Abstract
The use of implants that can electrically stimulate or record electrophysiological or neurochemical activity in nervous tissue is rapidly expanding. Despite remarkable results in clinical studies and increasing market approvals, the mechanisms underlying the therapeutic effects of neuroprosthetic and neuromodulation devices, as well as their side effects and reasons for their failure, remain poorly understood. A major assumption has been that the signal-generating neurons are the only important target cells of neural-interface technologies. However, recent evidence indicates that the supporting glial cells remodel the structure and function of neuronal networks and are an effector of stimulation-based therapy. Here, we reframe the traditional view of glia as a passive barrier, and discuss their role as an active determinant of the outcomes of device implantation. We also discuss the implications that this has on the development of bioelectronic medical devices.
There are more connections between neurons in the human brain than there are stars in our galaxy1, and there are at least a dozen specific neuronal subtypes in the brain that are recognized as unique on the basis of their distinctive functional and morphological characteristics2,3. There is also growing recognition that non-neuronal supporting cells are more diverse and dynamic than previously appreciated, with distinct classes and subclasses of glia actively shaping the structure and function of neural circuitry4. Although such complexity is a likely requisite for the ability to internalize, integrate and respond to the continuous streams of information that the brain must process, it also makes the effective treatment of neurological disorders especially challenging. In recent years, the development and design of new implantable-device technologies to read-out and write-in electrical and chemical signals to and from the nervous system have created unprecedented opportunities to understand normal brain function and to ameliorate dysfunction resulting from disease or injury.
Although research and clinical applications of implanted electrode arrays continue to experience rapid growth, their usage has outpaced the clear understanding of the mechanisms underlying their benefits, side effects and modes of failure. Originally a precision academic-research tool to measure and modulate neural circuitry at subsecond and submillimetre resolution, implanted electrode arrays have increasingly been used in the clinic to treat an expanding array of medical conditions. Reports in the late 1980s and early 1990s demonstrated compelling preliminary clinical efficacy of deep brain stimulation (DBS) for tremor as a safer alternative to thalamotomy or pallidotomy in medically intractable Parkinson’s disease5. Although the mechanisms underlying its benefits remain the subject of debate6, DBS has since been approved by the US Food and Drug Administration for Parkinson’s disease, essential tremor, obsessive compulsive disorder, dystonia and refractory epilepsy5. Therapeutic indications currently being pursued in clinical studies are rapidly expanding, and include Alzheimer’s disease, depression, Tourette’s syndrome, deafness, blindness, and strategies to promote plasticity in cases of severe stroke or tinnitus6. Electrophysiological and neurochemical recordings have gained traction as a diagnostic tool, as an enabling technology for brain/machine interfaces in paralysis patients and as biomarkers to inform strategies for closed-loop stimulation devices7.
The successful use of chronically implanted neuroprostheses is predicated on the ability to reliably modulate or record signals from surrounding neurons over time (preferably, for many years). This is true for the broad range of clinical and research applications pursued, and for the variety of methods of read-out or write-in of neural activity employed (such as optical or electrical)8. However, problems arising from small signal amplitudes and from signal instability plague implanted recording arrays, limiting their long-term function9–13. Signal amplitudes typically shift on a daily basis14, compromising the likelihood that spike detection crosses the required threshold. This can, in turn, affect apparent firing rates, contributing to the non-stationarity that burdens the use of these signals for prosthetic control13. Studies across animal models often report progressive losses in signal detection in the weeks following implantation14,15. In recordings taken from human subjects, significant changes in unit amplitudes were observed on an intraday basis13. Many of these shifts seem to be related to device micromotion (based on simultaneous effects observed across electrode sites)13, but the vast majority were attributed to a physiological origin (85%). Likewise, in applications that stimulate the central nervous system, desensitization can occur following chronic microstimulation, and inexplicably large placebo effects can follow implantation of non-functional devices16,17. A variety of factors, both biological and non-biological, have been proposed to contribute to observations of instability in neural recordings and to the variable thresholds of neurostimulation10,18. Among these, suboptimal biocompatibility and suboptimal integration with surrounding tissue remains a significant limitation to reliably transfer information to and from the brain through implanted electrode arrays.
Astrocytic responses to device insertion
Historically, neurons have been viewed as the information-processing cells of the central nervous system (CNS), because of their specialized capability to generate transient spikes in membrane potential (so-called action potentials). The presence or absence of these spikes serve as the putative ones and zeros of the neural code, where the detection or stimulation of these signals by implanted electrode arrays is the primary mode of device–neuron communication. However, neurons are outnumbered three-to-one by supporting glial cells in the brain19, and recent data has suggested that glia are capable of both transmitting and receiving synaptic signals as well as of producing profound effects on the local neurochemical environment20. These observations of complex functional roles belie the simple structural role implied by the origin of the term glia (Greek for ‘glue’)21. The foreign-body response to electrode arrays implanted in the brain is typified by glial encapsulation surrounding the device, where reactive glia ensheath the implant in a layered structure that can measure tens to hundreds of micrometres in thickness (Figs. 1 and 2). Heterogeneous types of glia respond to injury (Box 1), with reactive astrocytes being notable for their effects on the health, function and connectivity of neural networks.
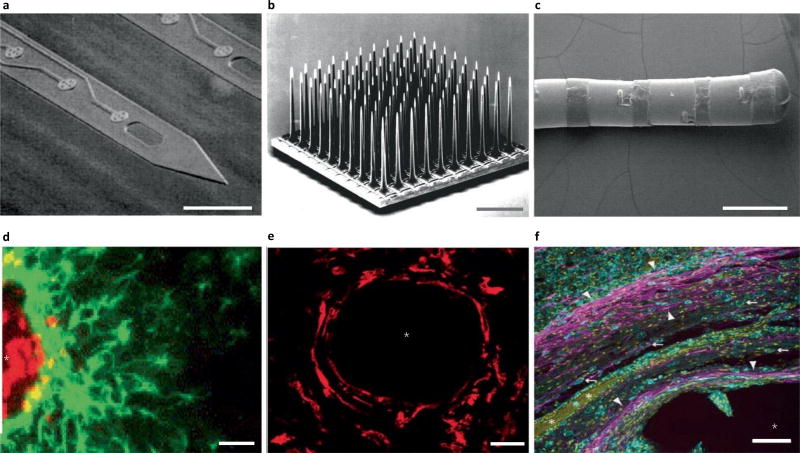
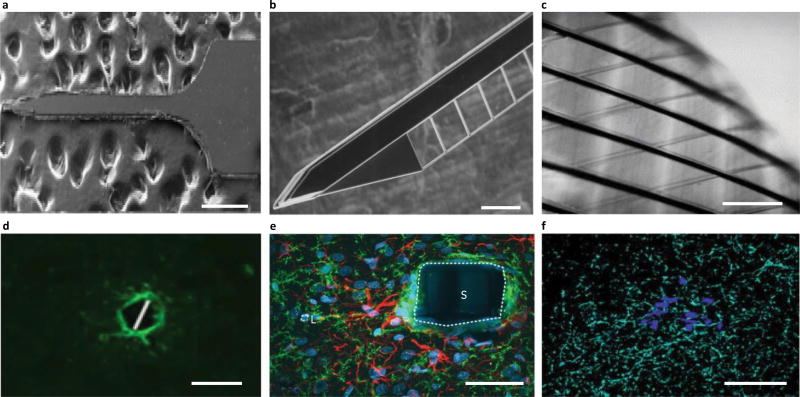
Fig. 1.
Traditional electrode arrays incite gliosis. a–f, Devices (a–c) are shown above the associated histology images (d–f). a, Michigan-style array217. b, Utah-style array218. c, DBS lead219. d, Rat histology from a Michigan-style multielectrode array (four weeks), with labelled astrocytes (GFAP, green) and microglia (ED1, red)80. e, Histology from a primate with Utah array implanted, with microglia labelled (IBA1, red)65, at 17 weeks. f, Human DBS lead implant at ~38 months, with labelled astrocytes (GFAP, magenta; white arrowheads), microglia (IBA1, cyan; white arrows) and all cell nuclei (CyQUANT, yellow)121. Scales bars: a,d,f, 100 µm; b, 1 mm; c, 2 mm; e, 28 µm. The asterisks in d–f indicate injury. ED1, antibody to cluster of differentiation 68; IBA1, ionized calcium binding adaptor molecule 1. Figure reproduced from: a, ref.217, IEEE; b, ref.218, Elsevier; c, ref.219, Oxford Univ. Press; d, ref.80, Elsevier; e, ref.65, IOP Publishing; f, ref.121, Springer.
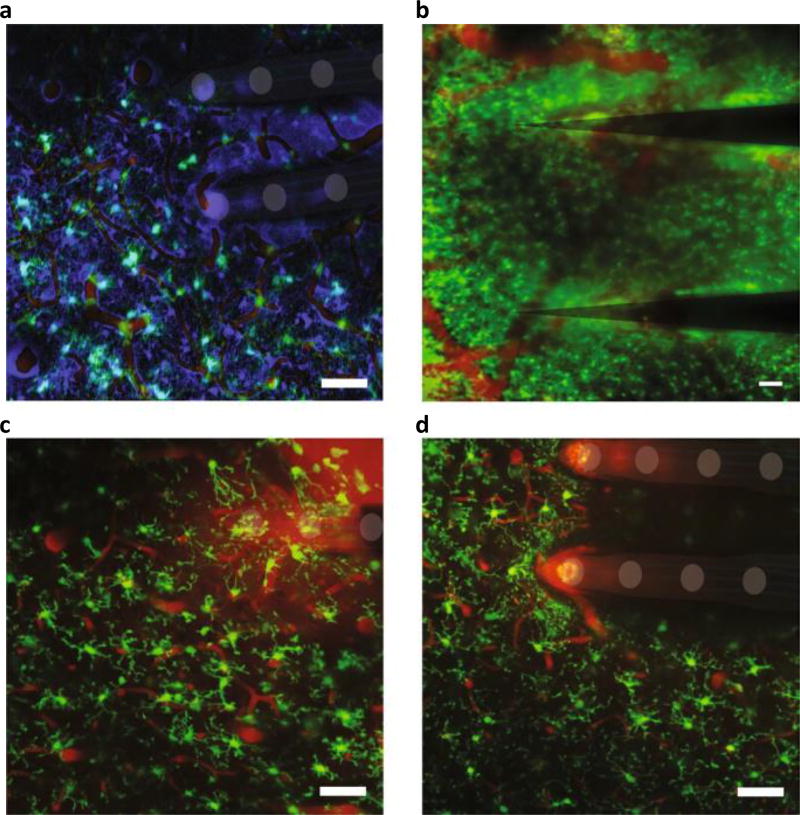
Fig. 2.
In vivo multiphoton imaging of the glial response to the implantation of a multielectrode array. Astrocytes and oligodendrocytes (sulfarhodamine99, false-coloured purple in a), neurovasculature (intravascular sulfarhodamine99, red in all panels) and microglia (the transgenic line CX3CR1-GFP, green in all panels) are shown. a, Microglia display an amoeboid morphology and encapsulate two shanks of a 4 × 4 NeuroNexus array six hours following implantation220. b, Microglia form a compact scar around two shanks of a 1 × 3 Blackrock array at two months post-implantation. c, Microglia activation and lamellipodia ensheathment of an implanted silicon/silicon-oxide microelectrode. d, Microglia avoid the silicon/silicon-oxide microelectrode surface when covalently coated with neurocamouflage protein L1CAM. Scale bars, 100 µm. Figure adapted from: a, ref.220, IOP Publishing; b, ref.34, Elsevier; c,d, ref.208, Elsevier.
Box 1. Non-neuronal responses to brain injury.
Non-neuronal cells in the brain include specialized supportive cells (such as astrocytes, microglia, oligodendrocytes and NG2-glia) and neurovascular cells (such as pericytes and ependymal cells), and structural connective tissue cells (such as meningeal fibroblasts). Of these, the most studied in response to brain implants are microglia and astrocytes, because of their prominent role in the encapsulation of devices. Pericytes and NG2-glia have also gained recent attention because of their suggested reparative potential following injury222–226. Also, blood-borne myeloid cells (such as monocytes and leukocytes) can infiltrate on BBB disruption, and their involvements around devices have been investigated and discussed elsewhere34,36. The foreign-body response to neural implants has been comprehensively discussed in refs26,35,156.
Pericytes, which form the basement membrane of capillaries and regulate blood flow, immediately respond to injury by disrupting the permeability of the BBB and by constricting cerebral blood flow, which can lead to larger volumes of cellular damage142,227. It has been proposed that these cells have reparative potential through association with neurogenic niches225,226.
NG2-glia were historically recognized as oligodendrocyte precursors found to be inhibitory to axonal outgrowth228. More recent evidence of functional synapses between neurons and NG2-glia has reframed this view: NG2-glia are now regarded as a unique class of glia that can actively participate in neural-network formation222,224. NG2-glia actively proliferate and arrive at injury sites within 24 hours of the insult, their responses are pronounced around devices at one week, and subsequently return to baseline responses at approximately four weeks following injury197,224. These cells can be a source of gliogenesis222,224,229 and may also have neurogenic potential222–224–229.
Microglia are the resident macrophages in the brain responsible for initiating the foreign-body response through the release of excitatory and inflammatory factors. They are the first responders to injury, rapidly adopting an activated, amoeboid morphology, then proliferating, migrating and encapsulating the device220.
Astrocytes activated by microglial signalling visibly respond to injury at approximately one week by proliferating, hypertrophying, upregulating intermediate filaments (such as GFAP), encapsulating the device and releasing factors to further promote the foreign-body response. By approximately four to six weeks, they begin to form a dense scar around the device that can last for years26,65,121.
Astrocytes are the most abundant cell in the brain22 and are so-named for their stellate morphology. They are responsible for regulating neurovascular blood flow, neurotransmitter activity and the composition of the extracellular environment, and provide metabolic support under physiological and pathological conditions23. They participate in communication as a third member of the traditional synapse (the ‘tripartite synapse’), through the release of gliotransmitters (glutamate, adenosine triphosphate (ATP), D-serine) in response to hundreds of synaptic inputs. Hence, they are responsible for the storage, processing and transfer of synaptic information across neuronal networks in the brain20. The diversity of their roles is reflected in the recent identification of distinct subclasses of astrocytes that are characterized by differences in gene expression, function and reactive states during CNS injury4,22–24. Gradients of damage-associated cues regulate the expression of extracellular-signalling molecules, of intracellular transducers and of transcription factors that instruct subtype specification25. Heterogeneous subtypes range from inflammatory phenotypes, which produce cytokines and chemokines, to phenotypes with an active role in interneuronal signal transmission (such as neurotransmitter release, sensing or re-uptake)22 and in blood-flow regulation26. Therefore, differential responses arising as a consequence of astrocyte reactivity, in addition to their physiological roles in the uninjured brain, need to be considered when evaluating the effects of astrogliosis on therapeutic outcomes and device performance. Brain injury, pathology and electrical stimulation generate considerable modifications to the physiological nature and consequences of glial signalling, with reactive astrogliosis implicated in both neuroprotective and neurodegenerative outcomes25,27.
Disruption of the blood–brain barrier (BBB) is inevitable during device implantation28 (Fig. 2). The influx of blood-serum proteins (including albumin and fibronectin) activate inflammatory pathways of nearby glial cells, including microglia and astrocytes26 (Fig. 2a). Microglia become activated, divide and migrate to the implant to release proinflammatory cytokines. This activation of microglia and the loss of ramified processes prevent these cells from undertaking their important ‘resting state’ activities, such as normal modulation of synapses26,29. In turn, the upregulation of proinflammatory cytokines drives nearby neurons towards excitotoxicity and neurodegeneration. Simultaneously, the loss of nearby oligodendrocyte precursor cells (also called NG2-glia) leads to the proliferation, migration and differentiation of distant NG2-glia into astrocytes30, increasing the activated astrocyte population (Box 1). Astroglial reactivity around the implant leads to increased expression of connexon-43 (Cx43), an astroglial hemichannel and gap junction known to facilitate the spread of inflammation31–33. In turn, inflammation leads to the recruitment of blood-borne monocytes and neutrophils through the intact BBB, and to the formation of multinucleated giant cells34. In addition, this inflammation alters the expression level of matrix metalloproteinases, together leading to further breakdown of the BBB and facilitating the influx of blood-serum proteins, red blood cells and leukocytes26. BBB disruption also leads to lower oxygen and nutrient delivery, as well as to impaired removal of neurotoxic waste products, including reactive oxygen species generated during the breakdown of red blood cells in the parenchyma26. This increase in metabolic, oxidative and osmotic stress further drives inflammation in nearby cells26. As expected, there is growing literature pointing to the idea that lasting BBB disruption around electrodes is implicated in long-term signal instability26,35–37. Together, this underscores an important role for BBB disruption in attracting and sustaining gliosis following device implantation.
After arrival, astrocytes can act as either effectors or affectors of device function. In the wake of the discovery of DBS and of expanding applications for similar devices, there has been growing interest in the role of glial cells in the effects and side effects of therapeutic stimulation, as well as in the progressive deterioration of the ability of electrodes to stimulate and record effectively38–42. Historically, the evaluation of the glial contribution to performance outcomes has been limited to the formation of an encapsulating scar around implanted electrodes (Fig. 1). For stimulation therapies, this often led to the simplistic view that the encapsulating scar was a passive physical barrier, where one could simply ‘turn up the current’ to offset any impact of the glial response, until hitting a threshold limit for safe electrical stimulation. For diagnostics and therapies depending on recording electrodes, the impact of the glial response was typically assessed by correlating measured tissue–electrode impedance to the quality of recorded neuronal activity43. However, more recent data have associated the chronic glial response to functional changes in neural circuit behaviour and to progressive neurodegeneration within the vicinity of implanted electrodes27,30,44, painting a more complicated picture of the glial contribution to the injury response. Likewise, newer data suggest that glia are an effector in stimulation-based therapeutic outcomes38,45. Isolating the structure–function relationship between glial reactivity and the remodelling of local neuronal circuits is central to understanding the fundamental mechanisms underlying therapeutic effects and device-failure modes. Here, we consider the influence of astroglia on device function, both as a passive barrier to device–tissue communication and as an active influence on neuronal signalling.
Consequences of glial encapsulation
The barrier nature of gliosis has traditionally been assessed through in vivo measurements of the impedance of the tissue/electrode interface, and modelled using static circuit elements. However, the electrode/tissue interface, especially in the presence of reactive gliosis, cannot be fully defined by these traditional methods.
Neurostimulation
In vivo impedance measurements are sensitive to a variety of factors in addition to glial encapsulation, including potential cellular encapsulation of the reference electrode, protein adsorption on electrode sites and the characteristics of the ionic environment at the electrode/electrolyte interface (such as diffusion, resistance to charge transfer and double-layer capacitance)46,47. Likewise, impedance can be especially difficult to interpret for emerging biomaterials with high ratios of electrochemical surface area to geometric surface area, for which the surface topography and chronic glia–surface interaction remain difficult to characterize48. Even for simple surfaces, faradaic reactions such as platinum dissolution occur at low levels of stimulation49, and increase as a function of increasing stimulation intensity50. Charge transfer via faradaic reactions risks damage to both electrodes and neighbouring tissues51. Similarly, the extracellular tissue resistance between cells comprising the glial scar, and the combined resistance and capacitance of their cellular membranes, can be altered as a function of stimulation intensity41,52,53. Given the nonlinear contributions of these elements, the accuracy of the volume of neural-tissue activation of a chronically healed-in electrode predicted by computational models is difficult to verify. Moreover, the impacts of these nonlinear elements in chronic settings on stimulation strategies, such as high-frequency stimulation to induce neural block54, on asymmetrical waveforms to inactivate neural tissue close to the electrode55, and on thresholding techniques to activate specific neural classes/elements remains unclear56.
For stimulation applications, the barrier nature of gliosis is reflected in models of the effective volume of tissue activated, where greater gliosis reduces the number of neurons stimulated55. The stimulation paradigm affects the impact of the glial barrier: in constant-voltage stimulation, voltage is controlled and the actual current delivered to tissue varies as the tissue response evolves (increased impedance due to gliosis reduces the stimulation delivered). Although glial encapsulation is known to change in the weeks immediately following implantation, it is generally assumed that reactive gliosis reaches a steady state three to six months post-implantation42,57. For constant-voltage stimulation paradigms, the day-to-day changes in impedance caused by consolidation of the glial scar during the first three to six months post-implantation may dramatically alter the effective current reaching neural tissue39. As a result, most device manufacturers have moved towards constant-current stimulation paradigms where the charge density delivered by the stimulating electrode does not depend on day-to-day changes in impedance of the tissue/electrode interface58,59.
Extracellular recordings
The impact of glial encapsulation on the quality of signals recorded in vivo remains ill-defined, as studies that investigate histology, impedance and recording quality for the same system are rare. Nevertheless, a few lines of indirect evidence support the idea that glial encapsulation acts as a barrier to signal detection by implanted electrodes37,43,60. Astrogliosis, as identified by increased glial fibrillary acidic protein (GFAP) immunoreactivity, was associated with reduced recording quality of Utah-style arrays implanted in the rat cortex in a study that investigated the relationship between histology and recording quality37. Another study found a correlation between increased impedance and the presence of GFAP-positive astrocytes (signal quality was not assessed, however)61. An inverse relationship between recording quality and impedance measurements over a chronic time course was also observed, but a direct assessment of histology was not reported43. In a study that assessed impedance, recording quality and quantitative histology within the same set of chronically implanted animals40, the data revealed a negative correlation between non-neuronal density (NND) and signal quality, and a relatively weaker, positive correlation between NND and 1 kHz impedance (Fig. 3).
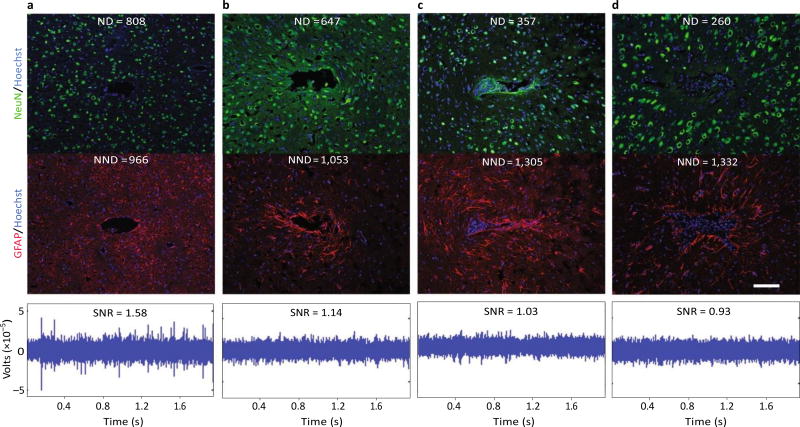
Fig.3.
Evidence for a negative impact of increased gliosis on recording quality. a–d, Representative images from four animals demonstrate the range of endpoint histological outcomes (from ‘good’ to ‘poor’, left to right). The figure has been generated after additional analysis on data collected in a previous study40. Neuronal nuclei (NeuN, green) and astrocytes (GFAP, red) surrounding probe tracts are shown, and the associated average neuronal and non- neuronal density data are listed (area binned cell counts, neuronal density (ND) and non-neuronal density (NND), in cells mm−2). Recording segments with signal-to-noise-ratio (SNR) values representative of the average value for each animal are depicted (the SNRs calculated from peak-to-peak noise result in lower values than those calculated from root-mean-square noise)40,221. Recording quality improved with decreased NND and increased ND/NND (P < 0.05, Spearman’s ρ, n = 6). Impedance increased with increased NND (P < 0.05, Spearman’s ρ, n = 6). Animals in a and c were drug-treated while b and d correspond to the controls. Scale bar, 100 µm. Figure adapted from ref.40, Elsevier.
These data suggest that glial encapsulation is an underlying cause of both increased impedance measurements and a concomitant reduction in recording quality (in support of a barrier role). However, the relationship between impedance and recording quality is complex, with multiple potential confounders and often inconsistent correlation between metrics62,63. For example, interanimal and intraday variability in recorded signal and impedance correlations have been reported, where a ‘simple’ relationship between impedance and unit activity could not be defined63. Loss of insulation integrity is an important factor in determining measured impedance values, and several results underscore the potential contribution of device integrity in determining performance outcomes63,64. Furthermore, drug treatments that reduce glial activation and decrease impedance do not necessarily translate to an improvement in recording quality40. In addition, modelling data suggest that glial scarring may have a large impact on impedance values but minimal impact on signal amplitude65. Interpreting impedance values measured in vivo and their relationship to recorded signal quality and histology is then confounded by the dual influence of mechanical integrity and glial encapsulation on recorded values. Also, the assimilation of reported effects across studies is undermined by inconsistencies in the analysis methods used. Moreover, impedance measurements are an imperfect surrogate for the measurement of action potentials generated by a nearby neuron. Impedance measurements are typically taken at 1 kHz or across a frequency spectrum, and consist of continuous sinusoids in the 5–25 mV range delivered by backend instrumentation. In contrast, extracellular potentials are generated by the movement of ions across a cellular membrane and are caused by the gating of ion channels during an action potential, are not continuous in nature, consist of multiple frequency components and are in the range of tens to hundreds of microvolts, depending on the distance and orientation with respect to the extracellular recording electrode66,67. New methods of assessing the barrier effect of gliosis in vivo, in concert with complementary approaches to assess individual glial-encapsulated sites (such as in vivo imaging, controlled perturbations of glial reactivity surrounding sites and improved computational models) will be required to determine the impact on long-term recording quality. Similarly, the view of the glial sheath as a passive barrier needs to be reconciled with an expanding body of evidence for direct action of glia on neuronal health and excitability.
Neurochemical sensing
In addition to electrically isolating devices from neuronal signals, glial encapsulation may pose a communication barrier between implanted sensors and the local neurochemical environment. Neurochemical sensing has become a commonplace application of implanted electrodes in research studying synaptic transmission68,69 and is an emerging approach in clinical diagnostics of neurological disease70. When coupled with implanted drug-delivery or neuromodulation devices, it can serve as a source of feedback, enabling personalized and smart neuroprosthetic therapies7,70, and providing a foundation for future closed-loop applications (for example, low neurotransmitter levels triggering the delivery of electrical or chemical-based therapy). Although the spatiotemporal resolution of these devices is superior to the alternative approach of microdialysis71, their lifetime is limited due to factors such as the electrochemical stability of the interface and the reliability of the transduced output measurement over time71.
Relatively limited histological examination has been reported for neurochemical sensors71, and further studies are necessary to clarify the impact of glia on the function of neurochemical sensing26. Given that effective diffusion of the chemical species to the electrode is a rate-limiting factor in the performance of neurochemical sensors72, the diffusion barrier posed by astrogliosis73 could be a key factor limiting the temporal resolution achievable by these devices. Also, although neurons are typically assumed to be both effector and affected cells of neurotransmitter release, an increasing body of evidence demonstrates that glia are capable of neurotransmitter release and uptake22. For example, an investigation into the source of glutamate in neurochemical sampling by microelectrode arrays reported only ~40–50% of measured glutamate to be of neuronal origin in the rat prefrontal cortex74. Likewise, non-vesicular glial mechanisms accounted for the majority of extracellular glutamate detected in the rat prefrontal cortex using microdialysis75. Glia can influence the local neurochemical environment and produce related effects on the excitability of local neurons, affecting the interpretation and quality of data collected from implanted sensors and stimulators.
Glia as an active modulator of signal transmission
An increasing body of literature demonstrates that reactive glia directly influence the signal-generating capabilities of local neurons by influencing the excitability of individual cells, the synaptic transmission of signals between them and the broader population activity detected within a network. In this section, we explore the mechanisms of these effects and consider the potential influence on the signals detected or generated by implanted devices.
Modulation of neuronal excitability
Neuronal signalling is enabled by the conduction of ionic charge carriers across the cell membrane through specialized transmembrane proteins known as ion channels76. The function and expression of ion channels is shaped by a variety of factors, including the ionic composition of the intracellular and extracellular environments as well as events occurring during individual stages of protein synthesis (such as transcription, translation, post-translational modification, assembly with ancillary subunits and alternative splicing). The glial–neuronal signalling pathways, in which autocrine/paracrine amplification loops for cytokine release are generated following injury, have the potential to affect these processes in several ways, ultimately influencing the excitability of individual neurons.
A downstream influence of glial–neuronal signalling is the efflux of potassium and the accumulation of glutamate in the extracellular environment surrounding neurons. Astrocytes have a primary role in maintaining the homeostasis of the ionic and chemical composition of the extracellular environment; their active clearance of potassium and glutamate from the extracellular space produces a net inhibitory effect on nearby neurons that dampens excitability20,77. In a mouse model deficient in astroglial connexins and astroglial coupling, hyperexcitability, synaptic unsilencing and increased synaptic release arose within the local neuronal network32. Glial scar tissue bears upregulated expression of connexins78, indicating tight astroglial network formation in the wake of the injury response to neural prostheses. By extension, astroglial scar formation may favour enhanced buffering of excitatory accumulation of extracellular potassium and glutamate, ultimately ‘quieting’ the local neuronal population surrounding a device.
In addition, glia are known to release cytokines in response to injury, which may influence neuronal function through direct impacts to ion-channel expression and physiology. Reactive glia, including astrocytes and microglia, release potentially neurotoxic, inflammatory cytokines following device implantation79,80, including interleukins 1 and 6 (IL-1 and IL-6), tumour necrosis factor alpha (TNFα) and monocyte chemoattractant protein 1 (MCP-1)81. These events may initiate cell death pathways and impair recording performance44, where preventing IL-1β activation showed significant improvement in neuroprosthesis performance82. Released cytokines can also result in a change in neuronal function, since alterations in ion-channel expression have been shown to follow exposure to inflammatory cytokines (IL-1β, TNF-α, IL-6) in models of traumatic brain injury83,84. Alterations in channel currents may occur on both short- and long-term timescales, where short-term effects are most probably attributed to alterations in gating characteristics or post-translational modifications to channel proteins, whereas longer-term impacts may be related to changes in channel expression84. Acute effects (within 24 hours) tend to favour hyperexcitability, whereas longer-term impacts (days or weeks after exposure or injury) tend to favour loss of excitatory sodium85,86 and calcium currents87,88 in the CNS, a trend that has been interpreted as the progressive dampening of the excitability of affected neurons to promote neuroprotection and prevent excitotoxicity89. Impaired excitability would limit the detection of neuronal activity by investigational recording devices and elevate the stimulation thresholds required for clinical neuromodulation devices. Relating the underlying inflammatory pathways to performance outcomes of implanted devices will require further efforts, and targeted intervention strategies will be necessary for restoring network-level excitability to maintain long-term function in implanted devices.
Modulation of synaptic transmission
Device implantation necessarily disrupts the connectivity of the surrounding network, and can remodel synaptic organization through multiple mechanisms. Gliosis and related changes in the local neurochemical environment can affect synapse formation and function following injury, influencing signal generation by the interfaced network. The impact on synaptic transmission mirrors that of intrinsic excitability, favouring a shift from hyperexcitability to hypoexcitability over time.
Synaptogenesis and silencing
Astrocytes can direct the formation and maintenance of synapses through multiple signalling pathways90. However, the influence of reactive glia on the synaptic remodelling surrounding implanted devices is only beginning to be explored. In the surroundings of electrode arrays implanted in rat brains, initially heightened excitatory synaptic transporters precede a chronic elevation in markers of inhibitory transmission91. BBB breach due to device insertion may be an initiating signal for these events, on the basis of evidence that astrocyte-induced excitatory synaptogenesis follows injury92. Furthermore, heightened glutamatergic transmission subsequently activates astrocytic release of transforming growth factor beta 1 (TGF-β1) to induce inhibitory synaptogenesis93; this parallels the observed excitatory-to-inhibitory shift surrounding implanted devices91.
An alternative mechanism of injury-induced synaptogenesis is related to a class of matrix-associated glycoproteins, known as thrombospondins (TSPs), produced by reactive astrocytes and microglia94,95 (Fig. 4). Purinergic signalling and mechanical stimulation, which are both relevant in device implantation, increase TSP production96. Here, TSP release is responsible for the formation of ultrastructurally normal yet functionally silent synapses97,98, which are characterized by altered expression of glutamate receptors. Silent synapses display normal postsynaptic N-methyl-D-aspartate receptor (NMDAR) density but an absence of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). Without AMPARs, these excitatory synapses are silent due to magnesium ion blockage of conductive NMDARs, unless they are artificially depolarized to remove the block97. Notably, TNF-α release by astrocytes can compensate for long-term silence via AMPAR insertion into all synapses of a given neuron98,100 (a mechanism of network-level homeostatic plasticity known as synaptic scaling99). However, upregulation of connexins, as occurs in the astroglial scar78, has been shown to limit AMPAR insertion and to maintain silent synapses through down-scaling mechanisms that prevent excitotoxicity32. Therefore, synapses formed near the injury scar may be likely to exhibit depressed activity. However, variability in the functional consequences of reactive signalling is to be expected, especially in the context of a chronic, indwelling implant where surrounding gliosis may be aggravated by chronic inflammation, ongoing micromotion or repetitive stimulation.
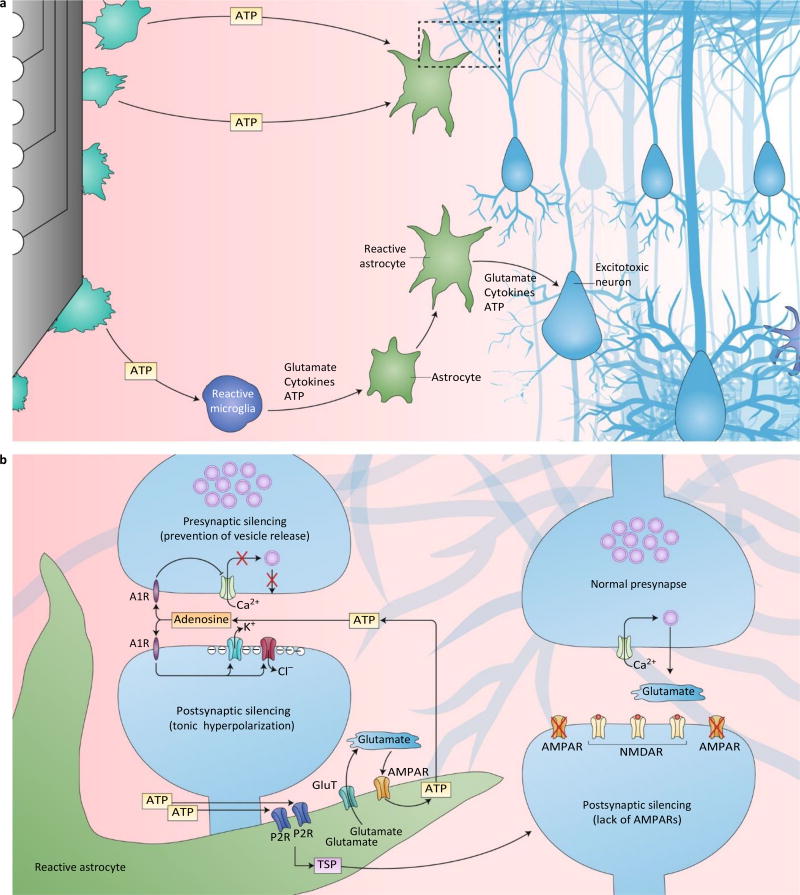
Fig.4.
Potential mechanisms of the active modulation of neurotransmission by glia. a, Insertional trauma incites reactive gliosis and impacts neuronal function through modifications to the local neurochemical environment. Punctured cellular membranes release ATP into the local extracellular space, whereby activated microglia and astrocytes are recruited to release glutamate, cytokines and ATP. The resulting signalling cascades ultimately reinforce reactive gliosis and impact local neuronal health and function. The dashed box indicates the region of synaptic silencing depicted in b. Neuronal excitotoxicity is another potential consequence of reactive signalling. b, As injured cells and reactive microglia release excess ATP, activated astrocytes are able to silence neuronal activity through two synaptic mechanisms. (1) Glutamate and ATP release, which generate a positive-feedback loop; ATP is rapidly hydrolysed to adenosine in the synapse, where adenosine is able to act on presynaptic A1Rs to inhibit Ca2+ channels and prevent vesicle release (presynaptic silencing), and to act on postsynaptic A1Rs to open K+ and Cl− channels and prevent the generation of action potentials (postsynaptic silencing). (2) TSP production and release, which forms ultrastructurally normal, but functionally silent synapses. These postsynaptic terminals lack AMPARs, which are required to alleviate the Mg2+ block on NMDARs, therefore preventing effective signal transfer from the presynapse (postsynaptic silencing). A1R, adenosine A1 receptor; P2R, purinergic P2 receptor; GluT, glutamate transporter.
Synaptic remodelling is shaped by the local neurochemical environment, which is in turn affected by device implantation. Electrode insertion induces significant increases in neurotransmitters in the extracellular environment (glutamate, ATP and adenosine)101, where likely sources include punctured cellular membranes and mechanoactivation of astrocytes and microglia (Fig. 4a)23. The resulting gradient serves as a beacon for attracting and reinforcing reactive gliosis22,23, and necessarily affects local synaptic plasticity20. Therefore, glial-derived changes in the local neurochemical environment are both neuron-affecting and self-sustaining27,30. Microglia mobilized by extracellular ATP withdraw their processes to assume an amoeboid morphology and converge on the site of injury to release cytokines, glutamate and ATP23. Adenosine is produced when ATP is rapidly hydrolysed in the synapse102, and has been demonstrated to play a key role in the suppression of synaptic activity. Activation of adenosine receptors inhibits presynaptic calcium-dependent release of neurotransmitters103 and opens postsynaptic K+ and Cl− channels104,105. These events collectively hyperpolarize the post-synapse and prevent synaptic transmission (Fig. 4b). A reduction in synaptic activity will affect synaptic strength and plasticity106, resulting in further alterations to the local synaptic network. To summarize, glial neurotransmitter release may underlie synaptic-silencing mechanisms as an origin of injury-induced synaptogenesis or of adenosine-mediated suppression of pre- and post-synaptic transmission. Combined with evidence of increased markers of inhibitory synaptic transmission in the chronic setting, device implantation is likely to favour dampened signal transmission in the long term. These plastic silencing mechanisms are likely to be maladaptive for the effective stimulation and recording of neurons near devices. However, they may be adaptive for confining the spread of excitotoxicity and neuronal loss in the wake of implant injury and for reducing the potential for excessive synchrony within the local network.
Modulation of network activity
Beyond their influence at the level of a single synapse or neuron, astrocytes coordinate activity across broader cohorts of neurons and the connections between them, resulting in network-level modulation. Interconnected astroglia are able to orchestrate synchrony through the integration of signalling within neuronal circuits and across functional regions of the brain20. The stimulated actions of a single astrocyte could dictate functional consequences on an entire network of neurons20. Artificial synchronous depolarization of astrocytes using optogenetic stimulation resulted in global suppression of neuronal activity in the subthalamic nucleus107, providing direct evidence of network coordination by glia. Computational models support the available empirical evidence, where astrocytes were identified as critical determinants of the level of synchrony between neighbouring neurons in simulated data108.
Gap junctions and hemichannels subserve this function through the rapid trafficking of ions, solutes and metabolites along astroglial networks, coordinating efforts across distributed spatial domains and providing a framework for modulating synchrony and plasticity in complete neuronal ensembles20. Given evidence for astrocyte coordination of neuronal networks20 (albeit controversial109), reactive gliosis likely impacts not only the generation and transmission of action potentials between single neurons, but also the broader population activity detected and stimulated by electrodes implanted in the brain.
Neuronal synchrony
The analysis of complex networks has revealed guiding principles for the emergence of synchrony within a network of oscillators, where both the dynamics of the individual oscillators and the architecture of their connectivity are key determinants of function: homogeneity of the oscillators and high coupling strength tend to favour synchrony110. Astroglial glutamate release is able to strengthen excitatory coupling between neurons by acting on pre- and postsynaptic receptors111,112, resulting in a robust propagation of synchronous activity across networks113,114. Moreover, computational modelling has supported glial mechanisms for synchronizing neuronal activity, where simulated presynaptic targeting of glutamate release by astrocytes was sufficient for initiating hypersynchronization and seizure activity115. In contrast, computational models have also demonstrated the importance of astrocytes in the desynchronization of neuronal activity, by providing activity-dependent stabilization, as neighbouring neurons are prone to hypersynchrony through their intrinsic excitatory coupling108.
This is supported by the observation that astroglial adenosine release desynchronizes network activity112. However, these apparently opposing results may be reconciled by considering the reactive state of the astrocyte: astrocytes in an activated, proinflammatory state4 may lose their ability to desynchronize local neuronal networks. It was suggested that a loss in the ability of astrocytes to desynchronize neuronal firing may underlie abnormalities in the oscillatory activity associated with brain pathology (such as Parkinson’s disease, Alzheimer’s disease and epilepsy)116,117. Therefore, therapeutic effects of stimulation may evoke astrocyte-mediated changes in network synchrony and plasticity that would otherwise occur under physiological conditions20. Taken together, this evidence suggests that glia are a central determinant of network-level activity and may be underutilized as a target cell of neuromodulation therapies that interrupt pathological oscillations.
Glial-activation challenges and design considerations
Glia are increasingly recognized as cells that influence the efficacy of therapy as well as the stability and longevity of device performance. Future device development should consider the potential impacts of design features and material effects on glial physiology.
Glia as an effector of clinical devices
The serendipitous discovery of DBS to alleviate the symptoms of Parkinson’s disease preceded understanding of the mechanisms of therapy. Subsequently, the role of glia as a cellular target of DBS treatment118–120 has emerged among several candidate mechanisms. Gliosis is commonly observed in post-mortem brain tissue from DBS patients121,122 and can be more pronounced when surrounding active devices123 (Fig. 1). Several DBS models using high-frequency stimulation have suggested that astrocytes are effectors for interrupting pathological oscillations in the thalamus45 and for attenuating tremor38. The release of adenosine or glutamate by high-frequency stimulation101 can modulate neuronal oscillations from non-synaptic sources38,45, with corresponding astrocytic Ca2+-wave propagation occurring in a frequency- and amplitude-dependent manner38. Likewise, neurochemical measurements taken from DBS patients have correlated adenosine release with both tremor arrest124 and seizure termination125. Although still at an early stage, evidence is mounting for glial contributions to clinical device efficacy, spanning from neurochemical mediators on implantation to direct effectors of neuromodulation devices. Even in the absence of stimulation, device implantation results in insertional trauma and in ensuing inflammation that can directly modulate network activity and affect clinical outcomes. This is known as the microthalamotomy effect101, where implantation results in a window of therapeutic efficacy that can last for as long as a year124, implying injury-induced plasticity. Astrocyte-mediated plasticity is a tightly regulated interaction between glutamate, ATP and cytokine signalling106,126 (Fig. 4), and can lead to either potentiation or depression after injury23,106,126. As an example, reactive inflammatory signalling can alter AMPAR/NMDAR ratios and ion-channel expression/function, and excessive glutamate release can alter the excitatory coupling strength of synaptic networks20,23,32,106. In turn, the resulting plasticity (including synaptogenesis and long-term potentiation or depression90) shapes long-term network function90,106, where potentiation favours hyperactivity (seizure activity) and depression results in network silencing. For this, recent evidence suggests immediate, local upregulation of markers of glutamatergic transmission surrounding devices after insertion, suggesting a potential mechanism of heightened synchrony and activity detected by recording electrodes91. However, later upregulation of inhibitory neurotransmission (driven by the release of gamma aminobutyric acid (GABA)) suggests a shift towards network silencing and limited signal detection91. This shift from elevated glutamatergic to GABAergic tone around implanted electrodes is likely astrocyte induced. In this regard, heightened glutamatergic transmission has been shown to activate astroglial release of TGF-β1 to induce GABAergic synaptogenesis93. Therefore, glial signalling after insertion can directly remodel the structure and function of surrounding circuitry, likely affecting the long-term performance of recording devices and the activation thresholds of stimulating devices. These factors will need to be further explored to uncover their impact on device efficacy.
A growing body of literature supports the important role of glial cells during electrical stimulation. Several models have demonstrated the direct modulation of plasticity, inflammation, neurogenesis and cerebrovascular functions by glial cells following neurostimulation118,127. For example, stimulation evokes astrocyte-induced cortical plasticity, as demonstrated in studies using transcranial direct current stimulation (tDCS)128. Also, optogenetic depolarization of astrocytes led to the release of glutamate, which directly modulates synaptic plasticity (long-term depression) and motor behaviour129. Inflammation is likewise modified by stimulation: tDCS can both incite inflammation in the uninjured brain and modulate it following injury130. Implanted-electrode stimulation upregulates inflammatory receptors (toll-like receptors) in microglia131, favouring a shift to a proinflammatory state117. However, the timing132 and intensity133 of stimulation may differentially affect reactivity and inflammation, suggesting a gradient of glial responses127. In the context of neurogenesis, neuromodulation is gaining traction as a reparative tool for brain injury and disease118,134. Neurostimulation stimulates neural progenitor proliferation135–137, directs the migration (galvanotaxis) of neuronal and glial precursors134,138–140 and promotes their differentiation136,137,140,141. Interestingly, tDCS-polarized proinflammatory microglia accompany NG2-precursor migration to promote functional recovery after stroke130. This suggests that the modulation of reactivity and inflammation could potentially be harnessed for guiding endogenous repair around active electrodes. Finally, astrocytes are key constituents of the neurovascular unit142, where they release neurochemicals to modulate vasodilation or constriction and provide activity-dependent metabolic support (as demonstrated with electrical143,144 and optogenetic145 stimulation). In turn, evidence points to astrocytes as important DBS effectors for improved cerebral blood flow and metabolism in drug-refractory epilepsy146. Taken together, glia represent important effectors of clinical devices, where their responses to electrical stimulation are gaining utility as targets to modulate regeneration or repair, cerebrovascular function and inflammation.
Consequences of higher-density arrays and multiple implants
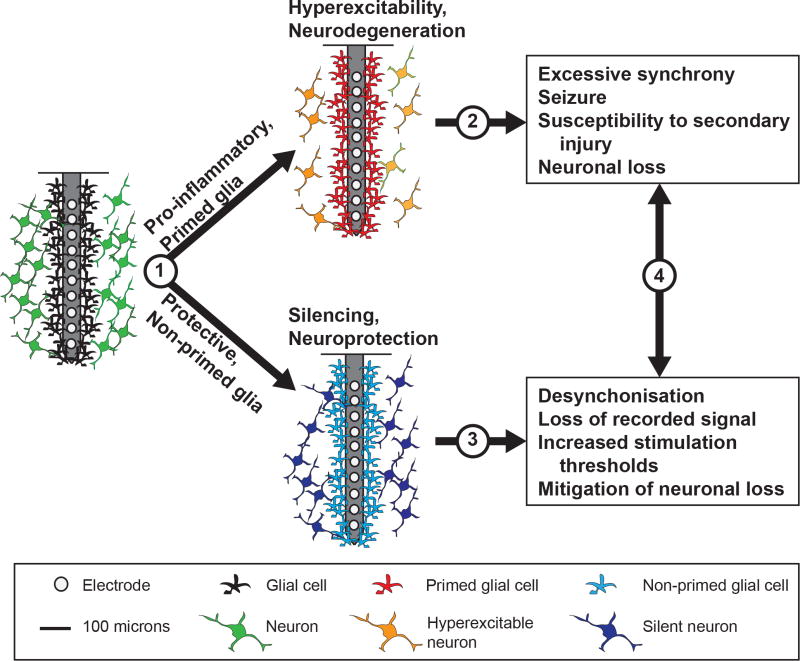
Monitoring the electrical activity of large numbers of neurons simultaneously with single-cell resolution is an ongoing challenge in neural engineering147, and has motivated the design of increasingly high-density electrode arrays with smaller individual electrode site sizes147. Furthermore, as neuromodulation strategies become increasingly sophisticated (as exemplified by closed-loop systems), multiple implants within a single patient or research subject are becoming more common148. The potential for injuries induced by multiple implants and/or multi-shank devices to exacerbate inflammation and gliosis should be considered as the field moves towards more distributed sampling approaches. Successive brain injuries engage a state known as glial priming: a condition where glia remain in an activated proinflammatory state with upregulated inflammatory markers, heightened sensitivity, resistance to negative feedback mechanisms and a predisposition to releasing excessive amounts of inflammatory factors on subsequent activation117. Glial priming can develop over many years following CNS insults, including cortical stab-wound injury149,150, as well as in neurodegenerative conditions151,152 and ageing153,154. Subsequent (secondary) insults exacerbate glial responses through excessive release of proinflammatory IL-1β, TNF-α and IL-6 (refs117,149,155), which can lead to prolonged inflammation and progressive degeneration151,152. These cytokines can also elicit hyperexcitability and excitotoxicity under primed conditions117, and have been implicated in susceptibility to seizures and epileptogenesis83,113, all of which bear implications on side effects not only for experimental models, but also for clinical DBS treatments where patients are inherently predisposed to conditions of pathology, ageing and hyperexcitability before the implantation of devices. The extent of glial priming incurred from pathology (such as Parkinson’s disease, Alzheimer’s disease, epilepsy and stroke) and ageing will need to be considered before the implantation of devices that will necessarily exacerbate proinflammatory glial priming. Moreover, device-design considerations will need to evaluate the relationship between glial priming and implant-feature sizes, the quantity of sites in high-density arrays, and distributed injuries caused by multiple implants and/or shanks.
Biomaterials and glial activation
The physicochemical properties of electrode materials directly influence glial gene expression, inflammation and chronic gliosis156. Soft, nanoscale and bioactive materials have been incorporated into device design to produce electrode arrays with improved biointegration157. The broad strategies are to reduce the mechanical mismatch between device materials and brain tissue, reduce the footprint (and invasiveness) of the array, enhance surface porosity to mitigate immune responses, or create a biomimetic or bioactive coating that conceals the implant from the foreign-body response157.
Improved softness
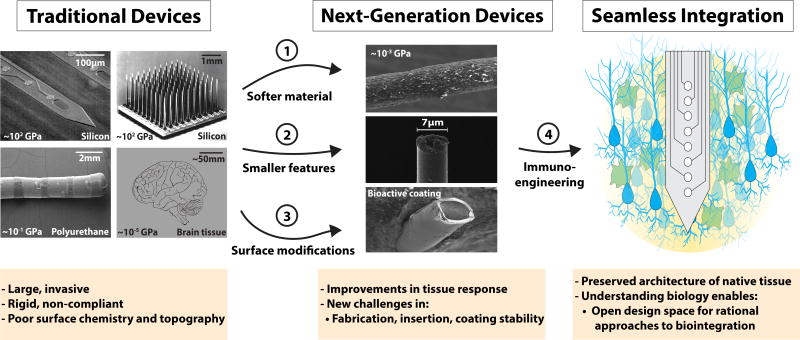
Stiff substrates, including silicon, exacerbate the activation of both astrocytes and microglia compared with softer materials158. Currently, silicon remains the most common material substrate for intracortical primate studies62,159 and clinical trials of brain/machine interfaces43,160, whereas DBS leads used in patients are primarily made of polyurethane (Fig. 1). Silicon and polyurethane are substantially stiffer than brain tissue (Young’s moduli for silicon, polyurethane and brain tissue are ~102, ~10−1 and ~10−5 GPa, respectively156). Minimizing the mechanical mismatch between the device and neural tissue improves gliosis, inflammation and neuronal preservation156,161,162, and next-generation devices incorporate flexible materials designed to more closely mimic the stiffness of brain tissue (Fig. 5). Mechanically adaptive materials (initially stiff materials that become compliant on contact with the physiological environment) significantly reduce glial scarring and inflammation161,163–165. Examples are mechanically compliant nanoparticle polymer substrates for stimuli-responsive designs inspired by the sea cucumber dermis161,163,166–168 (Fig. 5a), and shape-memory polymer substrates with similarly adaptive characteristics and tunable moduli164,165,169. Polymer blends of silicones and poly(3,4-ethylenedioxythiophene) are the softest reported materials to record extracellular units, with accompanied reductions in microglial attachment162. However, these materials introduce challenges for functional device design and minimally damaging deployment157.
Fig.5.
Next-generation arrays mitigate gliosis. a–f, Devices (a–c) are shown above the associated histology images (d–f). a, A mechanically adaptive nanocomposite microelectrode becomes compliant on implantation163. b, A hollow-architecture parylene-based microelectrode places sites away from the stiff penetrating shaft, along 4-µm-wide lateral support arms148. c, A syringe-injectable mesh electronics mimics brain parenchyma with sites featured along an interwoven structure172. d, Astrocytes labelled (GFAP, green) around mechanically compliant probe at eight weeks161. e, Astrocytes (GFAP, red), microglia (OX42, green), and all cells (Hoechst, blue) labelled around the stiff electrode-penetrating shaft (S) and lateral edge (L) at four weeks148. f, Astrocytes labelled (GFAP, cyan) around a syringe-injected mesh (blue) at one year171. Scale bars: a, 500 µm; b,d,f, 100 µm; c, 250 µm; e, 50 µm. Figure reproduced from: a, ref.163, IOP Publishing; b,e, ref.148, Elsevier; c, ref.172, American Chemical Society; d, ref.161, IOP Publishing; f, ref.171, Nature America Inc.
Both device architecture and its material composition affect flexibility, since bending stiffness is determined by both the Young’s modulus (E) and the dimensions of the material26,157,170. Bending stiffness is proportional to Et3 (where t is the thickness of a rectangular cross-section), meaning that reduced stiffness scales more rapidly with decreased device dimensions than reductions in modulus. Syringe-injectable, flexible mesh electronics have been shown to interpenetrate the brain parenchyma and record along interwoven neuronal networks (for up to one year, with minimal tissue response and sustained recordings)171,172 (Fig. 5c). And for the smallest chronically implantable extracellular microelectrode so far reported — an electrode with a 15 µm2 cross-sectional area173 — two-photon imaging surrounding the implant revealed a lack of astrogliosis and minimal disruption of the vasculature. However, reduced stiffness can make softer156,174 and subcellular devices48,68,175 difficult to implant, requiring the use of an insertion tool175,176 or dissolvable shuttle177,178. Since new device designs often employ both softer materials and reduced feature sizes, the relative impact of each of these factors on the tissue response can be difficult to interpret. Nonetheless, there is increased interest in the fabrication of electrode arrays with smaller features and softer materials, and potentially concomitant reduced gliosis (Fig. 5).
Smaller feature sizes
In addition to enhanced flexibility, reducing device dimensions may diminish gliosis48,179,180 by presenting an adhesive surface that is too small to allow cellular attachment181–185. For brain implants, feature sizes below 10 µm lead to reduced gliosis and preserved neuronal density186.
Reduced glial responses were observed with parylene-based Michigan-style arrays combined with the use of an open-architecture design (4-µm-wide feature sizes)49 (Fig. 5b). Open-architecture designs have also improved the integration of implanted planar arrays179. Ultrasmall, flexible carbon-fibre electrodes with subcellular features (<10 µm in diameter) have emerged as an approach to mitigate tissue response and to improve long-term recordings48. Devices are becoming both smaller and increasingly sophisticated. For example, injectable wireless electronics can carry out electrical recordings, optical stimulation, temperature sensing and photodetection175. Also, the immune response can be mitigated by decreasing implant volume and by increasing surface permeability or porosity, facilitating the dispersion of inflammatory cytokines and preventing their accumulation, as has been achieved with porous coatings187 and web-like mesh electronics188–190. Although advancements in material-based strategies to improve the neuron/electrode interface continue, there is a need to pursue basic-science studies to identify guiding biological principles for improved device design.
Surface modification
Electrode surface coatings have also become increasingly sophisticated for reducing the foreign-body response to brain implants156,191–193, where materials include hydrogel191,194, silk195,196, bioactive anti-inflammatory surface molecules193,197,198 and biodegradable polymer nanoparticles for the controlled delivery of anti-inflammatory therapeutics68,191,199,200. Coatings are designed to (1) reduce inflammation through drug release, (2) buffer or disperse inflammatory-cytokine accumulation, (3) increase the fractal dimensions of the site for reduced impedance and/or (4) present a biomimetic surface to mask the implant from being recognized as a foreign body. The controlled release of anti-inflammatories from coatings has shown promise in the reduction of glial encapsulation and impedance199,201–203, but the impact on neuronal health and recorded signal quality is less clear. In recent years, several strategies to increase the fractal dimensions of electrode sites with ‘fuzzy’ conductive material coatings have been developed to reduce impedance and improve tissue integration48,191,192,204,205. For example, carbon nanotube coatings for metallic-wire electrodes in vivo have led to improved impedance, recording and stimulation in both rats and monkeys206. Conductive polymer nanoparticles with hydrogel layers for decorating microfabricated electrode arrays with nano-structured surfaces offer the added advantages of improved charge transfer, greater compliance, reduced impedance and the precise delivery of bioactive species200,205,207. Strategies combining conductive polymer coatings and bioactive treatments lead to lower impedance and reduced gliosis156,191,192. However, the implementation of ‘stealth’ coatings, such as those based on neuronal cell-adhesion molecules, can alleviate the foreign-body response by both promoting neural growth and by reducing gliosis (Fig. 2c,d)162,193,208.
Effects at the molecular level
The characteristics of a material substrate can influence the signalling pathways associated with reactive glia (Fig. 4). Nanostructured topographical features can increase astroglial ATP release209, downregulate GFAP expression210, and increase the expression of glutamate transporters and the clearance of extracellular glutamate211. Stiffer substrates have been associated with the activation of microglia (amoeboid morphology, upregulation of CD11b) and of astrocytes (hypertrophy, upregulation of GFAP), as well as their proliferation, migration and adhesion158. With regard to gene expression, stiffer materials upregulate the molecular determinants of inflammatory signalling (toll-like receptors, IL-1β, TNFα) in glia158. Mediation of these pathways may improve device function; for instance, knock-out of caspase-1, which activates IL-1β, has demonstrated significant improvement to long-term functional recordings82. For smaller feature sizes, improvements in gliosis are broadly associated with reduced injury-related inflammation and BBB permeability along with reduced micromotion-related tissue strain187. Still, further details on the relationship between the material characteristics of an electrode and the inflammatory/molecular effects on reactive glia are needed to establish guiding principles to design fully integrated devices (including intervention strategies and their temporal influence). Future research directions will need to incorporate genetic tools to identify precise targets of design features. For instance, it would be useful to locally knockdown or upregulate specific glial pathways (such as receptor expression and transmitter production or release) to determine the consequences of gliotransmission on device performance (including plasticity, network function and neuronal health). Furthermore, advances in biomaterial science are producing new approaches to modify immune responses that could be leveraged to improve the tissue response to brain implants212–214. Uncovering the molecular pathways determining the relationship between glial responses and specific electrode features will facilitate targeted approaches to improved device design (Fig. 6).
Fig.6.
Opportunities for further enquiry in device design. Future work will need to uncover the effects of electrode properties on the molecular pathways that shape gliosis, including: (1) the degree of softness and corresponding inflammation from mechanoactivation of glia, and the evolution of the effect on gliosis over time (such as mechanical mismatch, micromotion, and the state of glial reactivity and ‘priming’); (2) the relationship between feature size and architecture on inciting and priming inflammatory gliosis around the injury, and the evaluation of the long-term consequences (such as hyperexcitability, excitotoxicity and degeneration) on device function; (3) the effects of surface modifications (chemistry and topography) on shaping reactive signalling at the interface (receptor activation and cytokine/gliotransmitter release) and the corresponding consequences on recording and stimulation performance; (4) targeted approaches to modify immune responses will need to be incorporated to achieve seamless integration, which should be guided by their impact on glial signalling, reactivity and device performance. Traditional devices images reproduced from refs217–219 (see Fig. 1 for credits). Next-generation devices reproduced from: top and bottom, ref.162, RSC; middle, ref.48, Macmillan Publishers Ltd.
Outlook
Although glia have been portrayed as acting as an encapsulating barrier to electrode integration and communication with surrounding neurons, this view does not capture the dynamic role of glia in the functional plasticity of neuronal networks following injury, and the implications of glia for the performance of microelectrode arrays implanted in the brain. A growing body of literature attests to the role of reactive gliosis in the remodelling and reshaping of neural circuitry during healing, yet relatively few reports have linked glial activity to the therapeutic effects of neuromodulation38,45 or explored the relationship between glial responses and recording quality62,63. Bridging this gap is a major opportunity for understanding the function and failure of microelectrode arrays in both research and clinical applications. Four major focus areas deserve further attention (Fig. 7). First, a better understanding of the glial role in shaping neural plasticity near devices (both at the cellular and network level). This is particularly relevant when interpreting results and developing methods to induce plasticity as a repair strategy. Targeted neurostimulation strategies can reorganize neural networks, potentially bypassing and overcoming neuronal damage or enhancing native function215,216. In addition, connecting well-described, known mechanisms for the glial influence on neuronal excitability and synaptic transmission to the performance of implants would create new opportunities for improved device design, stimulation protocols and tissue-integration strategies. Second, an in-depth study of glia as the effector of stimulation-based therapy, especially for reconciling the time course of therapeutic effects to potential glial-mediated underlying mechanisms (for instance, the slowly-emerging DBS outcomes that evolve over days and weeks6, or the stimulation-induced depression of neuronal excitability16). Third, the heterogeneity of glial responses, on the cellular scale (types and subtypes of glia, and their individual roles) and on spatiotemporal scales (the impacts of time post-implantation and the affected region relative to the electrode). Fourth, the development of electrodes for the seamless integration of clinical devices into brain tissue, including the identification of materials that are sufficiently stiff to allow for precise surgical placement and have the necessary balance of mechanical, chemical and electrical properties to reduce the inflammatory response and chronic gliosis (Fig. 6). Performance variability is a broad, ongoing challenge for both recording and stimulation applications in the research and clinical use of implanted electrode arrays, and understanding the biological underpinnings of inconsistent outcomes will inform the development of improved neuroprosthetic and neuromodulatory devices. As a regulator of the structural and functional remodelling of neuronal networks, glia are emerging as a dynamic, active determinant of device integration and performance.
Fig.7.
Opportunities for further biological enquiry. (1) The factors responsible for the ‘tipping point’ between reactive and non-reactive glial states, and the implications of glial priming on the safety of high-density arrays and of multiple implant strategies. (2) The contribution of hyperexcitability to neuronal loss and recorded signal quality, and the underlying relationship with a primed glial state. (3) Glial-mediated neuronal silencing surrounding implants and the relationship to recorded signals and stimulation thresholds. (4) The relationship between device performance and the time course of glial effects, for insights into the sources of performance variability, plasticity and placebo effects of device insertion, as well as therapeutic effects and side effects in a broad range of device applications.
Acknowledgments
J.W.S. was supported by National Institutes of Health (NIH) 1R21NS094900, T.D.Y.K. was supported by NIH 1R01NS094396, K.A.L. was supported by The Grainger Foundation, and E.K.P. was supported by NIH 1R21NS094900 and 5R03NS095202. The authors thank J. Eles for assistance collecting in vivo imaging data (Fig. 2a), D. Thompson and S. Yandamuri for assistance collecting data presented in Fig. 3, and M.-C. Senut of Biomilab, LLC, for providing feedback.
Footnotes
Author contributions
All authors contributed to researching the data and discussing the content of the manuscript, and to writing, reviewing and editing it.
Competing interests
The authors declare no competing financial interests.
References
- 1.Kasthuri N, Lichtman JW. Neurocartography. Neuropsychopharmacology. 2010;35:342–343. doi: 10.1038/npp.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberlaender M, et al. Three-dimensional axon morphologies of individual layer 5 neurons indicate cell type-specific intracortical pathways for whisker motion and touch. Proc. Natl Acad. Sci USA. 2011;108:4188–4193. doi: 10.1073/pnas.1100647108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubota Y. Untangling GABAergic wiring in the cortical microcircuit. Curr. Opin. Neurobiol. 2014;26:7–14. doi: 10.1016/j.conb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–171. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- 6.Herrington TM, et al. Mechanisms of deep brain stimulation. J. Neurophysiol. 2016;115:19–38. doi: 10.1152/jn.00281.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borton D, Micera S, Millán J, del R, Courtine G. Personalized neuroprosthetics. Sci. Transl. Med. 2013;5:210rv2. doi: 10.1126/scitranslmed.3005968. [DOI] [PubMed] [Google Scholar]
- 8.Jennings JH, Stuber GD. Tools for resolving functional activity and connectivity within intact neural circuits. Curr. Biol. 2014;24:R41–R50. doi: 10.1016/j.cub.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henze DA, et al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 2000;84:390–400. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- 10.Prasad A, et al. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012;9:56015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 11.Barrese JC, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013;10:66014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig KA, et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. J. Neural Eng. 2011;8:14001. doi: 10.1088/1741-2560/8/1/014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perge JA, et al. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J. Neural Eng. 2013;10:36004. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson A, Fetz EE. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J. Neurophysiol. 2007;98:3109–3118. doi: 10.1152/jn.00569.2007. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, et al. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans. Rehabil. Eng. 1999;7:315–326. doi: 10.1109/86.788468. [DOI] [PubMed] [Google Scholar]
- 16.McCreery DB, Yuen TGH, Agnew WF, Bullara LA. A characterization of the effects on neuronal excitability due to prolonged microstimulation with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 1997;44:931–939. doi: 10.1109/10.634645. [DOI] [PubMed] [Google Scholar]
- 17.McCreery DB, Agnew WF, Bullara LA. The effects of prolonged intracortical microstimulation on the excitability of pyramidal tract neurons in the cat. Ann. Biomed. Eng. 2002;30:107–119. doi: 10.1114/1.1430748. [DOI] [PubMed] [Google Scholar]
- 18.Prasad A, et al. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 2014;7:2. doi: 10.3389/fneng.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannasch U, Rouach N. Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 2013;36:405–417. doi: 10.1016/j.tins.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Virchow R. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. Meidinger; Frankfurt: 1856. [Google Scholar]
- 22.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016;275:305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sofroniew MV. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015;6:48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem. Pharmacol. 2010;79:77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Kozai TDY, et al. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J. Neural Eng. 2010;7:46011. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chew SSL, Johnson CS, Green CR, Danesh-Meyer HV. Role of connexin43 in central nervous system injury. Exp. Neurol. 2010;225:250–261. doi: 10.1016/j.expneurol.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Pannasch U, et al. Astroglial networks scale synaptic activity and plasticity. Proc. Natl Acad. Sci. USA. 2011;108:8467–8472. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JYW, et al. Neuropathology of the blood–brain barrier and pharmaco-resistance in human epilepsy. Brain. 2012;135:3115–3133. doi: 10.1093/brain/aws147. [DOI] [PubMed] [Google Scholar]
- 34.Kozai TDY, Eles JR, Vazquez AL, Cui XT. Two-photon imaging of chronically implanted neural electrodes: sealing methods and new insights. J. Neurosci. Methods. 2016;258:46–55. doi: 10.1016/j.jneumeth.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorfi M, Skousen JL, Weder C, Capadona JR. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2015;12:11001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxena T, et al. The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials. 2013;34:4703–4713. doi: 10.1016/j.biomaterials.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Nolta NF, Christensen MB, Crane PD, Skousen JL, Tresco PA. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials. 2015;53:753–762. doi: 10.1016/j.biomaterials.2015.02.081. [DOI] [PubMed] [Google Scholar]
- 38.Bekar L, et al. Adenosine is crucial for deep brain stimulation–mediated attenuation of tremor. Nat. Med. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 39.Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J. Neural Eng. 2009;6:46001. doi: 10.1088/1741-2560/6/4/046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell EK, Thompson DE, Ludwig KA, Kipke DR. Flavopiridol reduces the impedance of neural prostheses in vivo without affecting recording quality. J. Neurosci. Methods. 2009;183:149–157. doi: 10.1016/j.jneumeth.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Johnson MD, Otto KJ, Kipke DR. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Trans. Neural Syst. Rehabil. Eng. 2005;13:160–165. doi: 10.1109/TNSRE.2005.847373. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig KA, et al. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006;3:59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 43.Prasad A, et al. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J. Neural Eng. 2012;9:26028. doi: 10.1088/1741-2560/9/2/026028. [DOI] [PubMed] [Google Scholar]
- 44.McConnell GC, et al. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009;6:56003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 45.Tawfik VL, et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery. 2010;67:367–375. doi: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAdams ET, Lackermeier A, McLaughlin JA, Macken D, Jossinet J. The linear and non-linear electrical properties of the electrode–electrolyte interface. Biosens. Bioelectron. 1995;10:67–74. [Google Scholar]
- 47.Mercanzini A, Colin P, Bensadoun J-C, Bertsch A, Renaud P. In vivo electrical impedance spectroscopy of tissue reaction to microelectrode arrays. IEEE Trans. Biomed. Eng. 2009;56:1909–1918. doi: 10.1109/TBME.2009.2018457. [DOI] [PubMed] [Google Scholar]
- 48.Kozai TDY, et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012;11:1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robblee LS, McHardy J, Agnew WF, Bullara LA. Electrical stimulation with Pt electrodes. VII. Dissolution of Pt electrodes during electrical stimulation of the cat cerebral cortex. J. Neurosci. Methods. 1983;9:301–308. doi: 10.1016/0165-0270(83)90062-6. [DOI] [PubMed] [Google Scholar]
- 50.Wei XF, et al. Impedance characteristics of deep brain stimulation electrodes in vitro and in vivo. J. Neural Eng. 2009;6:46008. doi: 10.1088/1741-2560/6/4/046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Otto KJ, Johnson MD, Kipke DR. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 2006;53:333–340. doi: 10.1109/TBME.2005.862530. [DOI] [PubMed] [Google Scholar]
- 53.Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proc. Natl Acad. Sci. USA. 2004;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med. Biol. Eng. Comput. 2004;42:394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 55.McIntyre CC, et al. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 2002;88:1592–604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- 56.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. Exp. Brain Res. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- 57.Williams JC, Rennaker RL, Kipke DR. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 1999;4:303–313. doi: 10.1016/s1385-299x(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 58.Preda F, et al. Switching from constant voltage to constant current in deep brain stimulation: a multicenter experience of mixed implants for movement disorders. Eur. J. Neurol. 2016;23:190–195. doi: 10.1111/ene.12835. [DOI] [PubMed] [Google Scholar]
- 59.Timmermann L, et al. Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol. 2015;14:693–701. doi: 10.1016/S1474-4422(15)00087-3. [DOI] [PubMed] [Google Scholar]
- 60.McCreery D, et al. Correlations between histology and neuronal activity recorded by microelectrodes implanted chronically in the cerebral cortex. J. Neural Eng. 2016;13:36012. doi: 10.1088/1741-2560/13/3/036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007;4:410–423. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- 62.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2005;13:524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- 63.Jiang J, Willett FR, Taylor DM. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2014. Relationship between microelectrode array impedance and chronic recording quality of single units and local field potentials; pp. 3045–3048. [DOI] [PubMed] [Google Scholar]
- 64.Kozai TDY, et al. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials. 2015;37:25–39. doi: 10.1016/j.biomaterials.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malaga KA, et al. Data-driven model comparing the effects of glial scarring and interface interactions on chronic neural recordings in non-human primates. J. Neural Eng. 2016;13:16010. doi: 10.1088/1741-2560/13/1/016010. [DOI] [PubMed] [Google Scholar]
- 66.Humphrey DR, Schmidt EM. In: Neurophysiological Techniques. Boulton AA, Baker GB, Vanderwolf CH, editors. II. Humana Press; New York: 1990. pp. 1–64. [Google Scholar]
- 67.Shoham S, Nagarajan S. Neuroprosthetics: Theory and Practice. World Scientific; 2003. pp. 448–465. [Google Scholar]
- 68.Alivisatos AP, et al. Nanotools for neuroscience and brain activity mapping. ACS Nano. 2013;7:1850–1866. doi: 10.1021/nn4012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark JJ, et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grahn PJ, et al. A neurochemical closed-loop controller for deep brain stimulation: toward individualized smart neuromodulation therapies. Front. Neurosci. 2014;8:169. doi: 10.3389/fnins.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hascup ER, et al. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009;1291:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillips PEM, Wightman RM. Critical guidelines for validation of the selectivity of in-vivo chemical microsensors. Trends Anal. Chem. 2003;22:509–514. [Google Scholar]
- 73.Roitbak T, Sykov E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28:40–48. doi: 10.1002/(sici)1098-1136(199910)28:1<40::aid-glia5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 74.Hascup ER, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J. Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- 76.Hille B. Ion Channels of Excitable Membranes. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 77.Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat. Rev. Neurosci. 2014;15:379–393. doi: 10.1038/nrn3770. [DOI] [PubMed] [Google Scholar]
- 78.Haupt C, Witte OW, Frahm C. Up-regulation of connexin43 in the glial scar following photothrombotic ischemic injury. Mol. Cell. Neurosci. 2007;35:89–99. doi: 10.1016/j.mcn.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Karumbaiah L, et al. Relationship between intracortical electrode design and chronic recording function. Biomaterials. 2013;34:8061–8074. doi: 10.1016/j.biomaterials.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 80.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 81.Karumbaiah L, et al. The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials. 2012;33:5983–5996. doi: 10.1016/j.biomaterials.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 82.Kozai TDY, et al. Effects of caspase-1 knockout on chronic neural recording quality and longevity: insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials. 2014;35:9620–9634. doi: 10.1016/j.biomaterials.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 85.Zhou C, et al. Interleukin-1β inhibits voltage-gated sodium currents in a time- and dose-dependent manner in cortical neurons. Neurochem. Res. 2011;36:1116–1123. doi: 10.1007/s11064-011-0456-8. [DOI] [PubMed] [Google Scholar]
- 86.Li X, Chen W, Sheng J, Cao D, Wang W. Interleukin-6 inhibits voltage-gated sodium channel activity of cultured rat spinal cord neurons. Acta Neuropsychiatr. 2014;26:170–177. doi: 10.1017/neu.2013.49. [DOI] [PubMed] [Google Scholar]
- 87.Zhou C, Ye H-H, Wang S-Q, Chai Z. Interleukin-1β regulation of N-type Ca2+ channels in cortical neurons. Neurosci. Lett. 2006;403:181–185. doi: 10.1016/j.neulet.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 88.Ma S-H, Li B, Huang H-W, Peng Y-P, Qiu Y-H. Interleukin-6 inhibits L-type calcium channel activity of cultured cerebellar granule neurons. J. Physiol. Sci. 2012;62:385–392. doi: 10.1007/s12576-012-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z, Qiu Y-H, Li B, Ma S-H, Peng Y-P. Neuroprotection of interleukin-6 against NMDA-induced apoptosis and its signal-transduction mechanisms. Neurotox. Res. 2011;19:484–495. doi: 10.1007/s12640-010-9215-x. [DOI] [PubMed] [Google Scholar]
- 90.Baldwin KT, Eroglu C. Molecular mechanisms of astrocyte-induced synaptogenesis. Curr. Opin. Neurobiol. 2017;45:113–120. doi: 10.1016/j.conb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salatino JW, Winter BM, Drazin MH, Purcell EK. Functional remodeling of subtype-specific markers surrounding implanted neuroprostheses. J. Neurophysiol. 2017;118:194–202. doi: 10.1152/jn.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weissberg I, et al. Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol. Dis. 2015;78:115–125. doi: 10.1016/j.nbd.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diniz LP, et al. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia. 2014;62:1917–1931. doi: 10.1002/glia.22713. [DOI] [PubMed] [Google Scholar]
- 94.Lin T-n, et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- 95.Carsten Möller J, et al. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia. 1996;17:121–132. doi: 10.1002/(SICI)1098-1136(199606)17:2<121::AID-GLIA4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 96.Tran MD, Neary JT. Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc. Natl Acad. Sci. USA. 2006;103:9321–9326. doi: 10.1073/pnas.0603146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 98.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 100.Beattie EC, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 101.Chang S-Y, Shon YM, Agnesi F, Lee KH. 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2009. Microthalamotomy effect during deep brain stimulation: potential involvement of adenosine and glutamate efflux. In; pp. 3294–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fredholm B, Dunwiddie T. How does adenosine inhibit transmitter release? Trends Pharmacol. Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 104.Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J. Pharmacol. Exp. Ther. 1989;249:31–37. [PubMed] [Google Scholar]
- 105.Stone TW, Ceruti S, Abbracchio MP. In: Adenosine Receptors in Health and Disease. Handbook of Experimental Pharmacology. Wilson C, Mustafa S, editors. Vol. 193. Springer; Berlin, Heidelberg: 2009. pp. 535–587. [DOI] [PubMed] [Google Scholar]
- 106.Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem. Int. 2010;57:440–445. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 107.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amiri M, Montaseri G, Bahrami F. On the role of astrocytes in synchronization of two coupled neurons: a mathematical perspective. Biol. Cybern. 2011;105:153–166. doi: 10.1007/s00422-011-0455-5. [DOI] [PubMed] [Google Scholar]
- 109.Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- 110.Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- 111.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 112.Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 113.Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fellin T, et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 115.Silchenko AN, Tass PA. Computational modeling of paroxysmal depolarization shifts in neurons induced by the glutamate release from astrocytes. Biol. Cybern. 2008;98:61–74. doi: 10.1007/s00422-007-0196-7. [DOI] [PubMed] [Google Scholar]
- 116.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 117.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96:29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vedam-Mai V, et al. Deep brain stimulation and the role of astrocytes. Mol. Psychiatry. 2012;17:124–31. doi: 10.1038/mp.2011.61. [DOI] [PubMed] [Google Scholar]
- 119.McIntyre CC, Anderson RW. Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J. Neurochem. 2016;139:338–345. doi: 10.1111/jnc.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fenoy AJ, Goetz L, Chabardès S, Xia Y. Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci. Ther. 2014;20:191–201. doi: 10.1111/cns.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vedam-Mai V, et al. The national DBS brain tissue network pilot study: need for more tissue and more standardization. Cell Tissue Bank. 2011;12:219–231. doi: 10.1007/s10561-010-9189-1. [DOI] [PubMed] [Google Scholar]
- 122.Sun DA, et al. Postmortem analysis following 71 months of deep brain stimulation of the subthalamic nucleus for Parkinson disease. J. Neurosurg. 2008;109:325–329. doi: 10.3171/JNS/2008/109/8/0325. [DOI] [PubMed] [Google Scholar]
- 123.van Kuyck K, Welkenhuysen M, Arckens L, Sciot R, Nuttin B. Histological alterations induced by electrode implantation and electrical stimulation in the human brain: a review. Neuromodulation. 2007;10:244–261. doi: 10.1111/j.1525-1403.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 124.Chang S-Y, et al. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin. Proc. 2012;87:760–765. doi: 10.1016/j.mayocp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Gompel JJ, et al. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia. 2014;55:233–244. doi: 10.1111/epi.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santello M, Volterra A. TNFα in synaptic function: switching gears. Trends Neurosci. 2012;35:638–647. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Gellner A-K, Reis J, Fritsch B. Glia: a neglected player in non-invasive direct current brain stimulation. Front. Cell. Neurosci. 2016;10:188. doi: 10.3389/fncel.2016.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monai H, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 2016;7:11100. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sasaki T, et al. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl Acad. Sci. USA. 2012;109:20720–20725. doi: 10.1073/pnas.1213458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Braun R, et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp. Neurol. 2016;279:127–136. doi: 10.1016/j.expneurol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 131.Arsenault D, et al. A novel combinational approach of microstimulation and bioluminescence imaging to study the mechanisms of action of cerebral electrical stimulation in mice. J. Physiol. 2015;593:2257–2278. doi: 10.1113/jphysiol.2014.287243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsuo H, et al. Early transcutaneous electrical nerve stimulation reduces hyperalgesia and decreases activation of spinal glial cells in mice with neuropathic pain. Pain. 2014;155:1888–1901. doi: 10.1016/j.pain.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 133.Peruzzotti-Jametti L, et al. Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke. 2013;44:3166–3174. doi: 10.1161/STROKEAHA.113.001687. [DOI] [PubMed] [Google Scholar]
- 134.Iwasa SN, Babona-Pilipos R, Morshead CM. environmental factors that influence stem cell migration: an “electric field”. Stem Cells Int. 2017;2017:4276927. doi: 10.1155/2017/4276927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vedam-Mai V, et al. Increased precursor cell proliferation after deep brain stimulation for Parkinson’s disease: a human study. PLoS ONE. 2014;9:e88770. doi: 10.1371/journal.pone.0088770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J. Neurosurg. 2008;108:132–138. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- 137.Encinas JM, Hamani C, Lozano AM, Enikolopov G. Neurogenic hippocampal targets of deep brain stimulation. J. Comp. Neurol. 2011;519:6–20. doi: 10.1002/cne.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Babona-Pilipos R, Pritchard-Oh A, Popovic MR, Morshead CM. Biphasic monopolar electrical stimulation induces rapid and directed galvanotaxis in adult subependymal neural precursors. Stem Cell Res. Ther. 2015;6:67. doi: 10.1186/s13287-015-0049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rueger MA, et al. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS ONE. 2012;7:e43776. doi: 10.1371/journal.pone.0043776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao H, Steiger A, Nohner M, Ye H. specific intensity direct current (DC) electric field improves neural stem cell migration and enhances differentiation towards βIII tubulin+ neurons. PLoS ONE. 2015;10:e0129625. doi: 10.1371/journal.pone.0129625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khaindrava V, et al. High frequency stimulation of the subthalamic nucleus impacts adult neurogenesis in a rat model of Parkinson’s disease. Neurobiol. Dis. 2011;42:284–291. doi: 10.1016/j.nbd.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 142.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 144.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 145.Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 146.Ke B, Liu T-T, Liu C, Xiang H, Xiong J. Dorsal subthalamic nucleus electrical stimulation for drug/treatment-refractory epilepsy may modulate melanocortinergic signaling in astrocytes. Epilepsy Behav. 2014;36:6–8. doi: 10.1016/j.yebeh.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 147.Seymour JP, Wu F, Wise KD, Yoon E. State-of-the-art MEMS and microsystem tools for brain research. Microsyst. Nanoeng. 2017;3:16066. doi: 10.1038/micronano.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28:3594–607. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 149.Loane DJ, et al. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnson VE, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Perry VH, Nicoll JAR, Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 152.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain. Behav. Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frank MG, et al. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol. Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 155.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lacour SP, et al. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016;1:16063. [Google Scholar]
- 157.Wellman SM, et al. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 2017 doi: 10.1002/adfm.201701269. https://doi.org/10.1002/adfm.201701269 [DOI] [PMC free article] [PubMed]
- 158.Moshayedi P, et al. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials. 2014;35:3919–3925. doi: 10.1016/j.biomaterials.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 159.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 160.Collinger JL, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nguyen JK, et al. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 2014;11:56014. doi: 10.1088/1741-2560/11/5/056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kolarcik CL, et al. Elastomeric and soft conducting microwires for implantable neural interfaces. Soft Matter. 2015;11:4847–4861. doi: 10.1039/c5sm00174a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hess AE, et al. Development of a stimuli-responsive polymer nanocomposite toward biologically optimized, MEMS-based neural probes. J. Micromech. Microeng. 2011;21:54009. [Google Scholar]
- 164.Ware T, et al. Thiol-ene/acrylate substrates for softening intracortical electrodes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014;102:1–11. doi: 10.1002/jbmb.32946. [DOI] [PubMed] [Google Scholar]
- 165.Ware T, et al. Fabrication of responsive, softening neural interfaces. Adv. Funct. Mater. 2012;22:3470–3479. [Google Scholar]
- 166.Capadona JR, et al. Mechanically adaptive nanocomposites for neural interfacing. MRS Bull. 2012;37:581–589. [Google Scholar]
- 167.Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ, Weder C. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science. 2008;319:1370–1374. doi: 10.1126/science.1153307. [DOI] [PubMed] [Google Scholar]
- 168.Harris JP, et al. Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. J. Neural Eng. 2011;8:66011. doi: 10.1088/1741-2560/8/6/066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ware T, et al. Three-dimensional flexible electronics enabled by shape memory polymer substrates for responsive neural interfaces. Macromol. Mater. Eng. 2012;297:1193–1202. doi: 10.1002/mame.201200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies. Nat. Rev. Mater. 2017;2:16093. doi: 10.1038/natrevmats.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fu T-M, et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods. 2016;13:875–882. doi: 10.1038/nmeth.3969. [DOI] [PubMed] [Google Scholar]
- 172.Hong G, et al. Syringe Injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 2015;15:6979–6984. doi: 10.1021/acs.nanolett.5b02987. [DOI] [PubMed] [Google Scholar]
- 173.Luan L, et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017;3:e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim BJ, et al. 3D parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013;10:045002. doi: 10.1088/1741-2560/10/4/045002. [DOI] [PubMed] [Google Scholar]
- 175.Kim T, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park S., II Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015;33:1280–1286. doi: 10.1038/nbt.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Metallo C, Trimmer BA. Silk coating as a novel delivery system and reversible adhesive for stiffening and shaping flexible probes. J. Biol. Methods. 2015;2:e13. [Google Scholar]
- 178.Kozai TDY, et al. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials. 2014;35:9255–9268. doi: 10.1016/j.biomaterials.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 179.Seymour JP, Langhals NB, Anderson DJ, Kipke DR. Novel multi-sided, microelectrode arrays for implantable neural applications. Biomed. Microdevices. 2011;13:441–451. doi: 10.1007/s10544-011-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Skousen JL, et al. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog. Brain Res. 2011;194:167–80. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- 181.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 182.Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu. Rev. Biomed. Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 183.Turner AMP, et al. Attachment of astroglial cells to microfabricated pillar arrays of different geometries. J. Biomed. Mater. Res. 2000;51:430–441. doi: 10.1002/1097-4636(20000905)51:3<430::aid-jbm18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 184.Sanders JE, Stiles CE, Hayes CL. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J. Biomed. Mater. Res. 2000;52:231–237. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 185.Bernatchez S, Parks PJ, Gibbons DF. Interaction of macrophages with fibrous materials in vitro. Biomaterials. 1996;17:2077–2086. doi: 10.1016/0142-9612(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 186.Gällentoft L, et al. Size-dependent long-term tissue response to biostable nanowires in the brain. Biomaterials. 2015;42:172–183. doi: 10.1016/j.biomaterials.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 187.Skousen JL, Bridge MJ, Tresco PA. A strategy to passively reduce neuroinflammation surrounding devices implanted chronically in brain tissue by manipulating device surface permeability. Biomaterials. 2015;36:33–43. doi: 10.1016/j.biomaterials.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 188.Saxena T, Bellamkonda RV. Implantable electronics: a sensor web for neurons. Nat. Mater. 2015;14:1190–1191. doi: 10.1038/nmat4454. [DOI] [PubMed] [Google Scholar]
- 189.Xie C, et al. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015;14:1286–1292. doi: 10.1038/nmat4427. [DOI] [PubMed] [Google Scholar]
- 190.Liu J, et al. Syringe-injectable electronics. Nat. Nanotech. 2015;10:629–636. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fattahi P, Yang G, Kim G, Abidian MR. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014;26:1846–1885. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Green R, Abidian MR. Conducting polymers for neural prosthetic and neural interface applications. Adv. Mater. 2015;27:7620–7637. doi: 10.1002/adma.201501810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kolarcik CL, et al. In vivo effects of L1 coating on inflammation and neuronal health at the electrode–tissue interface in rat spinal cord and dorsal root ganglion. Acta Biomater. 2012;8:3561–3575. doi: 10.1016/j.actbio.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rao L, Zhou H, Li T, Li C, Duan YY. Polyethylene glycol-containing polyurethane hydrogel coatings for improving the biocompatibility of neural electrodes. Acta Biomater. 2012;8:2233–2242. doi: 10.1016/j.actbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 195.Kim D-H, et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010;9:511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tien LW, et al. Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes. Adv. Funct. Mater. 2013;23:3185–3193. [Google Scholar]
- 197.Zhong Y, Bellamkonda RV. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.He W, McConnell GC, Schneider TM, Bellamkonda RV. A novel anti-inflammatory surface for neural electrodes. Adv. Mater. 2007;19:3529–3533. [Google Scholar]
- 199.Mercanzini A, et al. Controlled release nanoparticle-embedded coatings reduce the tissue reaction to neuroprostheses. J. Control. Release. 2010;145:196–202. doi: 10.1016/j.jconrel.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 200.Abidian MR, Martin DC. Multifunctional nanobiomaterials for neural interfaces. Adv. Funct. Mater. 2009;19:573–585. [Google Scholar]
- 201.Bezuidenhout D, et al. Covalent incorporation and controlled release of active dexamethasone from injectable polyethylene glycol hydrogels. J. Biomed. Mater. Res. Part A. 2013;101A:1311–1318. doi: 10.1002/jbm.a.34438. [DOI] [PubMed] [Google Scholar]
- 202.Gutowski SM, et al. Protease-degradable PEG-maleimide coating with on-demand release of IL-1Ra to improve tissue response to neural electrodes. Biomaterials. 2015;44:55–70. doi: 10.1016/j.biomaterials.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control. Release. 2006;110:531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 204.Kim Y, et al. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature. 2013;500:59–63. doi: 10.1038/nature12401. [DOI] [PubMed] [Google Scholar]
- 205.Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Interfacing conducting polymer nanotubes with the central nervous system: chronic neural recording using poly(3,4-ethylenedioxythiophene) nanotubes. Adv. Mater. 2009;21:3764–3770. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Carbon nanotube coating improves neuronal recordings. Nat. Nanotech. 2008;3:434–439. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- 207.Abidian MR, Corey JM, Kipke DR, Martin DC. Conducting-polymer nanotubes improve electrical properties, mechanical adhesion, neural attachment, and neurite outgrowth of neural electrodes. Small. 2010;6:421–429. doi: 10.1002/smll.200901868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Eles JR, et al. Neuroadhesive L1 coating attenuates acute microglial encapsulation of neural electrodes as revealed by live two-photon microscopy. Biomaterials. 2017;113:279–292. doi: 10.1016/j.biomaterials.2016.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Singh AV, et al. Astrocytes increase ATP exocytosis mediated calcium signaling in response to microgroove structures. Sci. Rep. 2015;5:7847. doi: 10.1038/srep07847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Min SK, et al. Effect of topography of an electrospun nanofiber on modulation of activity of primary rat astrocytes. Neurosci. Lett. 2013;534:80–84. doi: 10.1016/j.neulet.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 211.Zuidema JM, et al. Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-l-lactic acid fibers. Biomaterials. 2014;35:1439–1449. doi: 10.1016/j.biomaterials.2013.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vishwakarma A, et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 2016;34:470–482. doi: 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 213.Webber MJ, Appel EA, Meijer EW, Langer R. Supramolecular biomaterials. Nat. Mater. 2016;15:13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 214.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat. Rev. Mater. 2016;2:16075. [Google Scholar]
- 215.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 216.Hampson RE, et al. Facilitation and restoration of cognitive function in primate prefrontal cortex by a neuroprosthesis that utilizes minicolumn-specific neural firing. J. Neural Eng. 2012;9:056012. doi: 10.1088/1741-2560/9/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2003;11:151–155. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 218.Kindlmann G, et al. Imaging of Utah electrode array, implanted in cochlear nerve. Digit. Biol. Emerg. Paradig. 2003:6–7. [Google Scholar]
- 219.Moss J, Ryder T, Aziz TZ, Graeber MB, Bain PG. Electron microscopy of tissue adherent to explanted electrodes in dystonia and Parkinson’s disease. Brain. 2004;127:2755–2763. doi: 10.1093/brain/awh292. [DOI] [PubMed] [Google Scholar]
- 220.Kozai TDY, Vazquez AL, Weaver CL, Kim S-G, Cui XT. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J. Neural Eng. 2012;9:066001. doi: 10.1088/1741-2560/9/6/066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ludwig KA, et al. Using a common average reference to improve cortical neuron recordings from microelectrode arrays. J. Neurophysiol. 2009;101:1679–1689. doi: 10.1152/jn.90989.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.tova M, Suzuki R, Zhu X. Polydendrocytes (NG2 Nishiyama, A., Komi cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 223.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–73. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 226.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 227.Hall CN, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fidler PS, et al. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J. Neurosci. 1999;19:8778–8788. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rivers LE, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]