either no benefits or small unexpected adverse effects of supra-physiological beta-carotene supplementation, including increased overall mortality and cancer incidence.15–18 A better understanding of the beta-carotene-mortality association, and test of dose-response, is clearly needed.
The present study examined the association between serum beta-carotene and risk of mortality from all causes, total CVD, heart disease, stroke, cancer, respiratory disease, diabetes, injuries/accidents, and other causes in a large prospective cohort of men with 30 years of follow-up and nearly 24,000 deaths.
METHODS
Because of cohort data use agreements in place between the U.S. and Finland, the data, analytic methods and study materials will not be made available to other researchers for the aim of reproducing the results.
Study population.
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study has been described in detail.19 The study was a 2×2 factorial, randomized, double-blind, placebo-controlled primary prevention trial that examined whether alpha-tocopherol and beta-carotene reduced incidence of cancer.19 Briefly, 29,133 male smokers from southwest Finland, aged 50–69 years, were eligible and randomly assigned to receive one of four supplements daily (alpha-tocopherol (50 mg), beta-carotene (20 mg), both or placebo) for 5–8 years between 1985 and 1988 until the end of the intervention (April 30, 1993). Self-administered questionnaires were completed at enrollment, including information regarding general health, lifestyle risk factors, and a validated food-frequency questionnaire, and height, weight, blood pressure, and heartrate were measured. Pre-supplementation fasting blood samples were collected and stored at −70 ⁰C until biochemically assayed. Written informed consent was provided by all participants, and the Study was approved by the institutional review boards at the Finnish National Public Health Institute and the U.S. National Cancer Institute.
Serum concentrations of beta-carotene, alpha-tocopherol and retinol were assayed using high-performance liquid chromatography,19, 20 and the coefficient of variation for serum beta-carotene was 6.5%.20 After excluding participants without a serum beta-carotene measurement (N=30), 29,103 were included in the final analysis (as a total of 29,133 participants being included in the ATBC parent trial cohort).
Outcome assessment.
Specific causes of death from baseline through the end of the follow-up (31 December 2015) were ascertained via linkage with the Causes of Death registry, Statistics Finland. We used the following codes from the 8th, 9th and 10th revisions of International Classification of Diseases (ICD-8, −9, −10, respectively) to classify the underlying cause of death: CVD (ICD-8, 390–458; ICD-9, 390–459; ICD10, I00-I99), heart disease (ICD-8 and ICD-9, 390–398, 401–404, 410–429, and 440–448; ICD10, I00-I13, I20-I51, and I70-I78), stroke (ICD-8 and ICD-9, 430–438; ICD10, I60-I69), cancer (ICD-8 and ICD-9, 140–239; ICD10, C00-D48), respiratory disease (i.e., pneumonia, influenza, chronic obstructive pulmonary disease, and other related conditions) (ICD-8, 470–474, 480–486, 490–493, and 518; ICD-9, 480–487 and 490–496; ICD-10, J10-J18 and J40-J47), diabetes (ICD-8 and ICD-9, 250; ICD-10, E10-E14), injuries and accidents (ICD-8, 800–978; ICD-9, E800-E978; ICD-10, V01-X59, Y85-Y86, U03, X60-X84, Y87.0, Y87.1, U01-U02, X85-Y09, Y35, and Y89.0), and all other causes combined.
Statistical analysis.
We calculated person-time of follow-up for each participant from the date of randomization to the date of death or the end of follow-up (31 December 2015), whichever occurred first. We used Cox proportional hazards regression models with attained age as the time metric to estimate the hazard ratio (HR) and 95% confidence interval (CI) for associations between serum beta-carotene (quintiles) and mortality risk, including overall and cause-specific mortality. In cause-specific models, mortality other than the event of interest was censored at the time of death. The proportional-hazards assumption was not violated based on inspection of the Schoenfeld residuals of exposure variables of interest. Tests for linear trend were constructed by assigning the median value of each quintile and entering it to the model as a continuous variable. The crude model was adjusted for age at blood collection (continuous variable). Multivariate models were additionally adjusted for number of cigarettes smoked per day, number of years of smoking, trial intervention group (beta-carotene or no beta-carotene, alpha-tocopherol or no alpha-tocopherol), systolic and diastolic blood pressure, serum total cholesterol and high-density lipoprotein (HDL) cholesterol. In the models with CVD, heart disease, and stroke as endpoints, we further adjusted for history of CVD. In addition, the following covariates were assessed but were not included in the final model as none altered the estimated main effect of serum beta-carotene by 10% or more: body mass index (BMI, kg/m2), physical activity, baseline vitamin A supplement use, history of diabetes, energy intake, educational status, and daily intakes of alpha-carotene, fruit, vegetables, fat, red meat, alcohol, total fiber, and cereals.
We constructed Kaplan-Meier survival curves according to serum beta-carotene quintiles compared differences for the composite endpoints across groups using the log-rank test. We used cubic restricted splines with 4-knots to account for possible non-linear associations between serum beta-carotene concentrations and mortality risk. Knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum beta-carotene concentration.
We performed stratified analyses by age at baseline (<54, 54–59 or ≥59 years), number of cigarettes smoked per day (<16, 16–20, or >20), number of years of smoking (<33, 33–40, or ≥40 years), daily alcohol consumption (<5.3, 5.3–20.4, or ≥20.4 g), BMI (<25, 25–28, or ≥28 kg/m2), intervention group assignment (beta-carotene or no beta-carotene, alpha-tocopherol or no alpha-tocopherol), and years of follow-up (<13, 13–23, or ≥23 years). P values for interactions were evaluated through likelihood ratio tests by comparing Cox proportional hazards models with and without the cross-product terms for each assessed factors and baseline serum beta-carotene (quintile). We conducted 8 interaction tests for each outcome, therefore differences with P<0.05 could be considered chance findings. To minimize the reverse causality bias, we performed a sensitivity analysis that excluded the first 5 years of follow-up. To decrease the potential bias from effects of preexisting illness on serum beta-carotene concentration, we performed sensitivity analyses that excluded participants who reported a history of CVD, diabetes or both at baseline.
All analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, NC), and statistical tests and reported P values were two-tailed.
RESULTS
Mean baseline serum beta-carotene concentration in the cohort was 212 μg/L, and mean beta-carotene in the fifth quintile was nearly seven times higher than that in the first quintile (Table 1). As compared to men in the lowest quintile, subjects in the higher quintiles of beta-carotene smoked less, were more physically active and likely to use vitamin supplements, and less likely to have a history of CVD or diabetes mellitus. Serum beta-carotene was positively associated with fruit and vegetable consumption, alpha-carotene and fiber intake, and serum total cholesterol (the latter likely due to beta-carotene transport in lipoproteins) (Table 1).
Table 1.
Baseline population characteristics by quintile of serum beta-carotene in the ATBC Study.*
| Quintile of serum beta-carotene | |||||
|---|---|---|---|---|---|
| Quintile 1 (n=5,797) | Quintile 2 (n=5,731) |
Quintile 3 (n=5,891) |
Quintile 4 (n=5,815) |
Quintile 5 (n=5,869) |
|
| Serum beta-carotene (μg/L) | 66.8 (21.8) | 120.9 (13.6) | 170.5 (15.8) | 238.8 (25.4) | 457.8 (273.0) |
| Age (y) | 57.0 (4.9) | 57.3 (5.1) | 57.3 (5.1) | 57.2 (5.1) | 57.3 (5.1) |
| Cigarettes/d | 22.2 (9.3) | 20.9 (8.8) | 20.4 (8.8) | 19.8 (8.6) | 18.8 (8.3) |
| Years smoked (y) | 36.4 (8.1) | 36.3 (8.4) | 35.9 (8.5) | 35.6 (8.7) | 35.4 (8.7) |
| Systolic blood pressure (mm Hg) | 146.5 (19.8) | 144.0 (19.4) | 141.4 (19.0) | 139.7 (19.1) | 138.3 (18.9) |
| Diastolic blood pressure (mm Hg) | 90.5 (11.1) | 88.7 (10.7) | 87.4 (10.5) | 86.2 (10.5) | 85.2 (10.5) |
| Serum total cholesterol (mmol/L) | 5.8 (1.2) | 6.1 (1.1) | 6.2 (1.1) | 6.4 (1.1) | 6.6 (1.2) |
| Serum HDL cholesterol (mmol/L) | 1.26 (0.39) | 1.18 (0.33) | 1.18 (0.30) | 1.17 (0.28) | 1.19 (0.28) |
| Serum alpha-tocopherol (mg/L) | 10.9 (4.6) | 11.6 (3.4) | 11.9 (3.1) | 12.2 (2.9) | 12.9 (3.2) |
| BMI (kg/m2) | 26.8 (4.3) | 26.9 (3.9) | 26.4 (3.7) | 26.0 (3.6) | 25.4 (3.3) |
| Physically active (%) | 15.0 | 19.3 | 20.8 | 23.2 | 25.5 |
| History of CVD (%)† | 46.8 | 44.3 | 40.6 | 39.2 | 36.6 |
| History of diabetes mellitus (%) | 7.2 | 4.9 | 3.6 | 2.9 | 2.7 |
| Vitamin A supplement use (%) | 8.0 | 9.1 | 9.0 | 10.0 | 15.6 |
| Vitamin E supplement use (%) | 8.0 | 9.3 | 9.0 | 9.9 | 14.5 |
| Daily dietary intake | |||||
| Energy (kcal) | 2,617 (768) | 2,676 (761) | 2,716 (739) | 2,719 (750) | 2,714 (746) |
| Alcohol (g ethanol) | 30.0 (28.0) | 20.5 (22.1) | 16.4 (19.0) | 13.2 (16.6) | 10.5 (14.4) |
| Fruit (g) | 106 (93.0) | 122 (97.5) | 128 (99.1) | 137 (104.8) | 150 (109.3) |
| Vegetables (g) | 96.3 (61.4) | 106.7 (65.2) | 111.2 (68.0) | 118.6 (71.8) | 134.1 (79.9) |
| Fat (triacylglycerol, g) | 98.8 (35.8) | 105 (35.8) | 107 (35.4) | 108 (36.2) | 109 (37.0) |
| Red meat (g) | 68.3 (34.1) | 70.9 (33.3) | 72.0 (34.3) | 72.4 (33.3) | 72.8 (34.3) |
| Alpha-carotene (μg) | 458(452) | 541 (501) | 596 (549) | 668 (597) | 855 (771) |
| Total fiber (g) | 17.0 (9.8) | 18.2 (9.9) | 19.0 (10.0) | 19.5 (10.0) | 20.0 (10.0) |
| Total cereal (g) | 194 (85) | 211 (85) | 212 (86) | 224 (86) | 229 (86) |
| Education (%, > elementary
school) |
21.0 | 21.3 | 19.6 | 20.2 | 23.1 |
Values are means (standard deviation) unless otherwise indicated. P values are based on ANOVA test for continuous variable and Chi-square test for categorical variable, respectively. All P value<0.002.
CVD, cardiovascular disease; includes a history of deep vein thrombosis, superficial venous thrombosis, lung infarction or embolus, hypertension, arterial obstruction, stroke, heart arrhythmia, enlarged heart, valvular heart disease, myocardial infarction, coronary heart disease, and heart failure.
During 31 years of follow-up (median, 18 years; total person-years, 514,271), 23,796 men died (81.8%), including 9,869 deaths from CVD disease (8,064 heart disease deaths and 1,764 stroke deaths), 7,692 deaths from cancer, 2,161 deaths from respiratory disease, 119 deaths from diabetes, 1,255 deaths from injuries and accidents, and 2,700 deaths from all other causes. Compared with men in the lowest quintile of serum beta-carotene, those in the higher quintiles experienced significantly lower age-adjusted mortality from all causes, CVD, heart disease, stroke, cancer, respiratory disease, diabetes, injuries and accidents, and all other causes, representing 34–83% risk reductions (all Ptrend <0.0001; Table 2). After multivariate-adjustment, the inverse associations remain statistically significant with the HRs attenuated slightly to 27–79% lower risk in the highest beta-carotene quintile (all Ptrend <0.0001; Table 2). Further adjustment for BMI, intakes of alcohol, alpha-carotene, fruits and vegetables, total fiber and cereal, and educational status, also did not change the association estimates appreciably (Online Tables I–III). Kaplan-Meier survival plots demonstrate that men in the lowest quintile of serum beta-carotene had significantly increased cumulative overall mortality than those in the higher quintiles (log-rank P value <0.0001; Figure 1A), and similar patterns were observed for cause-specific mortality (all log-rank P values <0.0001; Figure 1B-D and Online Figure I).
Table 2.
Hazard ratios (HRs) and 95% CI for all-cause and cause-specific mortality by quintile of serum beta-carotene in the ATBC Study *
| Serum beta-carotene | |||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| All-cause mortality | |||||
| Deaths (n) | 5,193 | 4,857 | 4,728 | 4,568 | 4,450 |
| Death rate† | 58.2 | 49.9 | 44.3 | 42.3 | 39.4 |
| Age-adjusted HR (95% CI) | 1.0 | 0.78 (0.75, 0.82) | 0.68 (0.65, 0.70) | 0.64 (0.61, 0.66) | 0.57 (0.55, 0.60) |
| Multivariate HR (95%CI) ‡ | 1.0 | 0.81 (0.78, 0.84) | 0.71 (0.69, 0.74) | 0.69 (0.66, 0.72) | 0.64 (0.61, 0.67) |
| CVD mortality | |||||
| Deaths (n) | 2,085 | 2,052 | 2,041 | 1,890 | 1,801 |
| Death rate† | 23.36 | 21.07 | 19.14 | 17.51 | 15.94 |
| Age-adjusted HR (95% CI) | 1.0 | 0.83 (0.78, 0.88) | 0.73 (0.69, 0.78) | 0.66 (0.62, 0.71) | 0.59 (0.55, 0.62) |
| Multivariate HR (95%CI) ठ| 1.0 | 0.84 (0.79, 0.89) | 0.76 (0.72, 0.81) | 0.71 (0.66, 0.75) | 0.64 (0.60, 0.68) |
| Heart disease | |||||
| Deaths (n) | 1,681 | 1,695 | 1,669 | 1,536 | 1,483 |
| Death rate† | 18.83 | 17.40 | 15.65 | 14.23 | 13.12 |
| Age-adjusted HR (95% CI) | 1.0 | 0.85 (0.80, 0.91) | 0.75 (0.70, 0.80) | 0.67 (0.63, 0.72) | 0.60 (0.56, 0.65) |
| Multivariate HR (95%CI) ठ| 1.0 | 0.85 (0.79, 0.91) | 0.76 (0.71, 0.82) | 0.70 (0.65, 0.75) | 0.64 (0.59, 0.68) |
| Stroke | |||||
| Deaths (n) | 395 | 347 | 364 | 342 | 316 |
| Death rate† | 4.43 | 3.56 | 3.41 | 3.17 | 2.80 |
| Age-adjusted HR (95% CI) | 1.0 | 0.72 (0.62, 0.83) | 0.67 (0.58, 0.77) | 0.61 (0.52, 0.70) | 0.52 (0.45, 0.60) |
| Multivariate HR (95% CI) ठ| 1.0 | 0.78 (0.68, 0.91) | 0.78 (0.67, 0.90) | 0.74 (0.64, 0.86) | 0.67 (0.57, 0.78) |
| Cancer mortality | |||||
| Deaths (n) | 1,584 | 1,543 | 1,501 | 1,546 | 1,518 |
| Death rate† | 17.75 | 15.84 | 14.07 | 14.32 | 13.43 |
| Age-adjusted HR (95% CI) | 1.0 | 0.83 (0.77, 0.89) | 0.72 (0.67, 0.77) | 0.72 (0.67, 0.77) | 0.66 (0.62, 0.71) |
| Multivariate HR (95%CI) ‡ | 1.0 | 0.85 (0.80, 0.92) | 0.75 (0.70, 0.81) | 0.78 (0.72, 0.84) | 0.73 (0.68, 0.79) |
| Respiratory disease | |||||
| Deaths (n) | 499 | 434 | 404 | 412 | 412 |
| Death rate† | 5.59 | 4.46 | 3.79 | 3.82 | 3.65 |
| Age-adjusted HR (95% CI) | 1.0 | 0.69 (0.61, 0.79) | 0.56 (0.49, 0.64) | 0.55 (0.48, 0.63) | 0.51 (0.44, 0.58) |
| Multivariate HR (95%CI) ‡ | 1.0 | 0.81 (0.71, 0.92) | 0.69 (0.60, 0.79) | 0.72 (0.63, 0.83) | 0.71 (0.62, 0.82) |
| Diabetes | |||||
| Deaths (n) | 41 | 28 | 20 | 19 | 11 |
| Death rate† | 0.46 | 0.29 | 0.19 | 0.18 | 0.10 |
| Age-adjusted HR (95% CI) | 1.0 | 0.56 (0.35, 0.91) | 0.35 (0.21, 0.60) | 0.32 (0.19, 0.56) | 0.17 (0.09, 0.34) |
| Multivariate HR (95%CI) ‡ | 1.0 | 0.57 (0.35, 0.93) | 0.37 (0.22, 0.65) | 0.37 (0.21, 0.64) | 0.21 (0.11, 0.42) |
| Injuries and accidents | |||||
| Deaths (n) | 301 | 286 | 254 | 222 | 192 |
| Death rate† | 3.37 | 2.94 | 2.38 | 2.06 | 1.70 |
| Age-adjusted HR (95% CI) | 1.0 | 0.84 (0.71, 0.99) | 0.69 (0.58, 0.81) | 0.59 (0.49, 0.70) | 0.48 (0.40, 0.57) |
| Multivariate HR (95%CI) ‡ | 1.0 | 0.93 (0.79, 1.10) | 0.78 (0.66, 0.93) | 0.69 (0.58, 0.83) | 0.57 (0.47, 0.69) |
| Other causes | |||||
| Deaths (n) | 683 | 514 | 508 | 479 | 516 |
| Death rate† | 7.65 | 5.28 | 4.76 | 4.44 | 4.57 |
| Age-adjusted HR (95% CI) | 1.0 | 0.61 (0.54, 0.68) | 0.51 (0.46, 0.58) | 0.47 (0.42, 0.53) | 0.46 (0.41, 0.51) |
| Multivariate HR (95% CI) ‡ | 1.0 | 0.64 (0.57, 0.72) | 0.55 (0.49, 0.62) | 0.51 (0.45, 0.58) | 0.51 (0.45, 0.58) |
Cause of death was missing for 47 subjects.
Crude death rate per 1000 person-years.
Adjusted for age, cigarettes smoked per day, years of smoking, intervention assignment, systolic and diastolic blood pressure, serum total and HDL cholesterol. P value for trend: based on statistical significance of the coefficient of the quintile variable (median value within each quintile).
P value for trend (all strata): <0.0001.
Further adjusted for history of CVD.
Figure 1.
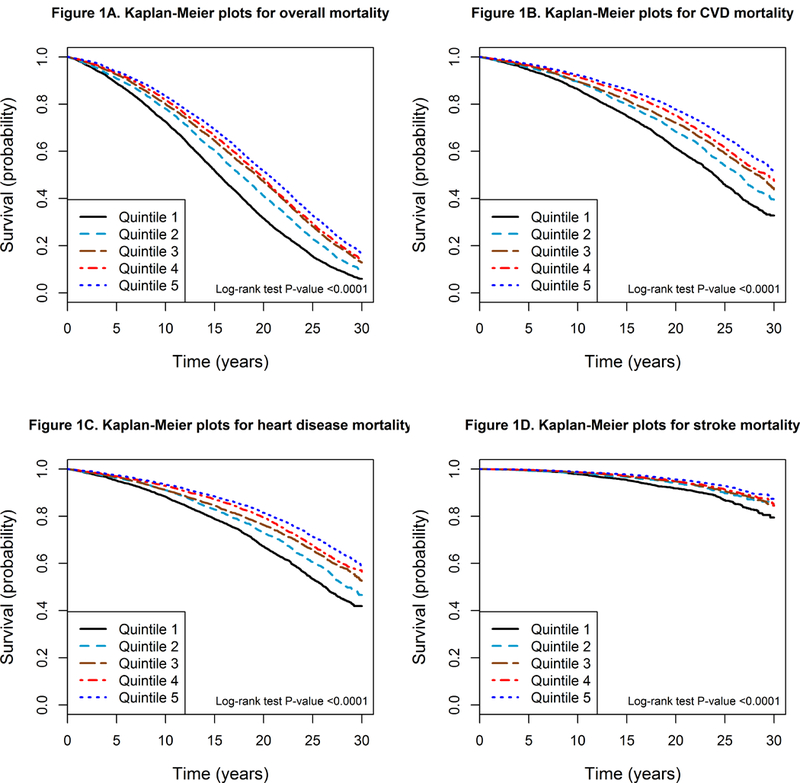
Kaplan-Meier plots of overall and cause-specific mortality by quintiles of serum beta-carotene in the ATBC Study. A) Overall mortality. B) Cardiovascular disease (CVD) mortality. C) Heart disease mortality. D) Stroke mortality.
To evaluate the serum beta-carotene-mortality dose-response relation, we used a restricted 4-knot cubic spline regression, modeling serum beta-carotene as a continuous variable. All-cause and cause-specific relative mortality increased as serum beta-carotene values decreased below the reference value of 98 μg/L (corresponds to the cutoff value of the first quintile), whereas significant reductions in risk were observed for increasing serum beta-carotene concentrations, with association plateaus suggested for CVD, heart disease and stroke mortality (Figure 2, and Online Figure II).
Figure 2.
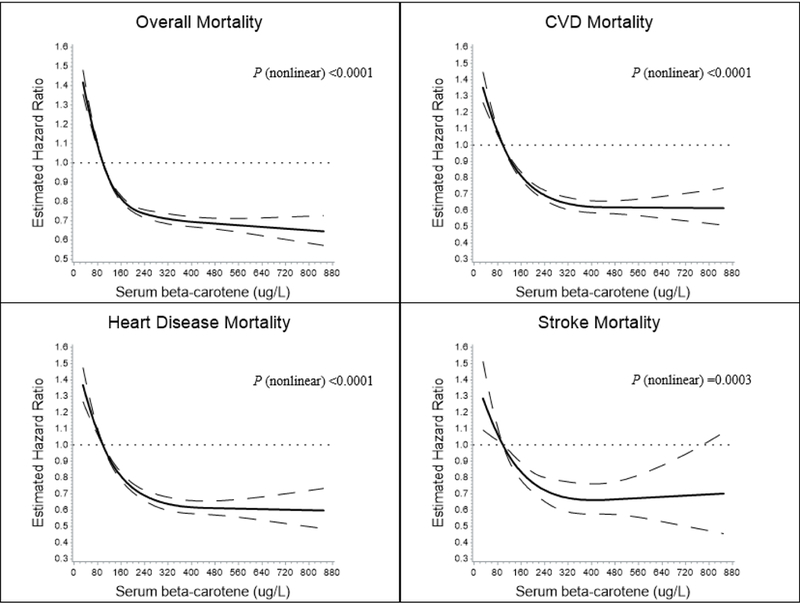
Cubic spline regression for estimated relative risk of overall and cause-specific death according to serum beta-carotene concentrations in the ATBC Study. The reference value (98 μl/L; relative risk = 1) corresponds to the cutoff value of the first quintile of serum beta-carotene concentration. A) Overall mortality. B) Cardiovascular disease (CVD) mortality. C) Heart disease mortality. D) Stroke mortality. The solid line indicates the relative risk for mortality and serum beta-carotene with a 4-knot spline (knots were selected at the 5th, 25th, 75th, and 95th percentiles of the serum beta-carotene); dashed lines indicated the 95% confidence intervals. Total No. of participants: 29,103. Event No. of overall-, CVD-, heart disease-, stroke mortality is 23,796, 9,869, 8,064, and 1,764, respectively.
Evaluating effect modification of the all-cause mortality association by baseline factors and duration of follow-up revealed generally similar findings for serum beta-carotene across subgroups of cigarettes smoked daily, alcohol consumption, trial intervention, and duration of follow-up. The beta-carotene association was, however, statistically significantly modified by baseline age (Pinteraction<0.0001), cumulative years of smoking (Pinteraction=0.0025), and BMI (Pinteraction=0.0009; Table 3) with stronger inverse associations for men <54 years old, those with <33 years of smoking, and those with BMI <25 kg/m2, the latter two findings being independent of age, based on the multi-variable-adjusted models. Associations between beta-carotene concentration and cause-specific mortality were also generally similar across categories of duration of follow-up (except for other causes of mortality), alcohol consumption, and trial intervention group (Online Tables IV–VII). The inverse association with CVD and heart disease mortality was stronger only among younger men (Pinteraction<0.0001 and Pinteraction =0.0012, respectively; Online Tables IV–V), while the inverse association with stroke and cancer mortality appeared stronger in those with <20 cigarettes smoked daily and those with BMI <25 kg/m2, respectively (Online Tables VI–VII, respectively). There were no significant subgroup differences for respiratory disease, injury/accidental deaths, or other causes, other than a strong inverse association in the first 13 years follow up for other causes (data not shown).
Table 3.
Hazard ratios (HRs) and 95% CI for all-cause mortality by quintile of serum beta-carotene in the ATBC Study, by baseline subgroups*
| Total numb er of subjects |
Serum beta-carotene |
P value
for interacti on ‡ |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||||||||
| Even ts/de ath rate† |
H R |
Even ts/de ath rate† |
HR (95% CI) | Even ts/de ath rate† |
HR (95% CI) | Even ts/de ath rate† |
HR (95% CI) | Even ts/de ath rate† |
HR (95% CI) | |||
| Age | ||||||||||||
| <54 y | 5437 | 1,355 /42.5 |
1. 0 |
1,124 /33.0 |
0.77 (0.71, 0.84) |
1,082 /28.8 |
0.67 (0.61, 0.72) |
977/ 25.6 |
0.61 (0.56, 0.66) |
899/ 23.7 |
0.56 (0.51, 0.62) |
<0.0001 |
| 54–59 y | 7791 | 1,816 /56.9 |
1. 0 |
1,597 /47.3 |
0.80 (0.75, 0.86) |
1,480 /40.3 |
0.67 (0.63, 0.72) |
1,493 /40.2 |
0.69 (0.64, 0.74) |
1,405 /35.0 |
0.61 (0.56, 0.65) |
|
| ≥59 y | 10568 | 2,022 /79.6 |
1. 0 |
2,136 /72.3 |
0.85 (0.80, 0.90) |
2,166 /67.0 |
0.78 (0.73, 0.83) |
2,098 /64.4 |
0.75 (0.70, 0.80) |
2,146 /61.4 |
0.72 (0.67, 0.76) |
|
|
Cigarettes
smoked/d |
||||||||||||
| <16 | 7620 | 1,311 /56.8 |
1. 0 |
1,480 /48.5 |
0.79 (0.73, 0.85) |
1,500 /43.1 |
0.70 (0.65, 0.76) |
1,583 /40.7 |
0.66 (0.62, 0.71) |
1,746 /37.2 |
0.60 (0.56, 0.65) |
0.31 |
| 16–20 | 8057 | 1,699 /58.3 |
1. 0 |
1,601 /49.6 |
0.81 (0.75, 0.87) |
1,669 /45.1 |
0.72 (0.67, 0.77) |
1,607 /42.8 |
0.69 (0.64, 0.74) |
1,481 /39.5 |
0.63 (0.58, 0.67) |
|
| >20 | 8119 | 2,183 /58.9 |
1. 0 |
1,776 /51.4 |
0.82 (0.77, 0.87) |
1,559 /44.8 |
0.71 (0.66, 0.76) |
1,378 /43.7 |
0.70 (0.66, 0.76) |
1,223 /42.8 |
0.69 (0.64, 0.75) |
|
|
Years
smoked |
||||||||||||
| <33 y | 5938 | 1,283 /44.2 |
1. 0 |
1,151 /34.9 |
0.78 (0.72, 0.84) |
1,171 /31.1 |
0.67 (0.62, 0.72) |
1,177 /29.1 |
0.64 (0.59, 0.69) |
1,156 /26.6 |
0.58 (0.53, 0.63) |
0.0025 |
| 33–40 | 6874 | 1,599 /52.4 |
1. 0 |
1,428 /45.6 |
0.84 (0.78, 0.90) |
1,333 /39.2 |
0.72 (0.67, 0.78) |
1,293 /38.1 |
0.70 (0.65, 0.76) |
1,221 /35.2 |
0.63 (0.58, 0.68) |
|
| ≥40 y | 10984 | 2,311 /77.8 |
1. 0 |
2,278 /68.8 |
0.81 (0.76, 0.86) |
2,224 /63.4 |
0.73 (0.69, 0.78) |
2,098 /62.5 |
0.72 (0.68, 0.76) |
2,073 /59.3 |
0.69 (0.64, 0.73) |
|
|
Daily
alcohol consumpti on |
||||||||||||
| <5.3 g | 7225 | 677/ 57.5 |
1. 0 |
1,176 /53.7 |
0.94 (0.85, 1.03) |
1,543 /47.0 |
0.80 (0.73, 0.88) |
1,778 /44.6 |
0.77 (0.70, 0.84) |
2,051 /40.3 |
0.71 (0.65, 0.78) |
0.12 |
| 5.3–20.4 | 7290 | 1,384 /58.8 |
1. 0 |
1,534 /47.7 |
0.76 (0.70, 0.82) |
1,496 /41.8 |
0.67 (0.62, 0.73) |
1,493 /40.1 |
0.64 (0.60, 0.69) |
1,383 /37.3 |
0.60 (0.56, 0.65) |
|
| ≥20.4 g | 7524 | 2,647 /57.1 |
1. 0 |
1,784 /48.3 |
0.81 (0.76, 0.86) |
1,358 /42.6 |
0.71 (0.66, 0.76) |
1,005 /40.3 |
0.70 (0.65, 0.75) |
730/ 37.8 |
0.64 (0.59, 0.70) |
|
|
BMI
(kg/m2) |
||||||||||||
| <25 | 9321 | 1,870 /63.0 |
1. 0 |
1,614 /53.4 |
0.79 (0.74, 0.85) |
1,795 /46.9 |
0.69 (0.64, 0.73) |
1,893 /44.3 |
0.65 (0.61, 0.70) |
2,149 /39.7 |
0.58 (0.54, 0.62) |
0.0009 |
| 25–28 | 7415 | 1,461 /55.2 |
1. 0 |
1,535 /47.5 |
0.81 (0.75, 0.87) |
1,526 /41.5 |
0.70 (0.65, 0.75) |
1,490 /39.8 |
0.69 (0.64, 0.74) |
1,403 /37.6 |
0.66 (0.61, 0.71) |
|
| ≥28 | 7042 | 1,858 /56.1 |
1. 0 |
1,706 /49.0 |
0.83 (0.78, 0.89) |
1,403 /44.4 |
0.75 (0.70, 0.81) |
1,180 /42.5 |
0.73 (0.68, 0.79) |
895/ 41.7 |
0.70 (0.64, 0.76) |
|
|
Trial
interventio n group |
||||||||||||
| Beta-carotene | 11867 | 2,620 /59.0 |
1. 0 |
2,397 /50.7 |
0.81 (0.76, 0.85) |
2,361 /44.5 |
0.70 (0.66, 0.74) |
2,272 /42.4 |
0.68 (0.64, 0.72) |
2,217 /39.2 |
0.62 (0.59, 0.66) |
0.51 |
| No beta-carotene | 11929 | 2,573 /57.4 |
1. 0 |
2,460 /49.1 |
0.81 (0.77, 0.86) |
2,367 /44.2 |
0.73 (0.69, 0.77) |
2,296 /42.2 |
0.70 (0.66, 0.74) |
2,233 /39.5 |
0.65 (0.62, 0.69) |
|
| Alpha-tocopherol | 11955 | 2,590 /58.2 |
1. 0 |
2,439 /49.8 |
0.82 (0.77, 0.87) |
2,398 /45.0 |
0.73 (0.69, 0.77) |
2,320 /43.5 |
0.72 (0.68, Y0.76) | 2,208 /39.4 |
0.65 (0.61, 0.69) |
0.34 |
| No alpha-tocopherol | 11841 | 2,603 /58.2 |
1. 0 |
2,418 /50.0 |
0.80 (0.76, 0.85) |
2,330 /43.7 |
0.70 (0.66, 0.74) |
2,248 /41.2 |
0.66 (0.62, 0.70) |
2,242 /39.4 |
0.62 (0.59, 0.66) |
|
|
Years of
follow-up |
||||||||||||
| 0–13 | 9625 | 2,466 /40.2 |
1. 0 |
2,036 /32.3 |
0.79 (0.74, 0.84) |
1,856 /28.0 |
0.70 (0.66, 0.75) |
1,691 /25.5 |
0.65 (0.61, 0.69) |
1,576 /23.2 |
0.60 (0.56, 0.64) |
0.02 |
| 13–23 | 9596 | 1,998 /88.6 |
1. 0 |
1,919 /71.2 |
0.80 (0.75, 0.85) |
1,897 /61.2 |
0.69 (0.65, 0.74) |
1,932 /60.9 |
0.71 (0.66, 0.75) |
1,850 /54.6 |
0.63 (0.59, 0.68) |
|
| ≥23 | 4575 | 729/ 135.0 |
1. 0 |
902/ 121.2 |
0.90 (0.82, 0.99) |
975/ 104.1 |
0.77 (0.70, 0.85) |
945 /95.9 |
0.73 (0.66, 0.80) |
1,024 /91.3 |
0.70 (0.63, 0.77) |
|
Adjusted for age, cigarettes smoked per day, years of smoking, intervention assignment, systolic and diastolic blood pressure, serum total and HDL cholesterol.
P value for trend: based on statistical significance of the coefficient of the quintile variable (median value within each quintile). P value for trend (all strata): <0.0001.
Crude death rate per 1,000 person years.
P value for interaction: according to the statistical significance of the cross-product term added to multivariate models.
The results were not materially different when the first five years of follow-up were excluded from the analysis (for all-cause mortality in the multivariate model, fifth versus first quintile: HR=0.64, 95% CI: 0.61, 0.67; Ptrend <0.0001; Online Table VIII). Our findings also remained unchanged after excluding from the analysis 12,488 participants who reported a history of CVD, diabetes, or both (for all-cause mortality in the multivariate model, fifth versus first quintile: HR=0.66, 95% CI: 0.62, 0.70; Ptrend <0.0001; Online Table IX).
DISCUSSION
The primary findings of this large cohort analysis of male smokers were significant inverse associations between prospectively measured serum beta-carotene concentrations and overall and cause-specific mortality, with men in the highest serum quintile experiencing 36% lower overall mortality compared with those in the lowest quintile. Of note, the associations were similar for deaths occurring in both the early (0 to <13 years) and later follow-up periods (13–23 and ≥23 years), as well as within most health risk factor subgroups examined, with the exception of somewhat stronger inverse associations in younger men, those with fewer years of smoking, and those with BMI <25 kg/m2 (e.g., 44%, 42% and 42% lower overall mortality for the highest beta-carotene quintile, respectively).
To the best of our knowledge, this is the largest investigation of beta-carotene biochemical status and mortality. Our findings are consistent with smaller studies of chronic disease mortality. The protective association is similar to a recent meta-analysis of all-cause mortality in 25,468 men and women (6,137 deaths), where a relative risk (RR) of 0.69 (95% CI: 0.59–0.80) was observed for those in the highest versus lowest serum beta-carotene category.5 The NHANES III study of 16,008 adults (4,225 deaths) found that those in the highest quintile of serum beta-carotene experienced a 25% lower risk of death compared to those in the lowest quintile (95% CI: 10–37%).6 The Swedish Uppsala cohort study of 2,301 older men with 630 deaths showed a RR of 0.76 for all-cause mortality (95% CI: 0.67, 0.85, P=0.0001) per 1-standard deviation increase in serum beta-carotene,9 with reduced risk observed for each major cause of death, while the mortality HR for high versus low quartile of beta-carotene among 2,646 white male asbestos-exposed insulators (984 deaths) was 0.63 (95% CI: 0.51–0.77).7 In addition, a lower HR of 8% per standard deviation increment in plasma beta-carotene (95% CI: +1%, −16%, P=0.08) was observed in the British National Diet and Nutrition Survey study of 1,054 older men and women (717 deaths).8 Cause-specific mortality findings have not been entirely consistent, with some studies reporting null associations for cancer deaths,7, 8 and several relatively small studies showing inverse CVD mortality associations with serum beta-carotene,9–13 but not all.6, 14 Moreover, there are few reports regarding respiratory disease,8 diabetes, and other causes. Given that fruits and vegetables are the primary dietary sources of beta-carotene, prior studies that show their higher consumption being associated with reduced risk of overall and cause-specific mortality are supportive of the present biochemical findings.21, 22
Large controlled trials have demonstrated that beta-carotene supplementation not only had no health benefits, but resulted in unanticipated harmful effects.15–18 The ATBC Study, the parent trial of the present cohort serum analysis, and the beta-Carotene and Retinol Efficacy Trial (CARET) found significantly increased overall mortality (8% and 17%, respectively) among participants who received beta-carotene (or beta-carotene plus retinyl palmitate in the latter trial) supplements.15, 16 By contrast, the Physicians’ Health Study and the Women’s Health Study controlled trials showed no effects of supplemental beta-carotene.17, 18 The beta-carotene dosages administered in these trials had increased the serum concentrations more than 10 times the average,15 raising concerns regarding natural dietary sources of beta-carotene versus supplementation,23 particularly given that at extremely high concentrations beta-carotene may lose its antioxidant function and instead have prooxidant effects.24 The aforementioned studies of fruit and vegetable consumption support a mortality benefit for greater dietary sources of beta-carotene,21 and the present findings combined with previous observational studies support inverse associations of overall and cause-specific mortality with higher serum beta-carotene concentrations within the normal range. For example, the cubic spline regression analysis suggested an optimal relative mortality reduction for older men whose serum beta-carotene values were at least 160 μg/L, with a sharper mortality excess for men with values below 160 μg/L. For overall and cause-specific mortality endpoints, our data may indicate that the decreased mortality is evident with beta-carotene concentrations in the 180–190 μg/L range, and is strongest between 200–700 μg/L. Our findings are in line with a previous report of the normal range being 70–680 μg/L, with a “healthy” average concentration of 190 μg/L.25 It should be noted, however, that the male smokers in our study had relatively low beta-carotene status (mean and median serum concentrations 212 μg/L and 170 μg/L, respectively), potentially providing a better examination of the dose-response relation in the lower range. The relationship between higher beta-carotene concentrations and lower mortality risk, should be investigated in additional studies that include nonsmokers and women.
Mammals do not synthesize carotenoids de novo, and the main sources of carotenoids (including beta-carotene) for humans are of dietary plant origin. For example, most fruits and vegetables contain beta-carotene, including carrots, red bell peppers, oranges, sweet potatoes, broccoli and all green leafy vegetables.26 Among those, carrots may provide an especially important source of dietary beta-carotene.26 As a short-term reflection of dietary intake, serum carotenoids are widely accepted as good biomarkers of fruit and vegetable consumption.26, 27 Therefore, the primary source for beta-carotene in this cohort was fruit and vegetable consumption and not supplements which were rarely used at baseline. Fruit and vegetables are the predominant sources of many other bioactive micronutrients including polyphenols, vitamin C, fiber, folacins, flavonoids, other carotenoids, and several minerals that have multiple biological mechanisms beyond anti-oxidation, including regulation of detoxification enzymes (CYP monooxygenases), activation of the immune system, modulation of hormone metabolism, and anti-bacterial and antiviral effects.28 Thus, serum beta-carotene may be serving as a biomarker of the beneficial effects of this dietary pattern on health outcomes. Adjustment for dietary fruit and vegetables in our serum beta-carotene models did not materially attenuate the estimated mortality associations, however (Supplementary Table I). Likely explanations for this include, 1) greater measurement error for self-reported fruit and vegetable intake based on the food frequency questionnaire than for serum beta-carotene measured using HPLC assays; 2) the carotenoid composition of fruits and vegetables and the carotenoid bioavailability based on cooking methods vary, and adjustment for total fruit and vegetable consumption may not have adequately reflected these effects; 3) host factors that influence serum biochemical status, including gut carotenoid absorption, metabolism and bioavailability that may confer inter-individual differences.26 On the other hand, it is also biologically plausible for a direct beneficial association for higher beta-carotene, which may include antioxidant or anti-inflammatory activity, as oxidative stress and inflammation are involved in diverse pathologies of chronic diseases, including insulin-resistance and beta-cell dysfunction.29 For example, carotenoids (including beta-carotene) inhibit the down-stream production of inflammatory cytokines through suppression the NF-κB pathway, which can translocate to the nucleus and then activate the inflammation-related genes.26 In addition, beta-carotene is a pro-vitamin A precursor of retinoids which exert pleiotropic effects regulating expression of greater than 500 genetic response elements through the retinoic acid and retinoid X nuclear receptors.30
It is noteworthy that the present study showed a stronger serum beta-carotene association for CVD mortality than for cancer mortality, in line with some studies of fruit and vegetable consumption.21, 31 Beta-carotene and other antioxidant compounds are known to inhibit lipid peroxidation in arterial walls, influencing plaque stability, vasomotor function, platelet aggregation, and thrombosis.32, 33 In addition, due to its lipophilic and non-polar properties, beta-carotene is exclusively transported by lipoproteins in the blood stream, and is primarily located in lipoprotein cores. Studies have shown that beta-carotene is mostly associated with low density lipoproteins (LDL),26, 34 and oxidative conversion of LDL is considered to have an important role in atherogenesis. Thus, as one of the antioxidants, beta-carotene may play a role in the modification of LDL oxidation and lipid peroxidation, consequently lowering CVD risk and mortality.35 Although biologically plausible reasons for the somewhat weaker cancer mortality association are not clear, it is possible that serum beta-carotene status is overshadowed by other risk factors such as cigarette smoking, something supported by our observation of a stronger inverse cancer mortality association among men with fewer cumulative years of smoking. Examination of specific cancer sites could shed light on this; i.e., is the serum beta-carotene association weaker in smoking-related malignancies such as lung, oropharynx, larynx, and bladder. Our results are consistent with previous studies, demonstrating inverse or positive associations of beta-carotene with diabetes,36 and lung function,37 respectively, and higher fruit and vegetable consumption has been associated with risk of chronic obstructive pulmonary disease among current and ex-smokers.38 Of note, we observed a strong inverse association between serum beta-carotene and diabetes mortality, despite the small number of diabetes deaths (i.e., 119), consistent with a meta-analysis showing greater green leafy vegetable consumption associated with lower type 2 diabetes mortality,22 possibly through reduced systemic oxidative stress. How higher serum beta-carotene might be related to lower risk of deaths from injuries and accidents could involve improved survival following the initial events, either directly (e.g., anti-inflammatory effects) or indirectly through better general health. However, we could not preclude the possibility that the association may be a chance finding or owing to residual confounding, although previous cross-sectional studies reported that a history of attempted suicide was associated with lower serum carotenoid levels.39
There are several notable strengths of our investigation. The large sample size and complete long-term ascertainment of cause-specific deaths through national registries provided validity and substantial power for detecting moderate serum beta-carotene associations with overall and cause-specific mortality, and for evaluating effect modification by other risk factors. High-quality isocratic HPLC biochemical assays conducted early in the study provided serum beta-carotene concentrations for over 29,000 men in our cohort that were more accurate than self-reported dietary food frequency questionnaire data. Study limitations include the homogenous smoker population of Finnish males which reduces generalizability of our findings to other populations, and the use of a single baseline serum beta-carotene measurement, with possible changes in biochemical status and diet during follow-up. This non-differential misclassification would, however, bias the findings toward the null. The correlation between serum concentrations at baseline and 3 years after randomization among those who were not supplemented with beta-carotene was very high, however (Spearman correlation coefficient=0.70, P<10−5), supporting its relative stability over time. Finally, although we carefully controlled for measured covariates and potential confounding factors, we still cannot rule out the possibility of residual confounding, including by unmeasured factors, that may have biased the observed associations.
In conclusion, we found significant inverse associations between serum beta-carotene concentrations and all-cause and cause-specific mortality, including death from CVD, heart disease, stroke, and cancer. The dose-response associations over a 30-year period were not attenuated by adjustment for important risk factors. Our results provide evidence to support an association between higher beta-carotene serologic status within the normal range and reduced mortality. Whether low dose beta-carotene supplementation could be similarly associated with lower mortality would require evidence from controlled trials. The association should be re-examined in other study populations that include women, nonsmokers, and other ethnic/racial participants.
Supplementary Material
NOVELTY AND SIGNIFICANCE
What Is Known?
• The phytochemical beta-carotene is found naturally in green leafy vegetables, fruits and other yellow/orange plants, and previous in vitro and in vivo studies demonstrated that it is an antioxidant capable of neutralizing free radicals that promote pathological processes related to chronic diseases.
• Beta-carotene, as the major pro-vitamin A carotenoid, is metabolized into bioactive retinol and other vitamin A compounds, which are critical for maintaining normal human development, physiology and homeostasis.
• Higher serum beta-carotene and consumption of carotenoid-rich diets have been related to lower overall mortality and chronic disease risk, whereas some controlled trials of beta-carotene supplementation have shown adverse effects on overall mortality and risk of cardiovascular disease (CVD) and cancer.
What New Information Does This Article Contribute?
• During 30 years of cohort follow-up, men with higher baseline serum beta-carotene experienced decreased risk of all-cause mortality, including mortality from CVD, heart disease, stroke, cancer, respiratory disease, diabetes, and other causes, compared to men with lower beta-carotene concentrations.
• The serum beta-carotene-mortality association appeared dose-response in nature, and it was not materially attenuated after adjustment for other important factors, including fruit and vegetable consumption.
Circulating beta-carotene concentrations have been reported to be inversely associated with overall mortality. Precise characterization of magnitude of the relationship and a systematic examination of the association with long-term overall and cause-specific mortality have not been reported, however. In a large, prospective cohort of 29,000 men, we found significant inverse, dose-response associations between baseline serum beta-carotene concentrations and overall, CVD, cancer and other cause-specific mortality during a 30-year period, that were not materially attenuated by adjustment for other important risk factors, including fruit and vegetable consumption. Our findings provide evidence to support greater carotenoid-rich fruit and vegetable consumption as means to increase beta-carotene status and promote longevity. The present findings should be re-examined in future studies of diverse populations that include women.
ACKNOWLEDGEMENTS
We appreciate all participants in the ATBC cohort for their contribution to the study.
SOURCES OF FUNDING
The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health, and by U.S. Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
Nonstandard Abbreviations and Acronyms
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HR
hazard ratios
- ICD
International Classification of Diseases
- LDL
low density lipoproteins
- RR
relative risk
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740:101–107 [DOI] [PubMed] [Google Scholar]
- 2.From Bendich A. 1989 to 2001: What have we learned about the “biological actions of beta-carotene”? J Nutr. 2004;134:225S–230S [DOI] [PubMed] [Google Scholar]
- 3.Erlinger TP, Guallar E, Miller ER 3rd, Stolzenberg-Solomon R, Appel LJ Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med. 2001;161:1903–1908 [DOI] [PubMed] [Google Scholar]
- 4.Higuchi K, Saito I, Maruyama K, Eguchi E, Mori H, Tanno S, Sakurai S, Kishida T, Nishida W, Osawa H, Tanigawa T. Associations of serum beta-carotene and retinol concentrations with insulin resistance: The toon health study. Nutrition. 2015;31:975–980 [DOI] [PubMed] [Google Scholar]
- 5.Zhao LG, Zhang QL, Zheng JL, Li HL, Zhang W, Tang WG, Xiang YB. Dietary, circulating beta-carotene and risk of all-cause mortality: A meta-analysis from prospective studies. Sci Rep. 2016;6:26983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal A, Terry MB, Siegel AB. Serum antioxidant nutrients, vitamin a, and mortality in u.S. Adults. Cancer Epidemiol Biomarkers Prev. 2013;22:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashim D, Gaughan D, Boffetta P, Lucchini RG. Baseline serum beta-carotene concentration and mortality among long-term asbestos-exposed insulators. Cancer Epidemiol Biomarkers Prev. 2015;24:555–560 [DOI] [PubMed] [Google Scholar]
- 8.Bates CJ, Hamer M, Mishra GD. Redox-modulatory vitamins and minerals that prospectively predict mortality in older british people: The national diet and nutrition survey of people aged 65 years and over. Br J Nutr. 2011;105:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of swedish 50-year-old men. Int J Epidemiol. 2001;30:1119–1126 [DOI] [PubMed] [Google Scholar]
- 10.Karppi J, Laukkanen JA, Makikallio TH, Ronkainen K, Kurl S. Low beta-carotene concentrations increase the risk of cardiovascular disease mortality among finnish men with risk factors. Nutr Metab Cardiovasc Dis. 2012;22:921–928 [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in japanese inhabitants. Asian Pac J Cancer Prev. 2006;7:533–546 [PubMed] [Google Scholar]
- 12.Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin c, are inversely related to 15-year cardiovascular mortality in dutch elderly men. J Nutr. 2008;138:344–350 [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Kurata M, Suzuki K, Hamajima N, Hishida H, Aoki K. Cardiovascular disease mortality and serum carotenoid levels: A japanese population-based follow-up study. J Epidemiol. 2006;16:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: Findings from the nutrition add-on study to the medical research council trial of assessment and management of older people in the community. Am J Clin Nutr. 2003;78:999–1010 [DOI] [PubMed] [Google Scholar]
- 15.Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035 [DOI] [PubMed] [Google Scholar]
- 16.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155 [DOI] [PubMed] [Google Scholar]
- 17.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149 [DOI] [PubMed] [Google Scholar]
- 18.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: The women’s health study. J Natl Cancer Inst. 1999;91:2102–2106 [DOI] [PubMed] [Google Scholar]
- 19.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. . Ann Epidemiol. 1994;4:1–10 [DOI] [PubMed] [Google Scholar]
- 20.Milne DB, Botnen J. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin Chem. 1986;32:874–876 [PubMed] [Google Scholar]
- 21.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ. 2010;341:c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryor WA, Stahl W, Rock CL. Beta carotene: From biochemistry to clinical trials. Nutr Rev. 2000;58:39–53 [DOI] [PubMed] [Google Scholar]
- 24.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez H Critical reviews of oxidative stress and aging advances in basic science, diagnostics and intervention. 2003 [Google Scholar]
- 26.Rodriguez-Concepcion M, Avalos J, Bonet ML, Boronat A, Gomez-Gomez L, Hornero-Mendez D, Limon MC, Melendez-Martinez AJ, Olmedilla-Alonso B, Palou A, Ribot J, Rodrigo MJ, Zacarias L, Zhu C. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62–93 [DOI] [PubMed] [Google Scholar]
- 27.Al-Delaimy WK, Slimani N, Ferrari P, Key T, Spencer E, Johansson I, Johansson G, Mattisson I, Wirfalt E, Sieri S, Agudo A, Celentano E, Palli D, Sacerdote C, Tumino R, Dorronsoro M, Ocke MC, Bueno-De-Mesquita HB, Overvad K, Chirlaque MD, Trichopoulou A, Naska A, Tjonneland A, Olsen A, Lund E, Skeie G, Ardanaz E, Kesse E, Boutron-Ruault MC, Clavel-Chapelon F, Bingham S, Welch AA, Martinez-Garcia C, Nagel G, Linseisen J, Quiros JR, Peeters PH, van Gils CH, Boeing H, van Kappel AL, Steghens JP, Riboli E. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Ecological-level correlations in the european prospective investigation into cancer and nutrition (epic). Eur J Clin Nutr. 2005;59:1397–1408 [DOI] [PubMed] [Google Scholar]
- 28.Lampe JW. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70:475S–490S [DOI] [PubMed] [Google Scholar]
- 29.Venkatasamy VV, Pericherla S, Manthuruthil S, Mishra S, Hanno R. Effect of physical activity on insulin resistance, inflammation and oxidative stress in diabetes mellitus. J Clin Diagn Res. 2013;7:1764–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin a metabolism: An update. Nutrients. 2011;3:63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz MN, Frei B, Vita JA, Keaney JF Jr., Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416 [DOI] [PubMed] [Google Scholar]
- 33.Asplund K Antioxidant vitamins in the prevention of cardiovascular disease: A systematic review. J Intern Med. 2002;251:372–392 [DOI] [PubMed] [Google Scholar]
- 34.Shete V, Quadro L. Mammalian metabolism of beta-carotene: Gaps in knowledge. Nutrients. 2013;5:4849–4868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll YL, Corridan BM, Morrissey PA. Lipoprotein carotenoid profiles and the susceptibility of low density lipoprotein to oxidative modification in healthy elderly volunteers. Eur J Clin Nutr. 2000;54:500–507 [DOI] [PubMed] [Google Scholar]
- 36.Arnlov J, Zethelius B, Riserus U, Basu S, Berne C, Vessby B, Alfthan G, Helmersson J, Uppsala Longitudinal Study of Adult Men S. Serum and dietary beta-carotene and alpha-tocopherol and incidence of type 2 diabetes mellitus in a community-based study of swedish men: Report from the uppsala longitudinal study of adult men (ulsam) study. Diabetologia. 2009;52:97–105 [DOI] [PubMed] [Google Scholar]
- 37.Grievink L, de Waart FG, Schouten EG, Kok FJ. Serum carotenoids, alpha-tocopherol, and lung function among dutch elderly. Am J Respir Crit Care Med. 2000;161:790–795 [DOI] [PubMed] [Google Scholar]
- 38.Kaluza J, Larsson SC, Orsini N, Linden A, Wolk A. Fruit and vegetable consumption and risk of copd: A prospective cohort study of men. Thorax. 2017;72:500–509 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhang J. Serum concentrations of antioxidant vitamins and carotenoids are low in individuals with a history of attempted suicide. Nutr Neurosci. 2007;10:51–58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


