Abstract
The present study describes how the development of a pair bond modifies social, sexual and aggressive behavior. Five heterosexual pairs of marmosets, previously unknown to each other, were formed at the beginning of the study. At the onset of pairing, social, sexual, exploratory and aggressive behaviors were recorded for 40 min. The animals were then observed for 20 min, both in the morning and afternoon for 21 days. The frequency and/or duration of behaviors recorded on Day 1 were compared to those recorded at later observations. The behavior displayed shortly after pairing should be completely unaffected by the pair bond, while such a bond should be present at later observations. Thus, it was possible to determine how the behavior between the pair was modified by the development of a pair bond. Social behaviors increased from Day 1 to Days 2–6 and all subsequent days observed. Conversely, other behaviors, such as open mouth displays (usually considered to be an invitation to sexual activity), had a high frequency during the early part of cohabitation but declined towards the end. Consequently, pair bonding manifests itself in an increased intensity of social behaviors. It is suggested that the intrinsically rewarding properties of grooming and perhaps other social behaviors turn the pair mate into a positive incentive, activating approach and further interactions when possible. Thus, the pair bond may be a motivational state activated by the conditioned incentive properties of the partner. This notion can explain all forms of pair bonds, including those occurring between individuals of the same sex and in promiscuous species.
Keywords: marmoset, pair bonding, social behavior
1. Introduction
Social bonds between individuals have been described in many species. One such presumptive bond is between the mother and her offspring and another is between members of a breeding pair (reviewed in Insel et al., 1995; Broad et al., 2006). Species in which this latter bond exists, or seems to exist, are frequently called monogamous although most explicit definitions of monogamy are not based only on the presence or absence of pair bonding (see Kleiman, 1977b; Dewsbury, 1988 for reviews). Nevertheless, the pair bond — usually operationalized as preference for the partner over an unfamiliar opposite sex conspecific — has been used as a hallmark of monogamy in many studies. This applies particularly to the numerous studies on the neurobiological bases of pair bonding in monogamous voles (e.g., Carter et al., 1995; Bales et al., 2007a; Mcgraw & Young, 2010).
Among primates, marmosets and other callitrichids are considered to be socially monogamous (e.g., Kleiman, 1977a,b), in the sense that they establish and maintain long-term male–female relationships, display intruder-directed aggression (Epple, 1977, 1978; French & Snowdon, 1981; French & Inglett, 1989), bi-parental care (Epple, 1975; Ross et al., 2007; Zahed et al., 2010) and other behavioral patterns that are considered typical of monogamy (Epple, 1990; Inglett et al., 1990; Buchanan-Smith & Jordan, 1992; Smith et al., 2010). In addition, pair bonding in marmosets has been characterized by a high frequency of partner-directed social interaction, including sexual activity (Abbott & Hearn, 1978; Evans & Poole, 1983).
Tests of preference for the pair mate over a strange individual have been used for studying pair bonding in primates (see, e.g., Mason, 1975). Among the callitrichids, a study of heterosexual pairs in the red bellied tamarin (Saguinus labiatus) showed that the pair mate was preferred over an unknown individual of the same sex, whereas there was no difference between the pair mate and an unfamiliar individual of the opposite sex (Buchanan-Smith & Jordan, 1992). In the golden lion tamarin (Leontopithecus rosalia), pair members approached and interacted more with an unknown individual than with the pair mate (Inglett et al., 1990) under conditions in which the pairmate was absent, but not when the pairmate was present during testing. In the saddle-back tamarin, Saguinus fuscicocollis, males preferred their female pair mate over an unfamiliar female, while females did not show any preference (Epple, 1990). Finally, neither male nor female black tufted-ear marmosets (Callithrix penicillata) showed any preference for their partner regardless of sex (Smith et al., 2010). These observations suggest that, in callitrichids at least, preference for the pair mate over other individuals cannot always be a reliable indicator of the presence or absence of a pair bond. In fact, it has been suggested that the expression of the pair bond is conditional, depending on the presence or absence of the mate (Inglett et al., 1990). It appears, then, that studies of partner preference are not enough for achieving a complete understanding of the behavioral and neurobiological mechanisms subjacent to the concept of pair bonding in callitrichids.
Although the exact nature of the notion of pair bond has yet to be resolved, it probably depends on emotional (Mason & Mendoza, 1998; Dunbar & Shultz, 2010) or motivational systems (Wickler, 1976). Regardless of the hypotheses concerning the bases of the bond, it can be assumed that the interactions between any pair of individuals should be modified whenever a pair bond becomes established. If pair bonding did not modify social interactions within the pair it would most likely have less functional consequences than if it did. The issue of behavioral changes following the establishment of a pair bond has been addressed in a few studies in marmosets. Data from Callithrix jacchus (Evans & Poole, 1984) and from the lion tamarin, Leontopithecus rosalia (Kleiman, 1977a; Ruiz, 1990), show that the frequency of affiliative behaviors is highest at the beginning of cohabitation. If it is assumed that the intensity of a pair bond manifests itself in the frequency of affiliative behaviors, then it would be concluded that the pair bond gets weaker as the duration of cohabitation increases. However, other studies have failed to reveal any systematic changes in affiliative behaviors during the first 10 weeks of cohabitation or longer in C. jacchus (Woodcock, 1982; Silva & Sousa, 1997) or Callithrix geoffroyi (Smith et al., 2011a), suggesting that the pair bond either does not affect affiliative behaviors at all or that it remained constant during the period of observation. Finally, a study in Wied’s black tufted-ear marmoset, Callithrix kuhli (Schaffner et al., 1995) showed that affiliative behaviors indeed increased during cohabitation. Whether the conflicting data can be attributed to differences among callitrichid species, different duration of cohabitation or other factors is unknown at present.
The studies mentioned in the preceding paragraph reported behavior patterns as weekly averages for the 10 weeks following pairing which is unfortunate since Abbott & Hearn (1978) suggested that increased affiliation becomes evident within hours of the initiation of cohabitation. Considering that a high frequency of affiliative behaviors are an important expression of the pair bond, this might mean that even most of the data for the first week after pairing reflects a situation where a pair bond is already present. Thus, weekly averages of affiliative behaviors are not enough for determining the behavioral changes associated with the initial stages of pair bond formation.
In the present study we determined if the intensity and types of sociosexual interactions during the initial period of cohabitation with an unknown, opposite sex conspecific in the black tufted-ear marmoset differed from that observed at a time when the pair bond should have been established. To that end, twice daily observations were performed during 3 weeks, the first observation starting within minutes of pairing. At that moment, there should be no pair bond between the members of the pair. Because the timeline for the formation of a pair bond in marmosets is not known, we based the choice of a 3 week observation period on the vole literature as well as on a study in the titi monkey (Callicebus cupreus). In voles, there are data showing that a pair bond develops between 6 and 48 h after pair formation, depending on the presence or absence of sexual activity (Williams et al., 1992). In C. cupreus, 48 h of cohabitation leads to changes in cerebral glucose uptake similar but not identical to those found in long-term pairs (Bales et al., 2007b). Considering that widely different species show significant manifestations of a pair bond within 48 h, 3 weeks of observation should be long enough for detecting behavioral changes associated with pair bond formation also in marmosets. We performed a detailed description of behavior combined with extensive statistical analyses. In that way, we were able to depict the behavioral manifestations of the developing pair bond.
2. Methods
2.1. Subjects
Five adult male and five adult female black tufted-ear marmosets (C. penicillata) were employed in this study. At the start of the study, animals were 2.5 ± 0.1 (mean ± SEM) years of age. They were housed in colony rooms at the Callitrichid Research Center at the University of Nebraska at Omaha, NE, USA. Room temperature was maintained at 19–22°C, and there was a 12:12 h light-dark cycle. All housing enclosures were wire-mesh cages (0.9 × 0.8 × 2.0 m) equipped with branches, nest boxes, wood shavings that covered the floor, and other assorted enrichment items. Opaque panels were placed between enclosures in each room to prevent any visual contact between pairs. The animals were fed every morning. These are the standard environmental conditions maintained for all marmoset species at the Callitrichid Research Center. Although the temperature is in the lower range of what is usual for marmosets (e.g., Vogt, 1978; Saltzman et al., 1994; Clara et al., 2008; Saltzman & Abbott, 2011), there is no evidence that this has any behavioral consequence. Dietary and husbandry information has been offered in considerable detail elsewhere (Schaffner et al., 1995).
All males had been surgically vasectomized prior to the study. To ensure that initial pairing occurred during the same phase of the ovarian cycle, all females received an intramuscular injection of cloprostenol (Estrumate®, Schering Plough, Kenilworth, NJ, USA), 37.5 μg in 0.15 ml of the commercial solution, 3 days before pairing. This dose assures luteal regression within a few days (Webley et al., 1991), and ovulation occurs around 10 days post-injection (Summers et al., 1985). The Institutional Animal Care and Use Committee of the University of Nebraska at Omaha/the University of Nebraska Medical Center approved all procedures for this study (Protocol 07-073-11-FC). The Callitrichid Research Center is a registered research facility with the United States Department of Agriculture, and is accredited by the Association of Zoos and Aquariums. All appropriate guidelines for housing and conducting research with animals were followed.
2.2. Procedure
In the morning of Day 1 (around 0900 h), one male and one female were introduced into an empty cage in a room different from the one where they had been housed previously. The animals were unknown to each other at the moment of pairing, and had been individually housed for one week before pairing. The first observation started immediately after the subjects had been introduced into the cage and lasted for 40 min. A second observation was made the same day sometime between 14 and 16 h. This, and all subsequent observations, lasted for 20 min. For the following 20 days, observations were made every morning and every afternoon at the times mentioned above.
At all observations, the marmosets were given several minutes to habituate to the presence of observers before we started recording of behavior. We recorded each occurrence of the following social behaviors: ‘Approach’ (moving to a distance of less than 10 cm from the partner); ‘leave’ (moving at least 10 cm away from the partner); ‘initiation of huddling’ (start to sit or lie side by side with the partner, maintaining bodily contact; ‘end of huddling’ (interruption of bodily contact); ‘allogrooming’ (manipulating another’s coat with teeth or hands); ‘grooming solicitation’ (lowering of the rump, thorax, or head in front of the potential groomer); ‘food sharing’ (offering or passively releasing food items to the partner). In addition to recording the frequency of these behaviors, the duration of proximity (time between an approach and the next leave) and huddling (time between initiation and termination of a huddling episode) as well as that of allogrooming was determined.
Sexual interactions were also recorded. These consisted of ‘mount attempts’ (one individual grasps the other’s back and assumes a position similar to a mount, but no pelvic thrusting is displayed); ‘mount’ (a mount attempt + pelvic thrusting but no penile insertion); ‘copulation’ (mount with penile insertion and ejaculation). Although not a behavior directly related to sex ‘open mouth display’ (lip-smacking or tongue-flicking) is included among the sexual behaviors. In both males and females, this behavior is considered as an invitation to sexual activity (Epple, 1967; Kendrick & Dixson, 1984; Stevenson & Rylands, 1988; Smith et al., 2011b). For example, it has been reported that ovariectomized marmosets showed few open mouth displays in response to a male, and treatment with estradiol caused a drastic increase. Most of the open mouth displays were associated with male mounting (Dixson, 1986). It should be noted, though, that tongue flicking, part of the open mouth display, also has been interpreted as a fear response (Barros et al., 2004). ‘Genital sniff’ (placing nose on or near the partner’s genital region) is another behavior not involving the genitals but it is nevertheless associated with sexual activities (Epple, 1967; Stevenson & Poole, 1976; Gerber et al., 2002).
In addition to social and sexual behaviors we recorded several other behavior patterns: The territorial and/or communicative behavior ‘scent marking’ (anogenital rubbing against any surface, often preceded by gnawing on the surface). This behavior has been reported to be either territorial or serving to label a food source (Lazaro-Perea et al., 1999; Oliveira & Macedo, 2010). ‘Phee calling’ (loud single- or multisyllable whistle). This call can be used for identification of the sex of the caller, among other things (Smith et al., 2009). In addition, we recorded ‘piloerection’ (erect pelage, frequently associated with an arched back) and ‘genital display’ (raising tail and displaying genitals while looking at the target of the display) as well as ‘food stealing’ (taking a food item from the partner or fighting over a food item). These latter behaviors have been attributed multiple functions (see, e.g., Stevenson & Poole, 1976; Barros et al., 2004; Ross et al., 2004) and are here grouped together under the heading Miscellaneous Behaviors. Genital display is sometimes considered an aggressive behavior (Cilia & Piper, 1997), but this is not evident in all contexts (Epple, 1978). Therefore, we abstained from including this behavior in any specific category. Finally we recorded the aggressive behaviors of ‘erh-erh’ (bouts of quick, guttural chucks as part of an aggressive interaction) and ‘fighting’ (biting, scratching, hitting, or chasing the partner).
Several observers participated in the study. The marmosets had been habituated to the presence of each of them on several occasions prior to pairing. Individual marmosets were recognized by distinctive physical characteristic, such as long or short tail in relation to the partner, shape of the ears or fur coloring. All observers had been carefully trained by an experienced technician to identify behavioral patterns during instructed observation sessions. Inter-observer reliability (based on correlations of frequency and/or duration) was above 95% for all behavior patterns. Behaviors were recorded with the Observer 5.0 software (Noldus, Wageningen, The Netherlands) and transferred to Excel sheets for analyses.
2.3. Data preparation and statistical analyses
To determine possible changes in frequency or duration of a behavior pattern during the 21 days of observation we arranged the data in blocks. From Day 2 and on, five-day averages were calculated for each behavior. Thereby we obtained 4 blocks (Days 2–6, Days 7–11, Days 12–16 and Days 17–21) in addition to Day 1. Since we were interested in possible behavioral changes caused by the establishment of a pair bond, it was considered important to maintain Day 1 as a separate block, since the pair bond should not be present at all at that time, or, during the afternoon session, just beginning to become formed. For the rest of the observations, five day blocks were chosen in order to reduce variability in behavioral measures and, thus, render analyses interpretable. The morning and afternoon observations were kept separate. It is possible that behavior differed between the morning and afternoon observations, partly because of possible circadian variations in some behaviors and partly because of the fact that attractive food items were present during the morning observation (the daily meal had been distributed shortly before the onset of morning observations), but not in the afternoon. Circadian variations in locomotor activity, scent marking and grooming have been described both in wild and captive marmosets (e.g., Azevedo et al., 1996; de Castro et al., 2003; de Sousa et al., 2006; Gonçalves et al., 2009). It is also likely that the pair bond on the afternoon on Day 1 would be weaker than on subsequent afternoons, again offering the possibility to detect behavioral changes between the afternoon of Day 1 and the following afternoons.
After having arranged the data as described above, each behavior pattern was analyzed separately by a three-factor ANOVA with repeated measurements on two factors. The within groups factors were block (5 levels) and time of day (morning, afternoon) while the between groups factor was sex. Previous studies of behavior in pair bonded marmosets have revealed some sex differences (Evans & Poole, 1984; Ruiz, 1990; Schaffner et al., 1995), suggesting that it would be inappropriate to pool data from males and females. Following significant main effect on block we used Tukey’s HSD test for pairwise comparisons. If the data deviated drastically from normality, as evaluated by the Shapiro–Wilk statistic, or if error variances were non-homogenous as determined by Hartley’s F max test, a non-parametric ANOVA was used. We employed the Friedman test for blocks. Separate tests were performed for the morning and afternoon observations. To evaluate differences between the morning and afternoon observations with a non-parametric test, the mean for the 21 days of observations was calculated and that mean was analyzed with the Wilcoxon test. Differences between sexes were analyzed with Mann–Whitney U-test, again based on the mean for the entire study. Following a significant effect of block, pairwise comparisons were made according to the procedures described by Conover (Conover, 1999). Independent of the test used, data in the text and figures are reported as mean ± SEM.
The relationship between behavior displayed on Day 1 and on later days was further evaluated by calculating the Spearman or Pearson correlation, whichever was appropriate, between the mean of the two observations performed on Day 1 and the mean of all observations performed between Day 2 and Day 21. These means were obtained by determining the sum of the observations on Day 1 and of the observations from Day 2 to 21, respectively, and then dividing that sum with the number of observations. The morning observation on Day 1 was counted as 2 observations. Correlations were calculated separately for each behavior, and each correlation was based on all 10 individuals.
To detect subtle behavioral changes associated with the development of a pair bond, we subjected the data to a series of multivariate analyses of correspondence (Greenacre, 2007; Le Roux & Rouanet, 2010). This technique allows for the arrangement of behaviors, of individuals or of time periods in a multidimensional space. The data points (behaviors, individuals, time periods) are then projected onto a two-dimensional plane and the proportion of the total variance explained by each of the two axes forming this plane is calculated. We used this procedure only for descriptive purposes. More detailed descriptions of this use of the analysis can be found elsewhere (Spiteri et al., 2000; Montaudouin & Le Pape, 2004).
3. Results
3.1. Social behavior during the cohabitation period
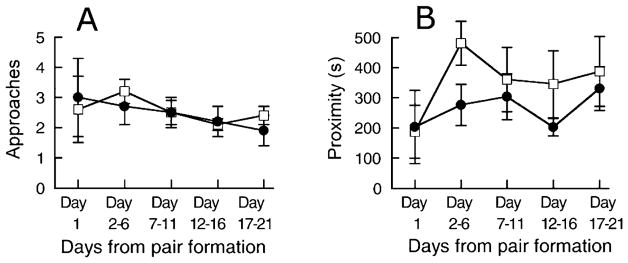
Most social behaviors require that the two members of the pair approach each other and remain in proximity. However, there was no systematic change in the frequency of approaching or leaving behavior or duration of proximity between the pair across the 5 blocks, no difference between morning and afternoon observations, no sex difference and no interaction (all p values > 0.17). Even though there was no significant effect, data are illustrated in Figure 1. The frequency of approach remained constant whereas the duration of proximity showed a tendency to be shorter on the morning of Day 1 than at later blocks.
Figure 1.
Frequency of approaches per 20 min (A) and duration of proximity per 20 min (B) at the morning (□) and at the afternoon (●) observations in marmosets observed for 21 days following pairing. Data are mean ± SEM from Day 1 and from the following 5 day periods. Durations are expressed in s.
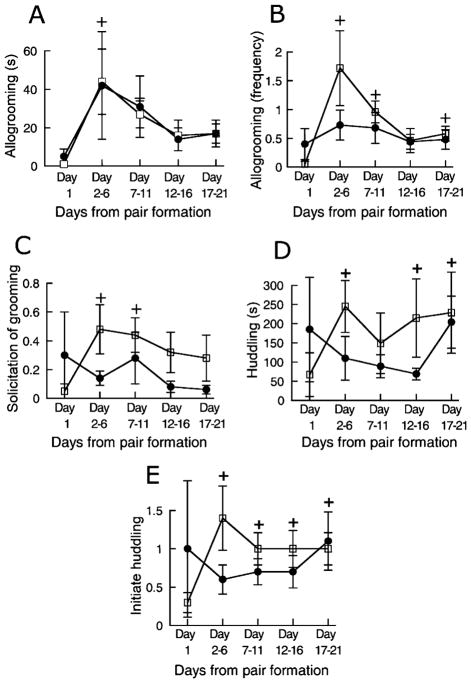
There was a difference in the duration of allogrooming between blocks in the morning ( , p < 0.05) but not in the afternoon ( , NS). Marmosets allogroomed for a shorter period of time on the morning of Day 1 compared to the mornings of Days 2–6 and 7–11, but no difference in allogrooming was noted between the morning of Day 1 and last two blocks. Data are shown in Figure 2A. When comparing the duration of allogrooming in the morning and in the afternoon with the Wilcoxon test, no difference was found (morning, 25 ± 5 s; afternoon, 25 ± 8 s; z = 0.15, NS). There was no sex difference in the morning (males, 32 ± 5 s; females, 18 ± 7 s; U = 6, NS) whereas there was in the afternoon (males, 42 ± 13 s; females, 8 ± 3 s; U = 2, p < 0.05).
Figure 2.
Duration (A) and frequency (B) of allogrooming, solicitation of grooming (C) and the duration of huddling (D) as well as the frequency of initiation of huddling (E) at the morning (□) and at the afternoon (●) observations in marmosets observed for 21 days following pairing. Data are mean ± SEM from Day 1 and from the following 5-day periods expressed as number per 20 min or seconds per 20 min. +, different from the morning of Day 1, p < 0.05. for less time on the morning of Day 1 than on the mornings on Days 2–6, Days 12–16 and Days 17–21 but not on Days 7–11. Data are illustrated in Figure 2D. The marmosets huddled as much in the morning as they did in the afternoon (morning, 203 ± 74 s; afternoon, 121 ± 25 s; z = 0.40, NS). Since huddling involves both individuals in the pair, no sex comparison could be made.
Concerning the frequency of allogrooming there was a difference between blocks with regard to the morning observations ( , p < 0.01). Further tests revealed that the frequency of allogrooming was higher on Days 2–6, 7–11 and 17–21 than on Day 1. For some reason, Days 12–16 did not differ from Day 1. The afternoon observations were not different ( , NS). Data are illustrated in Figure 2B. The marmosets had a higher frequency of allogrooming in the morning than in the afternoon (morning, 0.97 ± 0.16; afternoon, 0.72 ± 0.10; z = 2.29, p < 0.05), but there was no sex difference in either the morning or the afternoon observations (data not shown, p values > 0.07).
Marmosets not only groom each other, but they may also solicit grooming with the display of a specific behavior pattern. Analysis of the morning observations established that there was a difference between blocks ( , p < 0.05). A posteriori tests showed that the frequency of grooming solicitation was lower on Day 1 than during Days 2–6 and 7–11, while there was no difference between Day 1 and the last 2 blocks. There was no difference between blocks with regard to the afternoon observations ( , NS). Data are shown in Figure 2C. Males solicited grooming as much as the females both in the morning and in the afternoon (morning: males, 0.24 ± 0.12; females, 0.48 ± 0.15; U = 7, NS; afternoon: males, 0.06 ± 0.04; females, 0.24 ± 0.15, U = 7, NS). However, the number of grooming solicitations was higher in the morning than in the afternoon (morning, 0.36 ± 0.1; afternoon, 0.15 ± 0.08, z = 2.50, p < 0.05).
Huddling varied between blocks. There was a difference in the duration of huddling between blocks in the morning observations ( , p < 0.05) but not in the afternoon observations ( , NS). The pairs huddled
Although huddling is a pair behavior, each individual is free to initiate or terminate it. Analysis of the frequency of initiation of huddling at the morning observations revealed a difference between blocks ( , p = 0.001) with a lower frequency on the morning of Day 1 than on all other mornings. There was no difference between afternoon blocks ( , NS). Data are illustrated in Figure 2E. There was no difference in the frequency of initiation of huddling between morning and afternoon observations (morning, 1.1 ± 0.2; afternoon 0.8 ± 0.2; z = 1.12, NS) and no sex difference either in the morning (males, 1.0 ± 0.2; females, 1.1 ± 0.3; U = 11, NS) or in the afternoon (males, 1.0 ± 0.3; females, 0.6 ± 0.3; U = 4, NS). Termination of huddling showed a similar pattern (data not shown).
3.2. Sexual behaviors during the cohabitation period
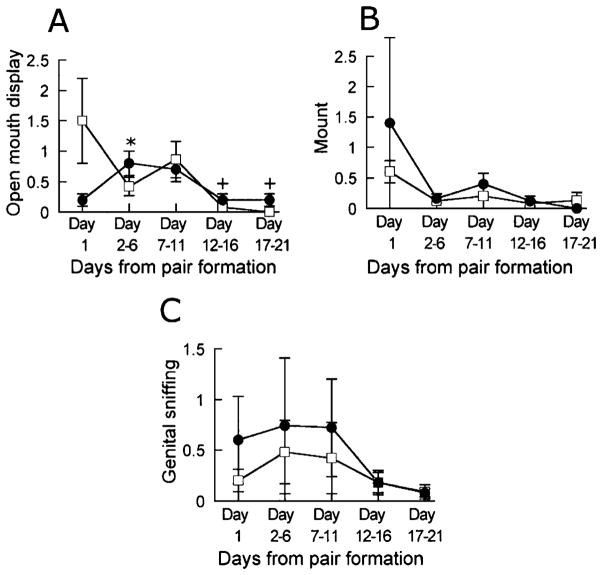
In addition to the social behaviors described above, the marmosets engaged in some sexual activity. Among the latter, we have included open mouth displays. Analysis of the morning observations revealed a difference between blocks ( , p < 0.001). Open mouth displays were more frequent in the morning of Day 1 than in the mornings of Days 12–16 and 17–21 but not in the mornings of Days 2–6 and 7–11. The afternoon observations also differed ( , p < 0.05) with more open mouth displays on the afternoons of Days 2–6 than on the afternoon of Day 1. Data are shown in Figure 3A. The females made more open mouth displays than did the males in the morning observations (males, 0.20 ± 0.05; females, 0.59 ± 0.18; U = 1.5, p < 0.05). There was no sex difference in the afternoon (males, 0.30 ± 0.08; females 0.60 ± 0.10; U = 5, NS), and there was no difference between morning and afternoon observations (morning, 0.39 ± 0.11; afternoon, 0.45 ± 0.08; z = 1.48, NS).
Figure 3.
Frequency of open mouth displays (A), number of mounts (B) and the frequency of genital sniffing (C) at the morning (□) and at the afternoon (●) observations in marmosets observed for 21 days following pairing. Data are mean ± SEM of number of occurrences per 20 min from Day 1 and from the following 5-day periods. +, different from the morning of Day 1, p < 0.05. *, different from the afternoon of Day 1, p < 0.05.
Males mounted the females with a rather low frequency throughout the 21 days of observation (Figure 3B). There was no significant difference between the morning observations ( , NS) or between the afternoon observations ( , NS). Likewise, there was no significant difference between observations performed in the morning and in the afternoon (morning, 0.15 ± 0.06; afternoon, 0.23 ± 0.09; z = 1.09, NS).
The number of ejaculations observed was very low. One male performed 3 ejaculations during the 21 days of observation, while 2 males made 2 ejaculations each, and 2 males were not observed to ejaculate during any of the observations. This small amount of data cannot be analyzed meaningfully.
Friedman’s ANOVA of genital sniffing showed that there was no significant difference between blocks in the morning ( , NS). This was also the case for the afternoon observations ( , NS). Males sniffed as much as females at the morning observations (males, 0.48 ± 0.36; females, 0.09 ± 0.06; U = 7.5, NS) whereas they had a higher frequency of genital sniffing in the afternoon (males, 0.84 ± 0.59; females, 0.04 ± 0.02; U = 0, p < 0.01). There was no significant difference between the frequency of genital sniffing in the morning and in the afternoon (morning, 0.29 ± 0.18; afternoon, 0.44 ± 0.31; z = 1.13, NS). Data are illustrated in Figure 3C.
3.3. Territorial/communicative behavior during the cohabitation period
Scent marking was performed by both males and females throughout the 21 days of observation. In fact, there was no difference in the frequency of scent marking between blocks and the marmosets scent marked as much in the morning as they did in the afternoon. There was no sex difference or interaction (all p values > 0.39). Thus, scent marking appears to be a remarkably stable behavior pattern, performed with the same frequency in both sexes regardless of the time of day or duration of cohabitation. The scent marking data are not illustrated. In addition, there was no difference between blocks with regard to phee calls, and the females emitted as many calls as the males. Likewise, there was no difference between morning and afternoon observations (all p values > 0.31, data not shown).
3.4. Miscellaneous behaviors
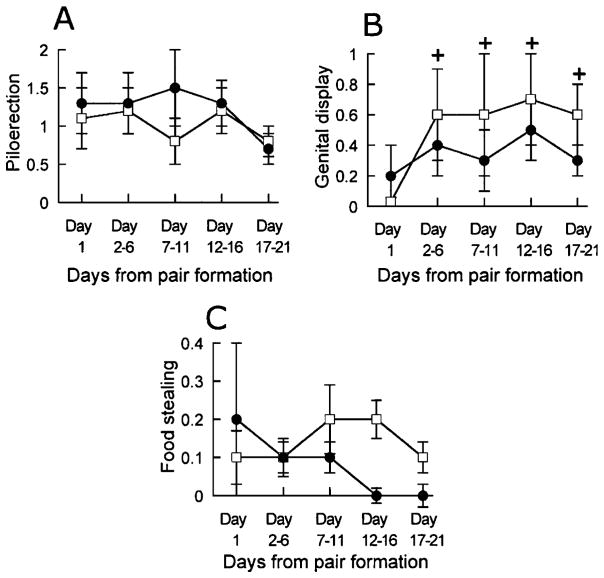
Piloerection ocurred with the same frequency during the 21 days of observation. There was no difference between blocks or between morning and afternoon observations, and no interaction approached significance (p values > 0.21). Data are shown in Figure 4A. There was a sex difference (F1,8 = 8.40, p < 0.05). Females showed a higher frequency of piloerection than the males (males, 0.62 ± 0.30; females 1.61 ± 0.25).
Figure 4.
The frequency of piloerection (A), genital display (B) and food stealing (C) at the morning (□) and at the afternoon (●) observations in marmosets observed for 21 days following pairing. Data are mean ± SEM of number of occurrences per 20 min from Day 1 and from the following 5-day periods. +, different from the morning of Day 1, p < 0.05.
Genital displays directed towards the partner had a low frequency. As can be seen in Figure 4B, the frequency of genital displays seemed to increase from the very low level on the morning of first day of observation. Friedman’s ANOVA confirmed a significant difference between blocks ( , p < 0.05). Subsequent tests established that the frequency of genital display was lower on the morning of Day 1 than at all subsequent morning observations. There was no differences between males and females (males, 0.65 ± 0.42; females, 0.56 ± 0.20; U = 11.5, NS). In the afternoon observations there was no difference between blocks ( , NS) and no sex difference (males, 0.45 ± 0.30; females, 0.25 ± 0.18; U = 11.0, NS). The frequency of genital displays was higher in the morning than in the afternoon (morning, 0.61 ± 0.22; afternoon, 0.35 ± 0.17; z = 2.24, p < 0.05).
Food stealing occurred with a low frequency at most sessions. As was the case with phee calls, there was no difference between blocks with regard to the morning observations ( , NS). Although the data illustrated in Figure 4C suggest that the frequency of food stealing was reduced at the afternoon observations, this was not confirmed by the statistical analysis ( , NS). There was no difference between morning and afternoon observations (morning, 0.12 ± 0.04; afternoon, 0.06 ± 0.02; z = 1.83, NS). However, there was a sex difference with males stealing food more frequently than females in the morning (males, 0.22 ± 0.05; females, 0.03 ± 0.02; U = 1, p < 0.05). In the afternoon, the sex difference only approached significance (males, 0.09 ± 0.03; females, 0.02 ± 0.02; U = 3.5, p = 0.05). Additional observed behaviors including food transfer, mount attempt, fight and erh-erh calls had all a total frequency < 20 for the 21 days of observation. Therefore, these behaviors were not analyzed.
Table 1 presents a summary of the behavioral data. The duration and frequency of allogrooming and huddling as well as the frequency of grooming solicitation, initiation and end of huddling and genital displays increased from the morning of Day 1 to later blocks while open mouth displays decreased. In contrast, when behavior patterns on the afternoon of Day 1 were compared to those displayed at later observations, the only difference obtained was a reduced number of open mouth displays at all observations following Day 1. Females showed a higher frequency of open mouth displays and piloerection than males at the morning observations, whereas the opposite was true for food stealing. In the afternoon, females allogroomed for a shorter time than males, and had fewer episodes of genital sniffing and food stealing than males. The frequency of allogrooming was higher in the morning than in the afternoon, whereas grooming solicitation showed the opposite pattern. Genital displays were more frequent in the morning than in the afternoon.
Table 1.
Summary of changes in frequency or duration of behavior patterns from Day 1 of pairing to the ensuing observations and differences between sexes and between morning and afternoon observations.
| Behavior | Morning
|
Afternoon
|
Time of day | ||
|---|---|---|---|---|---|
| Day 1–later periods | Sex | Day 1–later periods | Sex | ||
| Approach | 0 | 0 | 0 | 0 | 0 |
| Proximity duration | 0 | 0 | 0 | ||
| Leave | 0 | 0 | 0 | 0 | 0 |
| Allogrooming duration | + | 0 | 0 | −♀ | 0 |
| Allogrooming frequency | + | 0 | 0 | 0 | +§ |
| Grooming solicitation | + | 0 | 0 | 0 | −§ |
| Huddling duration | + | 0 | 0 | ||
| Initiate huddling | + | 0 | 0 | 0 | 0 |
| End huddling | + | 0 | 0 | 0 | 0 |
| Open mouth display | − | +♀ | − | 0 | 0 |
| Mount | 0 | 0 | |||
| Genital sniffing | 0 | 0 | 0 | −♀ | 0 |
| Scent marking | 0 | 0 | 0 | 0 | 0 |
| Piloerection | 0 | +♀ | 0 | 0 | 0 |
| Genital display | + | 0 | 0 | 0 | +§ |
| Phee calls | 0 | 0 | 0 | 0 | 0 |
| Food steal | 0 | −♀ | 0 | −♀ | 0 |
+, increase between Day 1 and later periods; −, decrease between Day 1 and later periods; +§, the frequency or duration of the behavior was higher or longer in the morning than in the afternoon; −§, the frequency or duration of the behavior was lower or shorter in the morning than in the afternoon; +♀, females showed a higher frequency or longer duration than males; −♀, females showed a lower frequency or shorter duration than males; 0, no significant difference.
3.5. Correlation between Day 1 and Days 2–21
Grooming on Day 1 was predictive for grooming throughout the 21 days of cohabitation. There was a significant correlation between the frequency of allogrooming on Day 1 and at later observations (r8 = 0.81, p < 0.01, as well as between the duration of allogrooming on Day 1 and on Days 2–21 (r8 = 0.77, p < 0.01). The mean duration of each allogrooming episode on Day 1 was correlated with the corresponding value for Days 2–21 (r8 = 0.75, p < 0.05). Approach was the only other behavior for which a significant correlation between Day 1 and later observations was obtained (r8 = 0.74, p < 0.05).
3.6. Multivariate analysis of behavior patterns
The univariate analysis of behaviors presented above provides valuable information as to variation over time of the individual behaviors, both during the 21 days of observation and between morning and afternoon observations. Likewise, they show sex differences in the frequency and/or duration of several behaviors. However, a complete description of sociosexual interactions requires that all behaviors are considered simultaneously. To that end, we conducted a series of correspondence analyses. Only the frequency of each behavior was included in these analyses, as durations are not appropriate.
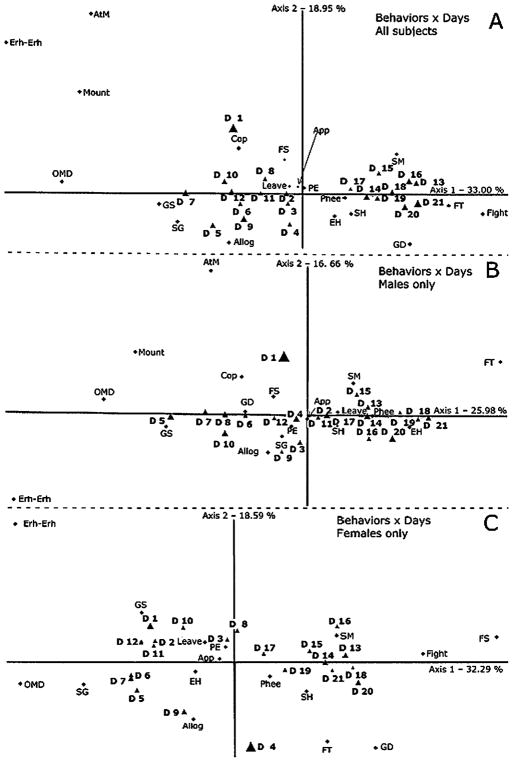
The first analysis employed day of observation in rows and behavior in columns. The data also included the subjects’ sex. As can be seen in Figure 5A, there is an orderly progression on axis 1 as the duration of cohabitation increases. This axis accounts for 33% of the variance, and seems to mainly represent social behaviors like genital sniffing, huddling, solicitation of grooming and food transfer. Axis 2 accounts for 19% of the variance. Sexual behaviors (mount attempts, mounts) as well as erh-erh calls scored high on this axes. Shortly after pairing, copulation and genital sniffing had high relative frequencies, whereas huddling and food transfer were more typical towards the end of the observation period in addition to scent marking, genital displays and even fighting. Based on a subsequent cluster analysis it became evident that the 21-days observation period could be divided in 3 clusters: Day 1, Days 2–12 and Days 13–21. We also performed separate analyses of the data from each sex. Results are remarkably similar to the initial analysis, suggesting that males and females modified their behavior in a similar way as the length of cohabitation increased (Figure 5B and 5C). One notable difference is that the males transferred more food and terminated huddling at the end of the observation period, whereas females stole more food and initiated huddling.
Figure 5.
(A) Results of the multivariate correspondence analysis based on the daily means of each behavior. Both males and females are included. (B, C) Multivariate correspondence analysis based on the daily means of each behavior in males and females, respectively. In all panels, axis 1 seems to be associated with social behaviors while axis 2 is associated with sexual behaviors in panels A and B. In panel C (females) axis 2 does not seem to be closely related to any particular behavior pattern, and it is unclear what it represents. This might be due to the fact that the females did not display any of the most frequent sexual behaviors, i.e., mount attempts and mounts. Nevertheless, the trajectories over days of both sexes are remarkably similar. Diamonds indicate the position of behaviors, and triangles indicate the position of the days of observation (D 1 to D 21). The size of the day symbols is proportional to their contribution to the axes. Abbreviations: Allog, allogrooming; App, approach; AtM, mount attempt; Cop, copulation; EH, end huddling; FS, food steal; FT, food transfer; GD, genital display; GS, genital sniff; OMD, open mouth display; PE, piloerection; Phee, phee call; SG, solicit grooming; SH, start huddling; SM, scent marking.
4. Discussion
The social behaviors allogrooming, solicitation of grooming, and huddling increased from Day 1 to the following observations. Considering that long term pair bonds are characterized by high levels of affiliative behavior, including allogrooming and huddling (Vogt, 1978; Schaffner et al., 1995), we suggest that the increase in these behaviors from the first day of cohabitation to later observations was caused by the formation of a pair bond. Although the main changes in behavior occurred between Day 1 and Days 2–6 the data do not allow for the conclusion that the putative bond had been fully established during the first six days of pairing. In fact, the results of the factorial analyses of correspondence show that behavior changed in an orderly way throughout the 21 days of observation. This suggests that the pair bond undergoes a constant development. An alternative explanation for the progressive change in social behaviors is that behaviorally important factors in addition to the pair bond change as a function of the length of cohabitation.
Even though none of the behaviors recorded in the present study was significantly modified after Day 6, there may be changes in the relative frequency of one or more behaviors. Because the factorial analysis of correspondence is based on the relative frequencies of all behaviors expressed on a particular day as well as on the frequency of a behavior on a particular day in relation to the sum of that behavior over all days, it is most suitable for detecting subtle changes in complex behavioral patterns over time. The fact that there was an orderly progression across days shows that the relative frequencies of one or several behaviors did not change randomly. Even though larger samples are needed for determining the specific behaviors that are responsible for the progressive change, the main conclusions of the present study should be robust enough.
The formation of a pair bond can probably not account for the changes observed in the frequencies of open mouth and genital displays. There is no reason to believe that there is an inverse relationship between pair bonding and open mouth displays. Since this display has been interpreted as an invitation to sexual activity we suggest that the decline in sexual motivation associated with increased duration of cohabitation in marmosets (see Introduction) as well as in humans (Klusmann, 2002) underlies this reduction. Likewise, the temporal coincidence between absence of a pair bond and low frequency of genital displays does not necessarily imply any causal relationship. It is more likely that both open mouth and genital displays are independent of the pair bond.
Besides the formation of a pair bond there are other possible explanations for the increase in affiliative behaviors from Day 1 to later observations. One is that social interaction on the morning of Day 1 was at a low level because of the stress associated with the transfer to a different cage and the encounter with an unknown individual. There are substantial data showing that these events indeed are stressful to marmosets (e.g., Smith et al., 1998; Gerber et al., 2002), and there are also data showing that social interaction is reduced in stressful situations (e.g., Gerber & Schnell, 2004). However, recent data from marmosets show that stress increases social proximity and grooming in males and females at pairing (Smith et al., 2011a). Furthermore, the behavioral data do not suggest the presence of significant stress at the observation on the morning of Day 1. For example, mounting was as frequent on the morning of Day 1 as at other observations, and mounting in marmosets is not considered a response to stress. Other behaviors that could be interpreted as behavioral manifestations of fear or stress, piloerection and scent marking (e.g., Stevenson & Poole, 1976; Cilia & Piper, 1997), did not occur more often on the morning of Day 1 than at later observations. Moreover, being placed together with an unknown individual of the opposite sex does not lead to any immediate increase in cortisol secretion in the common marmoset (Norcross & Newman, 1999), and exposure to a novel cage does not increase urinary cortisol concentrations for the first hours relative to more intense stressors (Smith & French, 1997). In view of all this, it is unlikely that social behavior was inhibited by stress during the first observation.
Another alternative explanation is that non-sexual social interaction during the first observation was reduced because of intense sexual activity. However, the intensity of sexual behavior displayed by the marmosets in the present study was low, and it is unlikely that it interfered with other social behaviors. It seems that an explanation in terms of pair bonding for the increased social behaviors observed here is more convincing than alternative explanations.
It should be noted that a basic assumption in this study is that a pair bond will develop in any heterosexual pair. This is a standard supposition in studies of pair bonds, since subjects almost always are paired randomly in experimental studies of this bond. Whether this assumption holds also in natural conditions is certainly debatable, but there are data suggesting that marmosets form pairs based on circumstance rather than on choice (reviewed in Snowdon & Ziegler, 2007). Nevertheless, it cannot be excluded that pairs established by choice could establish more intense pair bonds than pairs established by experimental design. Likewise, the changes in frequency and duration of some behaviors in the course of cohabitation might be different in pairs established by choice. It must also be noted that cohabitation leads to familiarity, and that familiarity could have effects on social interaction similar to those of the development of a pair bond. Social familiarity is an important contributor to the establishment of a pair bond (see Ricankova et al., 2007, and references therein). There are, however, important differences between the behavioral manifestations of a pair bond and of familiarity. For example, in species where a bond develops between pairs of individuals, the pair mate is preferred over an unfamiliar individual for mating. In species where pair bonding is not the rule, individuals prefer to mate with unfamiliar rather than with familiar conspecifics (Cheetham et al., 2008). Likewise, social investigation of conspecifics gets reduced with prolonged or repeated exposure both in monogamous and non-monogamous rodent species (Gheusi et al., 1994; Young, 2002) and in marmosets (Smith, 2006). These observations suggest that familiarity by itself does not necessarily increase sociosexual interaction. We suggest that the enhanced allogrooming and huddling observed in the present study is a result of a developing pair bond rather than of familiarity or some other factor unrelated to pair bonding.
Assuming that the behavioral manifestation of pair bonding is increased social behaviors we need to explain the mechanisms underlying the development of the bond. There is a substantial literature showing that social interactions of different kinds are rewarding. Play functions as a reward in conditioned place preference procedures in rats (e.g., Calcagnetti & Schechter, 1992; Thiel et al., 2008; Trezza et al., 2009), and rats prefer a place previously paired with encounters of a conspecific of the same age and sex (Douglas et al., 2004). Being housed with littermates has rewarding properties in mice (Panksepp & Lahvis, 2007). Social behavior has also been reported to be rewarding in primates. The possibility to groom the experimenter has successfully been used as reward in an operant task in the chimpanzee (Pan troglodytes) (Falk, 1958), and being groomed by the experimenter also functioned as a reward in the rhesus monkey (Macaca mulatta) (Taira & Rolls, 1996). These observations suggest that the act of grooming as well as being groomed have intrinsic rewarding properties. Support for this comes from data showing that grooming increased the concentration of β-endorphin in the cerebrospinal fluid of talapoin monkeys (Keverne et al., 1989). It is well established that β-endorphin and other opioids are rewarding (reviewed in van Ree et al., 2000).
Grooming has several physiological consequences in addition to promoting release of endorphins. In the human, heart rate is reliably reduced by touch (Wilhelm et al., 2001 and references therein), and allogrooming has a similar effect in non-human primates (Boccia et al., 1989; Aureli et al., 1999). Correlational data suggest that cortisol also is reduced by being groomed (Gust et al., 1993) or by grooming another (Shutt et al., 2007). These physiological responses may contribute to the rewarding effects of grooming. Furthermore, there are some indirect data suggesting that oxytocin release may either be caused by grooming or be a cause of grooming (e.g., Engelmann et al., 1994; Maestripieri et al., 2009). Perhaps that oxytocin released in association with grooming contributes to the rewarding properties of this behavior. In fact, intracerebroventricular oxytocin produces place preference (Liberzon et al., 1997), showing that this peptide indeed has rewarding properties.
The rewarding state associated with the act of grooming would become associated to salient contextual stimuli through simple contiguity. This, coincidentally, is the mechanism behind most classical conditioning, including conditioned place preference (see for example Tzschentke, 1998; Spiteri et al., 2000). The association between reward and contextual cues causes the subject to approach those cues on future occasions. In the context of grooming, one salient stimulus for the groomer is certainly the individual being groomed. Thus, in the future this individual will be approached because it has become associated with a reward. Likewise, the receiver of grooming will also experience reward, which will become associated with the grooming individual. In this way, each of the participants in the grooming episode will become associated with reward and thereby acquire conditioned incentive properties. These properties manifest themselves in a mutual attraction between the groomer and the recipient. One consequence of this is that each member of the pair will try to approach the other whenever possible. This may be accomplished through following, eventually leading to proximity and huddling, the maximal reduction of distance between the individuals. According to this reasoning, the pair bond develops because of an association between a reward and a stimulus present during the experience of reward. This stimulus is normally the partner.
It could be maintained that a conditioning model of pair bonding should lead to a continuous, progressive increase in rewarding social behaviors like grooming. However, present data show that social behaviors increased between Day 1 and Days 2–6, and thereafter remained at a stable level. It can be assumed that the conditioned incentive properties of the partner, hence the response of approach, had reached an asymptotic level by Day 6. Any further increase in response intensity would be offset because of habituation (McSweeney & Murphy, 2009; Carr & Epstein, 2011) or because of negative alliesthesia (Cabanac, 1971; Brondel et al., 2009; Kim et al., 2010). Thus, performance of conditioned responses stabilizes or gets reduced, as is the case with some sociosexual behaviors in long term marmoset pairs (see Introduction) and in human long term relationships (Klusmann, 2002).
Further support for the hypothesis that grooming is a key element for establishing the pair bond comes from the fact that the correlations between the frequency and duration of allogrooming on Day 1 and at later observations were high. This was also the case for approach but not for any other behavior. Thus, the number of approaches and the amount of allogrooming shown on Day 1 seem to predict the amount of these behaviors shown at the later observations, exactly as would be expected according to the hypothesis proposed above.
Grooming is assigned a prime role in the preceding reasoning. Indeed, grooming and play are the only non-sexual, social behaviors for which there is abundant evidence for rewarding properties. Furthermore, data from the assamese macaque (Macaca assamensis) show that grooming facilitated the establishment and maintenance of social bonds (Cooper & Bernstein, 2000). Explanations of grooming in terms of exchange of services or favors such as mating did not fit with the data. Likewise, data from the chimpanzee (Fedurek & Dunbar, 2009) and other primates (reviewed in Dunbar, 2010) suggest that grooming is crucial for all kinds of social bonds.
Needless to say, sexual behaviors are also rewarding (see Ågmo & Berenfeld, 1990; Paredes & Alonso, 1997; Tenk et al., 2009) and sexual reward may become associated with the partner, enhancing its incentive properties. Data from a study in male marmosets support this notion (Snowdon et al., 2011). An initially neutral odor caused approach behavior and erection after being associated with copulation, suggesting that sexual reward could become associated to cues emitted by the partner. However, the low level of sexual activity observed in the present study probably did not constitute an important element in pair bond formation. Unfortunately, the low number of animals available for this study precludes statistical comparisons between pairs with and without sexual activity.
The intrinsically rewarding properties of social behaviors like grooming or being groomed, or engaging in sexual interactions, are the bases of the pair bond according to the reasoning expressed above. The pair bond is, then, nothing more than an enhanced incentive value of the partner because of association with these rewards. This proposal has considerable implications regarding the behavioral and neurobiological properties of the pairbonding process. The most important is that the rather mysterious concept of bond becomes reduced to a motivational mechanism which is amenable for study with the experimental procedures employed in studies of other kinds of motivation. This facilitates behavioral as well as neurobiological analyses of bonds in different contexts, such as the formation of bonds between individuals of the same sex or multiple bonds, such as those described in meadow voles (Parker et al., 2001; Parker & Lee, 2003; Beery et al., 2009) or in several primate species (Henzi & Barrettt, 2007; Silk et al., 2010). Likewise, the mother–infant bond could be explained in terms of reward processes. As mentioned, grooming has been given a prime role in the establishment and maintenance of all kinds of bonds in non-human primates (Lazaro-Perea et al., 2004; Henzi & Barrettt, 2007) and touch has been given a similar importance in human relationships (Hertenstein et al., 2006; Dunbar, 2010; Smith et al., 2011b). Different forms of tactile stimulation also appears to be involved the reciprocal bonds between mothers and infants (Alberts & Brunjes, 1978; Kojima & Alberts, 2011).
At present, there is no direct experimental evidence showing that tactile stimulation is rewarding. However, pups have rewarding properties for female rats and can be used for reinforcing a bar pressing task (Lee et al., 1999) or for producing a place preference (Fleming et al., 1994). Also female marmosets learned to bar press to gain visual access to an infant replica combined with the cessation of infant distress calls (Pryce et al., 1993). These observations may suggest that the motivational basis for the mother–infant bond is not different from the bond between heterosexual partners described in this study (i.e., the pair bond). Whether interspecies bonds, for example those occurring between humans and their pets, also share this common basis is not known. The existence of reciprocal bonds between humans and pets has been firmly established (Prato-Previde et al., 2006; Parthasarathy & Crowell-Davis, 2006; Nagasawa et al., 2009a,b), but the role of tactile stimulation is unclear. In fact, a study in horses showed that grooming was not sufficient for the establishment of a bond, while feeding was (Sankey et al., 2010). Perhaps tactile stimulation in horses is not rewarding, whereas eating certainly is. The role of grooming or touch in dog-human bonds, probably the most common of all interspecies bonds, has not been experimentally determined, but we dare to propose that it is most important.
In addition to sharing common behavioral mechanisms, it is possible that all types of bonds share a common neurobiology. Oxytocin facilitates same-sex bonding in the meadow vole (Beery & Zucker, 2010) just as it facilitates heterosexual pair bonding in the monogamous prairie vole (Williams et al., 1994) and black tufted-ear marmoset (Smith et al., 2010). The importance of oxytocin for the mother–infant bond is well established both in rodents (reviewed in Leng et al., 2008) and primates (Maestripieri et al., 2009), including humans (Feldman et al., 2007, 2010; Levine et al., 2007). It is also important to note that sexual activity releases oxytocin in rats (Waldherr & Neumann, 2007), rabbits (Stoneham et al., 1985) and humans (Carmichael et al., 1987, 1994). In prairie voles, centrally administered oxytocin (Williams et al., 1994) or experimentally induced overexpression of the oxytocin receptor (Ross et al., 2009) facilitated pair bonding. Consequently, oxytocin released during sexual behaviors may contribute to the establishment of a pair bond. In voles, for example, copulation accelerated the formation of a pair bond (Insel et al., 1995). The neurobiology of human–pet bonds is not well known, but it has been reported that interspecies interaction between humans and their dogs increased the release of oxytocin and β-endorphin in both species (Odendaal & Meintjes, 2003). Release of oxytocin following interaction between dog and owner has been confirmed in more recent studies (Nagasawa et al., 2009a,b; Handlin et al., 2011). These observations suggest that there may be a similar neurobiological basis for bonds within a species and for the more unusual bonds occurring between species.
Unitary, basic mechanisms for all kinds of bonds would satisfy the principle of parsimony, although it might lack the poetical value of more romantic notions. Finally, the proposal presented here coincides with all available data concerning pair bonding, regardless of the species from which they come and whether the bond is between opposite sexes or within the same sex.
Acknowledgments
We thank Heather Jensen and Dr. Liz Gunkelman for providing excellence in colony management and veterinary care, respectively. Emily Harrison, Amanda Ciurej and Kayleigh Fehncke were able assistants in collecting behavioral data. The research was supported in part by funds to J.A.F. from the National Institutes of Health (HD-42882) and National Science Foundation (NSF) (IBN 00-91030) and A.S.S. from the NSF Graduate Research Fellowship Program, University Committee on Research and Creative Activity at the University of Nebraska at Omaha, and American Society of Primatologists. A.Å’s sabbatical at the Callitrichid Research Facility was financed by the Faculty of Health Sciences, University of Tromsø.
References
- Abbott DH, Hearn JP. Physical, hormonal and behavioral aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978;53:155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav Neurosci. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Alberts JR, Brunjes PC. Ontogeny of thermal and olfactory determinants of huddling in the rat. J Comp Physiol Psychol. 1978;92:897–906. doi: 10.1037/h0077533. [DOI] [PubMed] [Google Scholar]
- Aureli F, Preston SD, de Waal FBM. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J Comp Psychol. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- Azevedo CVM, Menezes AAL, Queiroz JW, Moreira LFS. Circadian and ultradian periodicities of grooming behavior in family groups of common marmosets (Callithrix jacchus) in captivity. Biol Rhythm Res. 1996;27:374–385. [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007a;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007b;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros M, de Souza Silva MA, Huston JP, Tomaz C. Multibehavioral analysis of fear and anxiety before, during, and after experimentally induced predatory stress in Callithrix penicillata. Pharmacol Biochem Behav. 2004;78:357–367. doi: 10.1016/j.pbb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Beery AK, Routman DM, Zucker I. Same-sex social behavior in meadow voles: multiple and rapid formation of attachments. Physiol Behav. 2009;97:52–57. doi: 10.1016/j.physbeh.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169:665–673. doi: 10.1016/j.neuroscience.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiol Behav. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother–infant bonding and the evolution of mammalian social relationships. Philos Trans Roy Soc B. 2006;361:2199–2214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondel L, Romer M, Van Wymelbeke V, Pineau N, Jiang T, Hanus C, Rigaud D. Variety enhances food intake in humans: role of sensory-specific satiety. Physiol Behav. 2009;97:44–51. doi: 10.1016/j.physbeh.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith HM, Jordan TR. An experimental investigation of the pair bond in the callitrichid monkey, Saguinus labiatus. Int J Primatol. 1992;13:51–72. [Google Scholar]
- Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Warburton VL, Dixen J, Davidson JM. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav. 1994;23:59–79. doi: 10.1007/BF01541618. [DOI] [PubMed] [Google Scholar]
- Carr KA, Epstein LH. Relationship between food habituation and reinforcing efficacy of food. Learn Motiv. 2011;42:165–172. doi: 10.1016/j.lmot.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cheetham SA, Thom MD, Beynon RJ, Hurst JL. The effect of familiarity on mate choice. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD, editors. Chemical signals in vertebrates. Vol. 11. Springer; New York, NY: 2008. pp. 271–280. [Google Scholar]
- Cilia J, Piper DC. Marmoset conspecific confrontation: an ethologically-based model of anxiety. Pharmacol Biochem Behav. 1997;58:85–91. doi: 10.1016/s0091-3057(96)00376-0. [DOI] [PubMed] [Google Scholar]
- Clara E, Tommasi L, Rogers LJ. Social mobbing calls in common marmosets (Callithrix jacchus): effects of experience and associated cortisol levels. Anim Cogn. 2008;11:349–358. doi: 10.1007/s10071-007-0125-0. [DOI] [PubMed] [Google Scholar]
- Conover WJ. Practical nonparametric statistics. 3. Wiley; New York, NY: 1999. [Google Scholar]
- Cooper MA, Bernstein IS. Social grooming in assamese macaques (Macaca assamensis) Am J Primatol. 2000;50:77–85. doi: 10.1002/(SICI)1098-2345(200001)50:1<77::AID-AJP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- de Castro CSS, de Menezes AL, Moreira LFD. Locomotor activity rhythm in free-ranging common marmosets (Callithrix jacchus) Biol Rhythm Res. 2003;34:23–30. [Google Scholar]
- de Sousa MBC, Moura SLN, Menezes AAD. Circadian variation with a diurnal bimodal profile on scent-marking behavior in captive common marmosets (Callitrhix jacchus) Int J Primatol. 2006;27:263–272. [Google Scholar]
- Dewsbury DA. The comparative psychology of monogamy. In: Leger DW, editor. Nebraska symposium on motivation. University of Nebraska Press; Lincoln, NE: 1988. pp. 1–50. [PubMed] [Google Scholar]
- Dixson AF. Proceptive displays of the female common marmoset (Callithrix jacchus): effects of ovariectomy and estradiol 17-beta. Physiol Behav. 1986;36:971–973. doi: 10.1016/0031-9384(86)90462-2. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Bondedness and sociality. Behaviour. 2010;147:775–803. [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Direct osmotic stimulation of the hypothalamic paraventricular nucleus by microdialysis induces excessive grooming in the rat. Behav Brain Res. 1994;63:221–225. doi: 10.1016/0166-4328(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Epple G. Vergleichende Untersuchungen über Sexual- und Sozialverhalten der Krallenaffen (Hapalidae) Folia Primatol. 1967;7:37–65. doi: 10.1159/000155095. [DOI] [PubMed] [Google Scholar]
- Epple G. The behavior of marmoset monkeys (Callitricidae) In: Rosenblum LA, editor. Primate behavior: developments in field and laboratory research. Vol. 4. Academic Press; New York, NY: 1975. pp. 195–239. [Google Scholar]
- Epple G. Notes on the establishment and maintenance of the pair bond in Saguinus fuscicollis. In: Kleiman DG, editor. The biology and conservation of the Callitrichidae. Smithsonian Institution Press; Washington, DC: 1977. pp. 231–237. [Google Scholar]
- Epple G. Lack of effects of castration on scent marking, displays, and aggression in a South American primate (Saguinus fuscicollis) Horm Behav. 1978;11:139–150. doi: 10.1016/0018-506x(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Epple G. Sex differences in partner preference in mated pairs of saddle-back tamarins (Saguinus fuscicollis) Behav Ecol Sociobiol. 1990;27:455–459. [Google Scholar]
- Evans S, Poole TB. Pair-bond formation and breeding success in the common marmoset Callithrix jacchus jacchus. Int J Primatol. 1983;4:83–97. [Google Scholar]
- Evans S, Poole TB. Long-term changes and maintenance of the pair-bond in common marmosets, Callithrix jacchus jacchus. Folia Primatol. 1984;42:33–41. [Google Scholar]
- Falk JL. The grooming behavior of the chimpanzee as a reinforcer. J Exp Anal Behav. 1958;1:83–85. doi: 10.1901/jeab.1958.1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedurek P, Dunbar RIM. What does mutual grooming tell us about why chimpanzees groom? Ethology. 2009;115:566–575. [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation. Plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- French JA, Inglett BJ. Female–female aggression and male indifference in response to unfamiliar intruders in lion tamarins. Anim Behav. 1989;37:487–497. [Google Scholar]
- French JA, Snowdon CT. Sexual dimorphism in responses to unfamiliar intruders in the tamarin, Saguinus oedipus. Anim Behav. 1981;29:822–829. [Google Scholar]
- Gerber P, Schnell CR. Behavioral and cardiophysiological responses of common marmosets (Callithrix jacchus) to confrontations with opposite-sexed strangers. Primates. 2004;45:187–196. doi: 10.1007/s10329-004-0086-8. [DOI] [PubMed] [Google Scholar]
- Gerber P, Schnell CR, Anzenberger G. Behavioral and cardiophysiological responses of common marmosets (Callithrix jacchus) to social and environmental changes. Primates. 2002;43:201–216. doi: 10.1007/BF02629648. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthé RM, Goodall G, Dantzer R. Social and individual recognition in rodents: methodological aspects and neurobiological bases. Behav Proc. 1994;33:59–87. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Gonçalves FB, Belísio AS, Azevedo CVM. Effect of nest box availability on the circadian activity rhythm of common marmosets (Callithrix jacchus) Folia Primatol. 2009;80:175–188. doi: 10.1159/000230035. [DOI] [PubMed] [Google Scholar]
- Greenacre MJ. Correspondence analysis in practice. 2. Chapman & Hall; Boca Raton, FL: 2007. [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between social factors and pituitary-adrenocortical activity in female Rhesus monkeys (Macaca mulatta) Horm Behav. 1993;27:318–331. doi: 10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- Handlin L, Hydbring-Sandberg E, Nilsson A, Ejdeback M, Jansson A, Uvnas-Moberg K. Short-term interaction between dogs and their owners: effects on oxytocin, cortisol, insulin and heart rate: an exploratory study. Anthrozoös. 2011;24:301–315. [Google Scholar]
- Henzi SP, Barrett L. Coexistence in female-bonded primate groups. Adv Stud Behav. 2007;37:43–81. [Google Scholar]
- Hertenstein MJ, Verkamp JM, Kerestes AM, Holmes RM. The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genet Soc Gen Psychol Monogr. 2006;132:5–94. doi: 10.3200/mono.132.1.5-94. [DOI] [PubMed] [Google Scholar]
- Inglett BJ, French JA, Dethlefs TM. Patterns of social preference across different social contexts in golden lion tamarins (Leontopithecus rosalia) J Comp Psychol. 1990;104:131–139. doi: 10.1037/0735-7036.104.2.131. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF. A quantitative description of copulatory and associated behaviors of captive marmosets (Callithrix jacchus) Int J Primatol. 1984;5:199–212. [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Kim BW, Kennedy DN, Lehar J, Lee MJ, Blood AJ, Lee S, Perlis RH, Smoller JW, Morris R, Fava M, Breiter HC. Recurrent, robust and scalable patterns underlie human approach and avoidance. Plos One. 2010;5:e10613. doi: 10.1371/journal.pone.0010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG. Characteristics of reproduction and sociosexual interaction in pairs of lion tamarins (Leontopithecus rosalia rosalia) during the reproductive cycle. In: Kleiman DG, editor. The biology and conservation of the Callitrichidae. Smithsonian Institution Press; Washington, DC: 1977a. pp. 181–190. [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977b;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Klusmann D. Sexual motivation and the duration of partnership. Arch Sex Behav. 2002;31:275–287. doi: 10.1023/a:1015205020769. [DOI] [PubMed] [Google Scholar]
- Kojima S, Alberts JR. Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Dev Psychobiol. 2011;53:813–827. doi: 10.1002/dev.20565. [DOI] [PubMed] [Google Scholar]
- Lazaro-Perea C, Arruda MF, Snowdon CT. Grooming as a reward? Social function of grooming between females in cooperatively breeding marmosets. Anim Behav. 2004;67:627–636. doi: 10.1016/j.anbehav.2003.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Perea C, Snowdon CT, Arruda MF. Scent-marking behavior in wild groups of common marmosets (Callithrix jacchus) Behav Ecol Sociobiol. 1999;46:313–324. [Google Scholar]
- Le Roux B, Rouanet H. Multiple correspondence analysis. Sage; Los Angeles, CA: 2010. [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Curr Opin Pharmacol. 2008;8:731–734. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Trujillo KA, Akil H, Young EA. Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology. 1997;17:353–359. doi: 10.1016/S0893-133X(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother–infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav. 2009;96:613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: strength and specificity of attraction between male–female cagemates. Folia Primatol. 1975;23:113–123. doi: 10.1159/000155664. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mcgraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Murphy ES. Sensitization and habituation regulate reinforcer effectiveness. Neurobiol Learn Mem. 2009;92:189–198. doi: 10.1016/j.nlm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Montaudouin S, Le Pape G. Comparison of the behaviour of European brown bears (Ursus arctos arctos) in six different parks, with particular attention to stereotypies. Behav Proc. 2004;67:235–244. doi: 10.1016/j.beproc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Kikusui T, Onaka T, Ohta M. Dog’s gaze at its owner increases owner’s urinary oxytocin during social interaction. Horm Behav. 2009a;55:434–441. doi: 10.1016/j.yhbeh.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Mogi K, Kikusui T. Attachment between humans and dogs. Jpn Psychol Res. 2009b;51:209–221. [Google Scholar]
- Norcross JL, Newman JD. Effects of separation and novelty on distress vocalizations and cortisol in the common marmoset (Callithrix jacchus) Am J Primatol. 1999;47:209–222. doi: 10.1002/(SICI)1098-2345(1999)47:3<209::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Odendaal JSJ, Meintjes RA. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet J. 2003;165:296–301. doi: 10.1016/s1090-0233(02)00237-x. [DOI] [PubMed] [Google Scholar]
- Oliveira DGR, Macedo RH. Functional context of scent-marking in Callithrix penicillata. Folia Primatol. 2010;81:73–85. doi: 10.1159/000313011. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J Comp Psychol. 2003;117:283–289. doi: 10.1037/0735-7036.117.3.283. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Lee TM. Development of selective partner preferences in captive male and female meadow voles, Microtus pennsylvanicus. Anim Behav. 2001;61:1217–1226. [Google Scholar]
- Parthasarathy V, Crowell-Davis SL. Relationship between attachment to owners and separation anxiety in pet dogs (Canis lupus familiaris) J Vet Behav Clin Appl Res. 2006;1:109–120. [Google Scholar]
- Prato-Previde E, Fallani G, Valsecchi P. Gender differences in owners interacting with pet dogs: an observational study. Ethology. 2006;112:64–73. [Google Scholar]
- Pryce CR, Dobeli M, Martin RD. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J Comp Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- Ricankova V, Sumbera R, Sedlacek F. Familiarity and partner preferences in female common voles, Microtus arvalis. J Ethol. 2007;25:95–98. [Google Scholar]
- Ross CN, French JA, Ortí G. Germ-line chimerism and paternal care in marmosets (Callithrix kuhlii) Proc Natl Acad Sci USA. 2007;104:6278–6282. doi: 10.1073/pnas.0607426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, French JA, Patera KJ. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiol Behav. 2004;83:437–445. doi: 10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JC. Comparison of affiliative behaviors between old and recently established pairs of golden lion tamarin, Leontopithecus rosalia. Primates. 1990;31:197–204. [Google Scholar]
- Saltzman W, Abbott DH. Hormonal and behavioral responses to stress in lactating and non-lactating female common marmosets (Callithrix jacchus) Physiol Behav. 2011;104:446–453. doi: 10.1016/j.physbeh.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav. 1994;56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Sankey C, Henry S, Gorecka-Bruzda A, Richard-Yris MA, Hausberger M. The way to a man’s heart is through his stomach: what about horses? Plos One. 2010;5:e15446. doi: 10.1371/journal.pone.0015446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in Wieds black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than to receive? Biol Lett. 2007;3:231–233. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Female chacma baboons form strong, equitable, and enduring social bonds. Behav Ecol Sociobiol. 2010;64:1733–1747. doi: 10.1007/s00265-010-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva HPA, Sousa MBC. The pair-bond formation and its role in the stimulation of reproductive function female common marmosets (Callithrix jacchus) Int J Primatol. 1997;18:387–400. [Google Scholar]
- Smith AS, Birnie AK, Lane KR, French JA. Production and perception of sex differences in vocalizations of Wied’s black-tufted-ear marmosets (Callithrix kuhlii) Am J Primatol. 2009;71:324–332. doi: 10.1002/ajp.20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiol Behav. 2011a;104:955–961. doi: 10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JCS, Vogel DL, Madon S, Edwards SR. The power of touch: nonverbal communication within married dyads. Couns Psychol. 2011b;39:764–787. [Google Scholar]
- Smith T. Individual olfactory signatures in common marmosets (Callithrix jacchus) Am J Primatol. 2006;68:585–604. doi: 10.1002/ajp.20254. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys (Callithrix kuhli) Physiol Behav. 1997;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, Greer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Horm Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Tannenbaum PL, Schultz-Darken NJ, Ziegler TE, Ferris CF. Conditioned sexual arousal in a nonhuman primate. Horm Behav. 2011;59:696–701. doi: 10.1016/j.yhbeh.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon CT, Ziegler TE. Growing up cooperatively: family processes and infant care in marmosets and tamarins. J Dev Process. 2007;2:40–66. [Google Scholar]
- Spiteri T, Le Pape G, Ågmo A. What is learned during place preference conditioning? A comparison of food- and morphine-induced reward. Psychobiology. 2000;28:367–382. [Google Scholar]
- Stevenson MF, Poole TB. An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim Behav. 1976;24:428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Stevenson MF, Rylands AB. The marmoset, genus Callithrix. In: Mittermeier RA, Rylands AB, Coimbra-Filho A, Fonseca GAB, editors. Ecology and behavior of neotropical primates. Vol. 2. World Wildlife Fund; Washington, DC: 1988. pp. 131–222. [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behavior in the male rat and rabbit. J Endocr. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Summers PM, Wennink CJ, Hodges JK. Cloprostenol-induced luteolysis in the marmoset monkey (Callithrix jacchus) J Reprod Fert. 1985;73:133–138. doi: 10.1530/jrf.0.0730133. [DOI] [PubMed] [Google Scholar]
- Taira K, Rolls ET. Receiving grooming as a reinforcer for the monkey. Physiol Behav. 1996;59:1189–1192. doi: 10.1016/0031-9384(95)02213-9. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progr Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Niesink RJM, van Wolfswinkel L, Ramsey NF, Kornet MMW, van Furth WR, Vanderschuren LJMJ, Gerrits MAFM, van den Berg CL. Endogenous opioids and reward. Eur J Pharmacol. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- Vogt JL. Social behaviour of a marmoset (Saguinus fuscicollis) group. III Spatial analysis of social structure. Folia Primatol. 1978;29:250–267. [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webley GE, Hodges JK, Given A, Hearn JP. Comparison of the luteolytic action of gonadotropin releasing-hormone antagonist and cloprostenol, and the ability of human chorionic gonadotropin and melatonin to override their luteolytic effects in the marmoset monkey. J Endocrinol. 1991;128:121–129. doi: 10.1677/joe.0.1280121. [DOI] [PubMed] [Google Scholar]
- Wickler W. The ethological analysis of attachment. Sociometric, motivational and sociophysiological aspects. Z Tierpsychol. 1976;42:12–28. [PubMed] [Google Scholar]
- Wilhelm FH, Kochar AS, Roth WT, Gross JJ. Social anxiety and response to touch: incongruence between self-evaluative and physiological reactions. Biol Psychol. 2001;58:181–202. doi: 10.1016/s0301-0511(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocr. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Woodcock AJ. The first weeks of cohabitation of newly-formed heterosexual pairs of common marmosets (Callithrix jacchus) Folia Primatol. 1982;37:228–254. doi: 10.1159/000156035. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- Zahed SR, Kurian AV, Snowdon CT. Social dynamics and individual plasticity of infant care behavior in cooperatively breeding cotton-top tamarins. Am J Primatol. 2010;72:296–306. doi: 10.1002/ajp.20782. [DOI] [PubMed] [Google Scholar]