Abstract
Stimulation of the mu-opioid receptor (MOR) on nociceptors with fentanyl can produce hyperalgesia (opioid-induced hyperalgesia, OIH) and hyperalgesic priming, a model of transition to chronic pain. We investigated if local and systemic administration of biased MOR agonists (PZM21 and TRV130), which preferentially activate G-protein over β-arrestin translocation, and have been reported to minimize some opioid side effects, also produces OIH and priming. Injected intradermally (100 ng), both biased agonists induced mechanical hyperalgesia and, when injected at the same site, 5 days later, prostaglandin E2 (PGE2) produced prolonged hyperalgesia (priming). OIH and priming were both prevented by intrathecal treatment with an oligodeoxynucleotide (ODN) antisense (AS) for MOR mRNA. Agents that reverse Type I (the protein translation inhibitor cordycepin) and Type II (combination of Src and mitogen-activated protein kinase [MAPK] inhibitors) priming, or their combination, did not reverse priming-induced by local administration of PZM21 or TRV130. While systemic PZM21 at higher doses (1 and 10 mg/kg) induced analgesia, lower doses (0.001, 0.01, 0.1, and 0.3 mg/kg) induced hyperalgesia; all doses induced priming. Hyperalgesia, analgesia and priming induced by systemic administration of PZM21 were also prevented by MOR AS-ODN. And, priming induced by systemic PZM21 was also not reversed by intradermal cordycepin or the combination of Src and MAPK inhibitors. Thus, maintenance of priming induced by biased MOR agonists, in the peripheral terminal of nociceptors, have a novel mechanism.
Keywords: hyperalgesic priming, hyperalgesia, mu-opioid receptor (MOR), opioid-induced hyperalgesia (OIH), biased agonist
Introduction
We previously demonstrated that the injection of DAMGO [(D-Ala2,N-MePhe4,Gly5-ol)-enkephalin], a potent highly selective mu-opioid receptor (MOR) agonist, at the site of nociceptive testing on the dorsum of the hind paw, can produce a decrease in nociceptive threshold, opioid-induced hyperalgesia (OIH) and, hyperalgesic priming (Type II), a model of transition to chronic pain (Araldi et al., 2015, 2017, 2018a). More recently, we have shown that local and systemic administration of fentanyl, a potent clinical MOR agonist, induces OIH and priming in nociceptors, at both the central (Type II priming) and peripheral (Type I priming) terminals (Araldi et al., 2018b).
The analgesia produced by clinical opioids is mediated by their action on MOR (Martin, 1983; Al-Hasani and Bruchas, 2011), an inhibitory (Gi) G-protein-coupled receptor (GPCR). The internalization of GPCRs, including MOR, as a means of receptor regulation, has been associated with conditions such as hyperalgesia (OIH), tolerance, addiction, constipation, and respiratory suppression induced by opioids (Alvarez et al., 2002; Bohn et al., 2004; Connor et al., 2004; Gainetdinov et al., 2004; Raehal et al., 2005; Morgan and Christie, 2011; Bao et al., 2018). While opioid agonists (e.g., DAMGO, fentanyl and morphine) stimulate MOR phosphorylation, βarrestin recruitment, and receptor internalization, the regulatory processes involved can vary, depending on the ligand binding to the receptor. For example, while β-arrestins are required for receptor internalization, only β-arrestin-2 is required for MOR internalization induced by morphine, whereas both β-arrestin-1 and −2 are required in DAMGO-induced MOR internalization (Bohn et al., 2000; Groer et al., 2011).
Based on the finding that β-arrestin-2 has been implicated in clinically important dose limiting adverse effects of opioid analgesics (e.g., respiratory depression, constipation, and dependence), biased MOR agonists are currently being developed that activate the G-protein signaling pathway much more than β-arrestin-2 recruitment (DeWire et al., 2013; Manglik et al., 2016). They would be expected to extend the therapeutic window, increasing the safety of opioid analgesics (DeWire et al., 2013; Manglik et al., 2016). In this study we evaluated if systemic or local administration of two well characterized biased MOR agonists, PZM21 and TRV130 (DeWire et al., 2013; Manglik et al., 2016), induce OIH or hyperalgesic priming.
Material and Methods
Animals
Experiments were performed on 240–400 g adult male Sprague–Dawley rats (Charles River Laboratories, Hollister, CA, USA). Rats were housed three per cage, under a 12-hour light/dark cycle, in a temperature- and humidity-controlled animal care facility at the University of California, San Francisco (UCSF). Food and water were available ad libitum. Nociceptive testing was performed between 9:30 A.M. and 5:30 P.M.. Experimental protocols were approved by the UCSF Institutional Animal Care and Use Committee and followed the National Institutes of Health Guide for the care and use of laboratory animals. Effort was made to minimize the number of animals used and their suffering.
Measuring mechanical nociceptive threshold
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter® (Randall-Selitto paw-withdrawal test, Stoelting, Chicago, IL, USA), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw, as previously described (Taiwo et al., 1989; Taiwo and Levine, 1989; Araldi et al., 2015; Ferrari and Levine, 2015; Araldi et al., 2017). Rats were placed in cylindrical acrylic restrainers designed to provide ventilation, allow extension of the hind leg from its lateral ports in the cylinder during the assessment of nociceptive threshold, and minimize restraint stress. To acclimatize rats to the testing procedure, they were placed in restrainers for 1 hour prior to starting each training session (3 consecutive days of training) and for 30 minutes prior to experimental manipulations. The nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw. Baseline mechanical nociceptive threshold for withdrawal was defined as the mean of the three readings taken before test agents were injected. Only one paw per rat was used. Each experiment was performed on a different group of rats. Individuals performing the behavioral experiments were blinded to experimental treatments.
Drugs and routes of administration
In this study, the following drugs were used: cordycepin 5′-triphosphate sodium salt (a protein translation inhibitor), prostaglandin-E2 (PGE2, a direct-acting hyperalgesic agent that sensitizes nociceptors), SU6656 (a Src family kinase inhibitor), and U0126 (a MAPK/ERK inhibitor), all from Sigma-Aldrich (St. Louis, MO, USA). PZM21 (a biased MOR agonist) was obtained from MedChemExpress (Monmouth Junction, NJ, USA), and TRV130, another biased MOR agonist was from AdooQ BioScience (Irvine, CA, USA).
The stock solution of PGE2 (1 μg/μL) was prepared in 10% ethanol and additional dilutions made with physiological saline (0.9% NaCl), yielding a final ethanol concentration <1%. Cordycepin was dissolved in saline. All other drugs were dissolved in 100% DMSO (SigmaAldrich) and further diluted in saline containing 1% DMSO. The final concentration of DMSO was ~2%.
Intradermal drug administration was performed on the dorsum of the hind paw, using a 30-gauge hypodermic needle adapted to a 50 μL Hamilton syringe by a segment of PE-10 polyethylene tubing (Becton Dickinson, Franklin Lakes, NJ, USA). The combination of SU6656 and U0126 was diluted to a concentration of 1 μg/2 μL each; the combination of cordycepin, SU6656, and U0126, was also diluted to a concentration of 1 μg/2 μL. All combinations of drugs were injected separated by an air bubble, to avoid mixing in the syringe. The intradermal administration of all drugs, except PGE2, PZM21, and TRV130, was preceded by distilled water to produce a hypotonic shock, to facilitate the permeability of the cell membrane to these agents (1 μL of distilled water separated by an air bubble, to avoid mixing in the same syringe), to enhance entry into the nerve terminal (Borle and Snowdowne, 1982; Burch and Axelrod, 1987). Importantly, control in vivo experiments have previously shown that the final concentration of ethanol (2%), used to prepare the solution of PGE2, had no effect on the mechanical threshold per se; saline containing 1% DMSO, used to dissolve PZM21, TRV130, SU6656 and U0126 did not produce an effect on the mechanical nociceptor threshold (Ferrari et al., 2016; Araldi et al., 2017).
Subcutaneous administration of PZM21
We also evaluated the effect of systemic (subcutaneous, s.c.) administration of PZM21, which was injected at the nape of the neck. Rats received an injection of PZM21 (0.001, 0.01, 0.1, 0.3, 1, or 10 mg/kg, s.c.) and mechanical nociceptive threshold evaluated 15, 30, 60 and 120 minutes later. PZM21 was dissolved in 100% DMSO and further diluted in saline containing 1% DMSO. The final concentration of DMSO was ~2%. Vehicle (saline containing 1% DMSO) and PZM21 were administered subcutaneously (100 μL/100 g body weight).
MOR antisense
To investigate the role of MOR in the hyperalgesia and priming induced by biased MOR agonists, an oligodeoxynucleotide (ODN) antisense (AS) for MOR mRNA (Khasar et al., 1996; Sanchez-Blazquez et al., 1997; Araldi et al., 2017; Araldi et al., 2018b) was used. The AS-ODN sequence for MOR, 5′-CGC-CCC-AGC-CTC-TTC-CTC-T-3’, was directed against a unique region of rat MOR (UniProtKB database entry P33535 [OPRM_RAT] antisense sequence to block translation and downregulate the gene expression of all 8 known isoforms [MOR]). The ODN mismatch (MM) sequence, 5′-CGC-CCC-GAC-CTC-TTC-CCT-T-3′ for MOR, was a scrambled version of the antisense sequence that has the same base pairs and GC ratio, with little or no homology to any mRNA sequences posted at GeneBank, with 4 mismatched bases (denoted by bold letters). A nucleotide BLAST search was performed to confirm that the mRNA sequence targeted by the AS-ODN, or its MM-ODN control, were not homologous to other sequences in the rat database. The oligodeoxynucleotides were synthesized by Invitrogen Life Technologies (Carlsbad, CA, USA).
Lyophilized ODNs were reconstituted in nuclease-free 0.9% NaCl and then administered intrathecally at a dose of 6 μg/μL in a volume of 20 μL (120 μg/20 μL). MM- or AS-ODNs were injected daily for 3 consecutive days, and on the 4th day, approximately 17 hours after the last injection of ODNs, MOR biased agonists (100 ng, intradermally [PZM21 or TRV130]; or 0.01 and 1 mg/kg of PZM21, systemically) were injected intradermally (5 μL) or systemically (100 μL/100 g body weight) and the mechanical nociceptive threshold evaluated 30 min later for both intradermal and systemic treatments. At the end of the 4th day, rats again received MOR MM- or AS-ODN. Twenty-four hours after the injection of a biased MOR agonist, PGE2 (100 ng/5 μL) was injected intradermally and mechanical nociceptive threshold evaluated 30 minutes and 4 hours later. As described previously (Alessandri-Haber et al., 2003), rats were anesthetized with isoflurane (2.5% in O2), and then ODN injected using a micro syringe (300 μL) with a 29-gauge needle, inserted into the subarachnoid space, between the L4 and L5 vertebrae. A total of 120 μg of ODN, in a volume of 20 μL, was then injected intrathecally. When anesthesia was turned off, rats regained consciousness, approximately 2 minutes after the intrathecal injection. Use of AS-ODN to attenuate the expression of proteins that play a crucial role in nociceptor sensitization, is well supported by studies from our group and others (Song et al., 2009; Su et al., 2011; Bogen et al., 2012; Quanhong et al., 2012; Sun et al., 2013; Araldi et al., 2015, 2016b; Ferrari et al., 2016; Araldi et al., 2017; Oliveira-Fusaro et al., 2017; Araldi et al., 2018a; Araldi et al., 2018b).
Statistical Analysis
Our data are presented as mean ± SEM of n independent observations; only 1 paw per rat was used in an experimental group. Statistical comparisons were made using GraphPad Prism 7.04 statistical software (GraphPad Software). A p-value < 0.05 was considered statistically significant. In our experiments, the dependent variable was change in mechanical pawwithdrawal threshold, expressed as percentage change from baseline. No significant difference in mechanical nociceptive threshold was observed before the injection of a biased MOR agonist and immediately before injection of PGE2 (average mechanical nociceptive threshold before biased MOR agonists [priming stimuli]: 139.02 ± 1.133 g; average mechanical nociceptive threshold before PGE2 injection: 138.93 ± 1.131 g; n = 234 rats; paired Student’s t test, t(233) = 0.2265, p = 0.8211). As specified in the figure legends, Student’s t test, one or two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, was performed to compare the magnitude of the hyperalgesia induced by MOR biased agonists or PGE2 injection in the different groups, or to compare the effect produced by different treatments on the prolongation of the PGE2-induced hyperalgesia (evaluated 4 hours after injection) with the control/vehicle groups.
Results
Intradermal biased MOR agonist induces hyperalgesia
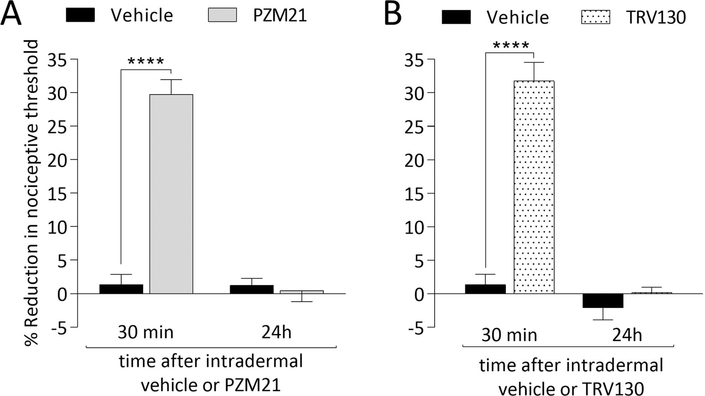
To verify if biased MOR agonists affect mechanical nociceptive threshold, we injected PZM21 and TRV130 intradermally, on the dorsum of the rat’s hind paw. PZM21 (Fig. 1A) and TRV130 (Fig. 1B), both 100 ng, decreased mechanical nociceptive threshold (hyperalgesia). Local administration of PZM21 or TRV130 did not, however, induce change in the nociceptive threshold in the contralateral hind paw (data not shown).
Figure 1. Mechanical hyperalgesia induced by intradermal administration of biased MOR agonists.
Rats received an intradermal injection of vehicle (5 μL of saline containing 2% DMSO; A and B, black bars), PZM21 (100 ng/5 μL; A, gray bars) or TRV130 (100 ng/5 μL; B, dotted bars), and mechanical nociceptive threshold was evaluated 30 min and 24 hours later. In both PZM21- and TRV130-treated groups a decrease in the mechanical nociceptive threshold was observed 30 min after their intradermal injection (F(1,10) = 148.1, *** p < 0.0001 [A]; F (1,10) = 84.56, **** p < 0.0001 [B], when vehicle-treated groups are compared with the PZM21- or TRV130-treated groups at 30 min after injection; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). By 24 hours after intradermal vehicle, PZM21 and TRV130 mechanical nociceptive threshold had returned to pre-treatment baseline. (n = 6 paws per group).
Intradermal biased MOR agonist induces prolongation of PGE2 hyperalgesia
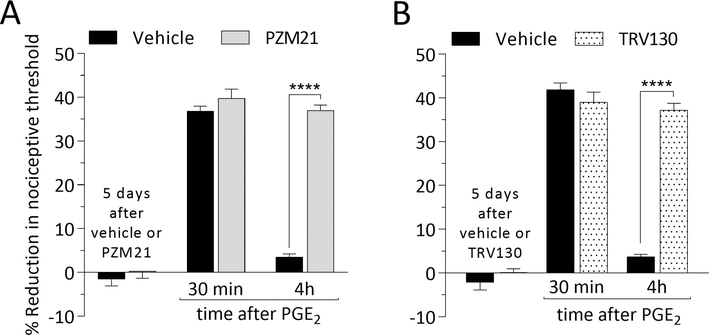
Five days after intradermal injection of PZM21 or TRV130, PGE2 was injected intradermally, at the same site, and the mechanical nociceptive threshold evaluated 30 min and 4 hours later. In groups treated with PZM21 (Fig. 2A) or TRV130 (Fig. 2B), hyperalgesia induced by intradermal PGE2 was prolonged, compatible with the presence of hyperalgesic priming (Joseph and Levine, 2010; Ferrari et al., 2013; Araldi et al., 2015; Ferrari et al., 2015; Kandasamy and Price, 2015; Araldi et al., 2017; Araldi et al., 2018b). Of note, when injected in the paw contralateral to the paw previously treated with PZM21 or TRV130, the hyperalgesia induced by PGE2 was not prolonged (data not shown).
Figure 2. Hyperalgesic priming induced by biased MOR agonists.
Rats were treated intradermally with vehicle (5 μL; A and B, black bars), PZM21 (100 ng/5 μL; A, gray bars) or TRV130 (100 ng/5 μL; B, dotted bars). Five days later, when the mechanical nociceptive threshold was not different from the pre-vehicle/agonist baselines (A: t(5) = 0.6984; p = 0.5160, for the vehicle-treated group and, t(5) = 0.2104; p = 0.8417, for the PZM21-treated group; B: t(5)= 0.4385; p = 0.6793, for the vehicle-treated group, and t(5) = 0.9068; p = 0.4061, for the TRV130-treated group, when the mechanical nociceptive threshold is compared before and after treatments; paired Student’s t test), PGE2 (100 ng/5 μL) was injected intradermally and the mechanical nociceptive threshold evaluated 30 min and 4 hours later. Measured 30 min after its injection, PGE2-induced hyperalgesia was present in all biased MOR agonist-treated groups. However, in the groups treated with PZM21 (A) and TRV130 (B), but not in the vehicle-treated group, PGE2 induced prolonged hyperalgesia, observed at the fourth hour after its injection (A: F(1,10) = 107.4, **** p < 0.0001; B: F(1,10) = 58.15, **** p < 0.0001; when vehicle-treated groups are compared with the PZM21- or TRV130-treated groups at the fourth hour after the injection of PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These findings support the suggestion that local/intradermal injection of biased MOR agonists induce hyperalgesic priming in the peripheral terminal of the nociceptor. (n = 6 paws per group)
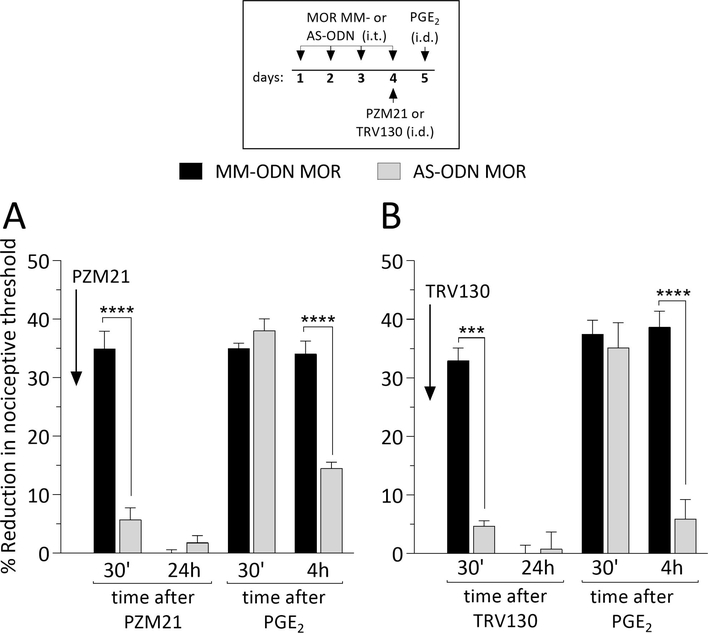
MOR-dependence of intradermal biased MOR agonist induces hyperalgesia and priming
To determine if the hyperalgesia and priming induced by biased MOR agonists, administered intradermally, is mediated by their action at MOR, on nociceptors, we evaluated whether MOR AS-ODN would attenuate the induction of hyperalgesia and priming by biased MOR agonists. MM- or AS-ODN against MOR mRNA was administered intrathecally, daily for 3 days. On the fourth day, PZM21 (Fig. 3A) or TRV130 (Fig. 3B) was injected intradermally on the dorsum of the hind paw and, the mechanical nociceptive threshold evaluated 30 min later. In the group treated with MOR AS-ODN, intradermal PZM21 or TRV130 was not able to induce hyperalgesia, when compared to MOR MM-ODN-treated group. Twenty-four hours later, PGE2 was injected intradermally at the same site as PZM21 and TRV130. Mechanical nociceptive threshold was evaluated 30 min and 4 hours later. Treatment with MOR AS-ODN markedly attenuated the prolongation of PGE2-induced hyperalgesia in both the PZM21- (Fig. 3A) and TRV130- (Fig. 3B) treated groups. Thus, both hyperalgesia and priming, induced by intradermal biased MOR agonists are MOR dependent.
Figure 3. MOR dependence of OIH and priming induced by biased agonists.
Rats were treated intrathecally with oligodeoxynucleotides (ODN) mismatch (MM-ODN; 120 μg/20 μL/day; black bars; A and B) or antisense (AS-ODN; 120 μg/20 μL/day, gray bars; A and B) against MOR mRNA, once a day, for 3 consecutive days. On the fourth day, approximately 17 hours after the last intrathecal administration of ODNs, PZM21 (100 ng/5 μL; A) or TRV130 (100 ng/5 μL; B) was injected intradermally, on the dorsum of the hind paw, and mechanical nociceptive threshold evaluated 30 min after injection. In MOR AS-ODN-treated groups, intradermal injection of PZM21 and TRV130 did not induce hyperalgesia, as observed in the MOR MM-ODN-treated groups (A: t(10) = 8.18, **** p < 0.0001; B: t(10) = 12.02, **** p = 0.0003; when the hyperalgesia in the MOR MM-ODN-treated group is compared to MOR AS-ODNtreated group 30 min after intradermal PZM21 or TRV130; unpaired Student’s t test). At the end of the 4th day, rats again received MOR MM- or AS-ODN. On the 5th day, approximately 24 hours after intradermal administration of PZM21 and TRV130, when the mechanical nociceptive threshold was not different from pre-MOR agonist baselines (A: t(5) = 0.3953; p = 0.7089, for the MOR MM-ODN-treated group and, t(5) = 1.472; p = 0.2009, for the MOR AS-ODN-treated group; B: t(5)= 0.237; p = 0.8220, for the MOR MM-ODN-treated group, and t(5) = 1.328; p = 0.2414, for the MOR AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and after biased MOR agonists; paired Student’s t test), PGE2 (100 ng/5 μL) was injected intradermally, and the mechanical nociceptive threshold evaluated 30 min and 4 hours later. In the group treated with MOR AS-ODN, which received intradermal PZM21, the prolongation of PGE2-induced hyperalgesia was markedly attenuated (A; F(1,10) = 47.62, **** p < 0.0001; when the hyperalgesia in the MM-ODN- and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test); however, the prolongation of PGE2-induced hyperalgesia was prevented in the MOR ASODN-treated group, which received intradermal TRV130 (B; F(1,10) = 64.88, **** p < 0.0001; when the hyperalgesia in the MM-ODN- and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These findings indicate that both OIH and priming, induced by intradermal injection of biased agonists, are MOR dependent. (n = 6 paws per group)
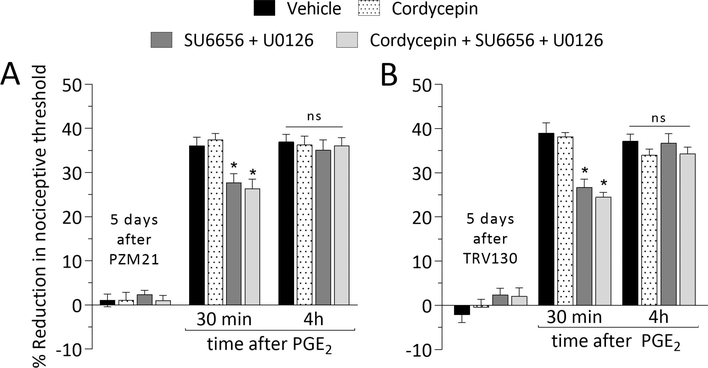
Hyperalgesic priming type
The maintenance mechanism of Type I priming is dependent on protein translation in the peripheral and central terminals of the nociceptor, being reversed by local administration of the protein translation inhibitor cordycepin (Ferrari et al., 2013). On the other hand, the maintenance of Type II priming is dependent on the simultaneous activation of Src and MAP kinases in the nociceptor terminals (Araldi et al., 2017; Araldi et al., 2018b). Five days after intradermal administration of PZM21 or TRV130, rats received, at the same site, vehicle, cordycepin, the combination of Src and MAPK inhibitors, or the combination of cordycepin, and Src and MAPK inhibitors. In all groups, prolongation of the hyperalgesia induced by PGE2 was observed (Fig. 4A and B), supporting the suggestion that biased MOR agonists induce a type of priming maintained by a mechanism different from that induced by signaling pathways involved in maintenance of Type I and II priming (Ferrari et al., 2013; Araldi et al., 2017; Araldi et al., 2018b). Of note, PGE2-induced hyperalgesia at 30 min was partially attenuated in the groups treated with the combination of Src and MAPK inhibitors, which was already demonstrated by us in a previous study for MOR agonist DAMGO (Araldi et al., 2017).
Figure 4. Type of priming induced by biased MOR agonists.
Rats received an intradermal injection of PZM21 (100 ng/5 μL; A) or TRV130 (100 ng/5 μL; B). Five days later, vehicle (5 μL; black bars), cordycepin (1 μg/5 μL; dotted bars), the combination (dark gray bars) of SU6656 (1 μg/2 μL) + U0126 (1 μg/2 μL), or the combination (light gray bars) of cordycepin (1 μg/2 μL) + SU6656 (1 μg/2 μL) + U0126 (1 μg/2 μL) were injected intradermally, followed 10 min later, by PGE2 (100 ng/5 μL), injected at the same site, on the dorsum of the hind paw. PGE2 induced hyperalgesia at 30 min after injection, was attenuated in groups treated with SU6656 + U0126 and cordycepin + SU6656 + U0126 (A: F(1,10) = 4.635, * p = 0.0128; B: F(1,10) = 3.132, * p = 0.0485; when the hyperalgesia in vehicle-, SU6656 + U0126-, and cordycepin + SU6656 + U0126-treated groups is compared 30 min after intradermal PGE2; two-way repeatedmeasures ANOVA followed by Bonferroni post hoc test). However, in all groups, PGE2 induced prolonged hyperalgesia, detected at the 4th hour after its injection (A: F(1,10) = 0.025, p = 0.9944; B: F(1,10) = 1.535, p = 0.1224; when the hyperalgesia in all treated-groups is compared at the 4th hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These data indicate that PZM21 and TRV130 induce a Type of priming that is not maintained by signaling pathways that maintain Type I or II priming. (n = 6 paws per group)
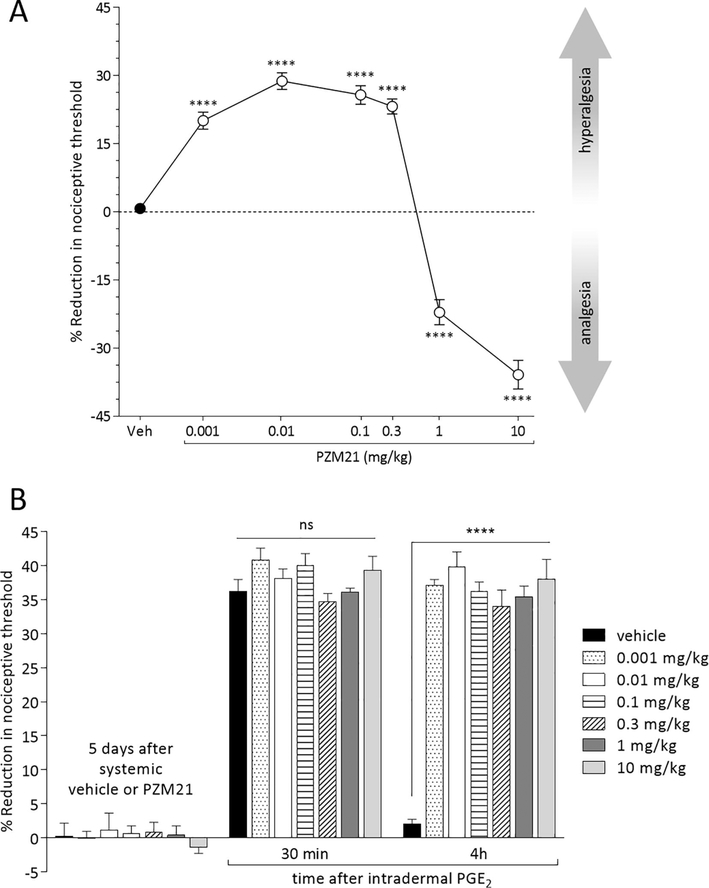
Systemic PZM21 induces dose-dependent hyperalgesia and analgesia, and priming
To study the effect of systemically administered biased MOR agonists we selected PZM21, since we did not observe a significant difference in our results between the effects of intradermally administered PZM21 and TRV130. Mechanical nociceptive threshold was evaluated 30 min after systemic (s.c.) injection of vehicle (saline containing 2% DMSO) or PZM21 (0.001, 0.01, 0.1, 0.3, 1 or 10 mg/kg; administered in a volume of 100 μL/100 g body weight). The doses of 0.001, 0.01, 0.1 and 0.3 mg/kg, injected systemically, induced hyperalgesia, detected at 30 min (Fig. 5A), returning to pre-PZM21 baseline at 60 min (data not shown). In contrast, systemic PZM21 at the doses of 1 and 10 mg/kg, induced an increase in the mechanical nociceptive threshold (analgesia), detected 30 min after its administration (Fig. 5A), returning to the prePZM21 baseline at 120 min (data not shown). When PGE2 (100 ng, diluted in 5 μL of saline) was injected intradermally on the dorsum of the hind paw, 5 days after systemic PZM21, prolongation of PGE2-induced hyperalgesia was observed in all PZM21-treated groups (Fig. 5B).
Figure 5. Effect of systemically administered biased MOR agonist.
Groups of rats were treated subcutaneously with vehicle (veh; saline containing 2% DMSO; black circle) or PZM21 (0.001, 0.01, 0.1, 0.3, 1 or 10 mg/kg; 100 μL/100 g body weight; white circles) and mechanical nociceptive threshold evaluated 30 min later. A. Thirty min after subcutaneous injection of PZM21, all groups treated with low doses of PZM21 (0.001, 0.01, 0.1, and 0.3 mg/kg) were hyperalgesic (F(6,47) = 119.7, **** p < 0.0001; when the groups treated with low doses of PZM21 were compared to the vehicle-treated group at 30 min after s.c. injection; one-way repeated-measures ANOVA followed by Bonferroni post hoc test), while in the groups treated with the high doses (1 and 10 mg/kg) analgesia was observed (F(6,47) = 119.7, **** p < 0.0001, when the groups treated with high doses of PZM21 were compared to the vehicle-treated group at 30 min after s.c. injection; one-way repeated-measures ANOVA followed by Bonferroni post hoc test). In all groups that received s.c. PZM21, at 120 min after injection, the mechanical nociceptive threshold was not different when compared to the vehicle-treated group (data not shown; F(6,47) = 1.062, ns, p > 0.9999 for 0.001-, 0.01-, and 0.1 mg/kg-treated groups; ns, p = 0.5634 for 0.3 mg/kg-treated group; ns, p = 0.4589 for 1 mg/kg-treated group; and ns, p = 0.9256 for 10 mg/kg-treated group, when all subcutaneous PZM21-treated groups are compared to the vehicle-treated group at 120 min after injection; one-way repeated-measures ANOVA followed by Bonferroni post hoc test). B. Five days after subcutaneous administration of vehicle and PZM21, a time at which the mechanical nociceptive threshold was not different from the pre-MOR agonist baseline (t(5) = 0.9337; p = 0.3933, for the vehicle-treated group, t(5) = 0.6956; p = 0.5177, for the 0.001 mg/kg-treated group, t(5) =1.225; p = 0.2752, for the 0.01 mg/kg-treated group, t(5) = 0.2832; p = 0.7884, for the 0.1 mg/kg-treated group, t(5) = 2.15; p = 0.0842, for the 0.3 mg/kg-treated group, t(5) = 0.2225; p = 0.8327, for the 1 mg/kg-treated group, and t(5) = 0.6143; p = 0.5659, for the 10 mg/kg-treated group, when the mechanical nociceptive threshold is compared before and after subcutaneous vehicle or PZM21; paired Student’s t test), PGE2 (100 ng/5 μL) was injected intradermally, on the dorsum of the hindpaw, and mechanical nociceptive threshold evaluated 30 min and 4 h after injection. In all groups treated with subcutaneous PZM21, PGE2 induced prolonged hyperalgesia (F(2,70) = 1024.0, **** p < 0.0001, when the hyperalgesia in the systemic PZM21-treated groups is compared with the vehicle-treated group at the 4th hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). (n = 6 paws per group)
MOR dependence of systemic PZM21 induced hyperalgesia, analgesia, and priming
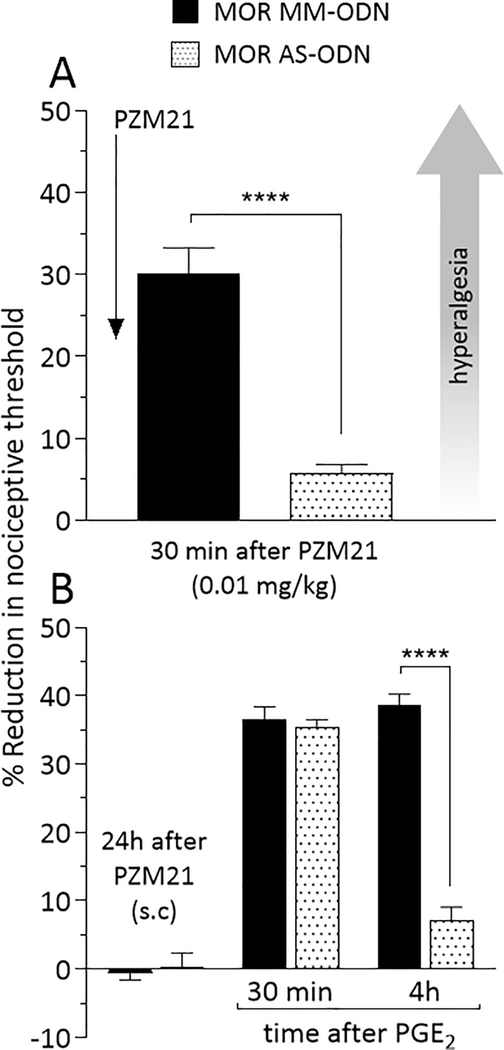
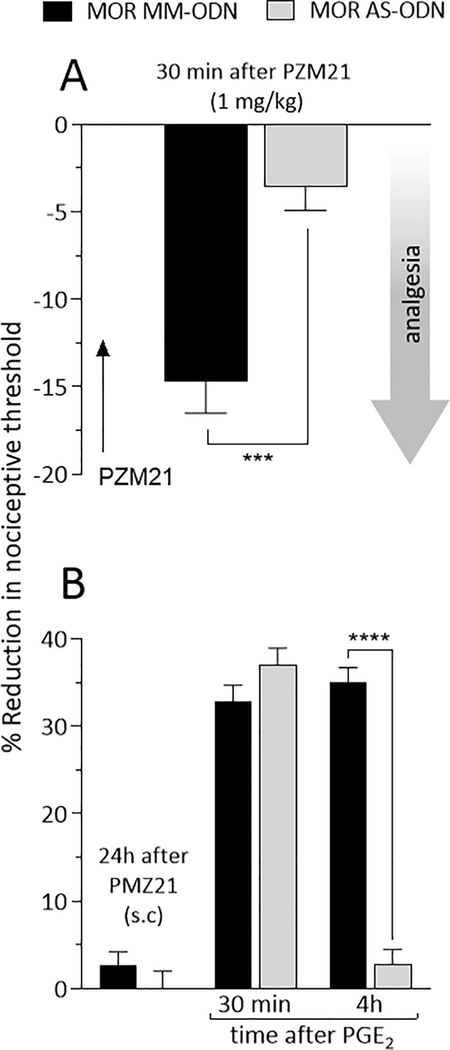
To verify if hyperalgesia, analgesia and priming induced by systemic administration of PZM21 is MOR dependent, rats were treated intrathecally, once a day, for 3 consecutive days with MOR MM- or AS-ODN. On the 4th day, approximately 17 hours after the last injection of ODNs, PZM21 was injected systemically at a dose of 0.01 mg/kg (Fig. 6A), which induces hyperalgesia, or 1 mg/kg (Fig. 7A), which induces analgesia. Mechanical nociceptive threshold was evaluated 30 min after systemic PZM21. In the group treated with MOR AS-ODN, PZM21 injected systemically at the dose of 0.01 mg/kg did not induce hyperalgesia (Fig. 6A), when compared to the MOR MM-ODN-treated group. In a different group of rats, PZM21 was injected systemically at the dose of 1 mg/kg, ~17 hours after the last intrathecal injection of MOR ODNs. PZM21-induced analgesia at the dose of 1 mg/kg, was also blocked in the group treated with MOR AS-ODN, compared to MOR MM-ODN-treated group (Fig. 7A). These findings support the suggestion that PZM21-induced hyperalgesia and analgesia, at the low and high doses, respectively, are MOR dependent.
Figure 6. MOR dependence of hyperalgesia and priming induced by systemically administered low dose PZM21.
Rats received intrathecal injections of MM-ODN (120 μg/20 μL/day; black bar) or AS-ODN (120 μg/20 μL/day; dotted bar) against MOR mRNA, daily for 3 consecutive days. A. On the fourth day, approximately 17 hours after the last ODN injection, PZM21 (0.01 mg/kg) was injected subcutaneously and the mechanical nociceptive evaluated 30 min after its injection. Subcutaneous PZM21 did not induce hyperalgesia in the group treated with AS-ODN for MOR (t(10) = 7.284, **** p < 0.0001, when the hyperalgesia in the MM-ODN- and the AS-ODN-treated groups is compared after subcutaneous PZM21; unpaired Student’s t test). At the end of the 4th day, rats received MOR MM- or AS-ODN, intrathecally. B. On the fifth day, approximately 24 hours after systemic PZM21, when the mechanical nociceptive threshold was not different from pre-PZM21 baseline (t(5) = 0.5423; p = 0.6109, for the MM-ODN-treated group, and t(5) = 0.6705; p = 0.5323, for the AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and after PZM21; paired Student’s t test), PGE2 (100 ng/5 μL) was injected intradermally and mechanical nociceptive threshold evaluated 30 min and 4 h later. Intradermal PGE2 did not induce prolonged hyperalgesia in the AS-ODN-treated group (F(1,10) = 55.86, **** p < 0.0001, when the hyperalgesia in the MM-ODN- and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), indicating that systemic low dose of PZM21-induced hyperalgesia and priming is MOR dependent. (n = 6 paws per group)
Figure 7. MOR dependence of analgesia and priming induced by high dose systemic PZM21.
Rats received intrathecal injections of MM-ODN (120 μg/20 μL/day; black bar) or AS-ODN (120 μg/20 μL/day; gray bar) against MOR mRNA, daily for 3 consecutive days. A. On the fourth day, approximately 17 hours after the last ODN injection, PZM21 (1 mg/kg) was injected subcutaneously and mechanical nociceptive evaluated 30 min after its injection. In the group treated with MOR AS-ODN, analgesia induced by subcutaneous injection of PZM21 was prevented (t(10) = 4.929, *** p = 0.0006, when the hyperalgesia in the MM-ODN- and the ASODN-treated groups is compared 30 min after subcutaneous PZM21; unpaired Student’s t test). At the end of the 4th day, rats received another dose of MOR MM- or AS-ODN. B. Approximately 24 hours after systemic PZM21 (1 mg/kg), when the mechanical nociceptive threshold was not different from pre-PZM21 baseline (t(5) =1.464; p = 0.2031, for the MM-ODN-treated group, and t(5) = 0.6956; p = 0.5177, for the AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and after PZM21; paired Student’s t test), PGE2 (100 ng/5 μL) was injected intradermally and mechanical nociceptive threshold evaluated 30 min and 4 h after injection. In the MOR AS-ODN-treated group, the prolongation of PGE2-induced hyperalgesia was prevented (F(1,10) = 101.2, **** p < 0.0001, when the hyperalgesia in the MM-ODN- and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These findings support the suggestion that analgesia and priming induced by high dose PZM21 (1 mg/kg), are both MOR dependent. (n = 6 paws per group)
All groups of rats also received MOR MM- or AS-ODN at the end of the 4th day. Twenty-four hours after systemic injection of PZM21, PGE2 was injected intradermally on the dorsum of the hind paw and, the mechanical nociceptive threshold evaluated 30 min and 4 hours later. In both 0.01 mg/kg (Fig. 6B) and 1 mg/kg (Fig. 7B) PZM21-treated groups, the prolongation of PGE2-induced hyperalgesia at the 4th hour was blocked in the group previously treated with MOR AS-ODN, when compared to the MOR MM-ODN-treated group (Fig. 6B and 7B).
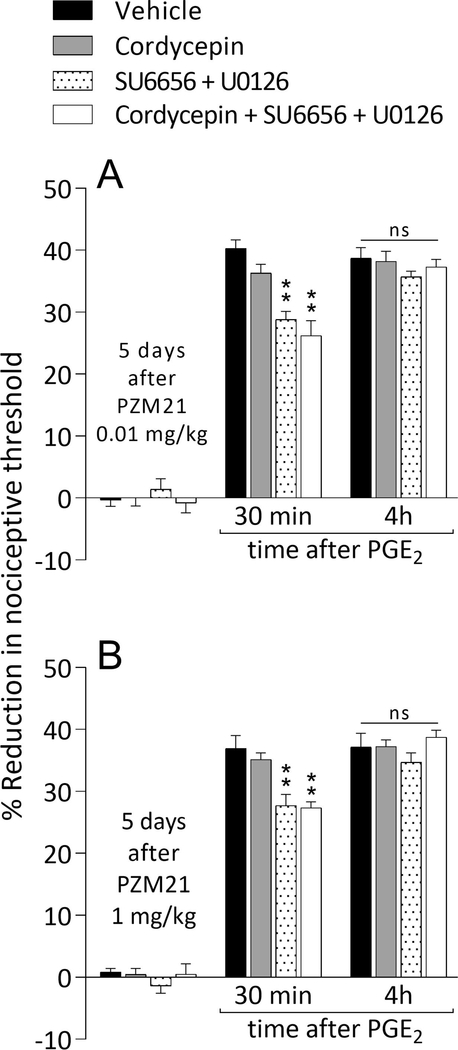
Type of priming induced by systemic PZM21
As shown previously, the maintenance of Type I priming involves protein translation at the nociceptor terminals (Ferrari et al., 2013), while maintenance of Type II priming involves the simultaneous activation of Src and MAPK, also at the terminals (Araldi et al., 2017; Araldi et al., 2018b). Five days after the systemic administration of 0.01 mg/kg (Fig. 8A) or 1 mg/kg (Fig. 8B) of PZM21, which induces hyperalgesia and analgesia, respectively, both groups of rats were treated intradermally with vehicle, cordycepin, the combination of Src and MAPK inhibitors (SU6656 + U0126, respectively), or the combination of cordycepin, SU6656, and U0126. In all groups, PGE2-induced hyperalgesia was still observed at the 4th hour (Fig. 8A and B), supporting the suggestion that biased MOR agonist-induced hyperalgesic priming is maintained by a mechanism distinct from those previously described for Types I and II priming.
Figure 8. Novel mechanism for maintenance of priming induced by systemically administered low and high dose PZM21.
Groups of rats were treated systemically with PZM21 (0.01 mg/kg [A] or 1 mg/kg [B]; 100 μL/100 g body weight). Five days later, vehicle (5 μL; black bars), cordycepin (1 μg/5 μL; gray bars), the combination (dotted bars) of SU6656 (1 μg/2 μL) + U0126 (1 μg/2 μL), or the combination (white bars) of cordycepin (1 μg/2 μL) + SU6656 (1 μg/2 μL) + U0126 (1 μg/2 μL) was injected intradermally, followed 10 min later by PGE2 (100 ng/5 μL), injected at the same site on the dorsum of the hind paw. PGE2-induced hyperalgesia, at 30 min, was attenuated in the SU6656 + U0126- and cordycepin + SU6656 + U0126-treated groups (A: F(3,20) = 5.633, ** p = 0.0058; B: F(3,20) = 6.805, ** p = 0.0024, when the hyperalgesia in the vehicle- and the inhibitors-treated groups is compared at 30 min after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). However, the prolongation of PGE2 hyperalgesia induced by a low (0.01 mg/kg, A) and a high dose (1 mg/kg, B) of PZM21 was not affected by cordycepin, the combination of SU6656 + U0126 or even by the combination of 3 inhibitors (A: ns, F(3,20) = 0.2403, p = 0.8672; B: ns, F(3,20) = 1.791, p = 0.1813, when the hyperalgesia in the vehicle- and the inhibitors-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni post hoc test), compatible with the suggestion that systemic PZM21 induces priming that does not involve signaling pathways involved in the maintenance of Type I and II priming. (n = 6 paws per group)
Discussion
Biased MOR agonists are designed to produce fewer and less severe side effects (e.g. addiction, constipation, and respiratory depression) than current clinical MOR agonists (e.g. morphine and fentanyl) (DeWire et al., 2013; Manglik et al., 2016). In the present experiments, we studied the ability of two well characterized biased MOR agonists, PZM21 and TRV130, to induce OIH and hyperalgesic priming. We found that both produce OIH and hyperalgesic priming, when injected at the peripheral terminal of the nociceptor, on the dorsum of the hind paw. When PZM21 was administered systemically, it induced hyperalgesia at low doses and analgesia at high doses, and 5 days later, when injected intradermally, PGE2 hyperalgesia was prolonged, indicating the presence of priming, in both low and high dose groups. Hyperalgesic priming, a model used by us and others (Joseph et al., 2003; Joseph and Levine, 2010; Ferrari et al., 2013; Araldi et al., 2015; Ferrari et al., 2015; Kandasamy and Price, 2015; Araldi et al., 2016a, b, 2017; Dai et al., 2017; Khomula et al., 2017; Araldi et al., 2018a; Araldi et al., 2018b; Paige et al., 2018; Wang et al., 2018) to study the transition to chronic pain, is produced in rats treated with DAMGO or fentanyl (Araldi et al., 2015, 2017, 2018a; Araldi et al., 2018b), both potent and highly selective MOR agonists. Recently, we have distinguished 2 types of hyperalgesic priming: Type I, maintained by protein translation in the peripheral and central terminals of nociceptors (Ferrari et al., 2013) and Type II, maintained by ongoing activity of Src and MAP kinases, also at the peripheral and central terminals of nociceptors (Araldi et al., 2017). When DAMGO is administered repeatedly, to the peripheral terminal of the nociceptor, it produced Type II priming (Araldi et al., 2015, 2017, 2018a). However, fentanyl when administered systemically, produces Type I priming in the peripheral terminal and Type II in the central terminal (Araldi et al., 2018b), compatible with different second messenger signaling pathways being activated by different MOR agonists.
Studies examining MOR signaling have identified β-arrestins to be key to diverse non-analgesic effects of opioids (Raehal et al., 2011; Raehal and Bohn, 2014). For instance, β-arrestin-2 contributes to opioid tolerance by binding to and desensitizing MOR to actions of opioid agonists, and β-arrestin-1 has been shown to promote ubiquitin-proteasomal degradation of MOR (Raehal et al., 2011). In mice lacking β-arrestin-2, chronic morphine did not induce tolerance, indicating that it is necessary for development of opioid tolerance (Bohn et al., 1999). Furthermore, in β-arrestin-2 knockout mice morphine also exhibited less respiratory depression, dependence and constipation, and the analgesic effect of morphine was enhanced (Raehal et al., 2011). Fentanyl exhibits a preference for β-arrestin over G-protein signaling and has been shown to cause respiratory depression even at low analgesic doses (Schmid et al., 2017). Therefore, βarrestin recruitment is associated with a narrower therapeutic window due to increased risk of adverse effects. Thus, biased MOR agonists, such as PZM21 and TRV130, which preferentially activate the G-protein signaling pathway with little or no β-arrestin-2 recruitment, might provide pain relief with lessened risks of respiratory depression, dependence, OIH and hyperalgesic priming.
The biased MOR agonist PZM21, has been reported to exhibit strong G-protein-dependent activity with no detectable β-arrestin-2 recruitment (Manglik et al., 2016). Also, PZM21 is highly specific for MOR, showing no kappa- and only weak delta-opioid receptor activation (Manglik et al., 2016). In mouse studies, PZM21 produced analgesia in a hotplate assay that assesses higher-level central nervous system and spinal nociceptive circuits, but no pain relief in the tail-flick assay, which assesses spinal reflexes (Manglik et al., 2016). These findings support the suggestion that PZM21 selectively blocks the affective component of pain, a novel distinction among opioids. Additionally, mice treated with PZM21 did not exhibit respiratory depression whereas at equi-analgesic doses morphine suppressed respiration (Manglik et al., 2016). In 2013, the biased MOR agonist TRV130, later named Oliceridine or OLINVO, was found to activate Gprotein-coupling to a similar extent compared to morphine but was 86% less efficient in recruiting β-arrestin-2 (DeWire et al., 2013). TRV130 was also found to be more potent than morphine, as an analgesic, and structurally different from other MOR agonists (DeWire et al., 2013). In a clinical trial TRV130 was reported to provide effective pain relief for patients who had undergone abdominoplasty surgery (Singla et al., 2017). These results support the hypothesis that PZM21 and TRV130 have wider therapeutic windows than currently available opioid analgesics.
Recently, we demonstrated that DAMGO and fentanyl, both potent and MOR selective unbiased opioid agonists, produce MOR dependent OIH and priming (Araldi et al., 2018a; Araldi et al., 2018b). In the present study, we observed that intradermal PZM21 and TRV130 were not able to produce hyperalgesia or prolongation of PGE2-induced hyperalgesia in rats treated intrathecally with MOR AS-ODN. Importantly, it has recently been demonstrated that MORs, expressed on primary afferent nociceptors, drive the initiation of adverse counter-adaptations to opioids responsible for the onset of OIH (Corder et al., 2017). These findings support the suggestion that biased MOR agonists, like unbiased agonists, can act at MOR on nociceptors to produce OIH and priming.
To test if the priming induced by biased MOR agonists is Type I or II priming, we used cordycepin, a protein translation inhibitor (Ferrari et al., 2013), or the combination of Src (SU6656) and MAPK (U0126) inhibitors (Araldi et al., 2017), respectively. Unexpectedly, the prolongation of PGE2 hyperalgesia induced by both PZM21 and TRV130, injected at the peripheral terminal of the nociceptor, was not inhibited by cordycepin, the combination of Src and MAPK inhibitors, or even by the combination of all three inhibitors, indicative of a novel mechanism for priming induced by biased MOR agonists, different from that previously established for Type I and II priming (Ferrari et al., 2013; Araldi et al., 2017).
The effect of the systemic administration of PZM21 was also evaluated. Similar to the effect of other opioids (van der Kooy and Nagy, 1985; Kayser et al., 1987; Crain and Shen, 2001), while systemic PZM21 at low doses (0.001, 0.01, 0.1 and 0.3 mg/kg) decreased mechanical nociceptive threshold (i.e., hyperalgesia), at high doses (1 and 10 mg/kg) it produced analgesia. Previous studies have shown that opioid agonists at doses far below those predicted to be effective as an analgesic, produce hyperalgesia (Kayser et al., 1987; Crain and Shen, 2001; Docquier et al., 2004). For example, when systemic morphine, at a dose approximately 3 orders of magnitude lower than that typically used to study antinociceptive effects, was administered in arthritic rats, it increased the sensitivity to noxious pressure in an arthritic paw (Kayser et al., 1987); a similar result was found using a thermal nociceptive test in mice (Crain and Shen, 2001). It has been suggested that the difference in the effect of ultra-low and high dose morphine may be a result of a bimodal dose-dependent effect of opioid receptor agonists in dorsal root ganglion (DRG) neurons (Shen and Crain, 1994), where opioid receptors activate an excitatory signaling cascade when exposed to very low opioid agonist concentrations, and inhibitory pathways when exposed to higher agonist concentrations (Shen and Crain, 1994).
Hyperalgesic priming was present in groups of rats treated systemically with low or high dose of PZM21 (0.01 and 1 mg/kg, respectively). When we evaluated the Type of priming induced by both doses of PZM21, the prolongation of PGE2-induced hyperalgesia was not inhibited by a protein translation inhibitor, the combination of Src and MAPK inhibitors or even by the combination of all three inhibitors, indicating that systemic PZM21-induced priming is neither Type I nor II. That is, priming induced by biased MOR agonists is dependent on a signaling pathway distinct from that mediating Type I and II priming. The signaling pathway, downstream of MOR, mediating biased MOR agonist-induced priming, remains to be established. Since biased MOR agonists produce analgesia, in vivo, yet in vitro fail to attenuate β-arrestin-2 recruitment (DeWire et al., 2013; Manglik et al., 2016; Kaye et al., 2018), we suggest that side effects induced by biased MOR agonists, shown in this study (e.g. OIH and priming), may be downstream of and not directly mediated by β-arrestin-2. Of note, results from earlier phase II clinical trials of TRV130 failed to demonstrate a reduction in opioid-associated side effects (Viscusi et al., 2016; Olson et al., 2017); and, a more recent study demonstrated that repeated administration of PZM21 induced tolerance similar to that seen with morphine, and some translocation of β-arrestin-2 (Hill et al., 2018).
Conclusion
In summary, biased MOR agonists, administered at the peripheral terminal of the nociceptor produce MOR dependent OIH and priming. Systemically administered, low doses of PZM21 decreased mechanical nociceptive threshold, while at high doses, it induced analgesia. Both hyperalgesia and analgesia induced by systemic PZM21 are MOR dependent. Finally, the hyperalgesic priming induced by local and systemic administration of the biased MOR agonist PZM21 does not include signaling pathways involved in the maintenance mechanisms of Types I and II priming. The opioid crisis has created a great societal burden, increasing opioid-related mortality and morbidity (Rudd et al., 2016; Vashishtha et al., 2017). While the design of biased MOR agonists is an important start to addressing the opioid crisis, undesirable side effects, such as OIH and hyperalgesic priming, are still present with their use.
Highlights.
Intradermal administration of biased MOR agonists produces opioid-induced hyperalgesia (OIH) and hyperalgesic priming;
While systemic administration of low doses of biased MOR agonist produces hyperalgesia, high doses induce analgesia;
OIH and hyperalgesic priming induced by local or systemic administration of biased MOR agonists are MOR dependent;
Biased MOR agonist-induced priming has a different maintenance mechanism than that established for Type I and II priming.
Acknowledgements:
The authors would like to thank Marie Kern for technical assistance. This study was funded by a grant from the National Institutes of Health (NIH), NS084545.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dionéia Araldi, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA.
Luiz F. Ferrari, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA
Jon D. Levine, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA
References
- Al-Hasani R, Bruchas MR (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39:497–511. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT (2002) mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2015) Repeated Mu-Opioid Exposure Induces a Novel Form of the Hyperalgesic Priming Model for Transition to Chronic Pain. J Neurosci 35:12502–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2016a) Gi-protein-coupled 5-HT1B/D receptor agonist sumatriptan induces type I hyperalgesic priming. Pain 157:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2016b) Adenosine-A1 receptor agonist induced hyperalgesic priming type II. Pain 157:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2017) Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain 158:1204–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2018a) Role of GPCR (mu-opioid)-receptor tyrosine kinase (epidermal growth factor) crosstalk in opioid-induced hyperalgesic priming (type II). Pain 159:864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Khomula EV, Ferrari LF, Levine JD (2018b) Fentanyl Induces Rapid Onset Hyperalgesic Priming: Type I at Peripheral and Type II at Central Nociceptor Terminals. J Neurosci 38:2226–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Li CL, Chen XQ, Lu YJ, Bao L, Zhang X (2018) Clinical opioids differentially induce co-internalization of mu- and delta-opioid receptors. Mol Pain 14:1744806918769492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD (2012) Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci 32:2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS (2004) Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol 66:106–112. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Borle AB, Snowdowne KW (1982) Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science 217:252–254. [DOI] [PubMed] [Google Scholar]
- Burch RM, Axelrod J (1987) Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A 84:6374–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ (2004) Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol 143:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G (2017) Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med 23:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF (2001) Acute thermal hyperalgesia elicited by low-dose morphine in normal mice is blocked by ultra-low-dose naltrexone, unmasking potent opioid analgesia. Brain Res 888:75–82. [DOI] [PubMed] [Google Scholar]
- Dai SP, Huang YH, Chang CJ, Huang YF, Hsieh WS, Tabata Y, Ishii S, Sun WH (2017) TDAG8 involved in initiating inflammatory hyperalgesia and establishing hyperalgesic priming in mice. Sci Rep 7:41415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD (2013) A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717. [DOI] [PubMed] [Google Scholar]
- Docquier MA, Lavand’homme P, Boulanger V, Collet V, De Kock M (2004) Questioning the cardiocirculatory excitatory effects of opioids under volatile anaesthesia. Br J Anaesth 93:408–413. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Levine JD (2015) Plasma membrane mechanisms in a preclinical rat model of chronic pain. J Pain 16:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Chu C, Levine JD (2013) Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain 14:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Reichling DB, Levine JD (2015) Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci 35:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Khomula EV, Araldi D, Levine JD (2016) Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat. Sci Rep 6:31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144. [DOI] [PubMed] [Google Scholar]
- Groer CE, Schmid CL, Jaeger AM, Bohn LM (2011) Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem 286:31731–31741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G (2018) The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD (2010) Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience 169:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD (2003) Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain 105:143–150. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Price TJ (2015) The pharmacology of nociceptor priming. Handb Exp Pharmacol 227:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye AD, Cornett EM, Hart B, Patil S, Pham A, Spalitta M, Mancuso KF (2018) Novel Pharmacological Nonopioid Therapies in Chronic Pain. Curr Pain Headache Rep 22:31. [DOI] [PubMed] [Google Scholar]
- Kayser V, Besson JM, Guilbaud G (1987) Paradoxical hyperalgesic effect of exceedingly low doses of systemic morphine in an animal model of persistent pain (Freund’s adjuvant-induced arthritic rats). Brain Res 414:155–157. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Dastmalchi S, Levine JD (1996) Selective attenuation of mu-opioid receptor-mediated effects in rat sensory neurons by intrathecal administration of antisense oligodeoxynucleotides. Neurosci Lett 218:17–20. [DOI] [PubMed] [Google Scholar]
- Khomula EV, Ferrari LF, Araldi D, Levine JD (2017) Sexual Dimorphism in a Reciprocal Interaction of Ryanodine and IP3 Receptors in the Induction of Hyperalgesic Priming. J Neurosci 37:2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR (1983) Pharmacology of opioids. Pharmacol Rev 35:283–323. [PubMed] [Google Scholar]
- Morgan MM, Christie MJ (2011) Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol 164:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Fusaro MC, Zanoni CI, Dos Santos GG, Manzo LP, Araldi D, Bonet IJ, Tambeli CH, Dias EV, Parada CA (2017) Antihyperalgesic effect of CB1 receptor activation involves the modulation of P2X3 receptor in the primary afferent neuron. Eur J Pharmacol 798:113–121. [DOI] [PubMed] [Google Scholar]
- Olson KM, Lei W, Keresztes A, LaVigne J, Streicher JM (2017) Novel Molecular Strategies and Targets for Opioid Drug Discovery for the Treatment of Chronic Pain. Yale J Biol Med 90:97–110. [PMC free article] [PubMed] [Google Scholar]
- Paige C, Maruthy GB, Mejia G, Dussor G, Price T (2018) Spinal Inhibition of P2XR or p38 Signaling Disrupts Hyperalgesic Priming in Male, but not Female, Mice. Neuroscience 385:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z, Wei J (2012) Intrathecal PLC(beta3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res 1472:38–44. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM (2014) beta-arrestins: regulatory role and therapeutic potential in opioid and cannabinoid receptor-mediated analgesia. Handb Exp Pharmacol 219:427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM (2005) Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314:1195–1201. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Schmid CL, Groer CE, Bohn LM (2011) Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev 63:1001–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L (2016) Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65:1445–1452. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Garcia-Espana A, Garzon J (1997) Antisense oligodeoxynucleotides to opioid mu and delta receptors reduced morphine dependence in mice: role of delta-2 opioid receptors. J Pharmacol Exp Ther 280:1423–1431. [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM (2017) Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell 171:1165–1175 e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, Crain SM (1994) Antagonists at excitatory opioid receptors on sensory neurons in culture increase potency and specificity of opiate analgesics and attenuate development of tolerance/dependence. Brain Res 636:286–297. [DOI] [PubMed] [Google Scholar]
- Singla N, Minkowitz HS, Soergel DG, Burt DA, Subach RA, Salamea MY, Fossler MJ, Skobieranda F (2017) A randomized, Phase IIb study investigating oliceridine (TRV130), a novel microreceptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res 10:2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Wang YQ, Wu GC (2009) Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull 78:335–341. [DOI] [PubMed] [Google Scholar]
- Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL (2011) Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci 12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M (2013) CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res 91:545–553. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD (1989) Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res 492:397–399. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD (1989) Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience 32:577–580. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Nagy JI (1985) Hyperalgesia mediated by peripheral opiate receptors in the rat. Behav Brain Res 17:203–211. [DOI] [PubMed] [Google Scholar]
- Vashishtha D, Mittal ML, Werb D (2017) The North American opioid epidemic: current challenges and a call for treatment as prevention. Harm Reduct J 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F (2016) A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain 157:264–272. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Gu HX, Eijkelkamp N, Heijnen CJ, Kavelaars A (2018) Low GRK2 Underlies Hyperalgesic Priming by Glial Cell-Derived Neurotrophic Factor. Front Pharmacol 9:592. [DOI] [PMC free article] [PubMed] [Google Scholar]