Abstract
Recent work has implicated spreading depolarization (SD) as a key contributor the progression of acute brain injuries, however development of interventions selectively targeting SDs has lagged behind. Initial clinical intervention efforts have focused on observations that relatively high doses of the sedative agent ketamine can completely suppress SD. However blocking propagation of SD could theoretically prevent beneficial effects of SD in surrounding brain regions. Selective targeting of deleterious consequences of SD (rather than abolition) could be a useful adjunct approach, and be achieved with lower ketamine concentrations. We utilized a brain slice model to test whether deleterious consequences of SD could be prevented by ketamine, using concentrations that did not prevent the initiation and propagation of SD. Studies were conducted using murine brain slices, with focal KCl as an SD stimulus. Consequences of SD were assessed with electrophysiological and imaging measures of ionic and synaptic recovery. Under control conditions, ketamine (up to 30μM) did not prevent SD, but significantly reduced the duration of neuronal Ca2+ loading, and the duration of associated extracellular potential shifts. Recovery of postsynaptic potentials after SD, was also significantly accelerated. When SD was evoked on a background of mild metabolic compromise, neuronal recovery was substantially impaired. Under compromised conditions, the same concentrations of ketamine reduced ionic and metabolic loading during SD, sufficient to preserve function after repetitive SDs. These results suggest that low concentrations of ketamine could be utilized to prevent damaging consequences of SD, while not blocking them outright and preserving potentially protective effects of SD.
Keywords: Spreading depression, brain slice, excitotoxicity, metabolic compromise, NMDA receptor, calcium loading, neuronal injury, excitatory postsynaptic potentials
Introduction
Spreading depolarization (SD) is a slowly propagating wave (2– 4 mm min−1) of near-complete neuronal and glial depolarization that has gained renewed interest as an important contributor to the progression of acute brain injuries (Lauritzen et al., 2011, Dreier et al., 2017, Hartings et al., 2017). SD can be initiated by stimuli that cause synchronous depolarization of a critical volume of brain tissue (Tang et al., 2014), and SD propagation across the brain is propelled by feed-forward release of glutamate and/or K+ (Somjen, 2001). In injured brain, the initiating depolarization is caused by ischemia, trauma, or other energetic supply-demand mismatches (von Bornstadt et al., 2015). The extent of ionic loading accompanying SD is extreme, with intracellular Ca2+ loads continuously exceeding 10s of micromolar for more than a minute (Somjen, 2001, Dietz et al., 2008). As such, the metabolic costs to recover from SD are much more demanding than other brain phenomena, such as seizures (Dreier et al., 2013), and thus are particularly challenging for the injured brain (Hartings et al., 2017).
Whether or not injury occurs after SD depends greatly on the capacity of tissues to re-establish ionic gradients in the aftermath of SD. This capacity is influenced by the degree of ionic loading during SDs, the baseline metabolic capacity, and the ability of a region to profoundly increase blood flow to match energy demands after SD (Dreier, 2011). This is exemplified during SD in the healthy brain (e.g. migraine aura), where metabolic and vascular perfusion reserves are adequate, and thus SD does not result in any permanent damage (Nedergaard and Hansen, 1988). In contrast, SDs that spontaneously occur following stroke (Dohmen et al., 2008), trauma (Hartings et al., 2011), or subarachnoid hemorrhage (Dreier et al., 2009) can underlie stepwise progression of injury (Busch et al., 1996, Hartings et al., 2003, Hartings et al., 2017).
The development of clinical interventions for SD has lagged behind efforts to demonstrate their incidence in different pathologic conditions. Initial efforts have concentrated on the application of agents such as NMDA receptor (NMDAR) antagonists that block the initiation and propagation of SD. The dissociative anesthetic ketamine is an NMDAR antagonist that prevents SD in animal models (Hernandez-Caceres et al., 1987, Marrannes et al., 1988) and shows effectiveness in case reports (Sakowitz et al., 2009, Schiefecker et al., 2015). A retrospective review of medications used in the intensive care unit (ICU) also shows that ketamine infusion can reduce the frequency of SDs in brain injured patients (Hertle et al., 2012). Prospective studies of ketamine would be useful to determine whether a reduction in SD frequency is associated with improved outcomes in the clinic. However, such studies are complicated by two potential problems. First, the high ketamine concentrations used to suppress SD also result in substantial sedation with attendant increases in risk of ICU complications (Abou-Chebl et al., 2010, Nichols et al., 2010). Secondly, SDs propagate widely in injured brain, including through tissue that may be distant from an injury core where intact metabolic capacity is retained. It is possible that SDs invading these distant regions cause protective preconditioning (Yanamoto et al., 2004, Viggiano et al., 2016), adaptive synaptic plasticity (Faraguna et al., 2010), and/or neurogenesis (Urbach et al., 2017) that may be beneficial to functional recovery (Nakamura et al., 2010, Dreier, 2011) These theoretical issues require further study, but suggest that different approaches to selectively target the deleterious consequences of SD could be a useful adjunct to the current focus on global block of SD events.
We tested here whether deleterious effects of SD could be limited by lower concentrations of ketamine that do not prevent SD outright. Our findings with measurements of Ca2+ loading and a model of metabolic vulnerability indicate that ketamine can be protective without blocking SD, and support a possible significant modification of therapeutic strategies for SD, based on blocking consequences of SD rather than incidence.
Materials and Methods
Animals and Preparations
All animal procedures were performed in accordance with protocols approved by the UNM Health Sciences Center Institutional Animal Care and Use Committee. Adult (4–8 weeks) male and female mice (C57Bl/6 and/or GCaMP5G) were used for all experiments. For Ca2+ imaging experiments, homozygous mice expressing the floxxed calcium indicator GCaMP5G under the CAG promoter (Gee et al., 2014) were purchased from The Jackson Laboratory (Stock No: 024477, B6;129S6-Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd/J), and bred with homozygous mice expressing Cre Recombinase under the CamK2a promoter (B6.Cg-Tg(Camk2a-cre)T29–1Stl/J, Jax Stock No: 005359). Offspring were utilized in experiments and had robust GCaMP5G expression in hippocampal pyramidal neurons (Wang et al., 2013).
Acute brain slices were prepared as previously described (Shuttleworth et al., 2003). Briefly, animals were deeply anaesthetized with 0.15mL (s.c.) injection of ketamine-xylazine (85 and 15 mg ml−1, respectively), decapitated, and brains were quickly removed into 150 mL oxygenated ice-cold cutting solution (in mM): sucrose, 220; NaHCO3, 26; KCl, 3; NaH2PO4, 1.5; MgSO4, 6; glucose, 10; CaCl2 0.2; equilibrated with 95% O2/5% CO2 supplemented with 0.2 ml ketamine (100 mg/ml, Putney Inc., Portland, ME), to limit excitotoxicity during the slice preparation as described in (Aitken et al., 1995). Coronal cortico-hippocampal slices (350 μm) were prepared with a Pelco 102 Vibratome (Ted Pella, Inc., Redding, CA), hemisected, and then allowed to recover in artificial cerebrospinal fluid (aCSF; containing (in mM): NaCl, 126; NaHCO3, 26; glucose, 10; KCl, 3; CaCl2, 2, NaH2PO4, 1.5; MgSO4, 1; equilibrated with 95% O2/ 5% CO2), at 35°C for 60 min. After 1h, the holding aCSF was replaced with chilled (20°C) aCSF and slices were allowed to equilibrate to room temperature until the start of recording sessions. These incubations and exchanges served to ensure effective wash out of residual ketamine from slices, as previously established with responsiveness to glutamate and NMDA (Shuttleworth et al., 2003, Hoskison and Shuttleworth, 2006, Vander Jagt et al., 2008).
Generation of SD
Individual brain slices were transferred to a submersion recording chamber with nylon slice supports (RC-27L, Warner Instruments, Hamden, CT), and continuously super fused with oxygenated (95% O2/95% CO2) aCSF at 2.2 ml min-1. Bath temperature was maintained at 32°C by an inline heater assembly (TC-344B, Warner Instruments). After placement of electrodes into the slice (See Electrophysiology methods) slices were allowed 20 minutes for equilibration. As described below (Results), modified aCSF with elevated K+ (8mM) was used for most experiments, in order to increase ability of single slices to support repetitive SDs and enable rigorous testing of drug effects (Funke et al., 2009, Zhang et al., 2015). SDs were evoked by pressure microinjection (40ms, 30 psi; Picospritzer; Parker Hannifin, OH, USA) of KCl (1M) via a glass micropipette ~3 MΩ) placed in hippocampal CA1 stratum radiatum. Repetitive SDs were initiated in each slice at 15 minute intervals to allow for full recovery between events. In experiments assessing the effect of ketamine antagonism during repetitive SDs (Figures 2–4 & Supplementary Figures), antagonist wash-in commenced following the second of two control SDs, and the second control SD was used for analyses (Footitt and Newberry, 1998). SD initiation and propagation, as well as slice viability (see Metabolic Challenge below), were examined by monitoring intrinsic optical signals (IOS) of submerged brain slices trans-illuminated with visible light (≥ 600nm) and collected using a 4X objective (Olympus, 0.10 NA). IOS data were captured at 0.5 Hz using a cooled CCD camera (Imago, Till Photonics) and analyzed with TillVision software (TillPhotonics, version 4.01). Data analysis involved normalizing transmitted light to baseline and expressing IOS as percent change in transmission (ΔT/T0 × 100) (Anderson and Andrew, 2002).
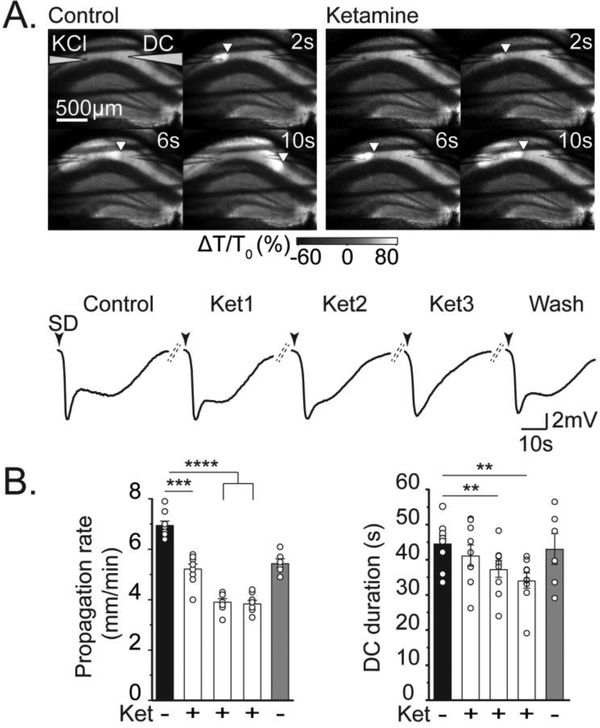
Figure 2. Ketamine reduced SD propagation rate and DC shift duration.
A: Representative example of effect of ketamine wash-in, during a series of repetitive SDs. Top panels show intrinsic optical signal changes to track SD propagation (as described in Figure 1) in control conditions (left), and during the third SD evoked in the presence of 30 μM ketamine (right). Note the delayed propagation of the SD wavefront in ketamine. The traces show DC potential recordings during this sequence of SDs in the same slice. Black arrowheads indicate DC shift onset, and dashed lines represent 15 minutes recovery between stimulations. The duration of the DC shift of SD was progressively reduced, and then recovered after ketamine washout. B: Summary data from 9 preparations as shown in A, demonstrating progressive decreases in both propagation rate and DC duration with partial recovery upon ketamine wash out in 6 experiments (gray bar). *P<.05, **P<0.01, ***P<0.001, **** P<0.0001.
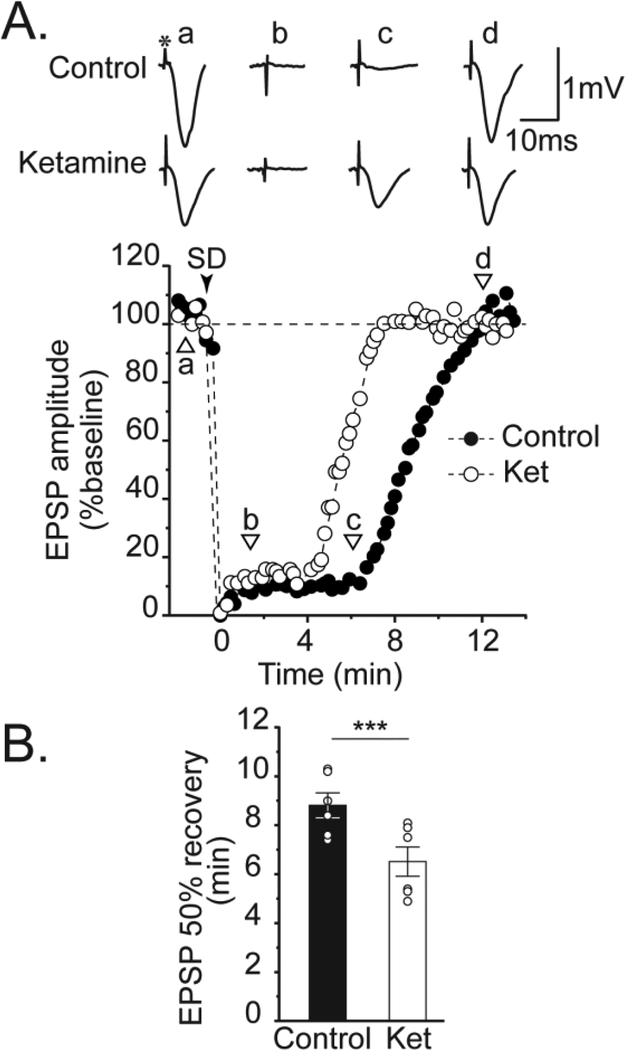
Figure 4. Ketamine accelerates recovery of evoked postsynaptic potentials after SD.
A: Representative example of suppression and recovery of evoked excitatory postsynaptic potentials (EPSPs) after SD. Control EPSPs (a) were abolished after SD (b,c), and slowly recovered to baseline amplitudes after ~12 min (d). The asterisk above control (a) trace indicates the bipolar stimulus artifact. The full time course of EPSP suppression and recovery in this same slice is plotted below (black circles). SD onset indicated by black arrowhead. Ketamine (30 μM) did not prevent EPSP suppression, but significantly accelerated recovery rate (lower set of traces, and white circles in plot). B: Summary data from 6 such experiments. The effect of ketamine on DC shift duration in this data set was consistent with prior observations in Figure 2 (45.9 ± 2.3 vs. 35.7 ± 1.1 s for control and ketamine, respectively; P = 0.0026). *** P<0.001
Electrophysiology
Extracellular recordings were acquired (1–10kHz) with an Axon MultiClamp 700A amplifier, digitized (Digidata 1332), and recorded using pCLAMP10.2 software (Molecular Devices, Sunnyvale, CA, USA). Glass recording microelectrodes were filled with aCSF (tip resistance ~3MΩ) and positioned at a depth of 50–100 μm in the CA1 stratum radiatum ≥200 μm from the KCl-filled glass ejection micropipettes. The durations of SDs were calculated from the extracellular potential shift (“DC shift” (Somjen, 2001)), measured at 20% of the peak maximum to 80% recovery. In experiments assessing synaptic recovery after SD, a concentric bipolar electrode (FHC, Bowdoin, ME, USA) was placed on the slice surface ofCA1 stratum radiatum, between the KCl ejection micropipette and recording electrode, for stimulation of Schaffer collateral inputs. Excitatory postsynaptic potentials (EPSPs) were recorded using test pulses (50 μs, 0.1Hz) delivered at intensities (80 – 400 μA) that gave 40 – 60% of the maximum EPSP amplitude. DC shifts and EPSPs were analyzed using Clampfit 10.2 software (Molecular Devices, Sunnyvale, CA, USA). Postsynaptic potentials were resolved from gap-free recordings with a high-pass filter (1 Hz cut-off). The duration of EPSP suppression after a single SD was measured from the time of the maximum negative potential of the DC shift to the time at which postsynaptic potentials first reached ≥ 50% of baseline values.
Fluorescence Imaging
Neuronal Ca2+ dynamics during SD reported by GCaMP5G were imaged with a 20X water-immersion objective (Olympus, 0.5 NA) and analyzed in TillPhotonics, version 4.01 software (Till Photonics GmBH, NY). GCaMP5G was excited at 480nm using a monochromator (Polychrome V, 2Hz); emission signals were passed through a dichroic mirror (515 DCLP) and captured using a cooled CCD camera (Imago, Till Photonics). Total Ca2+ accumulation in specific regions of interest during SD were calculated (GraphPad Prism 7.03) as the integral of the signals for 120 seconds or 200s following the peak of the SD transient. The duration of Ca2+ during SD was measured from the initial positive peak amplitude to the time point where fluorescence returned to ≤ 5% of baseline levels.
Autofluorescence signals during SD that could contaminate Ca2+ signals were evaluated in wild-type brain slices not expressing GCaMP5G. A small, but long-lasting (>200s) fluorescence decrease was observed, as described previously and attributed to flavoprotein autofluorescence (Shuttleworth, 2010). Autofluorescence decreases were similar in both somatic and dendritic compartments (−3.0 ± 0.9 and −2.3 ± 0.9 ΔF/F0 (%) decrease at 160s after SD for stratum pyramidale and stratum radiatum, respectively; n=3, Supplementary Figure 2B). Ca2+ signals were therefore corrected for background and autofluorescence changes during SD, and expressed as percent change from baseline values (ΔF/F0 × 100).
Metabolic challenge
Under control conditions, slices are normally held 0.5mm above the coverslip floor of the recording chamber to ensure continuous flow of aCSF on both sides of the slice. This is considered a nominally-healthy condition for the current study (see discussion in Frenguelli, 2017). However, in some experiments, we reduced aCSF flow to the bottom side of the slice, in order to intentionally reduce metabolic capacity and increase vulnerability to SD. This was accomplished by inverting the slice support insert (SS-3, Warner Instruments), to remove the flow channel under the slice. As opposed to complete oxygen-glucose deprivation, this partial metabolic compromise did not spontaneously initiate SD in any preparation (n = 44). Slice recovery after SD was evaluated from 1) intrinsic optical signals (Anderson and Andrew, 2002) 2) the ability of the tissue to generate a second SD (Koroleva and Bures, 1996) and 3) electrophysiologically with evoked extracellular postsynaptic potentials (Lindquist and Shuttleworth, 2012).
Drugs
Ketamine (100 mg/ml, racemic: R (−)/S (+)) was purchased from Putney, Inc. (Portland, ME), and solutions containing ketamine were prepared daily. All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Statistical analysis
Data are reported as mean ± SEM. Statistical analyses (repeated measure one-way analysis of variance (ANOVA), paired and unpaired t-tests) were calculated using GraphPad Prism (version 7.03; La Jolla, CA). Statistical significance was determined by P values < 0.05, with Bonferroni correction during multiple comparisons.
Results
Ketamine can reduce rate and duration of SD
We first examined the concentration-dependence of ketamine block of SD in brain slices (Figure 1). Under standard recording conditions (3mM K+ aCSF, see Methods), 100 μM ketamine invariably abolished SD. Consistent with previous observations using other NMDAR antagonists, the effectiveness of SD block was reduced by moderate elevations of baseline extracellular K+ that are similar to elevations in peri-infarct tissues in vivo (Petzold et al., 2005). From these initial experiments, we selected the highest concentration of ketamine that did not block SD for subsequent studies (30 μM, 8mM K+ aCSF).
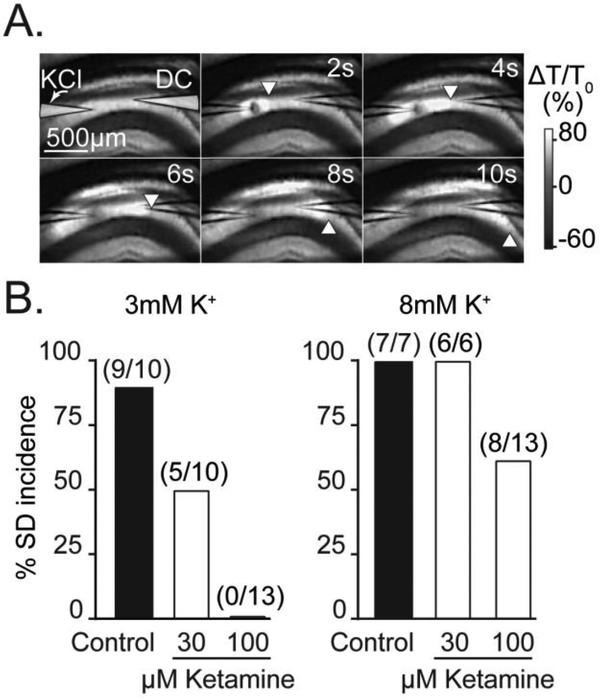
Figure 1. Basal extracellular K+ influences sensitivity to ketamine.
A: Representative intrinsic optical signals showing SD propagation through the hippocampal CA1 region of a brain slice. SD was triggered by micro injection of KCl from a micropipette on the left (labeled “KCl”) and SD is visualized as a slowly-propagating wave of increased light transmission. The location of the advancing wavefront is marked by white arrowheads and a second microelectrode (labeled “DC”) was used to confirm electrical responses of SD coincident with arrival of the optical signal (not shown). The upper right-hand values indicate time, in seconds, relative to the triggering of the KCl stimulus pulse. B: Effect of ketamine exposures on SD incidence under two different recording conditions (3mM vs 8mM bathing K+). Ketamine more potently prevented SD incidence in the lower basal K+ recording conditions. Values in parenthesis indicate number of preparations.
Figure 2 shows the time-dependent inhibition of SD propagation rate by ketamine. In these experiments, ketamine (10 minutes pre-exposure, and maintained throughout the experiment) immediately slowed SD propagation rate compared to that of control SDs initiated within the same slice, but the maximum effect was observed by the second SD following drug exposure (Figure 2B). In a separate set of experiments, brain slices were pre-exposed to ketamine for extended incubation times (> 3hrs) prior to recording sessions. In these preparations, the maximal slowing was achieved on the first SD trial in ketamine (3.4 ± 0.2 vs. 5.1 ± 0.3 mm min−1 for the first SD in ketamine during acute exposures (shown in Figure 2B)) and was not enhanced with successive stimulations (Supplementary Figure 1A; P = 0.27 for first vs. third SD in ketamine).
When recorded in the CA1 dendritic subfield, DC potential changes during SD have a prominent “inverted saddle-like” shape with a slower secondary phase involving NMDAR activation (Marrannes et al., 1988, Somjen, 2001, Aiba and Shuttleworth, 2012). SD duration was progressively reduced following acute ketamine exposures and reversed following wash out (Figure 2). Similar to effects on propagation rate, DC durations following long ketamine pre-exposures (> 3hrs, Supplementary Figure 1A), were maximally reduced upon the first SD trial (27.1 ± 2.2 vs. 41.2 ± 3.0s for the first SD in ketamine during acute exposures (shown in Figure 2B)), and were not enhanced by successive SDs in ketamine (Supplementary Figure 1A; P = 0.51 for first vs. third SD in ketamine).
Separate time-matched control studies verified that effects seen with ketamine on DC shift duration were not due to spontaneous rundown over time (Supplementary Figure 1B). Similarly, propagation rate showed no change during repetitive SDs in these control studies (Supplementary Figure 1B). A small decrease (~13%) in the amplitude of DC potential shifts was noted during repetitive SDs in ketamine (data not shown, control: 8.85 ± 0.43 mV vs. third ketamine trial: 7.71 ± 0.33 mV, n=6, P=0.02). However, time-matched control experiments (i.e. without ketamine) showed the same degree of run down, implying that this was not due to ketamine itself (data not shown, ~13% decrease; durations of second vs. fifth SD: 7.65 ± 0.78 mV vs. 6.37 ± 0.70 mV, P=0.01, n=8).
Ketamine reduces neuronal Ca2+ accumulation and accelerates postsynaptic recovery
Ionic disruption is massive during SD, and NMDAR activation is largely responsible for extended neuronal Ca2+ influx during the DC shift (See Introduction). We next tested whether ketamine reduced neuronal Ca2+ accumulation following SD (Figure 3). Since the maximum effect of ketamine was observed with successive SD stimulations, the 3rd SD following ketamine was used for experiments in Figures 3&4. Figure 3A shows large intracellular neuronal Ca2+ (GCaMP5G) transients during a control SD. Ca2+ rapidly increases during the SD wave front and returns to ≤5% baseline levels by ~2.5 minutes (Figure 3B). Ca2+ transients in pyramidal cell body regions (stratum pyramidale) had increased peak amplitudes compared to signals in dendrites (stratum radiatum), however Ca2+ elevations in dendrites were slightly longer in duration (Supplementary Figure 2A).
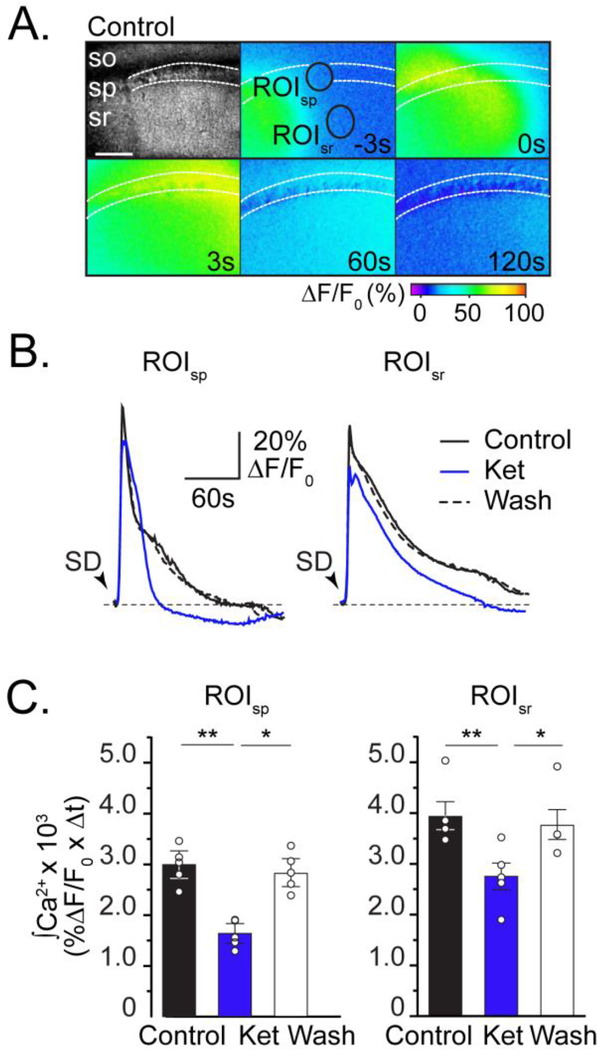
Figure 3. Ketamine reduces neuronal intracellular Ca2+ accumulation during SD.
A: Top left panel: Transmitted light image, showing stratum oriens (so), stratum pyramidale (sp), stratum radiatum (sr) in area CA1. Pseudo colored images show GCaMP5G fluorescence collected during SD in control conditions. Numbers in each frame indicate time (in seconds) in relation to peak Ca2+ during SD, and black circles are regions of interest surrounding predominately pyramidal cell bodies or dendrites in stratum pyramidale (ROIsp )or radiatum (ROIsr), respectively. Scale bar = 100μm. B: Data extracted from ROIsp and ROIsr show that Ca2+ transients during SD in ketamine (blue) recover faster than control (black), and are reversible after ketamine wash out (dashed). Black arrowheads indicate SD onset. C: Summary data (n=5), show that ketamine reversibly reduces total neuronal Ca2+ accumulation in both ROIs (integrals of 120s transients; see Methods). *P<0.05, **P<0.01
This resulted in an overall increase in total Ca2+ accumulation during control SDs in dendrites compared to cell bodies (black bars in Figure 3C, P=0.04) Ketamine reduced the peak amplitude and duration of Ca2+ transients (Supplementary Figure 2A). The rapid resolution of SD-induced Ca2+ transients in ketamine (Figure 3B) reveals a small, reversible underlying fluorescence decrease. Since these signals have been corrected for autofluorescence dynamics during SD (see Methods), residual undershoots revealed in ketamine are likely contributed to by light scattering changes during SD. Ketamine attenuated total Ca2+ accumulation during SD in stratum pyramidale and radiatum (Figure 3B&C). These data support the hypothesis that ketamine reduces the DC shift duration during SD, and thereby results in reduced intracellular Ca2+ dysregulation in neurons.
One consequence of SD is a long-lasting suppression of spontaneous and evoked synaptic transmission (Leao, 1944, Lindquist and Shuttleworth, 2012, 2017). We therefore determined whether shorter DC shifts and reduced neuronal Ca2+ dysregulation in ketamine were associated with accelerated synaptic recovery after SD. Figure 4 shows that ketamine reliably accelerated the recovery of evoked excitatory postsynaptic potentials (EPSPs) by ~25% compared to within-slice controls. Separate time-matched control experiments confirmed that changes in EPSP recovery time were due to antagonist exposure, rather than any other spontaneous changes. Together with Figures 2&3, these data suggest that (without blocking SD), ketamine can reduce SD propagation, duration, and ionic dysregulation thus enabling faster recovery of synaptic activity.
Ketamine improves recovery in metabolically vulnerable brain slices
We next examined whether ketamine, at a concentration that does not block the initiation or propagation of SD (i.e. 30 μM), can significantly protect against deleterious consequences of SD in metabolically vulnerable brain slices. As described above (Methods), partial reduction in metabolic substrate availability was achieved by restriction of aCSF flow under brain slices. As opposed to complete oxygen-glucose deprivation approaches, this partial metabolic compromise did not spontaneously initiate SD in any preparation tested (n = 44), but greatly impaired recovery after SD.
Figure 5 shows ketamine reduced excessive Ca2+ loading in vulnerable tissues, and was associated with significantly improved functional recovery. SD-induced Ca2+ transients were noticeably prolonged in vulnerable tissues, consistent with previous observations (Aiba and Shuttleworth, 2012), with residual intracellular Ca2+ remaining ~20–30% above baseline ~3.5 minutes after SD (Supplementary Figure 2C). Ketamine pre-exposure significantly reduced the integral of Ca2+ transients in both somatic and dendritic compartments (Figure 5B), and enabled generation of a second SD in vulnerable slices (Figure 5C). Likewise, recovery of EPSPs was substantially delayed after SD in vulnerable tissues, and ketamine enabled EPSPs to return to ~60% of baseline amplitude responses after SD (Figure 5D).
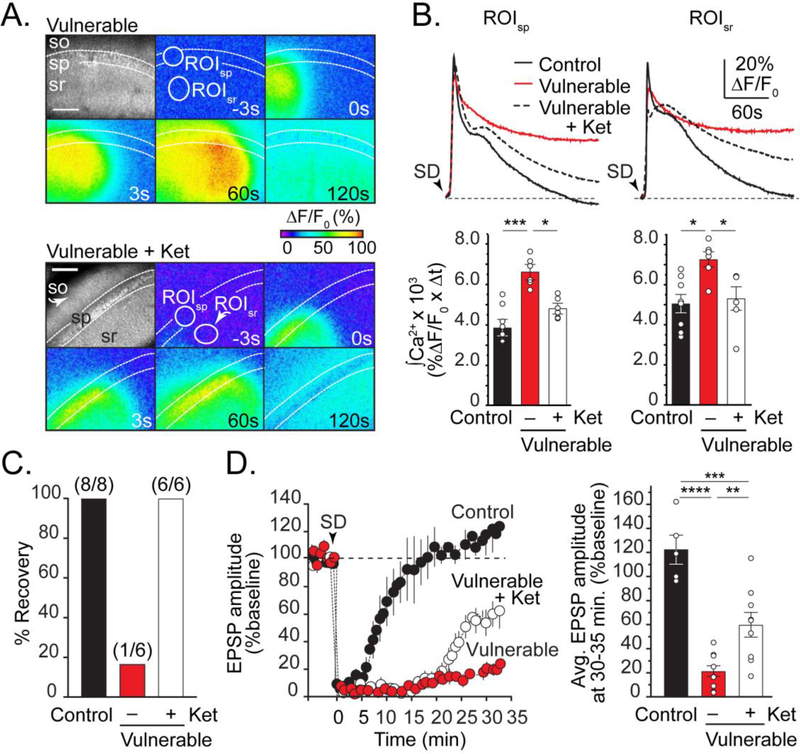
Figure 5. Ketamine improves recovery of neuronal Ca2+ loading and promotes functional recovery after SD in vulnerable brain slices.
A: Top montage: GCaMP5G imaging in vulnerable brain slices show considerably prolonged Ca2+ elevations compared to control conditions (compare with Figure 3). Lower montage shows reduced intracellular Ca2+ after SD in ketamine. Scale bar =100μm. B: Plots show Ca2+ transients from pyramidal cell bodies and dendrites (ROIsp and ROIsr, white circles in A) during SD in control (black), vulnerable (red), and vulnerable + ketamine (dashed). Summary data of Ca2+ transient integrals (200s after SD) from a set of such experiments confirm beneficial effects of ketamine (control, n=8; vulnerable, n=6; vulnerable + ketamine, n=6). C: Data from experiments in B, showing vulnerable slices exposed to ketamine recovered the ability to generate a second SD. Values in parentheses indicate number of preparations. D: Summary data of EPSP amplitude suppression and recovery after SD in control (black, n=5), vulnerable (red, n=10), and vulnerable with ketamine (white, n=9). Black arrowhead indicates SD onset and loss of postsynaptic responses. *P<0.05, **P<0.01,***P<0.001, ****P<0.0001.
Figure 6 shows the effects of metabolic compromise on intrinsic optical signals (IOS) during SD, and optical signals associated with ketamine protection. Under control conditions, a prominent light transmission increase is observed that recovers towards baseline. In contrast, SD in metabolically compromised conditions was invariably followed by a sustained decreases in IOS signals (~45% ΔT/T0; Figure 6A&B). Previous reports have attributed IOS decreases in metabolically compromised conditions to a combination of factors, including dendritic disruption and swelling of intracellular organelles (Obeidat and Andrew, 1998, Fayuk et al., 2002), and persistent astrocyte swelling observed in vulnerable tissues could also contribute (Risher et al., 2012). In the present study, ketamine effectively prevented decreased IOS signals associated with lack of functional recovery in vulnerable slices (see Figure 5). Thus, prolonged IOS decreases were prevented in almost all vulnerable preparations (Figure 6B). Together these findings support a role for extended NMDAR-dependent Ca2+ influx into neurons during SD in vulnerable tissues (Aiba and Shuttleworth, 2012), and suggest that sub-maximal concentrations of NMDAR antagonists can target this process to enable better functional recovery.
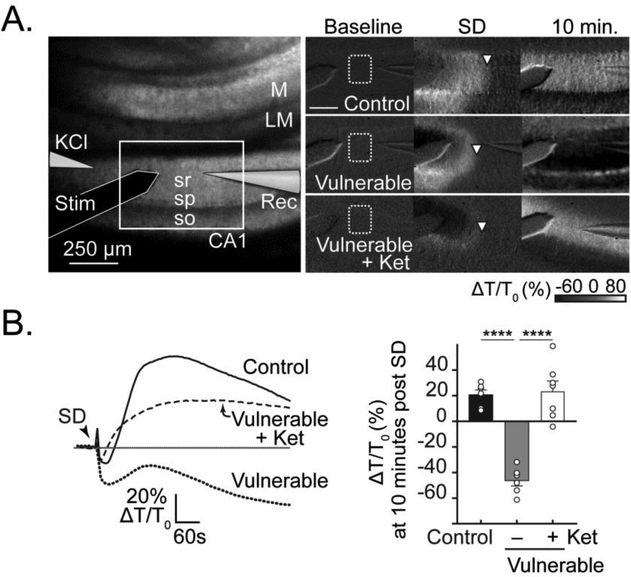
Figure 6. Ketamine protects against IOS decreases after SD in vulnerable slices.
A: Left hand panel shows the arrangement of recording electrodes on a transmitted light image. The white box outlines the imaging area shown in panels on the right; so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum; LM, stratum lacunosum moleculare; M, molecular layer of dentate gyrus. The set of images on the right show representative intrinsic optical (IOS, light transmittance) changes at baseline, during SD, and 10 minutes after SD in brain slices recorded under three different conditions: control, vulnerable, and vulnerable + ketamine. Note the difference in IOS seen at the 10-minute time point after SD. White arrowheads indicate the wave front of SD. Scale bar = 250 μm. B: Traces (left) show signals extracted from regions in stratum radiatum (dotted white box in A) during the three representative experiments shown in A. Summary data (n= 6–7, right hand panel) confirm substantially decreased light transmittance 10 minutes after the SD wavefront in vulnerable slices, and prevention by ketamine. ****P<0.0001
Discussion
General
The main new finding of the study is the demonstration that ketamine can be protective against SD-induced injury, even at concentrations that are insufficient to block the initiation or propagation of the SD event itself. Ketamine was able to significantly improve recovery from ionic loading of SD, and was shown to be sufficient to protect tissues from SDs in a model of metabolic vulnerability. These beneficial effects provide support for the notion that targeting consequences of SD could be effective in injured brain, as an adjunct or alternative to interventions intended to completely abolish SD events.
Mechanisms of ketamine actions
We focused on the NMDAR antagonist ketamine because of its current use in clinical settings and because of reports that ketamine sedation in the ICU was associated with reduced frequency of SD events (Sakowitz et al., 2009, Hertle et al., 2012, Schiefecker et al., 2015). In order to preserve NMDAR availability and potential beneficial outcomes of SD (see Introduction), we determined concentrations of ketamine that kept SD intact in healthy brain slices (Figure 1). Ketamine’s ability to block SD, even with high concentrations, was reduced when basal extracellular K+ was moderately increased. As previously discussed (Petzold et al., 2005), these K+ elevations may be similar to pathological ionic disturbances in peri-infarct tissues in animals (Nedergaard and Hansen, 1993) and in brain injured patients (Rogers et al., 2017). The ketamine concentration selected for most studies here (30 μM, Figures 2–6) allowed for repetitive SD initiation, while presumably leaving a portion of NMDARs available (Izumi and Zorumski, 2014, Khlestova et al., 2016). Since ketamine competes with Mg2+ for binding within the NMDAR channel pore, NMDAR subtypes with weaker Mg2+ block (i.e. GluN2C and GluN2D) are preferentially inhibited, whereas GluN2B and GluN2A-mediated currents are less sensitive to ketamine (Khlestova et al., 2016). If GluN2B and GluN2A NMDARs underlie residual NMDAR current in 30 μM ketamine, availability of these channel subtypes may be helpful for preserving synaptic plasticity in the recovering brain (Khlestova et al., 2016).
It is difficult to directly compare the ketamine concentration used here with prior clinical observations, in part because of species-dependent differences of in vivo ketamine distribution and metabolism, and brain concentrations following intravenous infusions were not determined in the ICU studies. Furthermore, while both the racemic mixture and the S (+)-isomer of ketamine are in clinical use, the racemic ketamine mixture used in the present study is approximately half as potent as the S-ketamine isomer (Peltoniemi et al., 2016) used in published clinical work with SD. The present results imply that brain concentrations effective at blocking SD clinically are higher than 30μM (for racemic ketamine, or ~15μM for S-ketamine), but more detailed studies are needed to determine whether infusions used for clinical sedation far exceed this value. Non-sedative concentrations that effectively prevent deleterious consequences of SD could be clinically valuable.
Ketamine’s efficacy was progressively enhanced during a series of repetitive SDs (Figure 2), or by prolonged (~3hr) ketamine pre-incubations (Supplementary Figure 1A). The time course of effects may be due in part to ketamine’s use-dependent mechanism of action at the Mg2+ - site of NMDARs (Johnson et al., 2015) and/or drug diffusion into brain slices. Time-dependent effects of ketamine have been noted previously in in vivo recordings in pigs (Sanchez-Porras et al., 2014). In the present study, the progressive decrease in the DC shift duration of SD was particularly notable (Figure 2). NMDAR activation is prominent during the secondary phase of the “inverted-saddle” - shaped DC shift. During the late-phase of SD, glutamate release probability is substantially enhanced for ~ 1 minute, at a time when postsynaptic neurons remain persistently depolarized. These conditions favor relief of Mg2+ from its binding site within the NMDAR pore, and lead to massive cationic influx. As such, targeted application of NMDAR antagonists (i.e. AP5) during the late-phase, can abolish the secondary component of the DC shift and reduce extended Ca2+ loading (Aiba and Shuttleworth, 2012). Use-dependency of block with ketamine may be particularly useful for targeting excessive glutamate accumulation during SD in injured tissues.
Our GCaMP5G imaging of neuronal Ca2+ accumulation showed significant reductions in total Ca2+ loading after ketamine (Figure 3 & Supplementary Figure 2A), associated with reduced DC shift durations. In addition, recovery of synaptic potentials after SD was significantly accelerated by ketamine exposures (Figure 4). The long-lasting suppression of evoked EPSPs in brain slice after SD (≥ 5 minutes) is largely a result of extracellular adenosine accumulation and activation of presynaptic adenosine-1-receptors (A-1R) and provides a measure of the metabolic burden imposed by SD (Lindquist and Shuttleworth, 2012, 2014). Taken together, these data show that a concentration of ketamine that does not block SD in nominally healthy tissues, decreases NMDAR-mediated Ca2+ influx during the late phase of the DC shift, reduces the metabolic burden of SD and enables faster recovery of EPSPs.
Protective effects of ketamine in vulnerable tissues
We used a novel brain slice model of metabolic insufficiency, in order to recapitulate the deleterious consequences that peri-infarct SDs have on viable, but vulnerable brain regions. By limiting aCSF superfusion to one side of the slice, metabolic capacity was sufficient for slices to remain viable for hours in the recording chamber. However, on this compromised baseline, an SD generated by focal KCl microinjection led to severely impaired 1) recovery of neuronal Ca2+ elevations, 2) recovery of synaptic potentials, 3) ability to generate a second SD, as well as signs of structural disruption (suggested from intrinsic optical signals) (Figures 5&6). This model of vulnerability to SD is fundamentally different from the oxygen-glucose deprivation (OGD) or hypoxia induced SD (HSD) paradigm that we and others have used to generate SD in brain slices (Somjen, 2001, Dietz et al., 2008). In the standard OGD or HSD models, severe oxygen and/or glucose reductions are used as the inciting stimulus for SD, as Na/K+/ATPase failure produces progressive loss of membrane potential and extracellular K+ accumulation. SD is usually triggered within ~10 minutes, and even if SD can be delayed or prevented by antagonists, cell damage invariably occurs if substrate removal is continued. The OGD or HSD paradigms are therefore useful for understanding initiation of events in infarct cores, but are not as well suited for understanding how at-risk penumbral tissue suddenly succumbs to injury when it is invaded by an SD. The partial inhibition model here addresses this concern and is straightforward and reproducible. It is also noted that the recording configuration tested here (with slices superfused on a single side) is common for many neurophysiology studies.
Ketamine exposures (30μM) provided substantial protection against the deleterious consequences of SD under these conditions of metabolic compromise. As described above for nominally healthy tissues, SD was not prevented by this concentration of ketamine, but the total Ca2+ loading after SD was significantly reduced, and both optical signals and functional recovery after SD in vulnerable conditions were significantly improved (Figures 5&6).
Potential beneficial effects of targeting consequences (rather than initiation/propagation) of SD
We expect the degree of NMDAR block in our experiments to leave a significant portion of the NMDAR pool available for plasticity and Ca2+-dependent synaptic signaling that is presumably beneficial for recovery and repair mechanisms required in the immediate aftermath of an injury (Shohami and Biegon, 2014). In addition to NMDAR availability, the fact that SDs still propagate may be directly beneficial effects to recovering brain. This is because SDs can travel long distances throughout the cortex, including to relatively healthy tissue that is remote from an injury site. While SDs can clearly cause damage when they propagate through metabolically compromised tissue, there is good evidence for protective effects of SD, when it is allowed to propagate through otherwise healthy brain regions (see Introduction). Whether or not substantial benefit could be derived from targeting the consequences of SD in vivo, could be tested by assessing SDs at different locations from an injury site and relating to outcome. In this scenario long-term functional outcomes, rather than lesion volume alone would be valuable to assess the importance of potential beneficial effects of SD on cortical plasticity remote from the infarct core.
Conclusions
Results from the current study suggest that SDs that occur in the presence of ketamine could be shorter in duration and less metabolically demanding. By reducing intracellular Ca2+ influx during SD, ketamine may minimize the amount of energy needed for recovery (exemplified by accelerated recovery of evoked synaptic activity). These results raise the possibility that ketamine may be effective clinically, even at lower concentrations that do not prevent SD, but may shorten the duration of ECoG suppression and minimize lesion development in patients with acute brain injuries. Our study focused on ketamine but the main pre-clinical findings here could be generalized to other interventions that improve recovery of neurons after the passage of SD. This includes other use-dependent NMDAR antagonists such as memantine, which are clinically well tolerated and may lack some of the negative side effects of ketamine (Johnson et al., 2015). Alternative approaches that improve neurovascular coupling after SD propagates through vulnerable tissues (Dreier, 2011) would be expected to be complementary or additive with ketamine effects.
Supplementary Material
Supplementary Figure 1.
A: Summary data from 6 preparations showing ketamine pre-exposure immediately reduced both SD propagation rate and DC shift duration and was not enhanced by successive SDs. B: DC potential recordings (traces) show that the DC shift duration is consistent during a sequence of SDs in a time-matched control slice not exposed to ketamine. Black arrowheads indicate DC shift onset, and dashed lines represent 15 minutes recovery between stimulations. Population data from 6 such experiments, confirm that SD propagation rate and DC shift duration are stable over consecutive SD stimulations.
Supplementary Figure 2.
A: Summary data of GCaMP5G imaging experiments shown in Figure 3 (n=5). Ketamine significantly and reversibly reduced the peak amplitude (top panel) of intracellular Ca2+ transients during repetitive SDs in cell body (pyramidale) and dendrite (radiatum) regions. Ketamine reduced the duration of transients (see Methods) resulting in decreased residual intracellular Ca2+ accumulation 60s after SD. Comparison of control SDs in pyramidale and radiatum (black bars) showed that peak Ca2+ was larger in cell bodies compared to dendrites (P=0.005). However, the duration of Ca2+ transients (middle panel) and residual intracellular fluorescence at 60s after SD were slightly increased in dendrites vs. cell bodies (P=0.06 and P=0.09 for duration and residual Ca2+, respectively). B: Representative Ca2+ traces from stratum pyramidale and radiatum regions from the same slice as shown in Figure 3B without background and autofluorescence correction. Gray traces in each plot represent autofluorescence (AF) decreases in cell body and dendrite regions during SD in a brain slice not expressing GCaMP5G. Black arrowheads indicate SD onset. C: Summary of GCaMP5G imaging from the same data set shown in Figure 5 (A-C). The peak amplitude of Ca2+ signals during SD in vulnerable slices tended to be reduced compared to control SDs, while residual intracellular Ca2+ fluorescence remained significantly elevated 200s after SD. Ketamine reduced residual Ca2+ accumulation 200s after SD in vulnerable slices. *P<.05, **P<0.01, ***P<0.001, ****P<0001.
Highlights.
Spreading depolarization can cause injury in metabolically compromised brain
Ketamine can prevent damaging effects of spreading depolarization
Calcium loading, synaptic recovery improved in vulnerable tissue
Effective at concentrations that do not block propagation of spreading depolarization
May limit injury expansion, while preserving potential beneficial effects
Acknowledgements
This study was supported by NIH grants NS051288, P20GM109089 and T32 HL007736. The authors are grateful to Russell Morton, Ph.D. and Donald Partridge, Ph.D. for helpful discussions and input throughout the course of the study, and for excellent pilot studies contributed by Kisa King.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, Yoo AJ, Hsu DP, Rymer MM, Tayal AH, Zaidat OO, Natarajan SK, Nogueira RG, Nanda A, Tian M, Hao Q, Kalia JS, Nguyen TN, Chen M, Gupta R (2010) Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke 41:1175–1179. [DOI] [PubMed] [Google Scholar]
- Aiba I, Shuttleworth CW (2012) Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. The Journal of physiology 590:5877–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken PG, Breese GR, Dudek FF, Edwards F, Espanol MT, Larkman PM, Lipton P, Newman GC, Nowak TS Jr, Panizzon KL, et al. (1995) Preparative methods for brain slices: a discussion. J Neurosci Methods 59:139–149. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Andrew RD (2002) Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol 88:2713–2725. [DOI] [PubMed] [Google Scholar]
- Busch E, Gyngell ML, Eis M, Hoehn-Berlage M, Hossmann KA (1996) Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J Cereb Blood Flow Metab 16:1090–1099. [DOI] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW (2008) Zn2+ influx is critical for some forms of spreading depression in brain slices. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:8014–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R (2008) Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol 63:720–728. [DOI] [PubMed] [Google Scholar]
- Dreier JP (2011) The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nature medicine 17:439–447. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Fabricius M, Ayata C, Sakowitz OW, William Shuttleworth C, Dohmen C, Graf R, Vajkoczy P, Helbok R, Suzuki M, Schiefecker AJ, Major S, Winkler MK, Kang EJ, Milakara D, Oliveira-Ferreira AI, Reiffurth C, Revankar GS, Sugimoto K, Dengler NF, Hecht N, Foreman B, Feyen B, Kondziella D, Friberg CK, Piilgaard H, Rosenthal ES, Westover MB, Maslarova A, Santos E, Hertle D, Sanchez-Porras R, Jewell SL, Balanca B, Platz J, Hinzman JM, Luckl J, Schoknecht K, Scholl M, Drenckhahn C, Feuerstein D, Eriksen N, Horst V, Bretz JS, Jahnke P, Scheel M, Bohner G, Rostrup E, Pakkenberg B, Heinemann U, Claassen J, Carlson AP, Kowoll CM, Lublinsky S, Chassidim Y, Shelef I, Friedman A, Brinker G, Reiner M, Kirov SA, Andrew RD, Farkas E, Guresir E, Vatter H, Chung LS, Brennan KC, Lieutaud T, Marinesco S, Maas AI, Sahuquillo J, Dahlem MA, Richter F, Herreras O, Boutelle MG, Okonkwo DO, Bullock MR, Witte OW, Martus P, van den Maagdenberg AM, Ferrari MD, Dijkhuizen RM, Shutter LA, Andaluz N, Schulte AP, MacVicar B, Watanabe T, Woitzik J, Lauritzen M, Strong AJ, Hartings JA (2017) Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: Review and recommendations of the COSBID research group. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37:1595–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Isele T, Reiffurth C, Offenhauser N, Kirov SA, Dahlem MA, Herreras O (2013) Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 19:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ (2009) Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 132:1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraguna U, Nelson A, Vyazovskiy VV, Cirelli C, Tononi G (2010) Unilateral cortical spreading depression affects sleep need and induces molecular and electrophysiological signs of synaptic potentiation in vivo. Cerebral cortex 20:2939–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayuk D, Aitken PG, Somjen GG, Turner DA (2002) Two different mechanisms underlie reversible, intrinsic optical signals in rat hippocampal slices. J Neurophysiol 87:1924–1937. [DOI] [PubMed] [Google Scholar]
- Footitt DR, Newberry NR (1998) Cortical spreading depression induces an LTP-like effect in rat neocortex in vitro. Brain Res 781:339–342. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG (2017) The Purine Salvage Pathway and the Restoration of Cerebral ATP: Implications for Brain Slice Physiology and Brain Injury. Neurochemical research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke F, Kron M, Dutschmann M, Muller M (2009) Infant brain stem is prone to the generation of spreading depression during severe hypoxia. Journal of neurophysiology 101:2395–2410. [DOI] [PubMed] [Google Scholar]
- Gee JM, Smith NA, Fernandez FR, Economo MN, Brunert D, Rothermel M, Morris SC, Talbot A, Palumbos S, Ichida JM, Shepherd JD, West PJ, Wachowiak M, Capecchi MR, Wilcox KS, White JA, Tvrdik P (2014) Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse. Neuron 83:1058–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Bullock MR, Okonkwo DO, Murray LS, Murray GD, Fabricius M, Maas AI, Woitzik J, Sakowitz O, Mathern B, Roozenbeek B, Lingsma H, Dreier JP, Puccio AM, Shutter LA, Pahl C, Strong AJ (2011) Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol 10:1058–1064. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Rolli ML, Lu XC, Tortella FC (2003) Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci 23:11602–11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Shuttleworth CW, Kirov SA, Ayata C, Hinzman JM, Foreman B, Andrew RD, Boutelle MG, Brennan KC, Carlson AP, Dahlem MA, Drenckhahn C, Dohmen C, Fabricius M, Farkas E, Feuerstein D, Graf R, Helbok R, Lauritzen M, Major S, Oliveira-Ferreira AI, Richter F, Rosenthal ES, Sakowitz OW, Sanchez-Porras R, Santos E, Scholl M, Strong AJ, Urbach A, Westover MB, Winkler MK, Witte OW, Woitzik J, Dreier JP (2017) The continuum of spreading depolarizations in acute cortical lesion development: Examining Leao’s legacy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37:1571–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Caceres J, Macias-Gonzalez R, Brozek G, Bures J (1987) Systemic ketamine blocks cortical spreading depression but does not delay the onset of terminal anoxic depolarization in rats. Brain Res 437:360–364. [DOI] [PubMed] [Google Scholar]
- Hertle DN, Dreier JP, Woitzik J, Hartings JA, Bullock R, Okonkwo DO, Shutter LA, Vidgeon S, Strong AJ, Kowoll C, Dohmen C, Diedler J, Veltkamp R, Bruckner T, Unterberg AW, Sakowitz OW (2012) Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain : a journal of neurology 135:2390–2398. [DOI] [PubMed] [Google Scholar]
- Hoskison MM, Shuttleworth CW (2006) Microtubule disruption, not calpain-dependent loss of MAP2, contributes to enduring NMDA-induced dendritic dysfunction in acute hippocampal slices. Exp Neurol 202:302–312. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF (2014) Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacology 86:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Glasgow NG, Povysheva NV (2015) Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol 20:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlestova E, Johnson JW, Krystal JH, Lisman J (2016) The Role of GluN2C-Containing NMDA Receptors in Ketamine’s Psychotogenic Action and in Schizophrenia Models. The Journal of neuroscience : the official journal of the Society for Neuroscience 36:11151–11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroleva VI, Bures J (1996) The use of spreading depression waves for acute and long-term monitoring of the penumbra zone of focal ischemic damage in rats. Proceedings of the National Academy of Sciences of the United States of America 93:3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ (2011) Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 31:17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao AAP (1944) Spreading depression of activity in the cerebral cortex. J Neurophysiol 7:359–390. [DOI] [PubMed] [Google Scholar]
- Lindquist BE, Shuttleworth CW (2012) Adenosine receptor activation is responsible for prolonged depression of synaptic transmission after spreading depolarization in brain slices. Neuroscience 223:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist BE, Shuttleworth CW (2014) Spreading depolarization-induced adenosine accumulation reflects metabolic status in vitro and in vivo. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34:1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist BE, Shuttleworth CW (2017) Evidence that adenosine contributes to Leao’s spreading depression in vivo. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrannes R, Willems R, De Prins E, Wauquier A (1988) Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res 457:226–240. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Strong AJ, Dohmen C, Sakowitz OW, Vollmar S, Sue M, Kracht L, Hashemi P, Bhatia R, Yoshimine T, Dreier JP, Dunn AK, Graf R (2010) Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain : a journal of neurology 133:1994–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ (1988) Spreading depression is not associated with neuronal injury in the normal brain. Brain Res 449:395–398. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ (1993) Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab 13:568–574. [DOI] [PubMed] [Google Scholar]
- Nichols C, Carrozzella J, Yeatts S, Tomsick T, Broderick J, Khatri P (2010) Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J Neurointerv Surg 2:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat AS, Andrew RD (1998) Spreading depression determines acute cellular damage in the hippocampal slice during oxygen/glucose deprivation. Eur J Neurosci 10:3451–3461. [DOI] [PubMed] [Google Scholar]
- Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI (2016) Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin Pharmacokinet 55:1059–1077. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Windmuller O, Haack S, Major S, Buchheim K, Megow D, Gabriel S, Lehmann TN, Drenckhahn C, Peters O, Meierkord H, Heinemann U, Dirnagl U, Dreier JP (2005) Increased extracellular K+ concentration reduces the efficacy of N-methyl-D-aspartate receptor antagonists to block spreading depression-like depolarizations and spreading ischemia. Stroke; a journal of cerebral circulation 36:1270–1277. [DOI] [PubMed] [Google Scholar]
- Risher WC, Croom D, Kirov SA (2012) Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia 60:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ML, Leong CL, Gowers SA, Samper IC, Jewell SL, Khan A, McCarthy L, Pahl C, Tolias CM, Walsh DC, Strong AJ, Boutelle MG (2017) Simultaneous monitoring of potassium, glucose and lactate during spreading depolarization in the injured human brain - Proof of principle of a novel real-time neurochemical analysis system, continuous online microdialysis. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37:1883–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, Unterberg AW, Dreier JP (2009) Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke; a journal of cerebral circulation 40:e519–522. [DOI] [PubMed] [Google Scholar]
- Sanchez-Porras R, Santos E, Scholl M, Stock C, Zheng Z, Schiebel P, Orakcioglu B, Unterberg AW, Sakowitz OW (2014) The effect of ketamine on optical and electrical characteristics of spreading depolarizations in gyrencephalic swine cortex. Neuropharmacology 84:52–61. [DOI] [PubMed] [Google Scholar]
- Schiefecker AJ, Beer R, Pfausler B, Lackner P, Broessner G, Unterberger I, Sohm F, Mulino M, Thome C, Humpel C, Schmutzhard E, Helbok R (2015) Clusters of cortical spreading depolarizations in a patient with intracerebral hemorrhage: a multimodal neuromonitoring study. Neurocrit Care 22:293–298. [DOI] [PubMed] [Google Scholar]
- Shohami E, Biegon A (2014) Novel approach to the role of NMDA receptors in traumatic brain injury. CNS Neurol Disord Drug Targets 13:567–573. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CW (2010) Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem Int 56:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW, Brennan AM, Connor JA (2003) NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci 23:3196–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG (2001) Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 81:1065–1096. [DOI] [PubMed] [Google Scholar]
- Tang YT, Mendez JM, Theriot JJ, Sawant PM, Lopez-Valdes HE, Ju YS, Brennan KC (2014) Minimum conditions for the induction of cortical spreading depression in brain slices. Journal of neurophysiology 112:2572–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach A, Baum E, Braun F, Witte OW (2017) Cortical spreading depolarization increases adult neurogenesis, and alters behavior and hippocampus-dependent memory in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37:1776–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Jagt TA, Connor JA, Shuttleworth CW (2008) Localized loss of Ca2+ homeostasis in neuronal dendrites is a downstream consequence of metabolic compromise during extended NMDA exposures. J Neurosci 28:5029–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano E, Monda V, Messina A, Moscatelli F, Valenzano A, Tafuri D, Cibelli G, De Luca B, Messina G, Monda M (2016) Cortical spreading depression produces a neuroprotective effect activating mitochondrial uncoupling protein-5. Neuropsychiatr Dis Treat 12:1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bornstadt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, Sandow N, Kura S, Eikermann-Haerter K, Endres M, Boas DA, Moskowitz MA, Lo EH, Dreier JP, Woitzik J, Sakadzic S, Ayata C (2015) Supply-Demand Mismatch Transients in Susceptible Peri-infarct Hot Zones Explain the Origins of Spreading Injury Depolarizations. Neuron 85:1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang C, Szabo G, Sun QQ (2013) Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain research 1518:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamoto H, Xue JH, Miyamoto S, Nagata I, Nakano Y, Murao K, Kikuchi H (2004) Spreading depression induces long-lasting brain protection against infarcted lesion development via BDNF gene-dependent mechanism. Brain research 1019:178–188. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Shuttleworth CW, Moskal JR, Stanton PK (2015) Suppression of spreading depolarization and stabilization of dendritic spines by GLYX-13, an NMDA receptor glycine-site functional partial agonist. Experimental neurology 273:312–321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1.
A: Summary data from 6 preparations showing ketamine pre-exposure immediately reduced both SD propagation rate and DC shift duration and was not enhanced by successive SDs. B: DC potential recordings (traces) show that the DC shift duration is consistent during a sequence of SDs in a time-matched control slice not exposed to ketamine. Black arrowheads indicate DC shift onset, and dashed lines represent 15 minutes recovery between stimulations. Population data from 6 such experiments, confirm that SD propagation rate and DC shift duration are stable over consecutive SD stimulations.
Supplementary Figure 2.
A: Summary data of GCaMP5G imaging experiments shown in Figure 3 (n=5). Ketamine significantly and reversibly reduced the peak amplitude (top panel) of intracellular Ca2+ transients during repetitive SDs in cell body (pyramidale) and dendrite (radiatum) regions. Ketamine reduced the duration of transients (see Methods) resulting in decreased residual intracellular Ca2+ accumulation 60s after SD. Comparison of control SDs in pyramidale and radiatum (black bars) showed that peak Ca2+ was larger in cell bodies compared to dendrites (P=0.005). However, the duration of Ca2+ transients (middle panel) and residual intracellular fluorescence at 60s after SD were slightly increased in dendrites vs. cell bodies (P=0.06 and P=0.09 for duration and residual Ca2+, respectively). B: Representative Ca2+ traces from stratum pyramidale and radiatum regions from the same slice as shown in Figure 3B without background and autofluorescence correction. Gray traces in each plot represent autofluorescence (AF) decreases in cell body and dendrite regions during SD in a brain slice not expressing GCaMP5G. Black arrowheads indicate SD onset. C: Summary of GCaMP5G imaging from the same data set shown in Figure 5 (A-C). The peak amplitude of Ca2+ signals during SD in vulnerable slices tended to be reduced compared to control SDs, while residual intracellular Ca2+ fluorescence remained significantly elevated 200s after SD. Ketamine reduced residual Ca2+ accumulation 200s after SD in vulnerable slices. *P<.05, **P<0.01, ***P<0.001, ****P<0001.