Summary.
Microtubule-organizing centers move from centrosomes to the nuclear envelope during muscle development. The KASH protein Nesprin-1α recruits pericentriolar material to the surface of myotube nuclei, which nucleates microtubules to evenly space nuclei apart in the developing myotube.
Mammalian skeletal muscles form when hundreds of myoblasts fuse into multi-nucleated myotubes, which differentiate into myofibers. The position of nuclei throughout muscle development is tightly controlled. Defects in nuclear positioning can lead to Emery-Dreifuss Muscular Dystrophy and centronuclear myopathies [1,2]. There are at least five mechanistically and temporally distinct nuclear movements during skeletal muscle development [3]. Shortly after a myoblast fuses with a multi-nucleated myotube, the nucleus rapidly migrates toward the center of the syncytia. Then, myotube nuclei spread apart. A key in the switch from myoblast to myotube nuclear behavior is a fundamental change in the microtubule-organizing center (MTOC). In myoblasts, microtubules are nucleated from the centrosome. In myotubes, the cytoplasmic surface of the nuclear envelope serves as the major MTOC of the cell [4,5]. MTOC switching from a centrosome to a non-centrosomal location is a hallmark of differentiation [6]. An exciting report in this issue of Current Biology [7] characterizes the mechanisms for how the MTOC is recruited to the cytoplasmic surface of the nuclei in developing myotubes. Gimpel et al [7] provide support for a new model for how nuclei are spaced apart in developing myotubes using microtubules nucleated from the cytoplasmic surface of the nuclear envelope.
The new microtubule-nucleation model requires Nesprin-1 at the surface of the nuclear envelope. Mammalian Nesprin-1 (encoded by the SYNE1 gene), its paralog Nesprin-2, and its orthologs ANC-1 in C. elegans and MSP-300 in Drosophila, encode some of the largest proteins ever identified. The full-length isoforms approach a mega Dalton in size [8]. They contain actin-binding domains at their N-termini, long extended regions made mostly of spectrin-like repeats, and a C-terminal KASH (Klarsicht, ANC-1 and Syne homology) domain that inserts into the outer nuclear membrane and interacts with SUN (Sad1, UNC-84 domain) proteins in the perinuclear space [8]. Thus, Nesprin-1 makes up the outer nuclear membrane half of LINC (linker of nucleoskeleton and cytoskeleton) complexes that bridge the nuclear envelope to connect the nucleus to the cytoskeleton.
There are many isoforms of Nesprin-1; 16 different 5’ UTRs and 14 different 3’UTRs have been identified [9]. A few of these isoforms have been validated in vivo, including the Nesprin-1α isoform of 115 kD, which contains its own 5’UTR, the last seven spectrin repeats, and the KASH domain [9]. Multiple labs have made mouse lines with Nesprin-1 mutations. Deletion of the KASH domain or a spectrin-repeat near the C-terminus of Nesprin-1 lead to nuclear positioning defects in skeletal muscles [10–12]. In contrast, mice with deletions in the N-terminal actin-binding domains of Nesprin-1 have no obvious muscle defects [13], suggesting that the C-terminal parts of Nesprin-1 are sufficient for nuclear positioning in muscles. A mouse was recently generated that specifically knocked out the 5’UTR of Nesprin-1α, presumably leaving other isoforms in tact. This mouse phenocopied the skeletal muscle nuclear positioning defect in Nesprin-1 KASH deletion mice, suggesting that the Nesprin-1α isoform mediates nuclear positioning in developing skeletal muscle [13]. Gimpel et al [7] therefore focus on the Nesprin-1α isoform. They first confirmed the recent results of others and showed that shortly after myoblasts are induced to fuse together and form myotubes, the Nesprin-1α isoform is turned on, highly expressed, and localized to the nuclear envelope [7,13–15]. Thus, Nesprin-1α is expressed at the correct time and place to mediate nuclear positioning in myotubes.
To elucidate the mechanism for how Nesprin-1α mediates nuclear spreading in myotubes, Gimpel et al [7] searched for potential Nesprin-1α binding patterns using a proximity assay called BioID [16]. They identified four centrosomal proteins, including pericentrin and Akap450, both of which have been shown to recruit the γ-tubulin ring complex to nucleate microtubules [17]. In support of the BioID assays, Nesprin-1α, pericentrin, and Akap450 co-localized, even using super-resolution microscopy. These findings led to a working model where Nesprin-1α recruits pericentriolar material to the cytoplasmic surface of the nuclear envelope to nucleate microtubules.
To test their model, Gimpel et al [7] examined the genetic requirement of Nesprin-1α to recruit pericentrin and Akap450 to the nuclear envelope. siRNA knockdown of either Nesprin-1 or both SUN partners led to a significant decrease of pericentrin at the nuclear envelope in cultured myotubes from two sources: primary mouse myoblasts or differentiated C2C12 cells. Similarly, both pericentrin and Akap450 failed to localize to the nuclear envelope of differentiated myoblasts when Nesprin-1 was knocked out of C2C12 cells by CRISPR. As a useful tool, the authors induced expression of Nesprin-1α in Nesprin-1 CRISPR C2C12 mutant cells after differentiation into myotubes and were able to then recruit pericentrin and Akap450. Furthermore, ectopic expression of Nesprin-1α in undifferentiated myoblasts was also sufficient to target peri-centriolar components to the nuclear envelope of some cells. One nice aspect of this study is that the authors confirmed their findings in differentiated myotubes from a clinical patient with a homozygous mutation in SYNE1 that truncates Nesprin-1α, causing muscular dystrophy [15]. Pericentrin, Akap450, and Pcm1 all failed to localize to the nuclear envelope in differentiated myotubes from the SYNE1 patient. Thus, Nesprin-1α is necessary and sufficient to recruit microtubule-nucleating proteins to the nuclear envelope in differentiating myoblasts.
The research next focused on determining whether disrupting the localization of peri-centriolar material to the nuclear envelope affected nuclear spreading. In both diseased patient and C2C12 cultured myotubes treated with siRNA against Nesprin-1 or Akap450, nuclei failed to spread efficiently. Thus, disrupting the MTOC at the nuclear envelope causes a nuclear localization effect. These results led to a microtubule-nucleation model for nuclear spreading.
To test the microtubule-nucleating activity of the myotube nuclear envelope, Gimpel et al [7] examined microtubule regrowth after depolymerization by nocodazole. Within five minutes of washing out the drug, microtubules rapidly grew from around the whole nuclear envelope of wild-type differentiating myotube nuclei. This regrowth failed in C2C12 cells depleted for Nesprin-1 or both SUN partners by siRNA and in SYNE1 mutant patient cells. These treatments did not disrupt longitudinal microtubules, suggesting that small puncta throughout the cytoplasm, as seen by pericentrin staining, served as MTOCs in the absence of Nesprin-1. Furthermore, the authors showed that Akap450, but not pericentrin, is the major player required for microtubule nucleation at the nuclear envelope. Thus, Nesprin-1α recruits pericentriolar material to the nuclear envelope, including Akap450 that functions through the γ-tubulin ring complex to nucleate microtubules.
How does the microtubule-nucleation model fit into other characterized mechanisms for nuclear spreading? Previously, two models were proposed to spread myotube nuclei apart. The first is a microtubule-sliding model. The Gomes and Baylies labs showed that the microtubule-associated protein MAP7/ensconsin crosslinks a microtubule to a tail of kinesin-1. Kinesin then reaches toward a second, anti-parallel microtubule and slides the two apart, resulting in nuclear spreading [18]. Since this functions at some distance from nuclei, it is likely that the microtubule-sliding model is compatible with the nuclear envelope microtubule-nucleation model. The additional model is the cargo model. The Holzbaur lab showed that Nesprin-1 and Nesprin-2 recruit kinesin-1 to cargo, the nucleus, through their LEWD motifs that binds directly to kinesin light chains [19]. This model is distinct from the microtubule-nucleation model because mutating the LEWD motif in Nesprin-1α did not disrupt the microtubule-nucleation activity of Nesprin-1α [7].
To further determine the relative contribution of microtubule nucleation vs. the cargo model, Gimpel et al [7] developed a computational model for nuclear spreading in myotubes. The model included known parameters for microtubules, MTOCs on the surface of nuclei as well as in smaller puncta away from nuclei, kinesin-1 and dynein motors on the surface of nuclei, and MAP7/kinesin-1 complexes for microtubule sliding. This model faithfully reproduced in silico simulations of nuclear spreading dynamics. When the microtubule nucleation activity of the nuclear envelope was removed from the model, nuclei were significantly less well spaced. When both microtubule-nucleation activity and kinesin-1 were removed from the nuclear envelope, nuclei became more clustered, suggesting these two mechanisms operate in parallel pathways and both are needed to fully position nuclei in myotubes.
In summary, this report [7] implicates a role for microtubule nucleation at the surface of the nuclear envelope for spreading nuclei in differentiating myotubes. Major questions remain to characterize the role of microtubule nucleation in the other nuclear movements throughout muscle development, including moving nuclei to the periphery of the myofiber [3]. The final major question is to understand how nuclear positioning defects relate to pathologies of human diseases. Since nuclear positioning defects are correlated with diseased or damaged muscles, it is expected that elucidating mechanisms for nuclear positioning will lead to future translational research for the treatment of human muscular diseases.
Figure 1. Nuclear spreading in developing myotubes.
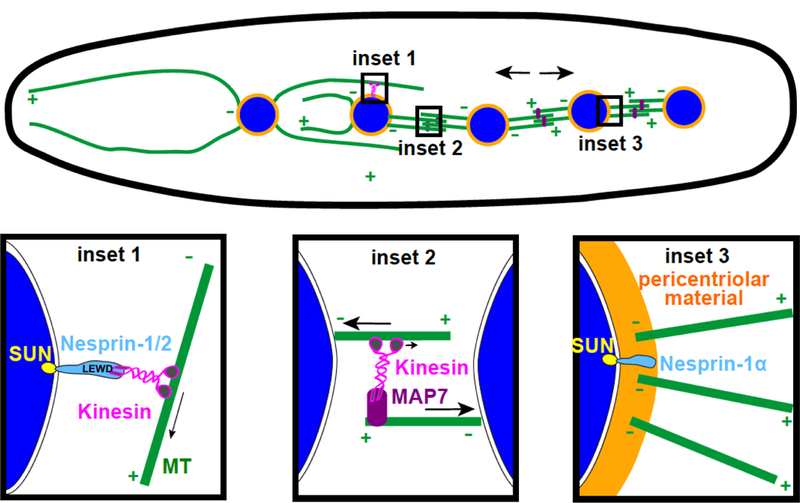
Nuclei (blue) spread apart in a developing myotube using three mechanisms. Nesprin-1/–2 functions as a cargo adaptor for kinesin-1 (inset 1). MAP7 and kinesin-1 slide antiparallel microtubules apart (inset 2). Nesprin-1α recruits peri-centriolar material to the nuclear envelope, which then nucleate microtubules (inset 3).
References
- [1].Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, et al. Nesprin-1 and −2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Human Molecular Genetics 2007;16:2816–33. doi:10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- [2].Romero NB. Centronuclear myopathies: a widening concept. Neuromuscul Disord 2010;20:223–8. doi:10.1016/j.nmd.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [3].Bone CR, Starr DA. Nuclear migration events throughout development. J Cell Sci 2016;129:1951–61. doi:10.1242/jcs.179788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bugnard E, Zaal KJM, Ralston E. Reorganization of microtubule nucleation during muscle differentiation. Cytoskeleton 2005;60:1–13. doi:10.1002/cm.20042. [DOI] [PubMed] [Google Scholar]
- [5].Tassin AM, Maro B, Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J Cell Biol 1985;100:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sanchez AD, Feldman JL. ScienceDirect Microtubule-organizing centers: from the centrosome to non-centrosomal sites. Current Opinion in Cell Biology 2016:1–9. doi:10.1016/j.ceb.2016.09.003. [DOI] [PMC free article] [PubMed]
- [7].Gimpel. Nesprin-1α-dependent microtubule nucleation from the nuclear envelope via Akap450 is necessary for nuclear positioning in muscle cells 2017:1–54. [DOI] [PMC free article] [PubMed]
- [8].Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 2010;26:421–44. doi:10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rajgor D, Shanahan CM. Nesprins: from the nuclear envelope and beyond. Expert Rev Mol Med 2013;15:e5. doi:10.1017/erm.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, et al. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development 2007;134:901–8. doi:10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- [11].Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Human Molecular Genetics 2009;18:607–20. doi:10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang J, Felder A, Liu Y, Guo LT, Lange S, Dalton ND, et al. Nesprin 1 is critical for nuclear positioning and anchorage. Human Molecular Genetics 2010;19:329–41. doi:10.1093/hmg/ddp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stroud MJ, Feng W, Zhang J, Veevers J, Fang X, Gerace L, et al. Nesprin 1α2 is essential for mouse postnatal viability and nuclear positioning in skeletal muscle. J Cell Biol 2017;115:jcb.201612128. doi:10.1083/jcb.201612128. [DOI] [PMC free article] [PubMed]
- [14].Espigat-Georger A, Dyachuk V, Chemin C, Emorine L, Merdes A. Nuclear alignment in myotubes requires centrosome proteins recruited by nesprin-1. J Cell Sci 2016;129:4227–37. doi:10.1242/jcs.191767. [DOI] [PubMed] [Google Scholar]
- [15].Holt I, Duong NT, Zhang Q, Lam Le Thanh, Sewry CA, Mamchaoui K, et al. Specific localization of nesprin-1-α2, the short isoform of nesprin-1 with a KASH domain, in developing, fetal and regenerating muscle, using a new monoclonal antibody. BMC Cell Biol 2016;17:26. doi:10.1186/s12860-016-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roux KJ, Kim DI, Burke B. BioID: A Screen for Protein-Protein Interactions. Curr Protoc Protein Sci 2013;74:19.23.1–19.23.14. doi:10.1002/0471140864.ps1923s74. [DOI] [PubMed] [Google Scholar]
- [17].Lin T-C, Neuner A, Schiebel E. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends in Cell Biology 2015;25:296–307. doi:10.1016/j.tcb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- [18].Metzger T, Gache V, Xu M, Cadot B, Folker ES, Richardson BE, et al. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature 2012;484:120–4. doi:10.1038/nature10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilson MH, Holzbaur ELF. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 2015;142:218–28. doi:10.1242/dev.114769. [DOI] [PMC free article] [PubMed] [Google Scholar]


