Abstract
Weakly basic drugs are predisposed to order of magnitude decreases in solubility and dissolution as pH increases from 1 to 7 along the gastrointestinal tract. Such behavior is known to be detrimental to drug absorption. The work presented here shows how cocrystals of basic drugs with acidic coformers can mitigate these negative effects. Cocrystals of ketoconazole (KTZ) with adipic, fumaric, and succinic acids exhibit a parabolic solubility dependence on pH such that with increasing pH, solubility decreases, reaches a minimum, and increases. Cocrystals exhibit pHmax values between 3.6 and 3.8, above which they generate supersaturation with respect to drug. Cocrystal supersaturation index (SA), defined as Scocrystal/Sdrug, changes from 1 (pHmax) to 10–30 (pH 5) to 800 – 3,000 (pH 6.5). SA represents the driving force for cocrystal conversion to the less soluble drug during dissolution. SA is not expected to be equal to the observed supersaturation, but it is of great value to classify cocrystals in terms of their risk of conversion. Cocrystal dissolution behavior was analyzed in terms of Cmax, σmax (maximum KTZ concentration and supersaturation), AUCdiss (KTZ concentration area under the curve during dissolution-precipitation), and SA. The three cocrystals studied achieved σmax values between 5 and 15 and sustained supersaturation for 1 to 3 h, resulting in AUCdiss advantage over drug in the range of 2 to 12. SA values as high as 800 were associated with enhanced drug exposure. SA of 3,000 led to limited exposure, very rapid conversion, and no measurable supersaturation. Since cocrystals may be more soluble than needed and/or too soluble to be developed, there is great value in recognizing the relationship between supersaturation threshold, cocrystal solubility, and SA. This becomes more important as cocrystal SA is dependent on pH and other environmental conditions.
Graphical abstract
Introduction
Solubility and permeability are the major factors that govern the oral absorption of a drug according to the Biopharmaceutical Classification System (BCS).1 For BCS class II drugs, which have low solubility and high permeability, drug dissolution in vivo is the rate controlling step in drug absorption.1 Much focus has been placed on the enhancement of drug solubility in order to improve dissolution and bioavailability, and some of the approaches include amorphous forms, salts, and cocrystals.2–6
These supersaturating drug delivery systems generate supersaturated solutions with respect to the crystalline parent drug, which can in turn enhance absorption and bioavailability if sustained over sufficient period of time.7 Cocrystals have gained much interest in recent decades due to their capability to incorporate both ionizable and non-ionizable drug/coformer components (unlike salts), their crystalline stability advantage over amorphous solids, and their ability to impart or alter solubility-pH dependence with coformers of different ionization properties.5, 8–10
While cocrystals are capable of increasing drug solubility by orders of magnitude, they often exhibit different ionization and solubilization behavior from their parent drugs, which alter the solubility enhancement by cocrystals based on solution conditions.11–14 Therefore, in order to comprehend cocrystal solubility, it is important to understand cocrystal solution phase interactions such as component ionization and solubilization by additives. Previous work by our laboratory has shown that cocrystals can exhibit different solubility-pH dependence from the parent drug, and this can sometimes lead to the existence of a pHmax.8–11, 15, 16 pHmax is defined as the solubility transition point based on solution pH, at which the cocrystal and drug solubilities are equal, and both solid phases are thermodynamically stable and coexist in equilibrium with solution.8, 11, 12, 17 The cocrystal solubility advantage over parent drug is therefore not a constant value, and it can be fine-tuned by changing solution pH.
Weakly basic drugs often rely on low gastric pH to dissolve prior to transfer to the small intestine for absorption into the systemic circulation.7, 18, 19 Elevated gastric pH due to disease state, food, or medication has a detrimental effect on KTZ absorption and efficacy.19–22 Ketoconazole (KTZ) is one such drug. KTZ is a lipophilic, BCS class II drug and is able to dissolve to a much higher extent under low pH conditions (< 3) compared to high or neutral pH conditions.1, 20, 23, 24 Its poor solubility at neutral pH (~7) and high solubility-pH dependence result in variable oral absorption due to pH effect.21, 23, 24 The drug label of oral KTZ tablets warns that reduction in gastric acidity caused by disease or medication can adversely affect the absorption of the drug.25 Considering its use as an antifungal agent which is prescribed to patients with underlying diseases such as gastric cancer and AIDS that can cause elevated gastric pH conditions, it is essential to address the solubility issue in order to ensure efficacy during treatment.26–28
Three cocrystals and a salt of KTZ with dicarboxylic acids were reported by Martin et al. in 2013.29 The cocrystals are ketoconazole-fumaric acid (KTZ-FUM), ketoconazole-succinic acid (KTZ-SUC), and ketoconazole-adipic acid (KTZ-ADP), all of which are of 1:1 stoichiometric ratio.29 These cocrystals were reported to generate up to 100 times higher KTZ concentrations during dissolution in water than pure drug dissolution.29 The solution pH was however not considered in their analysis, and this is important since pH is known to have profound effects on the solubility of ionizable drugs and cocrystals.29
This study focuses on the influence of pH on KTZ cocrystal solubility and dissolution. The study aims to (1) develop and validate mathematical models that predict solubility of KTZ cocrystals, (2) compare solubility-pH behavior of cocrystals and pure drug, (3) determine the dissolution advantage of cocrystals as a function of pH, and (4) relate the dissolution-precipitation behavior of cocrystals to their supersaturation index, SA = Scocrystal/Sdrug. SA is a theoretical supersaturation that represents the driving force for drug precipitation during cocrystal dissolution. SA is a valuable metric to classify cocrystals in terms of their ability to reach and sustain supersaturation. It is not expected to be equal to the experimental supersaturation, which is limited by nucleation.
Materials and Methods
Materials
Ketoconazole (lot # BS1203355108, 98% purity) was purchased from Bosche Scientific (New Brunswick, NJ) and used as received. Adipic acid (lot # 06807BE, 99% purity), succinic acid (lot # 037K0021, 99% purity), fumaric acid (lot # 09426EE, 99+% purity), acetic acid (lot # 074K3658, 99%), sodium acetate anhydrous (lot # 100K0272), dipotassium hydrogen phosphate (lot # 103H0287, ACS reagent), and sodium chloride (lot # 094K0183, ACS reagent) were purchased from Sigma-Aldrich (St. Louis, MO) and used as received.
HPLC grade methanol, HPLC grade 2-propanol, sodium phosphate monobasic (lot # 017316), and hydrochloric acid (lot # 2AJK15038, ACS grade) were purchased from Fisher Scientific (Fair Lawn, NJ). Acetone (ACS reagent 99.5%) and phosphoric acid (lot # B0506524, 85+%) were purchased from Acros Organics (NJ) and used as received. Trifluoroacetic acid (spectrophometric grade, 99%) was purchased from Aldrich Company (Milwaukee, WI). NaOH (pellets) was purchased from J.T. Baker (Philipsburg, NJ). Water used in this study was filtered through a double deionized purification system (Milli Q Plus Water System) from Millipore Co. (Bedford, MA).
Cocrystal Synthesis
1:1 cocrystals of KTZ and the dicarboxylic acid coformers were prepared by reaction crystallization method at room temperature.30, 31 KTZ-FUM and KTZ-SUC were synthesized in acetone. KTZ-ADP was synthesized in 2-propanol. Full conversion of drug to cocrystal was observed between 24 and 48 h. The solid phases were verified by X-ray powder diffraction (XRPD) and differential scanning calorimetry (DSC), and the stoichiometries were verified by HPLC.
Media Preparation
Solubility media
Phosphate buffers at pH 2.02 (± 0.02) and 8.04 (± 0.01) were prepared at concentrations of 12 mM and 100 mM, respectively, with the appropriate amount of phosphoric acid and dipotassium hydrogen phosphate. Acetate buffer at pH 5.00 (± 0.01) and concentration of 100 mM was prepared with sodium acetate anhydrous and acetic acid. pH 1.01 (± 0.01) HCl solution (100 mM) was prepared by diluting concentrated hydrochloric acid solution (~12 M). 1 M NaOH and 1 M HCl solutions were used to adjust the pH of the buffer to target pH.
Dissolution media
Dissolution media were prepared based on the conditions of fasted gastric, fasted intestinal, and fed intestinal pH published by Jantratid et al. without surfactants and pepsin.32 pH 1.60 (± 0.01) buffer (34 mM) was prepared with the appropriate amount of NaCl and HCl solution. pH 5.00 (± 0.03) acetate buffer (144 mM) was prepared with the appropriate amount of NaOH (pellets), acetic acid, and NaCl. pH 6.50 (± 0.04) phosphate buffer (29 mM) was prepared with appropriate amount of NaOH (pellets), sodium phosphate monobasic (NaH2PO4•H2O), and NaCl. The pH values of all dissolution media were adjusted to target pH with 1 M NaOH and 1 M HCl solutions.
Drug Solubility
Drug solubility was measured by adding excess solid to 3mL of solution media. The solutions were magnetically stirred and were kept in water bath at 25 ± 0.1°C over 96 hours. 0.5 mL aliquots of the suspension were sampled every 24 hours. Collected samples were filtered via centrifuge through a 0.45 µm pore cellulose acetate membrane, and the pH of the solutions was measured. The solution concentrations of KTZ were analyzed by HPLC.
Cocrystal Solubility
Method 1
Equilibrium solubility of the KTZ cocrystals can be directly measured when the solution pH is below 3. Excess solid for each cocrystal was added to 3 mL of solution media, and the solution was magnetically stirred in water bath at 25 ± 0.1°C up to 96 h. 0.5 mL aliquots of the suspension were sampled every 24 h and filtered via centrifuge through a 0.45 µm pore cellulose acetate membrane. The solid phases were analyzed by XRPD and DSC to ensure only cocrystal solid phases were present. The solution pH values were measured, and the cocrystal component concentrations were analyzed by HPLC.
Method 2
At solution pH above 3, the equilibrium solubility of the cocrystals was determined at the eutectic point, where the drug and cocrystal solid phases are in equilibrium with the solution.5, 8, 33 The eutectic points were approached by cocrystal dissolution, where 150 – 200 mg of cocrystal and 50 – 80 mg of KTZ were suspended in 3 mL of solution, and cocrystal precipitation, where 50 – 80 mg of cocrystal and 100 – 150 mg of KTZ were suspended in 3 mL of near saturated solution of coformer. The suspensions were kept in water bath at 25 ± 0.1°C and magnetically stirred for up to 96 h. Solution samples (0.5 mL) were collected every 24 h and were filtered via centrifuge through a 0.45 µm pore cellulose acetate membrane, and the pH values were measured. Solid phases were analyzed by XRPD and DSC to determine that both drug and cocrystal solid phases were present. The filtered solutions were then analyzed by HPLC.
Cocrystal and Drug Powder Dissolution
Powder dissolution of drug and cocrystals was conducted using an overhead stirrer with a glass propeller at 150 rpm over 3 hours. 30mg of KTZ drug or 30 mg KTZ-equivalent amount of cocrystal were added to 30 mL of dissolution media. Both drug and cocrystal powders were sieved through mesh screens and particle sizes between 106 and 125 µm were used. The dissolution experiments were conducted in a water bath at 24.5 (± 0.5) °C. Solution pH was measured at the beginning and at the end of each dissolution experiment. Aliquots of 0.5 mL were taken with syringe at appropriate time points for up to 180 min. The solution samples were filtered through syringe filter with PVDF membrane of pore size of 0.45 µm. The solution concentrations of drug and coformers were analyzed with HPLC.
High Performance Liquid Chromatography (HPLC)
KTZ and coformer concentrations were analyzed by a Waters HPLC equipped with a UV spectrometer detector. A Waters Atlantis C18 column with the dimension of 5µm, 250 × 4.6 mm was used for separation at ambient temperature. The mobile phase was composed of 60% methanol and 40% water with 0.1% trifluoroacetic acid (TFA), and the flow rate was set at 1mL/min. The injection volume was 20µL, and the wavelengths used for the analytes were as follows: 230 nm for KTZ, 220 nm for FUM, and 210 nm for SUC and ADP.
X-Ray Powder Diffraction (XRPD)
A Rigaku Miniflex X-ray diffractometer (Danverse, MA) using Cu-Kα radiation, a tube voltage of 30 kV, and a tube current of 15 mA was utilized for analysis and characterization of solid phases. Measurements were taken from 5° to 40° at a continuous scan rate of 2.5°/min.
Thermal Analysis
TA instrument DSC (Newark, DE) was used to analyze the collected solid phases from the solubility studies, after they were dried at room temperature. The heating rate was 10°C/min under dry nitrogen atmosphere. Standard aluminum sample pans and lids were used for these measurements.
Results and Discussion
The evaluation of cocrystal solubility as a function of pH is a three-step process. First, drug and coformer concentrations in equilibrium with cocrystal are measured at pH values of interest. Second, cocrystal solubility and solubility product (Ksp) are determined from the measured equilibrium concentrations. Third, the pH dependence of cocrystal solubility is calculated from cocrystal Ksp, cocrystal component pKa values, and corresponding solubility equations. In this work, the equation that describes the solubility-pH dependence of 1:1 cocrystals composed of a dibasic drug (KTZ) and diprotic acidic coformers (CF) is presented and applied to understand the effects of pH on cocrystal solubility and dissolution.
Cocrystal solubility and Ksp
The expression that relates the cocrystal solubility with cocrystal components in solution has been known for some time5, 9, 17 and is given by
| (1) |
where Scc,T is the total cocrystal solubility, the terms in brackets represent molar concentrations under equilibrium conditions, and the subscript T represents total concentration of all species. Cocrystal solubility refers to stoichiometric solubility unless otherwise noted.
Cocrystal component concentrations and pH values at equilibrium with solid phases are presented in Table 1. Initial pH is observed to change even in buffered solutions from pH 1.01 – 8.04 to equilibrium pH values of 2.03 – 5.05. These observations are explained by the concentrations and ionization of the basic drug and acidic coformers at equilibrium. For solubility measurements, it is the equilibrium pH values that are relevant and are therefore used in the analysis presented here.
Table 1.
Cocrystal solubilities determined from KTZ and CF concentrations in equilibrium with cocrystal and drug phases, or with cocrystal at corresponding pH.
| Cocrystal KTZ-X |
Initial pH |
Equilibrium pH |
Solid phase(s) at equilibrium |
[KTZ]T
a (mM) |
[CF]T (mM) |
Scc,Tb (mM) |
|---|---|---|---|---|---|---|
| ADP | 1.01±0.01 | 2.66±0.01 | KTZ-ADP | 56.5±0.7 | 49±1 | 53±1 |
| 2.02±0.02 | 3.37±0.02 | KTZ + KTZ-ADP | 15.2±0.9 | 6.6±0.3 | 10.0±0.7 | |
| 5.00±0.01 | 4.64±0.01 | 0.51±0.02 | 17.6±0.2 | 3.0±0.1 | ||
| 8.04±0.01 | 5.04±0.01 | 0.188±0.004 | 66.9±0.5 | 3.5±0.1 | ||
| FUM | 1.01±0.01 | 2.03±0.01 | KTZ-FUM | 49.09±0.04 | 47.0±0.6 | 48.0±0.6 |
| 2.02±0.02 | 3.35±0.01 | KTZ + KTZ-FUM | 15.5±0.5 | 1.48±0.08 | 4.8±0.3 | |
| 5.00±0.01 | 4.34±0.01 | 1.09±0.06 | 22.6±0.8 | 5.0±0.3 | ||
| 8.04±0.01 | 4.52±0.01 | 0.67±0.04 | 48±1 | 5.7±0.4 | ||
| SUC | 1.01±0.01 | 2.52±0.01 | KTZ-SUC | 53.1±0.4 | 53.5±0.5 | 53.3±0.6 |
| 2.02±0.02 | 3.36±0.01 | KTZ + KTZ-SUC | 15.35±0.08 | 6.12±0.06 | 9.7±0.1 | |
| 5.00±0.01 | 4.63±0.01 | 0.50±0.02 | 8±2 | 2.0±0.5 | ||
| 8.04±0.01 | 5.05±0.01 | 0.21±0.01 | 45±1 | 3.1±0.2 |
. Drug concentration is the drug solubility in solutions saturated with respect to cocrystal and drug phases.
. Determined from Equation 1.
Two types of solid/solution equilibria were studied. The solid phases in equilibrium with solution were either a single solid phase (cocrystal) or two solid phases (cocrystal and drug). The latter represents a doubly saturated solution with respect to drug and cocrystal and is the eutectic point for these two solids and solution at a given pH and temperature. Since we are concerned with conversions between drug and cocrystal, this is the eutectic point considered.
Results in Table 1 show that cocrystal solubility is less affected by pH than drug solubility. Cocrystal solubility decreased by a lower extent than drug solubility with increasing pH. Cocrystals decreased by a factor of 3 or less whereas drug solubility decreased by a factor of 80 to 20 as pH increased from 3 to 5. A reversal in cocrystal and drug solubilities between pH 3 and 4 is also observed for all cocrystals, suggesting the existence of pHmax.8, 11, 12, 15–17 At pH 5, cocrystals are 10 to 20 times more soluble than drug. Drug is 1.5 to 3 times more soluble than cocrystal at pH 3.
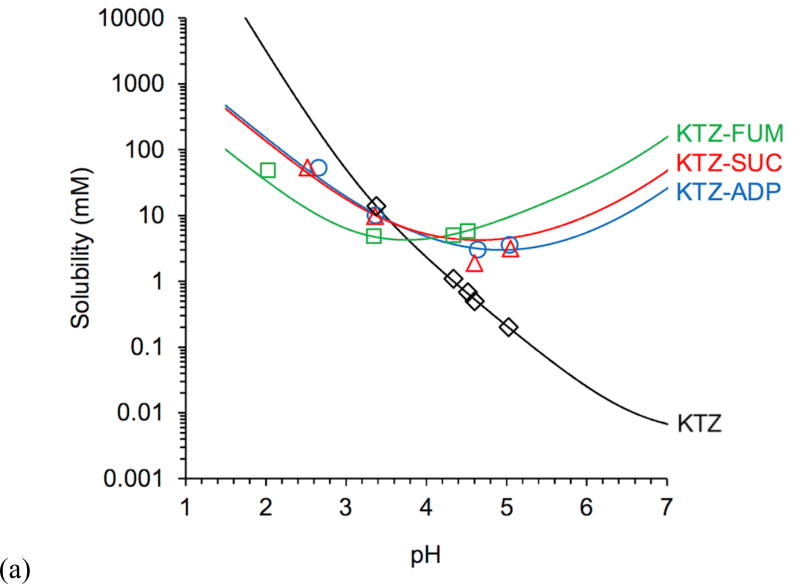
A plot of experimental and predicted cocrystal and drug solubility as a function of pH (Fig 1) shows that cocrystals changed the solubility-pH dependence from an exponentially decreasing curve for the drug to U-shaped curves for the cocrystals. Between gastric (1–3) and intestinal (6–7) pH,18, 20, 32 cocrystal solubility decreases by 3 to 6 fold, whereas KTZ solubility decreases by about 106 fold. This cocrystal solubility behavior has important implications for mitigating the pH effects on dependent bioavailability of poorly water-soluble (BCS class II) basic drugs like KTZ.1, 20, 23
Figure 1.
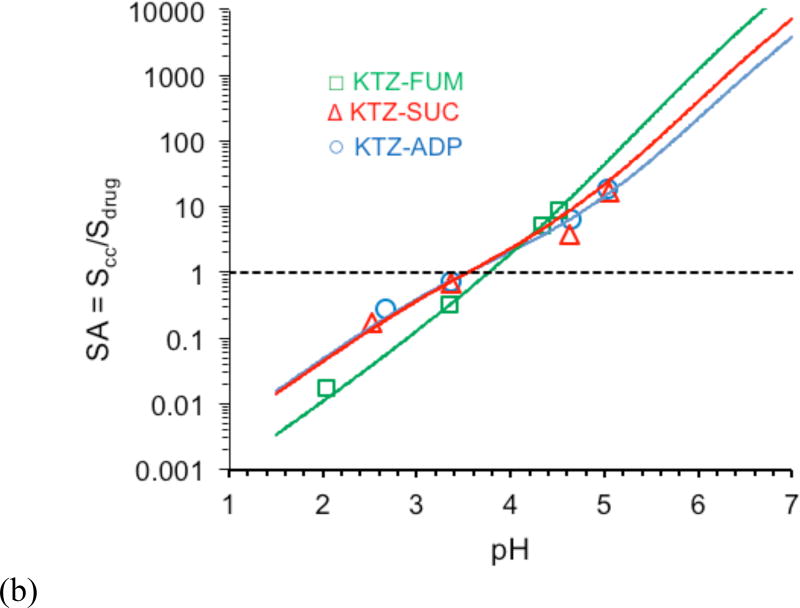
(a) Predicted (lines) and experimental (symbols) KTZ cocrystal and drug solubilities as a function of pH. Cocrystal solubility values were determined under equilibrium conditions from Equation 1. Solubility curves were generated from Equations 2 and 4 from cocrystal Ksp (Table 2), drug SKTZ,0 = 4.7 × 10−3 mM, and cocrystal component pKa values (Table 3). Several cocrystal solubility values are above KTZ solubility and are useful as supersaturation indicators. pH values correspond to equilibrium pH. The standard errors for experimental solubility values are less than 4% and are within the experimental data symbols. (b) Cocrystal solubility advantage over drug (SA = Scc/Sdrug) as a function of pH. Solid lines represent predicted SA based on Sdrug and Scc values calculated from equations 2 and 4 and appropriate parameters. The dotted line represents where the cocrystal solubility and drug solubility are equal and SA = 1. The standard errors for SA values are less than 7% and are within the experimental data points.
Figure 1b illustrates the strong influence of pH on cocrystal SA. SA is shown to increase by orders of magnitude with increasing pH. Clearly, such high supersaturations are not attainable but reveal the potential for cocrystal conversion to drug. This is applied to interpretation of cocrystal dissolution behavior of KTZ in a subsequent section.
An important feature of these plots is that they indicate the pH at which cocrystal solubility becomes equal to drug solubility or pHmax. This means that cocrystals are less soluble than drug at pH < pHmax, equally soluble to drug at pH = pHmax, and more soluble than drug at pH > pHmax. In other words, the cocrystal thermodynamic stability changes with pH. This is analogous to the well-known pHmax of salts, where the salt and free base or free acid solubility curves intersect, and the salt solubility is higher or lower than the non-ionized form of the drug depending on pH and pHmax.2, 34–37
The equation that describes cocrystal solubility (Equation 2) dependence on pH was derived by considering the equilibrium constants for cocrystal dissociation (solubility product or Ksp) and ionization (Ka) of its components as indicated by subscripts (derivation is presented in the supporting information).
| (2) |
The predictions show excellent agreement with experimental solubility behavior plotted in Figure 1.
The cocrystal solubility product
| (3) |
is the product of only the cocrystal components in the same molecular state as in the cocrystal, which for these cocrystals is the product of non-ionized species of KTZ and CF. Ksp values listed in Table 2 were obtained from linear regression analysis according to Equation 2 with Scc and pH values in Table 1 and reported pKa values in Table 3. Since Ksp values are very small, it is common to state them as pKsp values, where pKsp = −log Ksp. pKsp values of 1:1 cocrystals of BCS class II drugs have been reported to be in the range of 1 to 9, with higher values corresponding to lower Ksp.38
Table 2.
Ksp of KTZ cocrystals.
| Cocrystal | Ksp (M2) | pKspa |
|---|---|---|
| KTZ-ADP | 3.4(±0.2) × 10−8 | 7.5 |
| KTZ-SUC | 2.7(±0.1) × 10−8 | 7.6 |
| KTZ-FUM | 1.5(±0.2) × 10−9 | 8.8 |
. pKsp = −log(Ksp).
Table 3.
Cocrystal component pKa values.
Among the three cocrystals studied, KTZ-FUM was the least soluble cocrystal at pH < 4 and the most soluble above pH 4. This behavior is explained by Equation 2, as this cocrystal has the lowest Ksp and pKa values. Lower Ksp implies lower intrinsic solubility, but as coformer ionization increases so does cocrystal solubility. Lower pKa values of FUM mean greater coformer ionization at pH values lower than for the other coformers, and explain why the KTZ-FUM cocrystal becomes more soluble than KTZ-SUC and KTZ-ADP cocrystals above pH 4.
Drug solubility varies with pH according to the well-known equation
| (4) |
where Sdrug,T is the total KTZ solubility, the subscript 0 represents the intrinsic KTZ solubility (non-ionized drug solubility), and pKa,n represents the ionization constants of KTZ. KTZ solubility was experimentally measured at several pH values (Table 1, [KTZ]T at eutectic = Sdrug,T) and the intrinsic solubility (SKTZ,0) was determined to be 4.7 (±0.2) × 10−3 mM by fitting Equation 4.
Our findings on cocrystal and drug solubilities are not in agreement with those reported by Martin et al.29 Cocrystals were reported to be 75 to 100 times more soluble than KTZ, but pH values corresponding to these solubilities were not considered.29 Solubility studies for KTZ-FUM, KTZ-ADP, and KTZ-SUC cocrystals were carried out in DI water, and the final pH values were reported to be 3.8, 3.9, and 4.1, respectively.29 Unfortunately, the pH corresponding to KTZ solubility was not reported nor considered in the comparison of drug and cocrystals. A saturated solution of KTZ has a pH of 8. KTZ is a basic compound and will increase the pH of aqueous solutions, which is in contrast to the cocrystals that will lower the pH as they have acidic coformers. Therefore, the comparison between cocrystal and drug solubilities in these dissolution/solubility studies is not representative of the cocrystal true solubility advantage because they were conducted under different pH conditions.
The Martin et al. study29 also reported that in spite of the high cocrystal solubility enhancement, there was no conversion to the less soluble drug. The reason for this behavior is that their cocrystal studies were close to pHmax (Table 4), at which drug and cocrystal solubilities are equal. In fact, based on the final pH, KTZ-ADP and KTZ-SUC are only 1.7 and 2.5 times more soluble than the drug, respectively, while KTZ-FUM is equally soluble to the drug. The cocrystal solubility/dissolution advantages are therefore much lower than the enhancements originally suggested by the authors of this fine publication.
Table 4.
KTZ cocrystal pHmax and solubility at pHmax.
| Cocrystal | pHmax | Scc,pHmax (mM) |
|---|---|---|
| KTZ-ADP | 3.6 | 8.2 |
| KTZ-SUC | 3.6 | 7.8 |
| KTZ-FUM | 3.8 | 4.2 |
pHmax and Scc,pHmax
Table 4 shows the pHmax and corresponding Scc (Scc, pHmax) for KTZ cocrystals. While pHmax values are in a narrow pH range for all cocrystals, Scc, pHmax is higher for KTZ-ADP and KTZ-SUC cocrystals than for KTZ-FUM cocrystal.
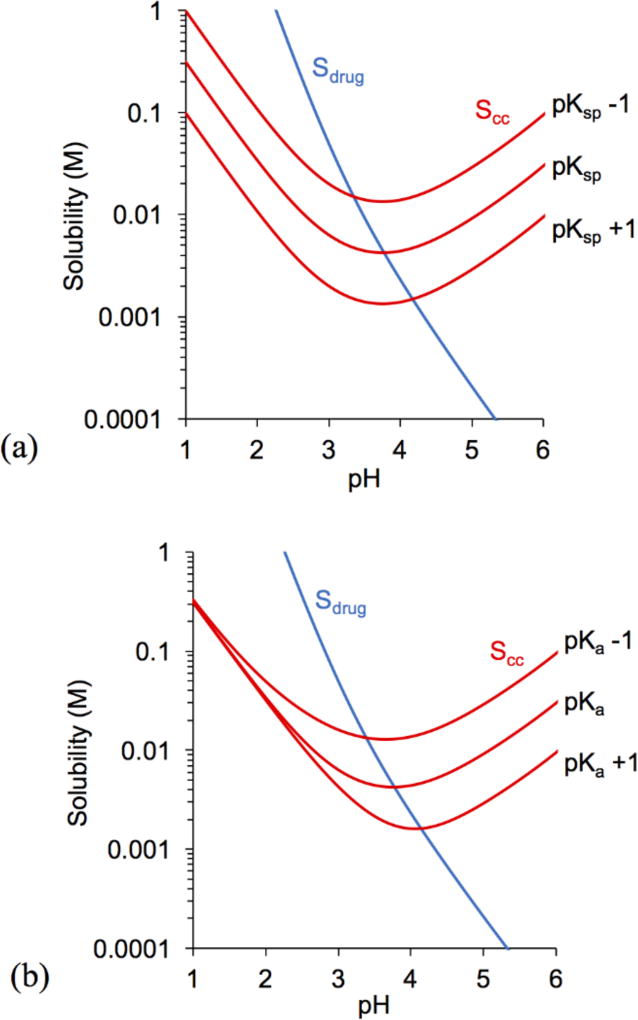
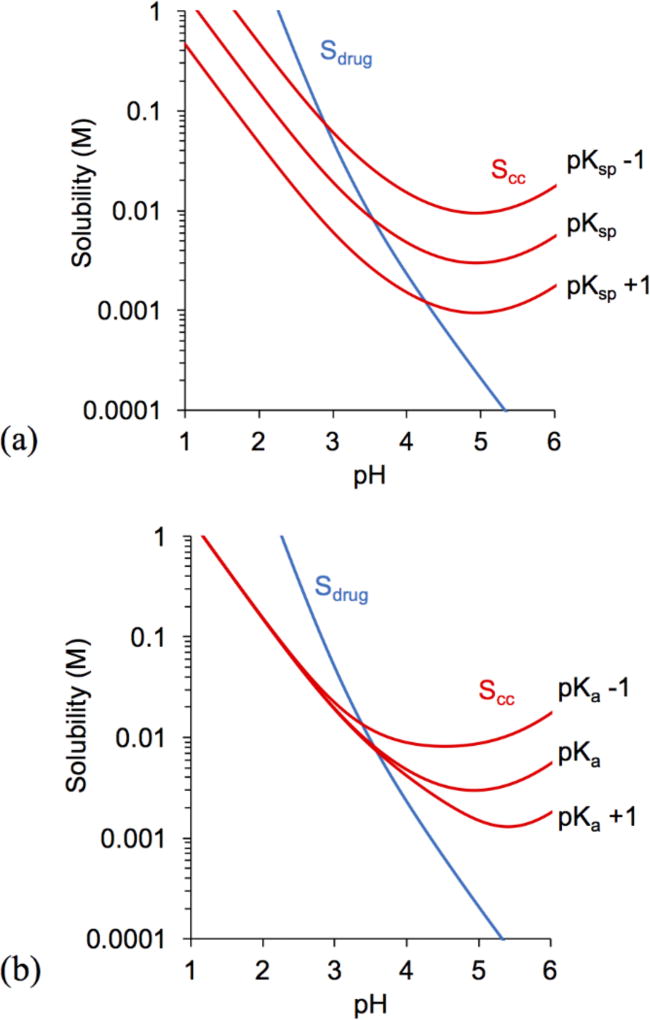
The influence of pKsp and pKa on cocrystal solubility and pHmax is illustrated in Figures 2 and 3 by considering the values in Tables 2, 3, and 4. Changing cocrystal pKsp resulted in parallel shifts in cocrystal solubility curves, whereas changing coformer pKa (pKa1,CF), altered the curvature. Scc,pHmax was found to exhibit an inverse relationship with pKsp and pKa, while pHmax was directly proportional.
The influence of cocrystal pKsp and coformer pKa on pHmax and Scc,pHmax was examined from simulations of cocrystal solubility equations for two hypothetical cocrystals with properties similar to KTZ-FUM and KTZ-ADP cocrystals. For a cocrystal with the properties of KTZ-FUM and its components (Fig. 2) each unit change in pKsp or in pKa1,CF predicts a change in pHmax of ~ 0.4 units and in Scc,pHmax of ~ 3 fold.
Figure 2.
Influence of (a) cocrystal pKsp and (b) coformer pKa (pKa1,CF) on KTZ-FUM solubility and pHmax. Drug and cocrystal solubility curves were generated using Equations 2 and 4 with the initial parameter values of SKTZ,0 = 4.7 × 10−6 M and KTZ-FUM pKsp, pKa,KTZ, and pKa,CF from Tables 2 and 3. pKsp changes by 1 unit for every magnitude (10 fold) change of Ksp. Only the first pKa of the coformer (pKa1,CF) was altered in plot (b) while pKa2,CF remained unchanged.
For cocrystals with pKsp and pKa similar to KTZ-ADP the solubility-pH dependence is shown in Fig. 3. One unit change in pKsp predicts a change in pHmax of ~ 0.7 units and in Scc,pHmax of 7 – 9 fold. Changes in pKa predict a smaller influence on pHmax and Scc,pHmax than changes in pKsp. Understanding the influence of cocrystal components on pHmax and Scc,pHmax is important to guide selection of the right cocrystal to meet desired drug exposure. Predictions for cocrystal KTZ-SUC (not shown) were similar to KTZ-ADP.
Figure 3.
Influence of (a) cocrystal pKsp, where, pKsp = −log(Ksp), and (b) coformer pKa (pKa1,CF) on KTZ-ADP solubility and pHmax. Drug and cocrystal solubility curves were generated using Equations 2 and 4 with the initial parameter values of SKTZ,0 = 4.7 × 10−6 M and KTZ-ADP pKsp, pKa,KTZ, and pKa,CF from Tables 2 and 3. pKsp changes by 1 unit for every magnitude (10 fold) change of Ksp.
Cocrystal eutectic constant and supersaturation index
The concept of cocrystal eutectic constant is usefully applied to determine the cocrystal to drug solubility ratio and supersaturation index (SA = Scc/Sdrug). The eutectic constant (Keu) has been defined as the ratio of coformer to drug concentration at the eutectic point, which for a 1:1 cocrystal is given by
| (5) |
Keu is a measure of the cocrystal SA according to
| (6) |
The significance of this relationship in obtaining cocrystal supersaturation index and thermodynamic stability has been demonstrated for numerous cocrystals in a wide range of solvents, ionization, complexation, and solubilization conditions.11, 12, 42 Keu <1, =1, or >1 corresponds to cocrystals that are less, equally, or more soluble than drug. Its relationship with SA provides a quantitative measure of the driving force for drug precipitation.
The ratio of equilibrium molar concentrations of coformer to drug (Table 1) is also observed to change with pH and is different from their molar ratio in the cocrystal (1:1). This observation has several implications. First, in solutions saturated with only cocrystal, a different ratio is a result of batch impurity, which was taken into account in the solubility determination according to Equation 1. Second, in solutions saturated with both cocrystal and drug phases (eutectic point), the molar ratio [CF]T/[drug]T is related to the cocrystal SA, according to equations 5 and 6 as shown in Figure 4.
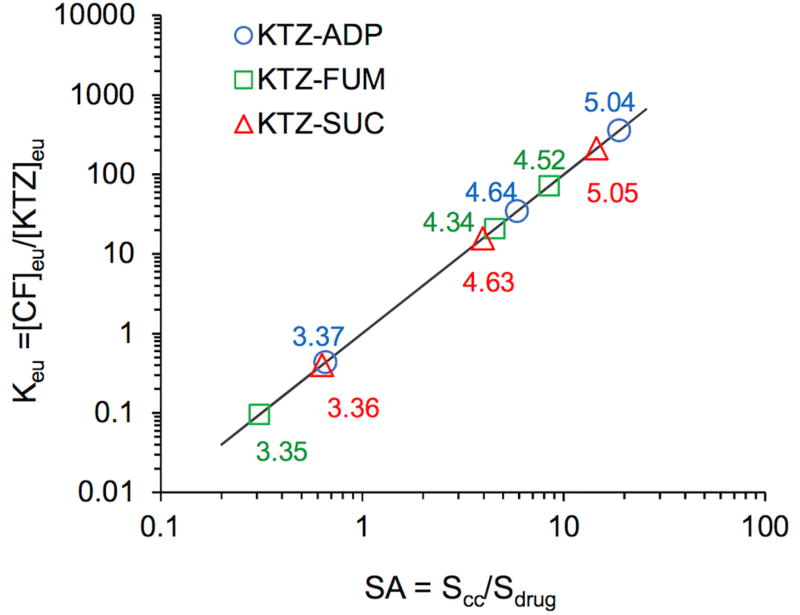
Figure 4.
Relationship between Keu and cocrystal solubility advantage or supersaturation index (SA=Scc/Sdrug) for KTZ cocrystals. The numbers by the symbols are equilibrium pH values. Line was generated from log form of Equation 6, log Keu = 2 log(Scc/Sdrug). Keu = 1 corresponds to pHmax. Standard errors of Keu values are less than 4% for all systems, except for KTZ-SUC at pH 4.63, which is 11%. Standard errors are within the experimental data points.
Figure 4 shows the dependence of Keu on SA for KTZ cocrystals and the importance of pH. Small changes in pH lead to order of magnitude changes in Keu and to changes in SA. Experimentally determined Keu values were <1 (0.1 to 0.4) at pH 3.4 and > 1 (16 to 400) at pH 4.3 – 5.0. The corresponding SA values were between 0.3 to 0.6 at pH 3.4, and 4 to 20 at pH 4.3 – 5.0. The existence of pHmax for all these cocrystals (at Keu = 1) is also obtained from this analysis. Measurement of Keu and calculation of SA provide a simple yet meaningful basis to quantitatively assess the risk of cocrystal conversions to the less soluble drug during cocrystal dissolution as presented in the following section.9, 15, 17
Cocrystal Dissolution
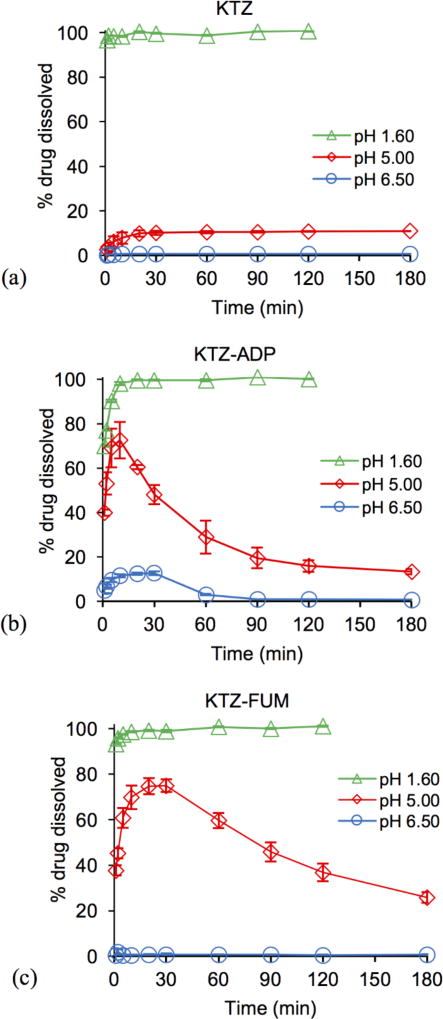
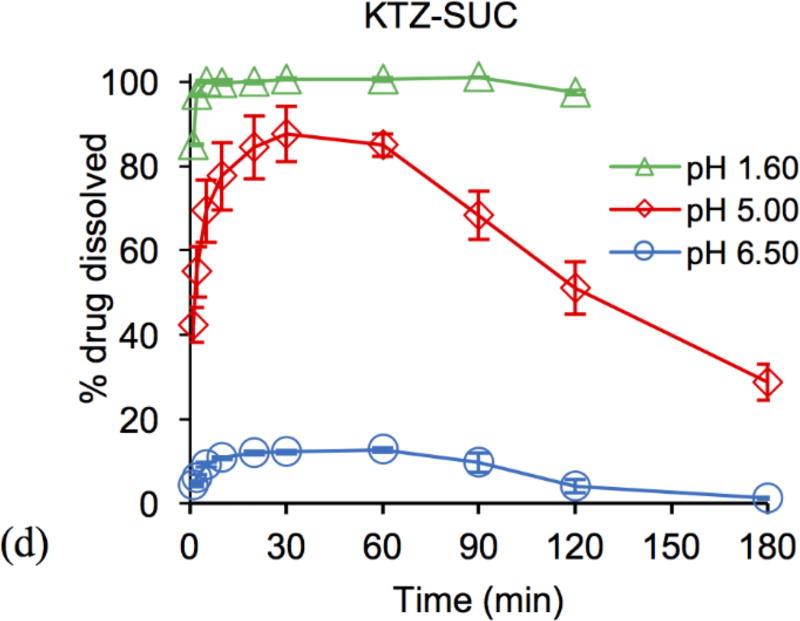
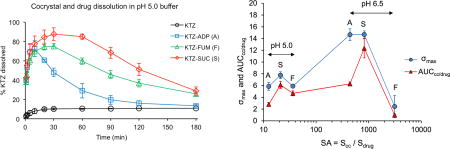
Cocrystal and drug dissolution studies were carried out under pH conditions relevant to those encountered in the gastrointestinal tract: pH 1.6, 5.0, and 6.5. Results in Figure 5 demonstrate that cocrystals achieve much higher drug concentrations than drug at pH of 5 and 6.5. The shape of the concentration-time profile curves for cocrystals and existence of Cmax indicates dissolution-precipitation behavior. Cocrystal dissolution generates supersaturation with respect to KTZ at levels that are sustained for up to 3 h at pH 5 and for about 1 h at pH 6.5. This finding suggests that cocrystals may increase drug exposure under similar conditions.
Figure 5.
Percent KTZ dissolved during drug (a) and cocrystal (b–d) dissolution at initial pH values relevant to the pH of the fluid in the gastrointestinal tract. % drug dissolved was calculated from the ratio of measured KTZ in solution as a function of time to the theoretical concentration from the initial mass added, 100 × [KTZ] dissolved / [KTZ] total cocrystal or pure drug added. Error bars represent standard errors.
In examining the dissolution results above we consider the dose and solubility of drug and cocrystals (Fig. 1). The mass of pure drug and cocrystals used was 1 mg KTZ equivalent per mL (1.9 mM), which corresponds to the oral dose of KTZ 200 mg if dissolved in 200 mL.1, 25, 43–45 The dose is below KTZ solubility at pH 1.6, but at pH 5 and 6.5 it is 9 and 173 times above drug solubility. The dose is below cocrystal solubility at all pH values studied and will generate supersaturation with respect to KTZ above pH 4. This means that drug will fully dissolve at pH 1.6, and cocrystals will fully dissolve at all pH values studied.
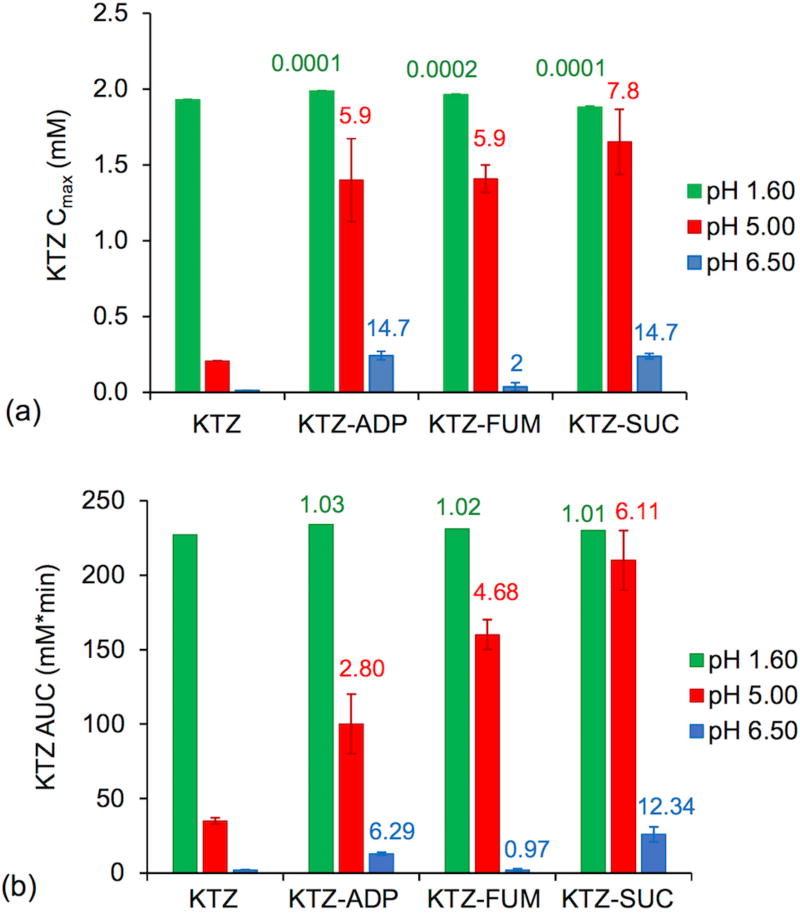
The maximum concentration is expressed in terms of supersaturation (σmax = Cmax/Sdrug) and represents the supersaturation above which drug precipitation is faster than cocrystal dissolution. Results in Figure 6a show that cocrystal Cmax values were generally higher than for drug. σmax increased with pH and so did its variability among cocrystals. At pH 5, σmax was in the range of 6 to 8 and at pH 6.5 in the range of 1–15. KTZ-FUM σmax was very low (1–2) compared to the other cocrystals (σmax 15), suggesting very fast drug precipitation that depleted KTZ levels to values close to drug solubility (σmax 1). The rapid cocrystal to drug conversion rates in this case may be a result of transient and higher supersaturation levels that eluded detection.
Figure 6.
(a) Cmax of KTZ during dissolution and (b) AUC of KTZ from 0 – 180 min for dissolution in pH 5.0 and 6.5 media, and from 0 – 120 min for dissolution in pH 1.6 media. Numbers on top of the columns represent (a) σmax and (b) AUC ratio of cocrystal to drug (AUCcc/drug). pH values in legend indicate initial media pH.
It is important to note that the buffer pH changed during dissolution (supporting information), and that although these changes appear to be small (less than 0.3 pH units in this study), they can lead to significant and sometimes substantial changes in solubility and supersaturation. The values of σmax were thus calculated from drug solubility values corresponding to the measured dissolution media pH.
The areas under the dissolution curves (AUCs) during pure drug and cocrystal dissolution are shown in Figure 6b. AUC represents the drug exposure and is determined by the interplay between cocrystal dissolution and drug precipitation rates. AUC is directly proportional to dissolution and inversely proportional to precipitation rates.
The results in Fig. 6 show that Cmax and AUC decreased with increasing pH for both drug and cocrystals, but the effect on cocrystals is weaker. Between pH 5 and 6.5, Cmax was 7 to 20 times higher than for drug and AUC was as high as 12 times. Even when cocrystals transformed to drug, they still outperformed pure drug dissolution in every case except for KTZ-FUM in pH 6.5, where the conversion to drug might have been too rapid to allow for concentration enhancements.
Comparing the behavior of cocrystals at pH 5 and 6.5, it is clear that lower Cmax and AUC values correspond to higher σmax values, with the exception of FUM cocrystal. At pH 5, σmax is in the range of 6 to 8, whereas at pH 6.5 it is 15 (ADP and SUC cocrystals) and 2 (FUM cocrystal). AUC reveals how long these supersaturated states were sustained (Fig. 6b). AUCs were found to be higher at pH 5 than at pH 6.5, suggesting that the cocrystal conversion rate to drug was faster at pH 6.5 for all cocrystals.
Since cocrystals will experience the highest supersaturation at the dissolving surface, where the solution is saturated with cocrystal,46, 47 we considered the supersaturation index as the driving force for cocrystal to drug conversion. As SA increases, the expected higher drug levels during cocrystal dissolution may be dampened by a faster precipitation to the less soluble drug.
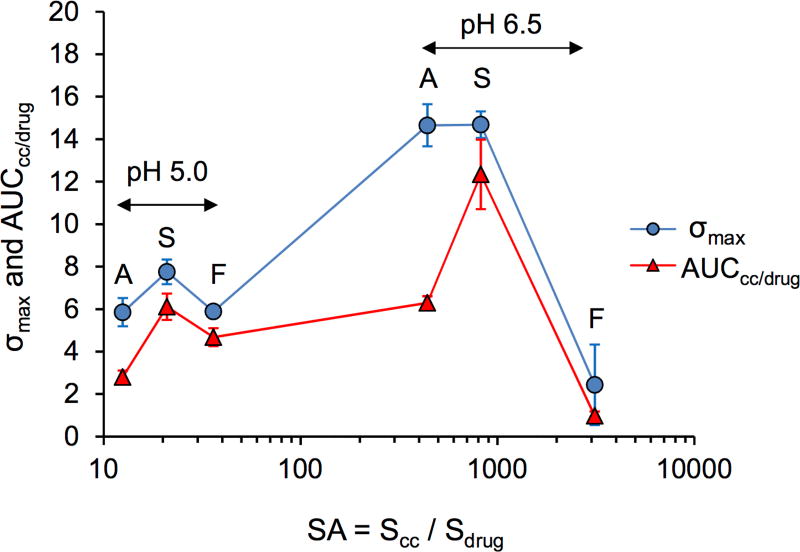
The significance of SA on the conversion rate to drug can be appreciated from the plot of σmax and AUCcc/drug vs SA in Fig. 7. AUCcc/drug is the ratio of cocrystal to drug dissolution AUC values, and it represents KTZ exposure from cocrystal relative to pure drug.
Figure 7.
Cocrystal σmax and AUCcc/drug as a function of cocrystal supersaturation index (SA). A, S, and F correspond to ADP, SUC, and FUM cocrystals, respectively.
The range of SA values was found to be as low as 13 (ADP cocrystal, pH 5) and as high as 3,100 (FUM cocrystal, pH 6.5). One would anticipate that such a high SA (3100) might not be sustained for very long if at all. In fact, this high SA led to the lowest σmax and AUCcc/drug of all cocrystals and pH conditions. SA values between 13 and 40 led to enhancements in both σmax and AUCcc/drug for all cocrystals, while SA values of 440 to 3,100 led to variable behavior. The SUC cocrystal achieved the highest σmax and AUCcc/drug at SA of 822. In contrast, a lower SA (440 for ADP cocrystal) reached a high σmax but a much lower AUCcc/drug. It appeared that the SUC cocrystal experienced the highest exposure levels with the slowest rate of conversion to drug among these cocrystals. This may be a consequence of coformer effect on KTZ precipitation or cocrystal surface influence on nucleation.
Conclusion
This work demonstrates that cocrystals of a weakly basic drug with acidic coformers can greatly reduce the negative effects of decreasing drug solubility and dissolution with increasing pH. Solubility-pH dependence of cocrystals, pHmax, and SA can be calculated from knowledge of cocrystal Ksp, component pKa(s), and drug intrinsic solubility. Cocrystal solubility increases over drug translated to huge dissolution advantage. SA provides a framework to interpret cocrystal dissolution-precipitation behavior, σmax and AUCdiss advantage over drug. In this limited number of cocrystals, SA values as high as 800 were associated with enhanced drug exposure during dissolution, whereas SA of 3,000 led to limited exposure, very rapid conversions, and no measurable supersaturation. SA is readily calculated from cocrystal and drug solubility equations and provides a useful metric to assess the risk of cocrystal conversions.
Supplementary Material
Synopsis.
Cocrystals of basic drugs with acidic coformers can mitigate the negative effects of high pH on drug solubility and dissolution. Ketoconazole cocrystals enhance the dissolution-pH dependence compared to drug. Cocrystal solubility advantage (SA) values are used to interpret drug exposure levels during dissolution and the potential for rapid conversions of cocrystal to the less soluble drug.
Acknowledgments
Research reported in this publication was partially supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM107146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also gratefully acknowledge partial financial support from the College of Pharmacy at the University of Michigan.
References
- 1.Amidon G, Lennernäs H, Shah V, Crison J. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharmaceutical Research. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Serajuddin ATM. Salt formation to improve drug solubility. Advanced Drug Delivery Reviews. 2007;59(7):603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Serajuddin ATM. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. Journal of Pharmaceutical Sciences. 1999;88(10):1058–1066. doi: 10.1021/js980403l. [DOI] [PubMed] [Google Scholar]
- 4.Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. Journal of Pharmaceutical Sciences. 1997;86(1):1–12. doi: 10.1021/js9601896. [DOI] [PubMed] [Google Scholar]
- 5.Good DJ, Rodríguez-Hornedo Nr. Solubility Advantage of Pharmaceutical Cocrystals. Crystal Growth & Design. 2009;9(5):2252–2264. [Google Scholar]
- 6.Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. International Journal of Pharmaceutics. 2013;453(1):101–125. doi: 10.1016/j.ijpharm.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 7.Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? Journal of Pharmaceutical Sciences. 2009;98(8):2549–2572. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]
- 8.Roy L, Lipert MP, Rodriguez-Hornedo N. Pharmaceutical Salts and Co-crystals. The Royal Society of Chemistry; 2012. Co-crystal Solubility and Thermodynamic Stability; pp. 247–279. [Google Scholar]
- 9.Alhalaweh A, Roy L, Rodríguez-Hornedo N, Velaga SP. pH-Dependent Solubility of Indomethacin–Saccharin and Carbamazepine–Saccharin Cocrystals in Aqueous Media. Molecular Pharmaceutics. 2012;9(9):2605–2612. doi: 10.1021/mp300189b. [DOI] [PubMed] [Google Scholar]
- 10.Maheshwari C, André V, Reddy S, Roy L, Duarte T, Rodríguez-Hornedo N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: effect of coformer ionization, pH max and solute–solvent interactions. CrystEngComm. 2012;14(14):4801–4811. [Google Scholar]
- 11.Bethune SJ, Huang N, Jayasankar A, Rodríguez-Hornedo Nr. Understanding and Predicting the Effect of Cocrystal Components and pH on Cocrystal Solubility. Crystal Growth & Design. 2009;9(9):3976–3988. [Google Scholar]
- 12.Huang N, Rodríguez-Hornedo N. Engineering cocrystal solubility, stability, and pHmax by micellar solubilization. Journal of Pharmaceutical Sciences. 2011;100(12):5219–5234. doi: 10.1002/jps.22725. [DOI] [PubMed] [Google Scholar]
- 13.Lipert MP, Rodríguez-Hornedo N. Cocrystal Transition Points: Role of Cocrystal Solubility, Drug Solubility, and Solubilizing Agents. Molecular Pharmaceutics. 2015 doi: 10.1021/acs.molpharmaceut.5b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang N, Rodríguez-Hornedo Nr. Effect of Micellar Solubilization on Cocrystal Solubility and Stability. Crystal Growth & Design. 2010;10(5):2050–2053. [Google Scholar]
- 15.Kuminek G, Rodriguez-Hornedo N, Siedler S, Rocha HVA, Cuffini SL, Cardoso SG. How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chemical Communications. 2016;52(34):5832–5835. doi: 10.1039/c6cc00898d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy LS, Bethune SJ, Kampf JW, Rodríguez-Hornedo N. Cocrystals and Salts of Gabapentin: pH Dependent Cocrystal Stability and Solubility. Crystal Growth & Design. 2009;9(1):378–385. [Google Scholar]
- 17.Kuminek G, Cao F, Bahia de Oliveira da Rocha A, Gonçalves Cardoso S, Rodríguez-Hornedo N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Advanced Drug Delivery Reviews. 2016;101:143–166. doi: 10.1016/j.addr.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dressman J, Berardi R, Dermentzoglou L, Russell T, Schmaltz S, Barnett J, Jarvenpaa K. Upper Gastrointestinal (GI) pH in Young, Healthy Men and Women. Pharmaceutical Research. 1990;7(7):756–761. doi: 10.1023/a:1015827908309. [DOI] [PubMed] [Google Scholar]
- 19.Hörter D, Dressman J. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Advanced Drug Delivery Reviews. 2001;46(1):75–87. doi: 10.1016/s0169-409x(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 20.Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of Various Dissolution Media for Predicting In Vivo Performance of Class I and II Drugs. Pharmaceutical Research. 1998;15(5):698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 21.Dressman JB, Reppas C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. European Journal of Pharmaceutical Sciences. 2000;11(Supplement 2):S73–S80. doi: 10.1016/s0928-0987(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 22.Kostewicz ES, Brauns U, Becker R, Dressman JB. Forecasting the Oral Absorption Behavior of Poorly Soluble Weak Bases Using Solubility and Dissolution Studies in Biorelevant Media. Pharmaceutical Research. 2002;19(3):345–349. doi: 10.1023/a:1014407421366. [DOI] [PubMed] [Google Scholar]
- 23.Zhou R, Moench P, Heran C, Lu X, Mathias N, Faria TN, Wall DA, Hussain MA, Smith RL, Sun D. pH-Dependent Dissolution in Vitro and Absorption in Vivo of Weakly Basic Drugs: Development of a Canine Model. Pharmaceutical Research. 2005;22(2):188–192. doi: 10.1007/s11095-004-1185-3. [DOI] [PubMed] [Google Scholar]
- 24.Van der Meer JWM, Keuning JJ, Scheijgrond HW, Heykants J, Van Cutsem J, Brugmans J. The influence of gastric acidity on the bio-availability of ketoconazole. Journal of Antimicrobial Chemotherapy. 1980;6(4):552–554. doi: 10.1093/jac/6.4.552. [DOI] [PubMed] [Google Scholar]
- 25.Nizoral (Ketoconazole) Tablets Drug Label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/018533s041lbl.pdf.
- 26.Yasui A, Hoeft S, Stein H, DeMeester T, Bremner R, Nimura Y. An Alkaline Stomach Is Common to Barrett’s Esophagus and Gastric Carcinoma. In: Nabeya K-i, Hanaoka T, Nogami H., editors. Recent Advances in Diseases of the Esophagus. Springer; Japan: 1993. pp. 169–172. [Google Scholar]
- 27.Welage LS, Carver PL, Revankar S, Pierson C, Kauffman CA. Alterations in Gastric Acidity in Patients Infected with Human Immunodeficiency Virus. Clinical Infectious Diseases. 1995;21(6):1431–1438. doi: 10.1093/clinids/21.6.1431. [DOI] [PubMed] [Google Scholar]
- 28.Geraghty J, Thumbs A, Kankwatira A, Andrews T, Moore A, Malamba R, Mtunthama N, Hellberg K, Kalongolera L, O’Toole P, Varro A, Pritchard DM, Gordon M. Helicobacter pylori, HIV and Gastric Hypochlorhydria in the Malawian Population. PLOS ONE. 2015;10(8):e0132043. doi: 10.1371/journal.pone.0132043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin FA, Pop MM, Borodi G, Filip X, Kacso I. Ketoconazole Salt and Co-crystals with Enhanced Aqueous Solubility. Crystal Growth & Design. 2013;13(10):4295–4304. [Google Scholar]
- 30.Rodríguez-Hornedo N, Nehm SJ, Seefeldt KF, Pagán-Torres Y, Falkiewicz CJ. Reaction Crystallization of Pharmaceutical Molecular Complexes. Molecular Pharmaceutics. 2006;3(3):362–367. doi: 10.1021/mp050099m. [DOI] [PubMed] [Google Scholar]
- 31.Nehm SJ, Rodríguez-Spong B, Rodríguez-Hornedo N. Phase Solubility Diagrams of Cocrystals Are Explained by Solubility Product and Solution Complexation. Crystal Growth & Design. 2005;6(2):592–600. [Google Scholar]
- 32.Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution Media Simulating Conditions in the Proximal Human Gastrointestinal Tract: An Update. Pharmaceutical Research. 2008;25(7):1663–1676. doi: 10.1007/s11095-008-9569-4. [DOI] [PubMed] [Google Scholar]
- 33.Good DJ, Rodriguez-Hornedo N. Cocrystal Eutectic Constants and Prediction of Solubility Behavior. Crystal Growth & Design. 2010;10(3):1028–1032. [Google Scholar]
- 34.Stephenson GA, Aburub A, Woods TA. Physical stability of salts of weak bases in the solid-state. Journal of Pharmaceutical Sciences. 2011;100(5):1607–1617. doi: 10.1002/jps.22405. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh Y-L, Merritt JM, Yu W, Taylor LS. Salt stability–the effect of pHmax on salt to free base conversion. Pharmaceutical research. 2015;32(9):3110–3118. doi: 10.1007/s11095-015-1691-5. [DOI] [PubMed] [Google Scholar]
- 36.Nie H, Byrn SR, Zhou Q. Stability of pharmaceutical salts in solid oral dosage forms. Drug Development and Industrial Pharmacy. 2017;43(8):1215–1228. doi: 10.1080/03639045.2017.1304960. [DOI] [PubMed] [Google Scholar]
- 37.Thakral NK, Kelly RC. Salt disproportionation: A material science perspective. International journal of pharmaceutics. 2017 doi: 10.1016/j.ijpharm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Kuminek G, Cavanagh K, Rodríguez-Hornedo N. Pharmaceutical Crystals: Science and Engineering. John Wiley & Sons; 2017. Measurement and mathematical relationships of cocrystal thermodynamic properties. Submitted. [Google Scholar]
- 39.Avdeef A. Absorption and drug development solubility, permeability, and charge state. 2. John Wiley & Sons; Hoboken, N.J.: 2012. p. xli.p. 698. [Google Scholar]
- 40.Serjeant EP, Dempsey B. Ionisation constants of organic acids in aqueous solution. Pergamon Press; Oxford; New York: 1979. p. xi.p. 989. [Google Scholar]
- 41.Smith R, Martell A. Critical Stability Constants. Vol. 6. Springer; US: 1989. Carboxylic Acids; pp. 299–359. [Google Scholar]
- 42.Lipert MP. Dissertations and Theses. University of Michigan; 2015. Predicting the Influence of Drug Solubilizing Agents on Cocrystal Solubility, Stability, and Transition Points. [Google Scholar]
- 43.Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. European Journal of Pharmaceutics and Biopharmaceutics. 2005;60(3):413–417. doi: 10.1016/j.ejpb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Molecular pharmaceutics. 2010;7(5):1388–1405. doi: 10.1021/mp100149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Männistö PT, M R, Nykänen S, Lamminsivu U, Ottoila P. Impairing effect of food on ketoconazole absorption. Antimicrobial Agents and Chemotherapy. 1982;21(5):730–733. doi: 10.1128/aac.21.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao F, Amidon GL, Rodriguez-Hornedo N, Amidon GE. Mechanistic Analysis of Cocrystal Dissolution as a Function of pH and Micellar Solubilization. Molecular Pharmaceutics. 2016;13(3):1030–1046. doi: 10.1021/acs.molpharmaceut.5b00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao F. Dissertations and Theses. University of Michigan; 2016. Mechanistic Analysis of Cocrystal Dissolution: Impact of Physicochemical Properties, pH, Surfactant and Buffer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.