Abstract
Background:
Bariatric procedures are on the rise. The risk of birth defects in pregnancies following such procedures may be increased (e.g. due to nutrient deficiencies) or decreased (e.g. due to decreased maternal body mass index, BMI).
Methods:
We conducted a systematic literature review of the association between bariatric surgery and birth defects using Ovid MEDLINE and PubMed (1946-2017). Information was abstracted on study design, exposures, outcomes, covariates, and estimates of association.
Results:
Fifteen studies met our inclusion criteria; 14 evaluated the outcome of any birth defect and one evaluated neural tube defects. Estimates of association between bariatric surgery and birth defects were available for nine studies and ranged from 0.6-1.9 (all 95% confidence intervals included 1.0). When studies were stratified by surgery type, there was no obvious pattern of association. When stratified by the approach used to account for BMI, positive associations were observed in studies that did not account for maternal prepregnancy BMI or used women with normal BMI as the reference group (range: 1.3-1.9). Estimates from studies that either matched or adjusted for prepregnancy BMI were closer to the null (range: 1.1-1.2) and studies that compared to morbidly obese women reported protective associations (range: 0.6-0.7).
Conclusions:
Studies of the association between bariatric surgery and birth defects vary with respect to the surgical procedures included, birth defects ascertainment methods, and approaches used to account for maternal BMI. Consequently, it is not possible to draw a conclusion regarding the association between bariatric surgery and birth defects. Additional studies are warranted.
Keywords: bariatric surgery, congenital abnormalities, neural tube defects, obesity, pregnancy
Introduction
Birth defects affect about 3% of live births in the United States and are a leading cause of infant and childhood morbidity and mortality.1–3 Primary prevention of these conditions is therefore an important goal. While there are several established risk factors for birth defects, only a few have provided the foundation for primary prevention strategies (e.g. folic acid fortification of the food supply, glucose control in women with pregestational diabetes).4 However, epidemiological studies have provided a relatively long list of factors for which there is evidence of an association with one or more birth defects.5 Hence, one approach for identifying new birth defect prevention strategies is to select potentially modifiable factors from this list, assess the evidence for the proposed association and, when appropriate, design and conduct studies that will ultimately confirm or refute the association.
Maternal obesity prior to pregnancy is one potentially modifiable factor for which there is evidence of an association with birth defect risk.6 Women with a body mass index (BMI) greater than 30 kg/m2 are at increased risk of having an infant with a birth defect and there is evidence that risk increases with increasing obesity class.7 Reducing BMI prior to pregnancy may reduce the risk of obesity-related birth defects. However, the use of specific weight loss strategies around the time of conception (e.g. restricted food intake, weight loss products) may also be associated with an increased risk of birth defects.8,9 Consequently, it is important to understand the potential impact of different weight loss strategies on the risk of birth defects.
Bariatric surgery is being used with increasing frequency as a method to treat obesity in individuals with BMI ≥40 kg/m2 or with BMI ≥35 kg/m2 and comorbid conditions such as Type II diabetes.10,11 Estimates from the American Society for Metabolic and Bariatric Surgery indicate that the number of bariatric procedures performed annually in the United States increased by 37% from 2011 through 2016, with 1.1 million procedures performed during this period.11 As approximately half of bariatric surgery procedures are performed among reproductive aged women,12 the outcome of pregnancies following bariatric surgery is of growing concern.13,14
Weight loss following bariatric surgery is associated with improved control of Type II diabetes and hypertension,15 which, in addition to obesity, are known risk factors for a range of adverse reproductive outcomes, including birth defects.16,17 In addition, bariatric surgery appears to have weight independent benefits, as remission of Type II diabetes can occur almost immediately following bariatric surgery, before any significant weight loss.18 Further, in reproductive age women, bariatric surgery is associated with increased fertility19 and improved pregnancy outcomes, including reduced rates of gestational diabetes, pregnancy-induced hypertension, preeclampsia, and macrosomia.20–23 Bariatric surgery could also be associated with reduced risk of birth defects, due to reductions in BMI, improvements in comorbid conditions (e.g. diabetes) or both. However, there is also evidence that the risk of birth defects may be increased in pregnancies that occur following bariatric surgery.24–26
There are several types of bariatric surgeries that may be differentially associated with the risk for birth defects. Historically, bariatric procedures have been classified as restrictive, malabsorptive, or combination (i.e. restrictive and malabsorptive). Restrictive procedures, such as gastric banding and sleeve gastrectomy limit the size of the gastric pouch. With such procedures, digestion and absorption are normal, and weight loss results from reduced food intake. Malabsorptive procedures, such as the biliopancreatic diversion, bypass the duodenum and jejunum, and weight loss results from decreased absorption. Combination procedures, such as the Roux-and-Y gastric bypass, limit both intake and absorption. However, these categories may not adequately capture differences in the weight-independent effects of bariatric surgery. Although the mechanisms underlying the weight-independent effects of bariatric surgery are not well understood, there is evidence that these effects vary across procedures. For example, remission of Type II diabetes is more common with biliopancreatic diversion than with other types of bariatric surgery.18 Hence, any of these procedures could increase the risk of birth defects as a result of a general reduction in nutrient availability. However, risk could also be influenced by specific micronutrient deficiencies and weight-independent effects, which may vary by surgery type.18–20
An increase in the risk of birth defects in the offspring of women who conceive after bariatric surgery was initially suggested by case-series: Savel et al. reported a series of 57 infants conceived following jejunoileal bypass, of which 4 (7%) had a major birth defect (2 hydrocephalus; 1 tracheo-esophageal fistula; 1 congenital heart defect) and Haddow et al. described three infants with neural tube defects conceived following gastric bypass surgery.24,25 However, subsequent cohort and case-control studies have provided inconsistent evidence for an association between bariatric surgery and birth defects. Further, although some of this literature has been included in broad systematic reviews of pregnancy outcomes following bariatric surgery, these reviews have not provided the details (e.g. number of cases, estimates of association) needed for a comprehensive evaluation of the evidence for an association between bariatric surgery and birth defects.20–22,27
Given that the number of bariatric procedures performed annually in the United States and other countries is on the rise11,22,28 and that reproductive-aged women are the largest group of bariatric surgery patients,12 it is important to understand whether bariatric surgery is associated with the risk of birth defects in subsequent pregnancies. Hence, there is a need for a comprehensive review of the literature on the association between birth defects and bariatric surgery.
Methods
To identify published studies of the association between birth defects and bariatric surgery, we conducted systematic searches of Ovid MEDLINE and PubMed, covering 1946 through April 13, 2017. We used keywords for pregnancy outcomes (e.g. pregnancy complications), birth defects (e.g. congenital abnormalities), and neural tube defects (e.g. anencephaly). In addition, we used keywords for bariatric surgery, including specific surgical procedures (e.g. gastric bypass, Roux-en-Y, biliopancreatic diversion). The search was limited to articles published in English. Appendix 1 (online supporting information) includes the complete Ovid MEDLINE search strategy.
Two authors independently screened the title and abstract of each identified article. These authors then reviewed the full text of each potentially relevant article identified by at least one of the screeners. We excluded review articles, case reports, case series, and commentaries. We also excluded articles that focused on specific sub-sets of women (e.g. women who developed gestational diabetes). Following the full text reviews, all authors met to review and resolve discrepancies in the conclusions to include or exclude an article in the systematic review. To identify additional potentially relevant articles, we reviewed the references cited in each included article and used Scopus to identify articles that cited the included articles. We reviewed articles identified through these searches as described above.
For articles selected for inclusion, two authors abstracted information on study design, location and timeframe, as well as the types of bariatric procedures evaluated, characteristics of the comparison group, outcome definitions, outcome frequencies, covariates and estimates of association (i.e. relative risk or odds ratio) with 95% confidence intervals (CI). We abstracted adjusted relative risks (aRR) or odds ratios (aOR) when available and otherwise abstracted unadjusted estimates (i.e. uRR or uOR). For articles that provided only count data, we used Stata 14 (Stata Corp, College Station, TX) to calculate unadjusted odds ratios and 95% confidence intervals, using the Woolf approximation to calculate standard errors. Estimates of association were not calculated when there were no birth defects observed in at least one group (exposed or unexposed). When an article included more than one adjusted measure, we abstracted the measure from the model that included the most covariates. Further, when an article included estimates based on different comparison groups (e.g., normal BMI and BMI-matched), we abstracted estimates based on each comparison group. We summarized study results by the type of surgery evaluated (e.g. any bariatric surgery, gastric bypass procedures), since birth defect risk may vary by procedure type. We also summarized study results by the methods used to control for BMI, which is an established risk factor for birth defects as well as both an indication for, and target of bariatric surgery.6
We assessed study quality for each included article using a modified Newcastle-Ottawa Quality Assessment Scale for the evaluation of nonrandomized studies (Appendix 2, online supporting information).29 Two authors scored each article and resolved discrepancies through discussion. Newcastle-Ottawa scores were based only on the information contained in the article and we did not use information from related publications to obtain supplemental details.
Results
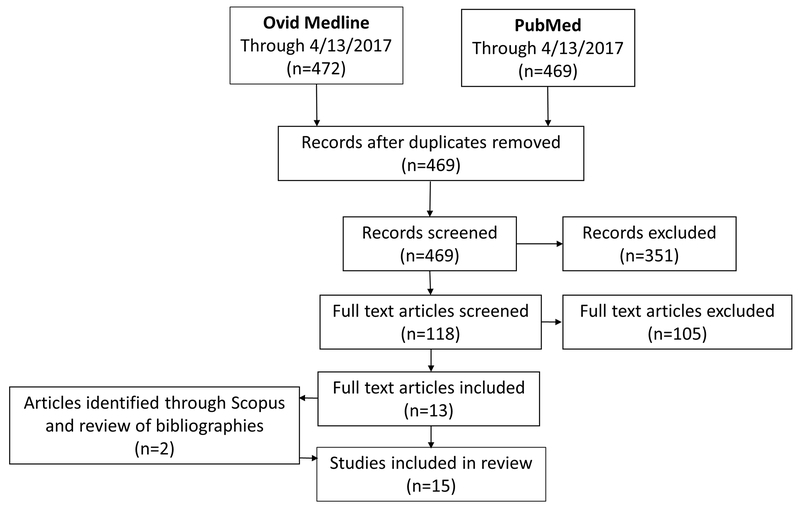
After removing duplicates, the Ovid MEDLINE and PubMed searches identified 469 unique records. Our review of titles and abstracts identified 118 articles for full text review. We identified 13 articles for inclusion through full text review and after checking the references and citing literature for these articles, we included two additional articles. Hence, 15 articles were included in the systematic review. Figure 1 summarizes the article selection process. One article30 evaluated only neural tube defects (NTDs). The remaining articles evaluated an outcome of any birth defect, which was defined differently in the individual studies. The studies that considered any birth defect generally did not specify the specific defects (e.g. spina bifida, cleft lip) that were observed.
Figure 1:
Flow diagram of data search and article selection.
The included articles described six population-based cohorts,31–36 eight hospital or clinic-based cohorts,30,37–43 and one nested case-control study.44 Two sets of studies likely overlapped: (1) Sheiner et al.31 and Weintraub et al.32 and (2) Josefsson et al.44 and Johansson et al.;34 however, results from both studies in each set were included in our review because they used different comparison groups. Sheiner et al.31 compared pregnancy outcomes for women who had bariatric surgery to women who did not have surgery and Weintraub et al.32 compared pregnancy outcomes to women prior to and after bariatric surgery. Josefsson et al.44 conducted a population-based nested case-control study comparing history of maternal bariatric surgery in infants with and without birth defects and statistically adjusted for early pregnancy BMI, whereas Johansson et al.34 compared women with and without a history of bariatric surgery with matching for the pre-surgery BMI of women who had bariatric surgery to the early pregnancy BMI of women without bariatric surgery.
Newcastle-Ottawa scores for included studies ranged from 4 to 7 out of 8 for the cohort studies. The single case-control study received a score of 8 (out of 9). Six studies had scores in the lower range (4 or 5). The authors of these studies did not adequately describe how birth defect outcomes were ascertained,31,40,41 relied on maternal report of birth defects,30,37,38 and/or did not consider potential confounders.30,31,37,38,40 Three studies had scores in the higher range (7 or 8). The authors of these studies used registry-based or hospital-based sources to identify birth defects (although little to no detail was provided regarding case confirmation procedures) and accounted for potential confounders in their study design or analyses.34,36,44
There was considerable variability across studies in the reported frequency of birth defects, likely due to differences in ascertainment and inclusion criteria. For example, Abenhaim et al. used hospital discharge data, did not describe birth defect inclusion criteria, and reported less than one percent of exposed and unexposed infants as having a birth defect.35 In contrast, Parent et al. defined birth defects as any malformation diagnosis on the birth certificate or delivery discharge diagnoses (International Classification of Diseases codes 740 to 756, excluding chromosomal abnormalities) and reported 22% and 16% of infants were affected in the exposed and unexposed groups respectively.36 Moreover, the majority of studies lacked details regarding the definition and ascertainment of birth defects, such as the specific pregnancy outcomes (e.g. livebirths, fetal deaths, terminations) that were included in the study, the timeframe for ascertainment of birth defects (e.g. at birth, through age 1 year), specific exclusion criterion (e.g. syndromes), and verification procedures (e.g. clinical review).
There were also differences across studies in the specific exposures (i.e. types of bariatric surgeries) that were evaluated. In several studies, the exposure was “any bariatric surgery,” whereas in other studies exposure was limited to a single type of procedure (e.g. gastric bypass, biliopancreatic diversion). Studies that considered any bariatric surgery as the exposure, either did not provide information on the specific surgeries or did not stratify results by surgery type.
The studies also differed with respect to the comparison groups that were employed. In some studies, pregnancy outcomes that occurred after bariatric surgery were compared to those that occurred prior to surgery, while in other studies the comparison group was pregnancy outcomes among women who had not had bariatric surgery. Among the latter studies, some used comparisons groups representing the general population, some restricted to normal weight women, and others restricted to obese women.
Of the fifteen studies that met our inclusion criteria, estimates of association could not be calculated for 6 studies. These studies were all small (24-110 post-surgical pregnancies) and either reported no birth defects in at least one group (5 studies30,39–42) or stated that there was no increased risk without providing the relevant numbers (1 study37). The remaining 9 studies were all larger (167-9,587 post-surgical pregnancies) and association estimated ranged from 0.6-1.9 (all 95% confidence intervals included 1.0). As these 9 studies differed with respect to several factors, including the exposure definition, characteristics of the comparison groups and consideration of maternal BMI, we further summarized these studies by the bariatric procedures included in the exposure definition (Table 1) and by the study design and statistical approaches used to control for maternal BMI (Table 2).
Table 1.
Summary of descriptive characteristics of included studies and reported associations between bariatric surgery and risk of birth defects.
| Reference Scorea | Location, Data Sources, and Study Details | Procedure(s) (Years)b | Outcome Assessment | BSurg # BD/total (%)c | Comparison # BD/total (%)c | OR or RR (95% CI)d | Variable used in Matching (MV) and Adjustment (AV) | |
|---|---|---|---|---|---|---|---|---|
|
Any Bariatric Surgery | ||||||||
| Sheiner et al. 200431,e 4 |
Southern Israel Soroka University Medical Center perinatal database Population-based birth cohort, 1988-2002 Compared births to women with and without prior BSurg |
Restrictive and malabsorptive | Reported by obstetrician | 15/298 (5.0) | 6,333/158,912 (4.0) | uOR 1.28f (0.76, 2.15) | MV: None AV: None |
|
| Weintraub et al. 200832,e 6 |
Southern Israel Soroka University Medical Center perinatal database Population-based sample of singleton deliveries, 1988-2006, to women who had BSurg Compared births before and after Bsurg |
Restrictive and malabsorptive | Reported by obstetrician | 40/507 (7.9) | 10/301 (3.3) | aOR 1.90 (0.88, 4.12) | MV: None AV: Age; Preterm delivery |
|
| Josefsson et al. 201344,g 8 |
Sweden Medical Birth, Total Population, National Patient and Birth Defects Registries Population-based, nested case-control study of first live born children, with birth dates before 2010, to women born 1973-1983 Compared infants with and without birth defects |
Gastroplasty, gastric banding and gastric bypass | Swedish Register of Birth Defectsh | 13/318 (4.1) | 8,282/244,294 (3.4) | aOR 1.09 (0.63, 1.91) | MV: None AV: Early pregnancy BMI; Age; Diabetes; HTN/Preeclampsia; Marital status; Smoking |
|
| Abenhaim et al. 201635 6 |
USA Health Care Costs and Utilization Project-Nationwide Inpatient Sample (HCUP-NIS) Birth cohort, 2003-2011 Compared births to women with prior Bsurg and obese (BMI>40 kg/m2 at delivery) women without BSurg |
Specific procedures not provided | HCUP-NIS database | 47/9,587 (0.5) | 1,196/221,580 (0.5) | aOR 0.74 (0.52, 1.04) | MV: None AV: Age; Cardiovascular disease; Diabetes; Hospital type; HTN; Income; Insurance type; Pulmonary disease; Race; Renal disease |
|
| Parent et al. 201736 7 |
Washington, USA Birth certificates and linked maternal discharge data from prior hospitalizations Population-based birth cohort, 1980-2013 Compared births to women with and without prior BSurg |
Banded gastroplasty, adjustable gastric band, sleeve gastrectomy and RYGB | Birth certificate and discharge diagnosis codesi | 403/1,859 (21.7) | 1,359/8,437 (16.1) | aRR 1.12 (0.99, 1.26) | MV: None AV: Prepregnancy BMI; Age; Birth year; Diabetes; Education; Household income; HTN; Parity; Race/Ethnicity |
|
|
Gastric Bypass | ||||||||
| Martin et al. 198830 4 |
Iowa, USA Self-administered questionnaire Patients of 4 bariatric surgeons Compared NTDs in pregnancies before and after BSurg |
Specific procedures not provided | Maternal report on questionnairej | 2/110 (1.8) NTDs only |
0/73 (0.0) | -- | MV: None AV: Not relevant |
|
| Wittgrove et al. 199837 5 |
California, USA Patient interviews and medical records Patients from one surgical group Compared pregnancies before and after BSurg |
Predominantly RYGB | Maternal report during interview | NR/36 | NR/23 | No increased risk of congenital anomalies | MV: None AV: Not relevant |
|
| Patel et al. 200839 6 |
Pennsylvania, USA Birth logs Women delivering at a single hospital, 2003-2006 Compared births to women with prior BSurg and 3 groups of women without BSurg: Non-obese (BMI <30 kg/m2); Obese (BMI 30-39.9 kg/m2) with no serious co-morbidities; and Morbidly or seriously obese with (BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with serious co-morbidities) |
RYGB | Not specified | 0/26 (0.0) | Non-obese: 2/188 1.1) Obese: 2/39 (5.1) Morbidly obese: 1/27 (3.7) |
-- |
MV: None AV: Not relevant |
|
| Wax et al. 200840 5 |
Maine, USA Data sources not specified Women delivering in a single maternal-fetal medicine unit Compared births to women with and without prior Bsurg |
RYGB | Not specified | 0/38 (0.0) | 4/76 (5.1) | -- | MV: Maternal age (<35 or ≥35 years); Prior cesarean AV: Not relevant |
|
| Santulli et al. 201042 6 |
France Louis Mourier Hospital’s database All births, 2004-2010, following Bsurg at a single institution and all deliveries at the same institution Compared births to women with prior BSurg to 2 groups of women without BSurg: normal BMI (18.5-24.9 kg/m2 ); and matched on prepregnancy BMI |
RYGB |
Not specified | NR/24 | Normal BMI: NR/120 BMI-matched: NR/120 |
No cases of birth defect | MV: Age; Parity AV: Not relevant MV: Prepregnancy BMI; Age; Parity AV: Not relevant |
|
| Berlac et al. 201433 6 |
Denmark National Health and National Birth Registries Population-based cohort Compared singleton births to women with prior BSurg and 2 groups of women without BSurg: normal BMI (20-24 kg/m2); and matched on prepregnancy BMI |
RYGB (1996-2011) | National Birth Registry | 30/413 (7.3) | Normal BMI: 44/827 (5.3) BMI-matched: 52/823 (6.3) |
uOR 1.39f (0.86, 2.25) uOR 1.16f (0.73, 1.85) |
MV: Age; Parity; Plurality; Year of delivery AV: None MV: Prepregnancy BMI; Age; Parity; Plurality; Year of delivery AV: None |
|
| Adams et al. 201543 6 |
Utah, USA Utah Population Database and birth certificates Women who had BSurg in a single surgical practice were linked to births after 1988 Compared births to women with prior BSurg and obese women without BSurg |
RYGB (1979-2011) | Birth certificates | 15/295 (5.1) | 12/295 (4.1) | uOR 1.26f (0.58, 2.74) | MV: Prepregnancy BMI prior to surgery; Age; Birth order; Parity; Race, Year of delivery AV: None |
|
| Johansson et al. 201534,g 7 |
Sweden Medical Birth, National Patient and Scandinavian Obesity Surgery Registries Population-based sample of singleton births, 2006-2011 Compared births to women with prior Bsurg to a matched group of women without Bsurg |
Predominantly RYGBk (2004-2011) | Medical Birth Registryl | All women (excluding chromosomal abnormalities): | MV: Pre-surgery BMI/Early pregnancy BMI; Age; Education; Parity; Smoking; Year of delivery AV: History of coexisting conditions; History of substance abuse; Mother’s country of birth |
|||
| 12/590 (2.0) | 79/2,334 (3.4) | aOR 0.63 (0.34, 1.18) | ||||||
| Restricted to one birth per women (excluding chromosomal abnormalities): | ||||||||
| 11/549 (2.0) | 67/2,110 (3.2) | aOR 0.68 (0.35, 1.31) | ||||||
|
Laparoscopic Adjustable Gastric Band (LAGB) | ||||||||
| Lapolla et al. 201041 5 |
Italy Databases of the Obesity Centers at Vicenza Regional Hospital and Padova University, phone interviews, and electronic database Compared pregnancies before and after BSurg and compared pregnancies to women with prior Bsurg to 2 groups of women without BSurg: BMI ≥ 40 kg/m2 and no diabetes or chronic hypertension; normal weight without gestational diabetes |
LAGB (1993-2005) | Maternal report and electronic database | NR/83 | Pre-surgery: NR/27 BMI≥40: NR/120 Normal: NR/858 |
No birth defects identified | MV: None AV: Not relevant |
|
|
Biliopancreatic Diversion (BPD) | ||||||||
| Marceau et al. 200438 5 |
Canada Laval Hospital Questionnaire Compared births before and after Bsurg |
BPD (1984-2000) | Maternal report | 7/167 (4.2) | 33/1,257 (2.6) | uOR 1.62f (0.71, 3.73) | MV: None AV: None |
|
Abbreviations: AV, variables used to derive adjusted effect estimates; BD, birth defects; BMI, body mass index; BPD, biliopancreatic diversion; Bsurg, bariatric surgery; CI, confidence interval; HTN, hypertension; ICD, International Classification of Diseases; LAGB, Laparoscopic adjustable gastric band; MV, variables used for matching; NR, not reported; NTDs, neural tube defects; RYGB, Roux-en-Y gastric bypass; uOR/aOR, unadjusted/adjusted odds ratio; uRR/aRR, unadjusted/adjusted rate ratio
The Newcastle-Ottawa Scale (NOS) score is out of a possible 8 for all studies except Josefsson, which is out of a possible 9 because it is a case control study
Years during which the bariatric procedures were performed, if specified in the article.
If counts were not provided, they were calculated from provided percentage.
Effect estimates were not calculated when 0 birth defects were reported in one or more groups. When specific birth defect counts were not specified in the article, a summary of the authors’ comments regarding the frequency of birth defects is provided.
There is likely to be overlap between the bariatric surgery cases included in Sheiner et al. 2004 and Weintraub et al. 2008. However, the two studies used different comparison groups.
uOR calculated from data provided in the article.
There is likely to be overlap between the study populations included in Josefsson et al. 2013 and Johansson et al. 2015.
Included ICD-10 codes starting with Q and ICD-9 codes 740.0-759.9.
Included ICD-9 codes 740-756.
Affected offspring verified by medical record review.
98% of procedures were gastric bypass, 2% were gastric banding, and 1% were another type of bariatric surgery.
ICD-10 codes for major non-chromosomal malformations.
Table 2.
Summary of study results by BMI adjustment method.a
| Comparison characteristics | Procedure(s) | OR or RR (95% CI) | Variable used in Matching (MV) and Adjustment (AV) |
|---|---|---|---|
| Unmatched/unadjusted for BMI | |||
| Sheiner et al. 200431 | Any bariatric surgery | uOR 1.28 (0.76, 2.15)b | MV: None AV: None |
| Marceau et al. 200438 | Biliopancreatic diversion | uOR 1.62 (0.71, 3.73)b | MV: None AV: None |
| Weintraub et al. 200832 | Any bariatric surgery | aOR 1.90 (0.88, 4.12) | MV: None AV: Age; Preterm delivery |
| Normal BMI | |||
| Berlac et al. 201433,c | Gastric bypass (RYGB) | uOR 1.39 (0.86, 2.25)b | MV: Age; Parity; Plurality; Year of delivery AV: None |
| Adjusted/matched BMI | |||
| Josefsson et al. 201344 | Any bariatric surgery (gastroplasty, gastric banding, and gastric bypass) | aOR 1.09 (0.63, 1.91) | MV: None AV: Early pregnancy BMI; Age; Diabetes; HTN/Preeclampsia; Marital status; Smoking |
| Berlac et al. 201433,c | Gastric bypass (RYGB) | uOR 1.16 (0.73, 1.85)b | MV: Prepregnancy BMI; Age; Parity; Plurality; Year of delivery AV: None |
| Parent et al. 201736 | Any bariatric surgery (gastroplasty, gastric banding, sleeve gastrectomy, and RYGB) | aRR 1.12 (0.99, 1.26) | MV: None AV: Prepregnancy BMI; Age; Birth year; Diabetes; Education; Household income; HTN; Parity; Race/Ethnicity |
| Obese (BMI>40 kg/m2) at delivery | |||
| Abenhaim et al. 201635 | Any bariatric surgery (specific procedures not provided) | aOR 0.74 (0.52, 1.04) | MV: None AV: Age; Cardiovascular disease; Diabetes; Hospital type; HTN; Income; Insurance type; Pulmonary disease; Race; Renal disease |
| Matched pre-surgery BMI/prepregnancy BMI | |||
| Johansson et al. 201534 All pregnancies Restricted to one pregnancy per women |
Gastric bypass (Predominantly RYGB) | aOR 0.63 (0.34, 1.18) aOR 0.68 (0.35, 1.31) |
MV: Pre-surgery BMI/Early pregnancy BMI; Age; Education; Parity; Smoking; Year of delivery AV: History of coexisting conditions; History of substance abuse; Mother’s country of birth |
BMI, body mass index; CI, confidence interval; OR, odds ratio; RR, risk ratio; RYGB, Roux-en-Y gastric bypass; uOR/aOR, unadjusted/adjusted odds ratio; uRR/aRR, unadjusted/adjusted rate ratio
Studies without association estimates30,37,39,40-42 were not included in this table and results from Adams et al.43 were not presented because the authors did not provide a clear description of their comparison group.
uOR calculated from data provided in the article.
Berlac et al.33 included two comparison groups: women with normal prepregnancy BMI and women matched on prepregnancy BMI.
Bariatric Procedures
Five studies evaluated the association between any bariatric surgery procedure and any birth defect.31,32,35,36,44 In these five studies, estimates of association ranged from 0.7 to 1.9, with four of the five studies reporting estimates greater than 1.0 (range: 1.1-1.9) (Table 1). However, the two largest studies (Josefsson et al. and Abenhaim et al.) provided little to no evidence for an association between bariatric surgery and birth defects (range: 0.7-1.1).35,44
Eight studies evaluated the association between gastric bypass procedures and either (1) NTDs30 or (2) any birth defect.33,34,37,39,40,42,43 The majority of these studies evaluated only or predominantly Roux-en-Y gastric bypass procedures. Estimates of association were not provided and could not be calculated for five studies.30,37,39,40,42 Four of these five studies included fewer than 50 exposed women and reported either no birth defects in the exposed group39,40,42 or stated that no increased risk was observed in the exposed group but did not provide the number (if any) of observed birth defects.37 The fifth study considered only NTDs and reported two affected infants born after surgery, as compared to no affected infants born before gastric bypass (1.8% versus 0.0%).30 Among studies that reported estimates of association or provided data from which estimates could be calculated, the association estimates ranged from 0.6 to 1.4.33,34,43
Two studies evaluated specific types of bariatric surgery other than Roux-en-Y gastric bypass. Lapolla et al. evaluated the association between laparoscopic adjustable gastric band and birth defects. Their study included only 83 exposed women and no birth defects were observed.41 Marceau et al. evaluated the association between biliopancreatic diversion and birth defects. They reported an elevated estimate of maternal reported birth defects among post-surgical women compared to the pre-surgical pregnancies of women who underwent BPD (uOR 1.6).38
Control for Maternal BMI
Of the 15 studies included in this review, eight provided a clear description of their comparison group(s) and either estimates of association or the data required to calculate such estimates (Table 2). In three of these eight studies, there was no attempt to control for maternal BMI.31,32,38 Specifically, the comparison groups were not matched for BMI and association estimates were not adjusted for BMI. In addition, one study compared exposed women to women with normal BMI.33 The association estimates reported in these studies ranged from 1.3 to 1.9.31–33,38
Three studies that either matched on or adjusted for prepregnancy BMI reported estimates of association between bariatric surgery and birth defects that were all closer to the null (range 1.1-1.2) than estimates from studies that did not account for prepregnancy BMI.33,36,44 Additionally, one study that used two comparison groups (Berlac et al.) reported an association estimate closer to the null in BMI-matched analyses (OR 1.2) as compared to analyses of post-surgical versus normal weight women (OR 1.4).33
Finally, two studies compared post-surgical women to obese women. One study included women with BMI greater than 40 at delivery as the comparison group35 and the other matched prepregnancy BMI among unexposed women to the pre-surgery BMI of exposed women.34 The association estimates reported in these studies ranged from 0.6 to 0.7.34,35
Conclusions
Over the past 20 years, the use of bariatric surgery to treat morbid obesity has increased rapidly. An estimated 216,000 bariatric procedures were performed in the United States in 2016, a 37% increase since 2011.11 Approximately half of these procedures were performed on women of reproductive age.12 Weight loss following bariatric surgery is associated with improvement in and, in some cases, remission of obesity-related comorbidities including Type II diabetes and hypertension.15 In addition, compared to obese women who have not had bariatric surgery, women who conceive following bariatric surgery have decreased rates of pregnancy complications, including gestational diabetes, pregnancy-induced hypertension, preeclampsia, and macrosomia.19–23 Since prepregnancy obesity, as well as diabetes and hypertension, are risk factors for several birth defects, post-surgical BMI reduction and control of comorbid conditions are mechanisms through which bariatric surgery might reduce the risk for birth defects.
Despite the known benefits of bariatric surgery in reproductive aged females, the potential for bariatric surgery to have adverse effects on reproductive outcomes is well recognized: women who undergo such procedures are generally advised to avoid conception for 12-18 months post-surgery and there are guidelines for assessing nutritional status and supplementation in pregnancies that follow bariatric surgery.10,14 Maternal nutritional compromise and micronutrient deficiencies, which have been associated with the risk of birth defects,45–48 are therefore mechanisms through which bariatric surgery might increase the risk for birth defects. Hence, there may be both risk increasing and risk decreasing consequences of bariatric surgery. Further, any association between bariatric surgery and birth defects may be moderated by BMI and obesity-related comorbidities at the time of pregnancy as well as adherence to recommendations regarding timing of conception and guidelines for nutritional monitoring and supplementation.
Based on our systematic review of the literature, there is insufficient evidence to draw definitive conclusions regarding an association between bariatric surgery and the risk of birth defects. This can be attributed to the relatively small number of studies that were identified (N=15), of which effect estimates could be obtained for only 9; differences in study design and analytic approach; and heterogeneity across studies for both exposure and outcome definitions. These limitations do not appear to be specific to studies of birth defects, as others have noted similar limitations for the literature on bariatric surgery and pregnancy outcomes in general.13,22
Although the studies included in our review were all designed to assess pregnancy outcomes following bariatric surgery, they differed in the specific comparison groups that were used and the approaches used to account for maternal BMI, which is a known birth defect risk factor and both an indication for and target of bariatric surgery. There was also wide variability in the number and types of additional covariates (e.g. diabetes, hypertension) that were accounted for in estimates of association. While these differences precluded direct comparisons across studies, contrasts across studies categorized by the comparison group and approach used to address BMI, provided some, limited, evidence that bariatric surgery may be associated with the risk of birth defects via its impact on BMI. Specifically, studies that compared women who had undergone bariatric surgery to obese women were suggestive of reduced risk of birth defects in the post-surgical women (aORs 0.6-0.7).34,35 Studies that matched or adjusted for prepregnancy BMI were close to the null (range: 1.1-1.2)33,36,44 and studies that compared post-surgical women to the general population or to women with normal BMI reported elevated association estimates (range: 1.3-1.9).31–33,38 This pattern is consistent with the observation that BMI tends to decrease following bariatric surgery, but remains in the overweight to obese range.49
Our findings must, however, be viewed with caution, given several additional limitations of the available literature. These limitations can be broadly classified as being related to the exposure or to the outcome of interest. With respect to the exposure of interest, several studies combined data across procedures, which may have obscured associations that are specific to individual procedures or categories of procedures. Further, with the exception of Roux-en-Y gastric bypass procedure, most individual procedures have been evaluated in only a single study (e.g. biliopancreatic diversion) or not at all (e.g. gastric sleeve). Finally, none of the studies accounted for the specific indication for surgery (e.g. BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with co-morbid conditions) or the timing of conception relative to surgery date.
There were also several limitations related to the outcome of birth defects. All but one study included a broad range of birth defects, which may have obscured associations with specific malformations (e.g. neural tube defects). Further, there were differences across studies in the criteria used to define birth defects. The latter is highlighted by the large range in the frequencies of birth defects across the studies (from less than 1% to 22%). Studies with high frequencies of defects likely included minor defects and suspected cases, some of which might not ultimately be confirmed. In general, studies provided little to no information on the pregnancy outcomes (e.g. live births, fetal deaths, terminations) that were considered, the period over which birth defects were ascertained (e.g. at birth, through the first year of life), specific exclusion criteria (e.g. chromosome abnormalities, genetic syndromes) and verification of reported conditions (e.g. clinical review).
In summary, our systematic review found insufficient evidence to draw conclusions regarding the association between bariatric surgery and the risk for birth defects in subsequent pregnancies. There is weak evidence, based on two studies, that bariatric surgery may be associated with a decreased risk of birth defects when comparing post-surgical women to morbidly obese women without surgery. If true, this association would appear to be mediated through post-surgical reductions in maternal BMI. However, definitive conclusions regarding the association of bariatric surgery and birth defects will require further evidence.
Optimally, future studies should focus on individual bariatric procedures, reflect current surgical practices, assess maternal nutrient intake and status as well as comorbidities through questionnaires and biological measures, and carefully consider the relationships between bariatric surgery, BMI, birth defect related comorbidities (i.e. diabetes and hypertension) and birth defects. Future studies should also identify birth defects using established data sources (e.g. birth defects registries, medical records), confirm cases via clinical review, report the specific defects observed, and consider individual birth defects to the extent possible, though some grouping may be necessary due to the low prevalence of most individual birth defects. Given that approximately 10% of US reproductive aged women have a BMI≥40 kg/m2 and reproductive aged women comprise approximately half of the patients undergoing bariatric procedures,12,50 it is important that we undertake additional studies of bariatric surgery and birth defects so that we can more fully understand the spectrum of reproductive risks and benefits associated with these procedures.
Supplementary Material
Acknowledgements (including details of funding)
This publication was supported by Grant Number U01 DD 001179 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention. The authors thank Dr. Sarah C. Tinker and Dr. A.J. Agopian for their comments and input on this manuscript.
Footnotes
Supporting Information
Additional Supporting Information may be found online at the publisher’s website.
References
- 1.Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978-2005. Morbidity and Mortality Weekly Report 2008; 57:1–5. [PubMed] [Google Scholar]
- 2.Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. National Vital Statistics Reports 2015; 64:1–30. [PubMed] [Google Scholar]
- 3.Arth AC, Tinker SC, Simeone RM, Ailes EC, Cragan JD, Grosse SD. Inpatient Hospitalization Costs Associated with Birth Defects Among Persons of All Ages - United States, 2013. Morbidity and Mortality Weekly Report 2017; 66:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris BS, Bishop KC, Kemeny HR, Walker JS, Rhee E, Kuller JA. Risk Factors for Birth Defects. Obstetrical & Gynecological Survey 2017; 72:123–135. [DOI] [PubMed] [Google Scholar]
- 5.Feldkamp ML, Botto LD, Carey JC. Reflections on the etiology of structural birth defects: Established teratogens and risk factors. Birth Defects Research Part A: Clinical and Molecular Teratology 2015; 103:652–655. [DOI] [PubMed] [Google Scholar]
- 6.Correa A, Marcinkevage J. Prepregnancy obesity and the risk of birth defects: an update. Nutrition Reviews 2013; 71 Suppl 1:S68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson M, Cnattingius S, Villamor E, Soderling J, Pasternak B, Stephansson O, et al. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. British Medical Journal 2017; 357:j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael SL, Shaw GM, Schaffer DM, Laurent C, Selvin S. Dieting behaviors and risk of neural tube defects. American Journal of Epidemiology 2003; 158:1127–1131. [DOI] [PubMed] [Google Scholar]
- 9.Hoang TT, Agopian AJ, Mitchell LE. Maternal Use of Weight Loss Products and the Risk of Neural Tube Defects in Offspring: A Systematic Literature Review. Birth Defects Research 2018; 110:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient−-2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring). 2013; 21 Suppl 1:S1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surgery for Obesity & Related Diseases 2018; 14:259–263. [DOI] [PubMed] [Google Scholar]
- 12.Maggard M, Li Z, Yermilov I, Maglione M, Suttorp M, Carter J, et al. Bariatric surgery in women of reproductive age: special concerns for pregnancy Evidence Report/Technology Assessment no. 169. Rockville, MD: Agency for Healthcare Research and Quality; 2008. http://purl.access.gpo.gov/GPO/LPS108434 [last accessed March 2018]. [PMC free article] [PubMed] [Google Scholar]
- 13.Carreau AM, Nadeau M, Marceau S, Marceau P, Weisnagel SJ. Pregnancy after Bariatric Surgery: Balancing Risks and Benefits. Canadian Journal of Diabetes 2017; 41:432–438. [DOI] [PubMed] [Google Scholar]
- 14.Kominiarek MA, Jungheim ES, Hoeger KM, Rogers AM, Kahan S, Kim JJ. American Society for Metabolic and Bariatric Surgery position statement on the impact of obesity and obesity treatment on fertility and fertility therapy Endorsed by the American College of Obstetricians and Gynecologists and the Obesity Society. Surgery for Obesity & Related Diseases 2017; 13:750–757. [DOI] [PubMed] [Google Scholar]
- 15.Puzziferri N, Roshek TB 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. Journal of the American Medical Association 2014; 312:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, et al. Diabetes mellitus and birth defects. American Journal of Obstetrics & Gynecology 2008; 199:237 e231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gelder MM, Van Bennekom CM, Louik C, Werler MM, Roeleveld N, Mitchell AA. Maternal hypertensive disorders, antihypertensive medication use, and the risk of birth defects: a case-control study. British Journal of Obstetrics and Gynaecology 2015; 122:1002–1009. [DOI] [PubMed] [Google Scholar]
- 18.le Roux CW, Heneghan HM. Bariatric Surgery for Obesity. Medical Clinics of North America 2018; 102:165–182. [DOI] [PubMed] [Google Scholar]
- 19.Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, Suttorp M, et al. Pregnancy and fertility following bariatric surgery: a systematic review. Journal of the American Medical Association 2008; 300:2286–2296. [DOI] [PubMed] [Google Scholar]
- 20.Guelinckx I, Devlieger R, Vansant G. Reproductive outcome after bariatric surgery: a critical review. Human Reproduction Update 2009; 15:189–201. [DOI] [PubMed] [Google Scholar]
- 21.Vrebosch L, Bel S, Vansant G, Guelinckx I, Devlieger R. Maternal and neonatal outcome after laparoscopic adjustable gastric banding: a systematic review. Obesity Surgery 2012; 22:1568–1579. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer MM, Nilas L. Pregnancy after bariatric surgery--a review of benefits and risks. Acta Obstetricia et Gynecologica Scandinavica 2013; 92:264–271. [DOI] [PubMed] [Google Scholar]
- 23.Willis K, Lieberman N, Sheiner E. Pregnancy and neonatal outcome after bariatric surgery. Best Practice & Research in Clinical Obstetrics & Gynaecology 2015; 29:133–144. [DOI] [PubMed] [Google Scholar]
- 24.Savel LE, Simon SR, Maxon WS. Pregnancy after jejunoileal bypass. A review and report of one case. Obstetrics & Gynecology 1978; 52:58S–60S. [PubMed] [Google Scholar]
- 25.Haddow JE, Hill LE, Kloza EM, Thanhauser D. Neural tube defects after gastric bypass. Lancet 1986; 1:1330. [DOI] [PubMed] [Google Scholar]
- 26.Pelizzo G, Calcaterra V, Fusillo M, Nakib G, Ierullo AM, Alfei A, et al. Malnutrition in pregnancy following bariatric surgery: three clinical cases of fetal neural defects. Nutrition Journal 2014; 13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jans G, Matthys C, Bogaerts A, Lannoo M, Verhaeghe J, Van der Schueren B, et al. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: a systematic review. Advances in Nutrition 2015; 6:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obesity Surgery 2013; 23:427–436. [DOI] [PubMed] [Google Scholar]
- 29.Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [last accessed May 2017].
- 30.Martin L, Chavez GF, Adams MJ Jr., Mason EE, Hanson JW, Haddow JE, et al. Gastric bypass surgery as maternal risk factor for neural tube defects. Lancet 1988; 1:640–641. [DOI] [PubMed] [Google Scholar]
- 31.Sheiner E, Levy A, Silverberg D, Menes TS, Levy I, Katz M, et al. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. American Journal of Obstetrics & Gynecology 2004; 190:1335–1340. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub AY, Levy A, Levi I, Mazor M, Wiznitzer A, Sheiner E. Effect of bariatric surgery on pregnancy outcome. International Journal of Gynaecology & Obstetrics 2008; 103:246–251. [DOI] [PubMed] [Google Scholar]
- 33.Berlac JF, Skovlund CW, Lidegaard O. Obstetrical and neonatal outcomes in women following gastric bypass: a Danish national cohort study. Acta Obstetricia et Gynecologica Scandinavica 2014; 93:447–453. [DOI] [PubMed] [Google Scholar]
- 34.Johansson K, Cnattingius S, Naslund I, Roos N, Trolle Lagerros Y, Granath F, et al. Outcomes of pregnancy after bariatric surgery. The New England Journal of Medicine 2015; 372:814–824. [DOI] [PubMed] [Google Scholar]
- 35.Abenhaim HA, Alrowaily N, Czuzoj-Shulman N, Spence AR, Klam SL. Pregnancy outcomes in women with bariatric surgery as compared with morbidly obese women. Journal of Maternal-Fetal & Neonatal Medicine 2016; 29:3596–3601. [DOI] [PubMed] [Google Scholar]
- 36.Parent B, Martopullo I, Weiss NS, Khandelwal S, Fay EE, Rowhani-Rahbar A. Bariatric Surgery in Women of Childbearing Age, Timing Between an Operation and Birth, and Associated Perinatal Complications. Journal of the American Medical Association Surgery 2017; 152:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Wittgrove AC, Jester L, Wittgrove P, Clark GW. Pregnancy following gastric bypass for morbid obesity. Obesity Surgery 1998; 8:461–464; discussion 465–466. [DOI] [PubMed] [Google Scholar]
- 38.Marceau P, Kaufman D, Biron S, Hould FS, Lebel S, Marceau S, et al. Outcome of pregnancies after biliopancreatic diversion. Obesity Surgery 2004; 14:318–324. [DOI] [PubMed] [Google Scholar]
- 39.Patel JA, Patel NA, Thomas RL, Nelms JK, Colella JJ. Pregnancy outcomes after laparoscopic Roux-en-Y gastric bypass. Surgery for Obesity & Related Diseases 2008; 4:39–45. [DOI] [PubMed] [Google Scholar]
- 40.Wax JR, Cartin A, Wolff R, Lepich S, Pinette MG, Blackstone J. Pregnancy following gastric bypass surgery for morbid obesity: maternal and neonatal outcomes. Obesity Surgery 2008; 18:540–544. [DOI] [PubMed] [Google Scholar]
- 41.Lapolla A, Marangon M, Dalfra MG, Segato G, De Luca M, Fedele D, et al. Pregnancy outcome in morbidly obese women before and after laparoscopic gastric banding. Obesity Surgery 2010; 20:1251–1257. [DOI] [PubMed] [Google Scholar]
- 42.Santulli P, Mandelbrot L, Facchiano E, Dussaux C, Ceccaldi PF, Ledoux S, et al. Obstetrical and neonatal outcomes of pregnancies following gastric bypass surgery: a retrospective cohort study in a French referral centre. Obesity Surgery 2010; 20:1501–1508. [DOI] [PubMed] [Google Scholar]
- 43.Adams TD, Hammoud AO, Davidson LE, Laferrere B, Fraser A, Stanford JB, et al. Maternal and neonatal outcomes for pregnancies before and after gastric bypass surgery. International Journal of Obesity 2015; 39:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Josefsson A, Bladh M, Wirehn AB, Sydsjo G. Risk for congenital malformations in offspring of women who have undergone bariatric surgery. A national cohort. British Journal of Obstetrics and Gynaecology 2013; 120:1477–1482. [DOI] [PubMed] [Google Scholar]
- 45.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. American Journal of Epidemiology 1998; 147:213–216. [DOI] [PubMed] [Google Scholar]
- 46.Carmichael SL, Yang W, Feldkamp ML, Munger RG, Siega-Riz AM, Botto LD, et al. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Archives of Pediatrics & Adolescent Medicine 2012; 166:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldkamp ML, Krikov S, Botto LD, Shaw GM, Carmichael SL, National Birth Defects Prevention S. Better diet quality before pregnancy is associated with reduced risk of gastroschisis in Hispanic women. The Journal of Nutrition 2014; 144:1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botto LD, Krikov S, Carmichael SL, Munger RG, Shaw GM, Feldkamp ML, et al. Lower rate of selected congenital heart defects with better maternal diet quality: a population-based study. Archives of Disease in Childhood Fetal Neonatal Edition 2016; 101:F43–49. [DOI] [PubMed] [Google Scholar]
- 49.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. Journal of the American Medical Association Surgery 2014; 149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Journal of the American Medical Association 2016; 315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.