Abstract
Background.
Normalization of medicinal and recreational marijuana use has increased the importance of fully understanding effects of marijuana use on individual- and population-level health, including prenatal exposure effects on child development. We undertook a systematic review of the literature to examine the long-term effects of prenatal marijuana exposure on neuropsychological function in children aged 1–11 years.
Methods.
Primary research publications were searched from Medline, Embase, PsychInfo, CINAHL EbscoHost, Cochrane Library, Global Health, and ERIC (1980–2018). Eligible articles documented neuropsychological outcomes in children 1–11 years who had been prenatally exposed to marijuana. Studies of exposure to multiple prenatal drugs were included if results for marijuana exposure were reported separately from other substances. Data abstraction was independently performed by two reviewers using a standardized protocol.
Results.
The eligible articles (n=21) on data from seven independent longitudinal studies, had high quality based on the Newcastle-Ottawa Scale. Some analyses found associations (p<0.05) between prenatal marijuana exposure and decreased performance on memory, impulse control, problem solving, quantitative reasoning, verbal development, and visual analysis tests; as well as increased performance on attention and global motion perception tests. Limitations included concurrent use of other substances among study participants, potential underreporting and publication biases, non-generalizable samples, and limited published results preventing direct comparison of analyses.
Conclusions.
The specific effects of prenatal marijuana exposure remain unclear and warrant further research. The larger number of neuropsychological domains that exhibit decreased versus increased psychological and behavioral functions suggest that exposure to marijuana may be harmful for brain development and function.
Keywords: cannabis, intrauterine, attention, memory, intellect, perception
Introduction
Marijuana is the most commonly used illicit drug in the United States, with an estimated 22.2 million past-month users aged 12 years or older in 2015.1 Since 1996, laws allowing medicinal marijuana use have been passed in 29 states, the District of Columbia (DC), Guam and Puerto Rico, and laws allowing recreational use and sales of marijuana for adults aged 21 and over have been passed in 8 states and DC since 2012.2,3 As more states consider legalizing marijuana use among adults, it is important to fully understand the effects of marijuana use on individual- and population-level health.3
The growing availability and use of marijuana is important to consider among women of reproductive age. Among US women aged 18–44 years, self-reported past-30-day use of marijuana has increased from 2002 to 2014 from 2.4% to 3.9% among pregnant and from 6.3% to 9.3% among non-pregnant women.4 Given the increasing trends of marijuana use among women of reproductive age including pregnant women and the changing landscape of legal and medical marijuana in the US, a more robust understanding of the consequences of prenatal marijuana exposure on children is critical to inform individual decision making and public health policy, planning, and practice.5,6
The use of marijuana during pregnancy could have implications for fetal brain development.7–12 Marijuana is lipid soluble and able to cross the placenta and blood-brain barrier to accumulate in fetal tissues including brain tissues.13,14 It is processed in the body through the endocannabinoid system, which may be involved in brain development through neurogenesis, differentiation, migration and neural circuit wiring.15,16 Data suggest that this system exists from the earliest stages of pregnancy, presenting multiple points of vulnerability to exposure of marijuana throughout gestation, although the exact processes of this system’s development are still not completely understood in humans.15,17 Additionally, there is evidence of several adverse effects on the brain and cognition, including structural damage, learning and memory deficits, and impaired motor function in adolescents and adults who are active marijuana users.18–25 Therefore, marijuana exposure has potential adverse effects on brain development in prenatally exposed children.26
The strongest evidence of adverse effects of prenatal marijuana exposure comes from animal studies.7–9 These studies demonstrated that even low doses of marijuana during pregnancy can result in adverse cognitive and developmental effects in offspring.7-9 In human studies, there are variations in the effect’s direction, degree and duration.11,12 Moreover, it is often difficult to discern whether the effects are due solely to marijuana or to combination of marijuana with another substance the mother may have used concurrently.27–29 Syntheses of studies that have examined prenatal marijuana effects on children’s brain development, while controlling for other substances use, are limited.6
Existing systematic reviews have partially examined consequences of prenatal marijuana exposure in children; however, they have certain limitations. Among infants, a 2016 review found increased irritability, tremors and startles, and decreased stability scores in exposed neonates compared to unexposed neonates.30 Two systematic reviews from 2007 and 2012 examining cognitive functions in children with prenatal exposures to marijuana, alcohol, cocaine, tobacco, lead and mercury found evidence for long-term damage to attention resulting from prenatal marijuana exposure, attempting to control for use of other substances; however, these studies involved adolescents.31,32 By adolescence, subjects may have been subject to other potential developmental insults, including their own substance use, and it is difficult to distinguish consequences resulting from prenatal exposure.33 A 2011 summary article focused mainly on the endocannabinoid system and animal studies supporting evidence of marijuana’s potential to interfere with the role of this system in development and did not employ systematic review methodology.15 Additionally, a recent consensus study by the National Academies of Sciences, Engineering, and Medicine noted the dearth of good or fair quality systematic reviews examining associations between maternal marijuana use and offspring’s cognition or academic achievement.34 Given the abovementioned gaps in the scientific literature, this study presents the findings of a systematic review of the impact of prenatal marijuana exposure on neuropsychological functioning in children aged 1–11 years.
Methods
Literature searches for this review were conducted by a librarian specializing in systematic reviews. An initial literature search took place in August 2014 in the following databases: Medline, Embase, PsychInfo, Cumulative Index to Nursing and Allied Health Literature (CINAHL), EbscoHost, Cochrane Library, Global Health, and Education Resources Information Center (ERIC). Supplementary searches using the same terms were conducted in April 2015, September 2016, July 2017, and August 2018. Additionally, a cited reference search was conducted to identify articles missed in the searches.35 Appendix A of the supplemental materials provides an example of terms used in Medline. Search terms included terms for marijuana (e.g., cannabis, hash, ganja), pregnancy (e.g., pregnancy, pregnant women, in-utero), and outcomes (e.g., cognitive disorders, intelligence, learning, executive functions, attention). All terms were entered as subject headings, text words, and Medical Subject Headings (MESH) terms per requirements of each database. Detailed overview of the search and selection strategy is available in the supplemental materials (Appendix B).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to track literature review results and to standardize the review process.36 The PRISMA flow diagram is displayed in Figure 1. Inclusion and exclusion criteria (the supplemental materials, Appendix C) were designed to include published or unpublished studies documenting neuropsychological outcomes in children aged 1–11 years who had been prenatally exposed to marijuana. Studies of prenatal exposure to multiple drugs were included if results for marijuana exposure and its associations with the outcomes were reported separately from results for other substance exposures. Grey literature, including conference abstracts, dissertations, white papers, and reports retrieved by the literature searches, was considered for eligibility. Reviewers identified 1 doctoral dissertation and 4 conference abstracts that met criteria for full text review. Authors of the conference abstracts were contacted in regards to potential pending publication of their studies. Full text review and further research lead to exclusion of these articles.
Figure 1. Flow chart of literature review.
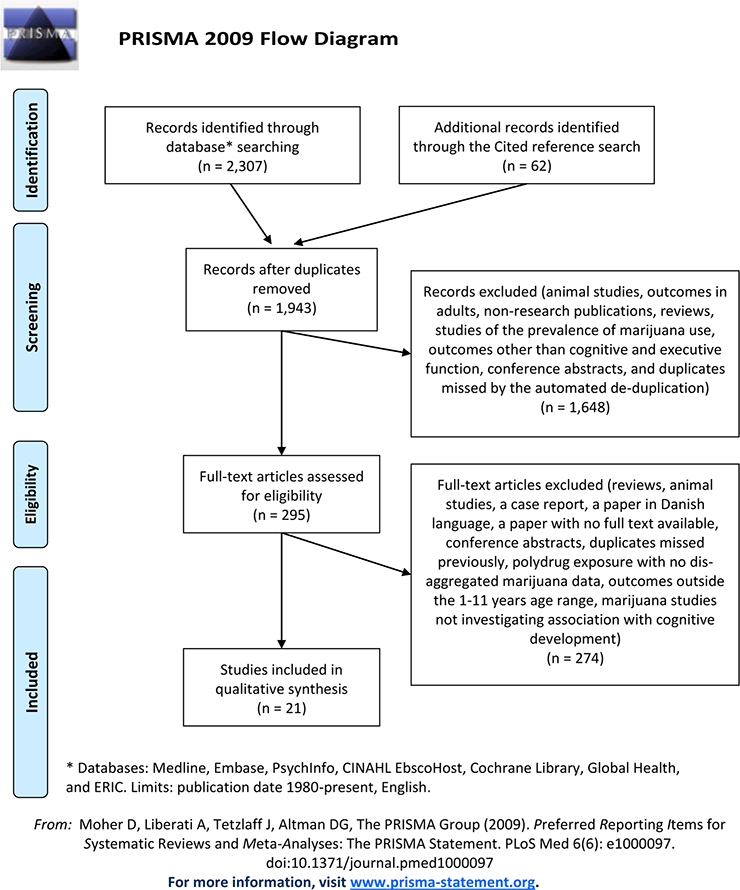
Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. (http://www.bmj.com/content/339/bmj.b2535)
The literature search and selection consisted of 2 steps: (1) title and abstract screening, and (2) full text and reference review. A primary and a secondary reviewer independently reviewed all articles retrieved from the literature search. The articles were divided between nine reviewers who were either subject matter experts in child development and/or substance abuse (authors: SRS, KS, JK, RL, IR) or public health scientists (acknowledged: KA, RP, AJ, LP) trained to perform the review by the subject matter experts. A primary and a secondary reviewer screened each reference to determine if the reference met inclusion or exclusion criteria.
All articles that were found eligible during the full text review reported data from longitudinal studies. Additionally, it was found that some articles utilized data from a single study reporting results from different analyses or from different time points of the study. The reviewers utilized the list of authors and the methodology description of each eligible article, including references to publications reporting study methodology, in order to determine if articles belonged to a particular study.
The data abstraction instrument used by the Community Preventive Services Task Force was used to abstract data from the eligible articles.37 As the data abstraction instrument had been originally designed to assess public health interventions, it was adapted for assessing reports from longitudinal studies. The articles selected for the review, were divided between three reviewers (SRS, RP, and AJ) for data abstraction. Two reviewers independently coded qualitative and quantitative data from each selected article.
Study quality was graded using the Newcastle-Ottawa scale designed to assess longitudinal studies.38 It took into account factors of representativeness, comparability, and outcome. The scale included assessment of the suitability of study design and quality of study execution to determine each study’s utility to answer the research questions. At any step of the review, when discrepancies occurred, primary and secondary reviewers discussed the discrepancy to achieve consensus. Additional reviewers were consulted if needed.
Results were synthesized qualitatively. Utilizing a conservative approach, only results that were statistically significant (p<0.05) in analyses adjusted for potential confounders were considered to be different from the null. Negative association was defined as association between prenatal marijuana exposure and diminished neuropsychological function (e.g., lower score for verbal development and higher score for inattention). Positive association was defined as association between prenatal marijuana exposure and enhanced neuropsychological function (e.g., higher score for attention and lower score for impulsivity). High heterogeneity of assessment tools, analytical approaches and reported effect sizes precluded a quantitative assessment of publication bias and meta-analysis.
Results
Twenty-one articles were eligible for review and analysis (Table 1); the review process determined that these articles were based on data from 7 distinct longitudinal studies. There were 4 US studies: Maternal Health Practices and Child Development Project (MHPCD; 1982–1997) in Pennsylvania, a study of prenatal cocaine exposure in Ohio (1994–2003), a study of developmental effects of prenatal substance exposure in New Jersey and Pennsylvania (1993–2004), and the National Maternal and Infant Health Survey (NMIHS; 1988–1991). The other 3 studies included were the Ottawa Prenatal Prospective Study (OPPS; 1978–1995) in Canada; the Infant Development, Environment and Lifestyle Study (IDEAL; 2001–2008) in New Zealand; and a study in Jamaica (1983–1990). All the studies were of high quality (ranked 7 to 9 out of 9 stars) based on the Newcastle-Ottawa Scale (Table 2).38
Table 1.
Methodology summary of the eligible articles.
| Author, year Study |
Sample sizes (at
follow-up/recruited) Age at follow-up |
Analyses comparison groups by marijuana exposure | Prenatal exposure to substances other than marijuana and other covariates that analyses controlled for. | Study biases and limitations (reported by the authors and additionally identified by the reviewers) |
|---|---|---|---|---|
| Hayes, 1991 Jamaica study |
54/59 4 and 5 years |
Non-users Light users (<10 marijuana cigars or ‘spliffs’ per week) Moderate (11–20) Heavy (21–70) |
Quality of Housing Index | (a) Results may not be generalizable as sample consisted of lower income rural women. (b) Impossible to distinguish between exposure effects of marijuana and child’s environment. (c) Authors do not provide results of the analyses for 5-year-olds. |
| Fried, 1988 OPPS |
153/217 1 year and 2 years |
Joints per week (range 0–153, mean 15
in 12-month sample, and 18 in 24-month sample) Heavy use (>5 joints/week). |
Cigarettes, alcohol Family income, maternal age and education, maternal caffeine, protein, and caloric intake during pregnancy, difficulties during pregnancy, maternal and paternal health history, exposure to X-rays or rubella, gestation, birth weight, parity, method of feeding, and the HOME scale. |
(a) Prenatal marijuana use was confounded by nicotine and alcohol use. (b) Study results may not be generalizable. |
| Fried, 1990 OPPS |
133/190 3 and 4 years |
Infrequent/ no use Moderate (> 1 - < 6 joints/week) Heavy (≥ 6 joints/week) |
Cigarettes, alcohol Family income, mother’s weight and pregnancy weight gain, age, education, nutrition, and the two drugs not of primary interest. Perinatal controls were sex, parity, gestation, birth weight, and HOME scale |
(a) Volunteer subjects are a low risk sample which may represent a conservative estimate of drug effects. (b) Variance explained by maternal drug use was relatively small compared with the HOME test. |
| Fried, 1992 OPPS |
139/190 5 and 6 years |
Infrequent/no use (≤1
joint/week) Moderate (>1 - <6 joints/week) Heavy (≥6 joints/week) |
Cigarettes, alcohol Family income, mother’s pregnancy weight and pregnancy weight gain, mother’s age at delivery, average level of parental education, parental relationship, predominant language spoken by the child, child’s gender, HOME scale, and the two drugs not of primary interest |
(a) Instruments that provide a general description of cognitive abilities may not be capable of identifying nuances in neuro-behavior that may discriminate between marijuana and non-marijuana exposed children. (b) Very low risk sample which may represent a conservative estimate of drug effects. (c) Potency of marijuana preparations has increased several fold since the entrance of pregnant women in the study. |
| Fried, 1992 OPPS |
127/190 6 years |
Infrequent/no use Moderate (>1 - <6 joints/week) Heavy (≥6 joints/week) |
Cigarettes, alcohol Family income, mother’s pre-pregnancy weight, mother’s caffeine intake and nutrition during pregnancy, pregnancy difficulties, mother’s age at delivery, average level of parental education, parity, child’s sex, predominant language spoken by child, parental relationship, the two drugs not of primary interest and the HOME scale |
(a) Fetal drug exposure measurements do not distinguish timing of exposure or account for sporadic heavy use during pregnancy. (b) Measures of home environment were not statistically associated with attention-related outcomes, other postnatal factors not assessed may influence child’s performance on these tasks. (c) Interaction between drug exposure and parenting and/or personality is currently being investigated in this sample. |
| O’Connell,
1991 OPPS |
56/56 6–9 years |
Non-users Users (>1 joint/week) Reported range 1.5–50 joints/week (mean=14.4, standard deviation=15) |
Cigarettes, alcohol Mother’s age at delivery, mother’s education level, father’s education level, highest occupational status of parents, family income, number of parents in the home, the number of parents working outside the home, the number of children in the family, the birth order of the subject child, the principal language of the home, the principal language of instruction in school, presence of problems in school, history of eye and/or ear infections, the need for visual correction, the presence of special conditions at the time of testing, and HOME scale |
(a) Home environment measures are viewed as legitimate outcomes, rather than potential confounders. (b) Low risk sample which may represent a conservative estimate of drug effects. (c) Potency of marijuana preparations has increased since the entrance of pregnant women in the study. |
| Fried, 1997 OPPS |
146/190 9–12 years |
Infrequent/no use (≤1
joints/week) Moderate (>1 - <6 joints/week) Heavy (≥6 joints/week) |
Cigarettes, alcohol Family income, mother’s age at delivery, mother’s weight before pregnancy, mother’s total pregnancy weight gain, average level of parental education, other maternal drug use, and prenatal passive smoke exposure. Postnatal variables: sex of the child, the home environment, the mother’s personality, child’s level of depression and anxiety, secondhand smoke exposure of child, and current maternal sociodemographic characteristics and marijuana use at the time of child’s testing. |
(a) Small number of subjects in the group of children exposed to moderate marijuana use limits confidence in the results. |
| Fried, 1998 OPPS |
146/190 9–12 years |
No use Infrequent/moderate (>0 - <6 joints/week) Heavy (≥6 joints/week) |
Cigarettes, alcohol Family income, mother’s age at delivery, mother’s weight before pregnancy, average level of parental education, other maternal drug use, and prenatal passive smoke exposure. Postnatal variables: sex of the child, home environment, mother’s personality, child’s level of depression and anxiety, secondhand smoke exposure of the child, current maternal sociodemographic characteristics and marijuana use at the time of child’s testing. |
(a) Some mothers continued to use marijuana after the pregnancy (b) Data had extreme univariate outliers (z score >4): 2 marijuana and alcohol, one nicotine value. |
| Fried, 2000 OPPS |
146/190 9–12 years |
No use Infrequent/moderate (>0 - <6 joints/week) Heavy (≥6 joints/week) |
Cigarettes, alcohol Average level of parental education, other maternal drug use, prenatal passive smoke exposure, and sex of the baby. Postnatal variables: home environment, current socioeconomic status, child’s gender, and the environmental tobacco smoke exposure of the child. |
(a) Could not categorize marijuana use into three levels due to inadequate cell size (delineated into heavy use and infrequent or moderate or no use). (b) Unclear whether deficits observed in visuoperceptual tasks are due to the perceptual demands of these tests or due to one or more non-perceptual requirements that are necessary for their successful performance. |
| Day, 1994 MHPCD |
672/763 3 years |
Average daily number of joints (ranges: 0–8.8 in first trimester, 0–6.5 in second trimester, and 0–9.4 in third trimester) | Alcohol, tobacco, amphetamines, tranquilizers,
heroin, cocaine Maternal education, current work status, family income, home environment, number and distance in age between siblings, maternal levels of: depression, anxiety, hostility, self-esteem, mother’s perception of how difficult the child was |
(a) Only 55% of the children completed the quantitative reasoning subscale. (b) Significant differences between non-completion and age at assessment were found, but not by prenatal marijuana exposure. (c) The effects reported are not clinically significant for an individual. |
| Leech, 1999 MHPCD |
608/763 6 years |
Abstainers >0 to <0.4 joint/day 0.4 to <1 joint/day ≥1 joint/day |
Alcohol, tobacco, amphetamines, tranquilizers,
heroin, cocaine Child Characteristics: age at assessment, gender, number of hospitalizations, number of illnesses, race Environmental Characteristics: Home Screening Questionnaire, male in household, maternal work/school status Maternal Characteristics: Hostility, life events, maternal age |
(a) The Continuous Performance Test varies across studies in terms of modality (visual, auditory), type of stimulus (color, letter, number, animal), and difficulty of task. It may not have been difficult enough, did not allow comparison of different types of commission errors, and did not include a measure of reaction time. (b) All subjects were assessed by Stanford-Binet test but results were not reported by marijuana exposure status. |
| Goldschmidt,
2008 MHPCD |
648/763 6 years |
Abstainers/light/ moderate (≥0 and <1 joint/day) Heavy (≥1 joint/day) |
Alcohol, tobacco, amphetamines, tranquilizers,
heroin, cocaine. Maternal variables: cognitive ability, age at delivery, ethnicity, current level of education, income, work status, marital status, depression, hostility, social support, number of life events Environmental variables: total number of people in the household, drug and alcohol problems of the man in the household, current home environment Child variables: sex, nutrition, number of siblings, poor speech/vision/hearing, number of injuries, hospitalizations, and illnesses |
(a) The sample was predominantly of lower socio-economic status. |
| Goldschmidt,
2000 MHPCD |
636/763 10 years |
First trimester
users: Abstainers Light/moderate (0–0.89 joints/day) Heavy (>0.89 joints/day) Second/third trimester users: No use Light use (0–0.4 joints/day) Moderate/heavy (>0.4 joints/day) |
Alcohol, tobacco, amphetamines, tranquilizers,
heroin, cocaine Maternal variables: Number of years of education, working/studying outside the home, monthly family income, race/ethnicity, presence of husband or boyfriend in the household, depression, hostility, and number of reported life events Child’s environment variables: cognitive stimulation and emotional support provided by the child’s family, level of overt aggression among family members, number of siblings, child in maternal custody, gender, age, number of illnesses, number of injuries |
(a) Mothers reported 21 children (3.3%) taking medication for attention attention-deficit/hyperactivity disorder. |
| Richardson,
2001 MHPCD |
636/763 10 years |
No use Light (0–0.4 joints/day) Moderate (>0.4–0.89 joints/day) Heavy (>0.89 joints/day) |
Alcohol, tobacco, amphetamines, tranquilizers,
heroin, cocaine Maternal variables: education, monthly family income, race Child characteristics: Age, anxiety, gender, cognitive stimulation and emotional support provided by the child’s family, uncorrected vision problems |
(a) Magnitude of marijuana effects was small and limited to only a few aspects of functioning. (b) Difficult to compare Continuous Performance Test measure of inattention to parental reports of inattention. (c) Possible that marijuana effects on these and additional domains may be found when the children reach 14 years of age. |
| Goldschmidt,
2004 MHPCD |
636/763 10 years |
Light/moderate (<1
joints/day) Heavy (≥1 joints/day). |
Alcohol, tobacco, amphetamines, tranquilizers,
heroin, cocaine Maternal variables: age, education, family income, presence of an adult male in the household, ethnicity, working status, depression, hostility, number of life events, and support from friends and relatives Child characteristics: home environment, child in maternal custody, number of siblings, age between oldest and youngest child, child’s gender |
(a) Variables such as motivation, social skills, and parent involvement in child’s education were not taken into account. (b) Generalizability is somewhat limited as cohort is low-income and only women who had received prenatal care. |
| Faden, 2000 NMIHS |
8285/9953 3 years |
No
use <1/month 1/month 2–3/month 1–2/week >3/week |
Alcohol, tobacco (cocaine use collected but
too rare to be analyzed) Birthweight, child’s exact age in months, child’s sex, mother’s race, mother’s level of education, and mother’s Hispanic status |
(a) Parental report and self-report of marijuana use may cause reporting bias. (b) Biased estimates of effects from exposure – important covariates left out or incorrectly modeled in the regression analysis. |
| Noland, 2003 Prenatal cocaine exposure study |
316/415 4 years |
Exposed Unexposed No/light use Heavier use (>5 joints per week) |
Cocaine, alcohol, tobacco Race, gender, birth mother characteristics (age, education, verbal ability, block design and picture completion, SES, psychiatric symptoms, and marital status), and current caregiver characteristics (education, verbal ability score, block design and picture completion, SES, psychiatric symptoms, marital status, and HOME interview). |
(a) Prenatal marijuana exposure effect on speeded and organized responding may not be apparent until subsystem develops more fully. (b) Atypical levels of gestational stress associated with sample may limit generalizability. |
| Noland, 2005 Prenatal cocaine exposure study |
330/415 4 years |
Exposed Unexposed No/light use Heavier use (>5 joints per week) |
Cocaine, alcohol, tobacco Gender, African-American ethnicity of birth mother, maternal age at birth, parity, prenatal care visit(s), maternal years of education, marital status, low socioeconomic status, biological and current caregiver mental functioning variables |
(a) Prenatal substance exposure accounts for a very small percent of the variance in performance. |
| Bennett, 2008 Developmental effects of prenatal substance exposure study |
185/231 4, 6 and 9 years |
0
joints/day 0.01–0.5/day 0.51–1/day >1/day |
Cocaine, alcohol, cigarettes, opiates, phencyclidine, tranquilizers, amphetamines, barbiturates Environmental risk, maternal verbal intelligence, gender, and neonatal health problems |
(a) Main focus of study was cocaine exposure. (b) Maternal marijuana use was associated with cocaine, alcohol and tobacco use. (c) Results are not generalizable as study enrolled a convenience sample of urban, predominantly African American and low socio-economic status population. (d) Other environmental factors may have affected children’s IQ but were not controlled for in this study. |
| Carmody, 2011 Developmental effects of prenatal substance exposure study |
210/321 6, 9 and 11 years |
Joints/day (range
0.022–0.497) |
Cocaine, alcohol, cigarettes, opiates, phencyclidine, tranquilizers, amphetamines, barbiturates Environmental risk, medical complications, and gender |
(a) Main focus of study was cocaine exposure. (b) Maternal marijuana use was associated with cocaine, alcohol and tobacco use. (c) Results are not generalizable as study enrolled a convenience sample of urban, predominantly African American and low socio-economic status population. (d) Other environmental factors may have affected children’s IQ but were not controlled for in this study. |
| Chakraborty,
2015 IDEAL |
165/170 4.5 years |
Frequency of use (days per
week): <1 1–4 5–7 Amount of drug (joint per occasion): Light (<1) Moderate (1–2) Heavy (>2) |
Methamphetamine,
nicotine, alcohol Sex, ethnicity, stereoacuity, visual acuity, and verbal IQ |
(a) Results cannot be extrapolated beyond global motion perception or interpreted as marijuana having beneficial effects on fetal development. (b) Average motion coherence thresholds reported for non-drug exposed children are slightly elevated (worse) compared to previous studies of global motion perception in preschool children. (c) Study has a small sample size in which the majority of participants were poly drug users. |
OPPS – Ottawa Prenatal Prospective Study, Canada
MHPCD – Maternal Health Practices and Child Development Project, USA, Pennsylvania
NMIHS – National Maternal and Infant Health Survey, USA
IDEAL – Infant Development, Environment, and Lifestyle study, New Zealand
IQ – Intelligence quotient
Table 2.
Newcastle-Ottawa scale assessment of the eligible studies.
| # (by study/year of publication) | Star cate-go-ries | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study or location | Jamaica | OPPSa | MHPCDa | NMIHS | OHa | NJ/PAa | IDEAL | |||||||||||||||
| Author, year | Hayes, 1991 | Fried, 1988 | Fried, 1990 | Fried, 1992 | Fried, 1992 | O’Connell, 1991 | Fried, 1997 | Fried, 1998 | Fried, 2000 | Day, 1994 | Leech, 1999 | Goldschmidt, 2008 | Goldschmidt, 2000 | Richardson, 2001 | Goldschmidt, 2004 | Faden, 2000 | Noland, 2003 | Noland, 2005 | Bennett, 2008 | Carmody, 2011 | Chakraborty, 2015 | |
| 1) Representativeness of the exposed cohort | ||||||||||||||||||||||
| a) Truly representative of the average demographics in the community | * | * | ||||||||||||||||||||
| b) Somewhat representative of the average demographics in the community | * |
* (Rural lower income community) |
* (Lower income population) |
* (Lower income) |
* (Lower income and educa-tion) |
|||||||||||||||||
| c) Selected group of users, e.g. nurses, volunteers | Volunteers responding to advertisement of the study, low-risk sample | Volun-teers | ||||||||||||||||||||
| d) No description of the derivation of the cohort | ||||||||||||||||||||||
| 2) Selection of the non-exposed cohort | ||||||||||||||||||||||
| a) Drawn from the same community as the exposed cohort | * | * | * | * | * | * | * | * | ||||||||||||||
| b) Drawn from a different source | ||||||||||||||||||||||
| c) No description of the derivation of the non-exposed cohort | ||||||||||||||||||||||
| 3) Ascertainment of exposure | ||||||||||||||||||||||
| a) Secure record (e.g. surgical records) | * | *b | * | * | * | |||||||||||||||||
| b) Structured interview | * | * | * | * | ||||||||||||||||||
| c) Written self-report | ||||||||||||||||||||||
| d) No description | ||||||||||||||||||||||
| 4) Demonstration that outcome of interest was not present at start of studyc | ||||||||||||||||||||||
| a) Yes | * | * | * | * | * | * | * | * | ||||||||||||||
| b) No | ||||||||||||||||||||||
| Comparability | ||||||||||||||||||||||
| 1) Comparability of cohorts on the basis of the design or analysis | ||||||||||||||||||||||
| a) Study controls for _exposure to tobacco_ | * | * | * | * | * | * | * | * | ||||||||||||||
| b) Study controls for any additional factor | * | * | * | * | * | * | * | * | ||||||||||||||
| Outcome | ||||||||||||||||||||||
| 1) Assessment of outcome | ||||||||||||||||||||||
| a) Independent blind assessment | * | * | * | * | * | * | * | * | ||||||||||||||
| b) Record linkage | * | |||||||||||||||||||||
| c) Self-report | ||||||||||||||||||||||
| d) No description | ||||||||||||||||||||||
| 2) Was follow-up long enough for outcomes to occur? | ||||||||||||||||||||||
| a) Yes | * | * (5y) | * (1–2y) | * (3–4y) | * (5–6y) | * (6y) | * (6–9y) | * (9–12y) | * (9–12y) | * (9–12y) | * (3y) | * (6y) | * (6y) | * (10y) | * (10y) | * (10y) | * (3y) | * (4y) | * (4y) | * (4–9y) | * (6–11y) | 8 (4.5y) |
| b) No | ||||||||||||||||||||||
| 3) Adequacy of follow up of cohorts | ||||||||||||||||||||||
| a) Complete follow up – all subjects accounted for | * | * | ||||||||||||||||||||
| b) Subjects lost to follow up unlikely to introduce bias – small number lost - > 80% follow up, or description provided of those lost | * | *(92%) | * (88%) | * (80%) | * (85%) | * (83%) | * (83%) | * (83%) | * (83%) | * (80%) | * (80%) | * (97%) | ||||||||||
| c) Follow up rate < 80% and no description of those lost | 71% | 70% | 73% | 67% | 77% | 77% | 77% | 76% | 65% | |||||||||||||
| d) No statement | ||||||||||||||||||||||
| Total score: | 8.5 | 7 | 7 | 7 | 7 | 8 | 7 | 7 | 7 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 9 | 8 | 7 | 7 | |
Article has met requirement to be awarded a star (1 score point)
Article’s standing in an assessment category, when no star is awarded
When all articles belonging to a single study received same score, the cells were combined to save space.
Study has met the requirement to be awarded the star, however, this information was not in the reviewed article, and was found in a different publication that did not meet eligibility criteria for this review (.5 score point)
As outcome of interest is affected cognitive development, all articles were awarded star for this question since all studies had enrolled participants at birth.
OPPS – Ottawa Prenatal Prospective Study, Canada
MHPCD – Maternal Health Practices and Child Development Project, Pennsylvania, USA
NMIHS – National Maternal and Infant Health Survey, USA
OH – Prenatal cocaine exposure study, Ohio, USA
NJ/PA – Developmental effects of prenatal substance exposure study, New Jersey/Pennsylvania, USA
IDEAL - Infant Development, Environment, and Lifestyle study, New Zealand
Each of the 7 studies utilized a variety of instruments to assess children’s neuropsychological outcomes (Table 3). Instruments varied from very specific, measuring only one function (e.g., pegboard test measuring manual dexterity) to complex multi-scale tools assessing intelligence and various cognitive domains (e.g., Stanford-Binet Intelligence Scale measuring intelligence quotient (IQ), memory, visual reasoning, quantitative reasoning, and verbal reasoning). Six studies applied one of the commonly used comprehensive intelligence or academic achievement tests administered by trained professionals who were blinded to children’s prenatal history. The tests included the following: Wechsler Intelligence Scale for Children (WISC) (OPPS, IDEAL and prenatal cocaine exposure study);39 the Stanford-Binet Intelligence Test (MHPCD and developmental effects of prenatal substance exposure studies);40 the McCarthy Scales of Children’s Abilities (OPPS, Jamaica study and prenatal cocaine exposure study);41 and the Wide Range Achievement Test (OPPS and MHPCD).42 One study (NMIHS) relied only on parental reports based on the Denver Developmental Scale.43
Table 3.
Scope of diagnostic tests and outcomes in the eligible studies.
| Diagnostic instrument | Outcomes measured | Reported associations with prenatal marijuana exposurea | Age at assessment | Article (Study, first author, year) |
|---|---|---|---|---|
| Bayley Scale of Infant Development | Mental Development Index: sensory/perceptual
abilities, acquisition of object constancy, memory, learning, problem
solving, vocalization and beginning of verbal
communication Psychomotor Development Index: degree of body control, large muscle coordination, finer manipulatory skills of the hands and fingers, dynamic movement, postural imitation, and the ability to recognize objects by sense of touch (stereognosis) |
No significant associations | 1 year and 2 years | OPPS, Fried, 1988 |
| Infant Behavior Record | Primary Cognition Composite Score: object orientation, goal directedness, attention span, reactivity, and vocalization | Positive association | 1 year | OPPS, Fried, 1988 |
| No results reported. | 2 years | OPPS, Fried, 1988 |
||
| Extraversion Score: social orientation to the
examiner, cooperativeness, and general emotional tone Visual and auditory sensory systems |
No associations | 1 year and 2 years | OPPS, Fried, 1988 |
|
| Reynell Developmental Language Scale | Comprehension | Negative association | 2 years | OPPS, Fried, 1988 |
| Expression | No associations | 2, 3, and 4 years | OPPS, Fried, 1988; 1990 | |
| Denver Developmental Scale | Gross motor development | Negative association | 3 years | NMIHS, Faden, 2000 |
| Adaptive functioning, language, and fine motor development | No associations | |||
| McCarthy Scales of Children’s Abilities | General Cognitive Index (memory, verbal development, perception, and quantitative abilities) | No associations | 3 years | OPPS, Fried, 1990 |
| Negative association | 4 years | OPPS, Fried, 1990 | ||
| No associations | 4, 5 and 6 years | Jamaica study, Hayes, 1991; OPPS, Fried, 1992 |
||
| Motor performance score | Positive association in moderately exposed children compared to unexposed and heavily exposed | 3 years | OPPS, Fried, 1990 |
|
| No associations | 4, 5 and 6 years | OPPS, Fried, 1990; 1992; Jamaica study, Hayes, 1991 |
||
| Memory score | No associations | 3 years | OPPS, Fried, 1990 | |
| Negative association | 4 years | OPPS, Fried, 1990 | ||
| No associations | 4, 5 and 6 years | Jamaica study, Hayes, 1991; OPPS, Fried, 1992 |
||
| Verbal score | No associations | 3 years | OPPS, Fried, 1990 | |
| Negative association | 4 years | OPPS, Fried, 1990 | ||
| No associations | 4, 5 and 6 years | Jamaica study, Hayes, 1991; OPPS, Fried, 1992 |
||
| Quantitative score | Negative association | 3 years | OPPS, Fried, 1990 | |
| No associations | 4 years | OPPS, Fried, 1990 | ||
| No associations | 4, 5 and 6 years | Jamaica study, Hayes, 1991; OPPS, Fried, 1992 |
||
| Perceptual score | No associations | 3 years | OPPS, Fried, 1990 | |
| Negative association | 4 years | OPPS, Fried, 1990 | ||
| No associations | 4, 5 and 6 years | Jamaica study, Hayes, 1991; OPPS, Fried, 1992 |
||
| McCarthy Scales of Children’s Abilities subset adapted for use with children 3 to 12 years of age, truncated. | Category fluency (language development) | No associations | 4 years | Prenatal cocaine exposure study, Noland, 2003 |
| Stanford-Binet Intelligence Scale, 4 Ed. | Composite score (IQ) | Negative association | 3 years | MHPCD, Day, 1994 |
| No associations | 4, 6 and 9 years | Developmental effects of prenatal substance exposure study, Bennett, 2008 | ||
| Not reported | 6 years | MHPCD, Leech, 1999 | ||
| Negative association | 6 years | MHPCD, Goldschmidt, 2008 | ||
| Short-term memory | Negative association | 3 years | MHPCD, Day, 1994 | |
| No associations | 4, 6 and 9 years | Developmental effects of prenatal substance exposure study, Bennett, 2008 | ||
| Negative association | 6 years | MHPCD, Goldschmidt, 2008 | ||
| Verbal reasoning | Negative association | 3 years | MHPCD, Day, 1994 | |
| No associations | 4, 6 and 9 years | Developmental effects of prenatal substance exposure study, Bennett, 2008 | ||
| Negative association | 6 years | MHPCD, Goldschmidt, 2008 | ||
| Quantitative reasoning | No associations | 3 years | MHPCD, Day, 1994 | |
| No associations | 4, 6 and 9 years | Developmental effects of prenatal substance exposure study, Bennett, 2008 | ||
| Negative association | 6 years | MHPCD, Goldschmidt, 2008 | ||
| Abstract/visual reasoning | Negative association | 3 years | MHPCD, Day, 1994 | |
| No associations | 4, 6 and 9 years | Developmental effects of prenatal substance exposure study, Bennett, 2008 | ||
| No associations | 6 years | MHPCD, Goldschmidt, 2008 | ||
| Wechsler Preschool and Primary Scale of Intelligence, III | Verbal IQ, attention | Not reported | 4 years | Prenatal cocaine exposure study, Noland, 2003; 2005 |
| No associations | 4.5 years | IDEAL, Chakraborty, 2015 | ||
| Finger sequencing task adapted by Welsh for use with children. | Motor planning | No associations | 4 years | Prenatal cocaine exposure study, Noland, 2003 |
| Pegboard test | Manual dexterity and bimanual coordination | No associations | 4 years | OPPS, Fried, 1990 |
| Picture deletion task for preschoolers-modified (Corkum) | Attention | Non-significant negative association | 4 years | Prenatal cocaine exposure study, Noland, 2005 |
| Tactile Form Recognition Task | Stereognosis | No associations | 4 years | OPPS, Fried, 1990 |
| Tapping Inhibition (test of frontal lobe functioning (Luria) adapted by Diamond and Taylor for use with children 3.5 through 7 years of age) | Inhibitory control (Ability to override their natural, habitual, or dominant behavioral response to a stimulus in order to implement more adaptive goal-oriented behaviors) | No associations | 4 years | Prenatal cocaine exposure study, Noland, 2003 |
| Peabody Picture Vocabulary Test | Vocabulary | Negative association | 4 years | OPPS, Fried, 1990 |
| No associations | 5 years, 6 years and 9–12 years | OPPS, Fried, 1992; 1997 | ||
| Random Dot Kinematograms | Global motor perception (higher-level processing in visual cortex) | Positive association in children who were not prenatally exposed to alcohol. | 4.5 years | IDEAL, Chakraborty, 2015 |
| Conners Parent Questionnaire |
Impulsivity hyperactivity | Non-significant negative association | 6 years | OPPS, Fried, 1992 |
| No associations | 6–9 years | OPPS, O’Connell, 1991 | ||
| Hyperactivity index, learning problems, and psychosomatic problems | No associations | 6–9 years | OPPS, O’Connell, 1991 | |
| Anxiety, conduct problems | Non-significant negative association | 6–9 years | OPPS, O’Connell, 1991 | |
| Gordon Diagnostic System | Sustained attention and self-control | Negative association | 6 years | OPPS, Fried, 1992 |
| Impulsivity | Negative association | 9–12 years | OPPS, Fried, 1998 | |
| Continuous Performance Task | Errors of commission
(impulsivity) |
Not reported | 4 years | Prenatal cocaine exposure study, Noland, 2005 |
| Negative association | 6 years and 10 years | MHPCD, Leech, 1999; Richardson, 2001 | ||
| Errors of omission (inattentiveness) | Not reported | 4 years | Prenatal cocaine exposure study, Noland, 2005 | |
| Positive association | 6 years | MHPCD, Leech, 1999 | ||
| The Sentence Memory Test | Immediate auditory memory and auditory attention for sentences | No associations | 6 years | OPPS, Fried, 1992 |
| The Target Test | Visual-spatial memory | No associations | 6 years | OPPS, Fried, 1992 |
| The Yale Child Study Center Attention Task | Attention and inhibitory control | No associations | 6, 9, and 11 years | Developmental effects of prenatal substance exposure study, Carmody, 2011 |
| Test of Visual-Perceptual Skills | Perceptual Quotient, Visual Discrimination, Visual Sequential Memory | Non-significant negative association | 6–9 years | OPPS, O’Connell, 1991 |
| No associations | 9–12 years | OPPS, Fried, 2000 | ||
| Visual Closure, Visual Figure Ground, Visual Form Constancy, Visual Memory, and Visual Spatial Relations | No associations |
6–9 and 9–12 years |
OPPS, O’Connell, 1991; Fried, 2000 | |
| Trail Making Test | Visual scanning, visuospatial sequencing, attention, mental flexibility, and motor function | Non-significant negative association | 6–9 years | OPPS, O’Connell, 1991 |
| No associations | 9–12 years | OPPS, Fried, 2000 | ||
| Wide Range Achievement
Test-revised |
Reading, arithmetic, spelling | No associations | 6–9 years | OPPS, O’Connell, 1991 |
| Negative association | 10 years | MHPCD, Goldschmidt, 2004 | ||
| Reading | No associations | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1997 | |
| Knox Cube Test | Visual attention, visual memory and visual sequencing | No associations |
6–9 and 9–12 years |
OPPS, O’Connell, 1991; Fried, 2000 |
| Woodcock Reading Mastery Test | Passage comprehension | No associations | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1997 |
| Beery Developmental Test of Visual Motor Integration | Visual-motor integration (copy geometric forms into a notepad) | No significant associations | 6–9 years | OPPS, O’Connell, 1991 |
| No associations | 9–12 years | OPPS, Fried, 2000 | ||
| Draw a man | Intelligence (score is based on detail, proportion and coordination) | No significant associations | 6–9 years | OPPS, O’Connell, 1991 |
| Finger tapping | Motor control, speed and lateral coordination | No significant associations | 6–9 years | OPPS, O’Connell, 1991 |
| Stroop Interference | Tests the ability to sort and selectively react to information: e.g. word ‘red’ is printed in green ink. The child must say loudly the color of the text and not the word. | Non-significant negative association | 6–9 years | OPPS, O’Connell, 1991 |
| Test of Language Development (Primary syntax quotient score) | Ability to generate acceptable sentences | Non-significant negative association | 6–9 years | OPPS, O’Connell, 1991 |
| Auditory Working Memory | Working memory | Non-significant negative association | 9–12 years | OPPS, Fried, 1998 |
| Category Test | Problem-solving capacity | Negative association | 9–12 years | OPPS, Fried, 1998 |
| Fluency Test | Verbal fluency (number of words starting with ‘C’ and ‘P’ produced in 60 sec) | No associations | 9–12 years | OPPS, Fried, 1997; 1998 |
| Oral Cloze Task | Ability to understand the basic grammatical structure of English based on auditory process | No associations | 9–12 years | OPPS, Fried, 1997 |
| Pseudoword Task | Reading and decoding abilities | Non-significant negative association in children with moderate exposure compared to children with no or heavy exposure | 9–12 years | OPPS, Fried, 1997 |
| Seashore Rhythm Test | Rhythm discrimination | No associations | 9–12 years | OPPS, Fried, 1997 |
| Tactile Performance Task | Motor abilities and motor memory (blindfolded, place wooden blocks into properly shaped holes) | No associations | 9–12 years | OPPS, Fried, 1998 |
| Wechsler Intelligence Scale for
Children, 3rd ed. |
Full scale IQ | No associations | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1997; 1998; 2000 |
| Information | No associations | 9–12 years | OPPS, Fried, 1997 | |
| Non-significant negative association | 9–12 years | OPPS, Fried, 1998 | ||
| Verbal IQ, Verbal Comprehension Index | No associations | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1997; 1998 | |
| Similarities, Vocabulary | No associations | 9–12 years | OPPS, Fried, 1997; 1998 | |
| Performance IQ | No associations | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1998 | |
| Arithmetic, Processing Speed Index | No associations | 9–12 years | OPPS, Fried, 1998 | |
| Freedom from Distractibility Index | No associations | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1998; 2000 | |
| Coding (discrimination and memory of visual symbols); Symbol Search (visual scanning), and Digit Span (memory) | No associations | 9–12 years | OPPS, Fried, 1998; 2000 | |
| Comprehension | Negative association | 9–12 years | OPPS, Fried, 1998 | |
| Mazes (rudimentary
planning) |
Negative association | 9–12 years | OPPS, Fried, 1998 | |
| No associations | 9–12 years | OPPS, Fried, 2000 | ||
| Perceptual Organization Index (a summary index of picture completion, picture arrangement, block design, and object assembly) | No association | 6–9 and 9–12 years | OPPS, O’Connell, 1991; Fried, 1998 | |
| Negative association | 9–12 years | OPPS, Fried, 2000 | ||
| Object Assembly | Negative association | 9–12 years | OPPS, Fried, 1998; 2000 | |
| Block Design | Negative association | 9–12 years | OPPS, Fried, 1998; 2000 | |
| Picture Arrangement | No associations | 9–12 years | OPPS, Fried, 1998; 2000 | |
| Picture Completion | No associations | 9–12 years | OPPS, Fried, 1998; 2000 | |
| Child Behavior Checklist | Attention problems | No associations | 10 years | MHPCD, Goldschmidt, 2000 |
| Severe discrepancy between ability (Stanford-Binet Intelligence Scale, 4ed.) | Underachieving | Negative association | 10 years | MHPCD, Goldschmidt, 2004 |
| Swanson, Noland, and Pelham Assessment | Impulsivity, hyperactivity, inattention symptoms | Negative association | 10 years | MHPCD, Goldschmidt, 2000 |
| Teacher’s Report Form | Attention problems | No associations | 10 years | MHPCD, Goldschmidt, 2000 |
| Teacher’s assessment of the child in language arts, history, math, and science. | Educational performance | Negative association | 10 years | MHPCD, Goldschmidt, 2004 |
| Peabody Individual Achievement Test-revised | Reading comprehension | Negative association | 10 years | MHPCD, Goldschmidt, 2004 |
| Wide Range Assessment of Memory and Learning | Design memory, screening index | Negative association | 10 years | MHPCD, Richardson, 2001 |
| Story memory, verbal learning | No association | 10 years | MHPCD, Richardson, 2001 |
Negative associations were defined as statistically significant association in analyses adjusted for potential confounders between prenatal marijuana exposure and diminished neuropsychological function, e.g. lower scores on reading comprehension or memory; or higher scores on errors, impulsivity, inattention, or underachievement (p<0.05), regardless of the trimester of exposure. Positive associations were defined as statistically significant associations in adjusted analyses between prenatal marijuana exposure and enhanced neuropsychological function, e.g. higher scores on reading comprehension or memory; or lower scores on errors, impulsivity, inattention, or underachievement (p<0.05), regardless of the trimester of exposure. Non-significant negative and positive associations were defined as statistically significant negative or positive associations found in bivariate analyses but not in adjusted analyses (p≥0.05). No association the analyses did not find associations between prenatal marijuana exposure and neuropsychological functions in bivariate and adjusted analyses.
Most analyses found no associations between prenatal marijuana exposure and children’s outcomes or found associations that were significant in bivariate analyses but not in adjusted analyses (Table 3). Table 1 lists comparison groups and covariates that each article used for adjusted analyses.
Ottawa Prenatal Prospective Study
Eight of the articles reported on results of OPPS (Tables 1, 2 and 3). This was a longitudinal study of the effects of prenatal marijuana, cigarette, and alcohol use in offspring in a mostly low-risk, middle class population of the Ottawa area, Ontario, Canada.44–51 Recruitment took place through advertisement in media and obstetricians’ offices. Analyses of the children at ages 1 and 2 years found no associations of prenatal marijuana exposure and cognitive outcomes, but found that prenatal marijuana use was associated (p<0.05) with higher scores on the 1-year-old Primary Composite score of the Infant Behavior Record that assessed interests and attitudes (i.e., that children exposed in utero had higher developmental levels than children who were not exposed).44 At ages 3 and 4 years, McCarthy quantitative scores were lower among children with heavy prenatal marijuana exposure before adjustment for confounding, but moderate marijuana exposure correlated to superior motor performance on the McCarthy test, even after adjustment for confounders.45 There were no differences on a series of cognitive tasks (e.g., memory, verbal and perceptual scores) between 5–6 year-old children with and without prenatal marijuana exposure. 46,47 For children aged 6–9 years, there was no statistically significant relationship after adjustment between prenatal marijuana exposure and parental ratings of behavior problems, visual-perceptual tasks, language comprehension, or distractibility.48 Prenatal marijuana exposure was not associated with deficits in reading, language, or psychometrically determined intelligence in children aged 9–11 years.49,50 Prenatal marijuana exposure was negatively associated with performance in visual problem solving situations as measured by WISC Perceptual Organization Index in children aged 9–11 years.51 The Perceptual Organization Index assesses non-verbal reasoning and hypotheses testing drawing upon visual-perceptual skills.
Maternal Health Practices and Child Development Project
MHPCD findings were reported in 6 articles (Tables 1, 2 and 3).52–57 Participants in MHPCD were women of lower socioeconomic status, recruited from an outpatient prenatal clinic in Pittsburgh, Pennsylvania. Day et al. found no associations between prenatal marijuana exposure and the Stanford-Binet Intelligence Test performance at age 3 years.52 Goldschmidt et al. found heavy prenatal marijuana use statistically significantly associated with lower verbal and quantitative reasoning and with decreased short-term memory at age 6 years.53 Leech et al. found a statistically significant negative association between prenatal marijuana exposure and measures of impulsivity at age 6 years using a continuous performance task, but a positive association (p<0.05) with attention using this same task.54 At age 10 years, there were associations (p<0.05) between prenatal exposure to marijuana and child behavior problems and school achievements. Specifically, first and third trimester exposure to marijuana was associated with increased hyperactivity, inattention and impulsivity, and heavy second and third trimester exposure was associated with increased delinquency and externalizing behavior problems.55 Associations were reported between first trimester prenatal marijuana exposure and lower predicted reading and spelling scores, and between second trimester exposure and deficits in reading comprehension and underachievement, all at age 10 years (p<0.05).56 A similar analysis by Richardson and colleagues57 suggested that prenatal marijuana exposure was associated with increased impulsivity in ten-year-olds based on continuous performance task.
Prenatal cocaine exposure study
Two eligible articles used data from a longitudinal prospective study of the developmental effects of prenatal cocaine exposure conducted in Ohio (Tables 1, 2 and 3).58,59 Study participants were patients of a large urban hospital who had clinical indications of illicit drug use and had no private health insurance. At age 4 years, there was no relationship between prenatal marijuana exposure and performance on the tapping inhibition test, a measure of ability to resist acting impulsively;58 however, heavier prenatal marijuana use was associated with lower ability to maintain sustained attention.59
Developmental effects of prenatal substance exposure study
Two articles reported on results of the study of developmental effects of prenatal substance exposure with the focus on maternal cocaine use. This study recruited women from hospital-based prenatal clinics or hospitals in Trenton, NJ, or at the Medical College of Pennsylvania Hospital in Philadelphia, PA (Tables 1, 2 and 3).60,61 Neither article found statistically significant associations between marijuana exposure and child IQ, attention, or impulsivity at ages 6, 9 and 11 years.60,61
Jamaica study
One article examined data from a longitudinal study of children born to mothers living in rural areas of Jamaica and having low income. The women were recruited through fieldwork with the assistance of nurses from the Jamaican Ministry of Health antepartum clinics (Tables 1, 2 and 3).62 This study differed from the others as marijuana use was not confounded by use of other substances. In a sample of 4 and 5 year olds, Hayes and colleagues62 found no association between prenatal marijuana exposure and McCarthy Scales of Children’s Abilities scores measuring IQ, memory, verbal development, perception, and quantitative abilities.
National Maternal and Infant Health Survey
One article by Faden and colleagues analyzed data from NMIHS.63 This longitudinal follow-up survey was conducted by the Centers for Disease Control and Prevention. The survey sampled participants from live births occurring in 1988, based on race and/or birthweight strata, to look at poor pregnancy outcomes.64 Women from the 1988 survey were re-contacted and interviewed in 1991. Faden’s study differed from the others included in this review as child outcome was determined by self-report from the mothers via detailed questionnaires mailed after the birth and when the child reached age 3 years rather than by direct assessment (Tables 1, 2 and 3).63 Prenatal marijuana use was associated with increased fear, poorer gross motor development and shorter length of play at age 3 years, which impeded overall ability to get along with peers.63
Infant Development, Environment, and Lifestyle (IDEAL) Study
IDEAL is a prospective, controlled longitudinal study of prenatal methamphetamine exposure from birth to 36 months, conducted in the US and New Zealand (Tables 1, 2 and 3).65 Independent and hospital-based midwives recruited mothers. Among 4.5- year-olds from the New Zealand study population, prenatal marijuana exposure was found to be associated with improved global motion perception compared to non-exposed children (p=0.001).66 Global motion perception is ability to recognize speed and direction of moving objects and is linked to cognitive skills and social competence.67
Comment
Principal findings
Among the 21 reports completed from seven longitudinal studies, results varied on the association between prenatal marijuana exposure and child’s neuropsychological functioning. Several analyses found statistically significant associations between prenatal marijuana exposure and both decreased and increased neuropsychological functions, while others found no significant associations. These findings indicate that the specific effects of prenatal marijuana exposure remain unclear. However, while more research is warranted to clarify the specific effects of prenatal marijuana exposure, there were more instances of negative than positive associations among the articles, suggesting that exposure to marijuana may be harmful to neuropsychological functioning.18–21
The analyses that found positive associations suggested improved aspects of attention and perceptive abilities in exposed children aged 1 to 6 years. While the positive findings were statistically significant, it is important to note that cognitive testing on children aged ≤5 years, is typically not as reliable as testing performed when children are older and better able to communicate and understand the tasks presented to them.68,69 In contrast, the significant negative associations were mostly drawn from testing of children over 6 years old, and the majority of studies without statistically significant results still showed decrease in neuropsychological functions. These results suggest some potential adverse effects of prenatal marijuana exposure on attention and perceptive abilities, in addition to decreased general cognitive function, memory, impulse control, IQ, and reading comprehension especially in children aged >6 years.
Strengths of the study
While majority of data on prenatal marijuana exposure and neuropsychological outcomes in children come from only a few longitudinal studies, they are methodologically sound, with standardized outcome assessment, and high response and participant retention rates.44–63,66,70,71 Each study provides some higher level measure of the marijuana exposure: as average marijuana use per day/week (MHPCD, NMIHS, and developmental effects of prenatal exposure study) or smoking frequency (OPPS, prenatal cocaine study, and IDEAL). One study additionally provided timing of the exposure by trimester of pregnancy (MHPCD). These measures allowed distinction of a dose-response relationship of marijuana use. Heavy marijuana use had stronger associations and larger effect sizes compared to moderate and light use.45,53,55
Limitations of the data
However, despite these strengths, all of the studies used in this review were subject to several limitations. First, concurrent use of other substances was present among study participants, except the single Jamaican study of participants who used marijuana almost exclusively.62,72 Tobacco and alcohol were the most frequent. Prenatal nicotine exposure is a known determinant of negative health outcomes for children, and tends to be a significant confounder for marijuana research.73,69 Smoking tobacco during pregnancy can cause tissue damage affecting fetal brain development, and has been associated with negative behavioral and cognitive outcomes throughout the lifetime, including conduct disorder, attention-deficit/hyperactivity disorder, poor academic achievement, and cognitive impairment.74–77 Alcohol use may also be a source of confounding in this research, as fetal alcohol spectrum disorders (FASDs) can cause a variety of physical and cognitive impairments.78 Prenatal alcohol exposure is associated with deficits in memory, attention span, verbal learning, motor function, and a lower overall IQ.79 The prenatal cocaine exposure study and the developmental effects of prenatal substance exposure study examined concurrent use of cocaine, and the IDEAL study examined methamphetamine. Only three articles reported that self-reported drug use was confirmed by toxicology tests.58,59,61 While all of the articles in the review attempted to control for other substances use in their analyses, variation in measurements of other substance exposures, such as tobacco use, may skew outcomes attributable to marijuana, and statistical controls might not account for all potential confounding of the other substances since the interactive effects of exposures to different or multiple substances are not fully understood.
A second limitation is not controlling for postnatal maternal marijuana use and thus potentially mixing effects of prenatal and postnatal exposures. Only 6 of the reviewed articles explicitly stated adjusting analyses for postnatal maternal marijuana use.49,50,54,55,58,59 Five more articles listed postnatal maternal marijuana use as a potential confounder that did not meet requirements for inclusion into final analytic models.51–53,56,57 However, in future research postnatal maternal marijuana use might be better conceptualized as a mediator rather than a confounder. Temporal and causal relationships between prenatal and postnatal maternal marijuana use prenatally and neuropsychological functioning in children are plausible for mediation conceptualization.
A third limitation is the potential for bias arising from sample selection and response. For example, the MHPCD study consisted of mostly low-income women and the OPPS study consisted of a low-risk predominantly middle class sample. Moreover, with the exception of NIMS, no samples were representative of the general US population. Additionally, the pregnancies took place when recreational use was illegal and medicinal use was illegal in the locations of data collection, thus potentially resulting in underreporting.
A fourth limitation is publication bias due to possible selective publication of results. More specifically, comparability of the test results is limited by several factors, including the fact that tests were administered selectively utilizing subscales and adaptations in different age groups, and test results were not reported in a consistent manner. Moreover, at least three studies conducted WISC; however, not all presented the results of this particular test in association with marijuana exposure. Publishing WISC measures from all or most of the analyses, would have allowed for an individual patient data (IPD) meta-analysis. Employing IPD meta-analysis would have enabled researchers to more reliably compare individual outcomes of prenatal marijuana exposure across the different studies, independent of the specific intent of the 21 published works.80 Finally, we were unable to conduct a formal assessment of publication bias due to heterogeneity of the data. However, as with any systematic review, issues of publication bias may have influenced the results and led to overestimates of effect. Although we allowed for the inclusion of non-peer-reviewed papers, none met the inclusion criteria. The results are thus reflective of the published literature.
Finally, there are additional concerns about the reported growing potency of marijuana and increasing variety of marijuana products and modes of administration that may potentially increase severity of dependence and have stronger effects on the brain.81–84
Interpretation of findings
When interpreting the findings of this review, it is important to note that neuropsychological functioning is a multidimensional construct. The children in our review were tested at a wide variety of ages. Testing at different ages changes the tools available to measure ability, as younger children will not be able to complete the same tasks that older children can. While there are some effects of prenatal marijuana exposure on neuropsychological functions in children, one has to exercise caution interpreting these effects. On one hand, though cognitive function effects due to prenatal marijuana exposure may be small in magnitude and often are not statistically significant, they may still have a significant impact on social outcomes for an individual in later life.85,86 Thus, it is important to fully understand the risks of exposure in light of the changing culture and political climate surrounding marijuana. On the other hand, additional factors, including genetics, maternal cognitive abilities,87 medical conditions, such as preterm birth or nutritional deficits, and environmental influences, such as parenting, preschool attendance, or lead exposure, may influence the detectible effects of prenatal marijuana exposure.56,62
Conclusions
This systematic review suggest possible negative associations of prenatal marijuana exposure and neuropsychological functions, such as attention, memory, and impulse control in older children. However, the available literature shows mixed results and does not allow us to confidently exclude other explanations, including confounding and publication bias. More mixed results were found for the association with prenatal marijuana exposure and language development, reading, and composite IQ scores. More complete reporting of the findings made by existing studies could facilitate data accumulation and meta-analyses, allowing for a more robust assessment of these associations. More recent data capturing the effects of marijuana in the absence of polysubstance use and changing dynamics in use could also be beneficial. While data are beginning to accumulate, educating the public about potential dangers of marijuana use during pregnancy is warranted.
Supplementary Material
Acknowledgements
We are grateful to Joanna Taliano for her invaluable contribution to composing the literature search strategy, running literature searches and keeping us updated on new publications. We also acknowledge Paul Cheh, Sara Kennedy and Derrick Beasley for conceptualizing the systematic review and preparing a foundation for this project. We thank Kat Asman for reviewing literature to summarize the state of the science. Roshni Patel, and Amal Jama have our gratitude for their assistance with screening thousands of titles retrieved by the literature searches, collecting full text articles and abstracting data. We also would like to thank Linda Pederson for helping to sort and review titles and abstracts from the literature search.
References
- 1.Center for Behavioral Health Statistics and Quality Key substance use and mental health indicators in the United States: results from the 2015 National Survey on Drug Use and Health. 2016. HHS Publication No. SMA 16–4984, NSDUH Series H-51. [Google Scholar]
- 2.National Conference of State Legislatures. National Conference of State Legislatures. State medical marijuana laws. 2016; http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Accessed August 19, 2017.
- 3.Carliner H, Brown QL, Sarvet AL, Hasin DS. Cannabis use, attitudes, and legal status in the U.S.: A review. Prev Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA. 2017;317(2):207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasnoff IJ. Medical marijuana laws and pregnancy: implications for public health policy. Am J Obstet Gynecol 2017;216(1):27–30. [DOI] [PubMed] [Google Scholar]
- 6.Mark K, Terplan M. Cannabis and pregnancy: Maternal child health implications during a period of drug policy liberalization. Prev Med 2017. [DOI] [PubMed] [Google Scholar]
- 7.Benevenuto SG, Domenico MD, Martins MA, et al. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: An experimental study in mice. Toxicology. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Calvigioni D, Hurd YL, Harkany T, Keimpema E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry. 2014;23(10):931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Salas-Quiroga A, Diaz-Alonso J, Garcia-Rincon D, et al. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc Natl Acad Sci U S A 2015;112(44):13693–13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang GS. Pediatric Concerns Due to Expanded Cannabis Use: Unintended Consequences of Legalization. J Med Toxicol 2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter RC, Wainwright H, Molteno CD, et al. Alcohol, Methamphetamine, and Marijuana Exposure Have Distinct Effects on the Human Placenta. Alcohol Clin Exp Res 2016;40(4):753–764. [DOI] [PubMed] [Google Scholar]
- 12.Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol 2016. [DOI] [PubMed] [Google Scholar]
- 13.Blackard C, Tennes K. Human placental transfer of cannabinoids. N Engl J Med 1984;311(12):797–797. [DOI] [PubMed] [Google Scholar]
- 14.Lee CC, Chiang CN. Maternal-fetal transfer of abused substances: pharmacokinetic and pharmacodynamic data. NIDA Res Monogr 1985;60:110–147. [PubMed] [Google Scholar]
- 15.Wu CS, Jew CP, Lu HC. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol 2011;6(4):459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobrian SK. Developmental cannabinoid exposure: New perspectives on outcomes and mechanisms. Neurotoxicol Teratol. 2016;58:1–4. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Compton WM, Wargo EM. The risks of marijuana use during pregnancy. JAMA 2017;317(2):129–130. [DOI] [PubMed] [Google Scholar]
- 18.Batalla A, Bhattacharyya S, Yucel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS one. 2013;8(2):e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill SY, Sharma V, Jones BL. Lifetime use of cannabis from longitudinal assessments, cannabinoid receptor (CNR1) variation, and reduced volume of the right anterior cingulate. Psychiatry Res 2016;255:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakabek D, Yucel M, Lorenzetti V, Solowij N. An MRI study of white matter tract integrity in regular cannabis users: effects of cannabis use and age. Psychopharmacology (Berl) 2016;233(19–20):3627–3637. [DOI] [PubMed] [Google Scholar]
- 21.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287(9):1123–1131. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzetti V, Alonso-Lana S, Youssef GJ, et al. Adolescent cannabis use: What is the evidence for functional brain alteration? Curr Pharm Des 2016. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez R, Swanson JM. Long-term effects of adolescent-onset and persistent use of cannabis. Proc Natl Acad Sci U S A 2012;109(40):15970–15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev 2008;1(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A 2012;109(40):E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpar A, Di Marzo V, Harkany T. At the Tip of an Iceberg: Prenatal Marijuana and Its Possible Relation to Neuropsychiatric Outcome in the Offspring. Biol Psychiatry. 2016;79(7):e33–45. [DOI] [PubMed] [Google Scholar]
- 27.Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:45–52. [DOI] [PubMed] [Google Scholar]
- 28.Irner TB, Teasdale TW, Nielsen T, Vedal S, Olofsson M. Substance use during pregnancy and postnatal outcomes. J Addict Dis 2012;31(1):19–28. [DOI] [PubMed] [Google Scholar]
- 29.Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Marijuana: Current concepts. Frontiers in Public Health. 2013;1:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunn JK, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ open. 2016;6(4):e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irner TB. Substance exposure in utero and developmental consequences in adolescence: a systematic review. Child Neuropsychology. 2012;18(6):521–549. [DOI] [PubMed] [Google Scholar]
- 32.Williams JH, Ross L. Consequences of prenatal toxin exposure for mental health in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2007;16(4):243–253. [DOI] [PubMed] [Google Scholar]
- 33.Hasin DS, Wall M, Keyes KM, et al. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry. 2015;2(7):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Academies of Sciences, Engineering, Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 35.Reavie KT. Mining the research literature in systems biology. Bioinformatics for Systems Biology. 2009:369–383. [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;339 Accessed 2009–07–21 10:46:49. [PMC free article] [PubMed] [Google Scholar]
- 37.Zaza S, Wright-De Agüero LK, Briss PA, et al. Data collection instrument and procedure for systematic reviews in the guide to community preventive services. Am J Prev Med 2000;18(1, Supplement 1): 44–74. [DOI] [PubMed] [Google Scholar]
- 38.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014;http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 21, 2015.
- 39.Wechsler D Wechsler Preschool and Primary Scale of Intelligence. Pearson; 2002. [Google Scholar]
- 40.Thorndike R, Hagen E, Sattler J. The Stanford-Binet intelligence scale. Chicago: Riverside Publishing; 1986. [Google Scholar]
- 41.MacCarthy D Manual for the McCarthy scales of children’s abilities. Psychological Corporation; 1972. [Google Scholar]
- 42.Wilkinson G Wide range achievement test administration manual. Wilmington, DE: Wide Range. In: Inc; 1993. [Google Scholar]
- 43.Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89(1):91–97. [PubMed] [Google Scholar]
- 44.Fried PA, Watkinson B. 12- and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicol Teratol 1988;10(4):305–313. [DOI] [PubMed] [Google Scholar]
- 45.Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr 1990;11(2):49–58. [PubMed] [Google Scholar]
- 46.Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol 1992;14(5):299–311. [DOI] [PubMed] [Google Scholar]
- 47.Fried PA, O’Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr 1992;13(6):383–391. [PubMed] [Google Scholar]
- 48.O’Connell CM, Fried PA. Prenatal exposure to cannabis: a preliminary report of postnatal consequences in school-age children. Neurotoxicol Teratol 1991;13(6):631–639. [DOI] [PubMed] [Google Scholar]
- 49.Fried PA, Watkinson B, Siegel LS. Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol 1997;19(3):171–183. [DOI] [PubMed] [Google Scholar]
- 50.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 1998;20(3):293–306. [DOI] [PubMed] [Google Scholar]
- 51.Fried PA, Watkinson B. Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 2000;22(1):11–20. [DOI] [PubMed] [Google Scholar]
- 52.Day NL, Richardson GA, Goldschmidt L, et al. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol 1994;16(2):169–175. [DOI] [PubMed] [Google Scholar]
- 53.Goldschmidt L, Richardson GA, Willford J, Day NL. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47(3):254–263. [DOI] [PubMed] [Google Scholar]
- 54.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol 1999;21(2):109–118. [DOI] [PubMed] [Google Scholar]
- 55.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol 2000;22(3):325–336. [DOI] [PubMed] [Google Scholar]
- 56.Goldschmidt L, Richardson GA, Cornelius MD, Day NL. Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol 2004;26(4):521–532. [DOI] [PubMed] [Google Scholar]
- 57.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol 2002;24(3):309–320. [DOI] [PubMed] [Google Scholar]
- 58.Noland JS, Singer LT, Arendt RE, Minnes S, Short EJ, Bearer CF. Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcohol Clin Exp Res 2003;27(4):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noland JS, Singer LT, Short EJ, et al. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol 2005;27(3):429–438. [DOI] [PubMed] [Google Scholar]
- 60.Bennett DS, Bendersky M, Lewis M. Children’s cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol 2008;44(4):919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carmody DP, Bennett DS, Lewis M. The effects of prenatal cocaine exposure and gender on inhibitory control and attention. Neurotoxicol Teratol 2011;33(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayes JS, Lampart R, Dreher MC, Morgan L. Five-year follow-up of rural Jamaican children whose mothers used marijuana during pregnancy. West Indian Med J 1991;40(3):120–123. [PubMed] [Google Scholar]
- 63.Faden VB, Graubard BI. Maternal substance use during pregnancy and developmental outcome at age three. J Subst Abuse. 2000;12(4):329–340. [DOI] [PubMed] [Google Scholar]
- 64.US Department of Health and Human Services, National Center for Health Statistics. National Maternal and Infant Health Survey, 1988: Longitudinal Follow-up, 1991. In: Inter-university Consortium for Political and Social Research (ICPSR) [distributor]; 1995. [Google Scholar]
- 65.LaGasse LL, Wouldes T, Newman E, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol 2011;33(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakraborty A, Anstice NS, Jacobs RJ, et al. Prenatal exposure to recreational drugs affects global motion perception in preschool children. Scientific Reports. 2015;5:16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pavlova MA. Biological motion processing as a hallmark of social cognition. Cereb Cortex 2012;22(5):981–995. [DOI] [PubMed] [Google Scholar]
- 68.Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychology. 2002;8(2):71–82. [DOI] [PubMed] [Google Scholar]
- 69.Riley AW. Evidence that school-age children can self-report on their health. Ambul Pediatr 2004;4(4):371–376. [DOI] [PubMed] [Google Scholar]
- 70.Kane-Wineland M The relationship between prenatal substance exposure and neuromotor development in children from birth to three years. Dissertation Abstracts International Section A: Humanities and Social Sciences. 2002;63(3-A): 860. [Google Scholar]
- 71.Stein MT, Drahota A, Chavira DA. Ian: a 7-year old with prenatal drug exposure and early exposure to family violence. J Dev Behav Pediatr 2008;29(6):512–515. [DOI] [PubMed] [Google Scholar]
- 72.Dreher MC, Hayes JS. Triangulation in cross-cultural research of child development in Jamaica. West J Nurs Res 1993;15(2):216–229. [DOI] [PubMed] [Google Scholar]
- 73.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 2006;30(1):24–41. [DOI] [PubMed] [Google Scholar]
- 74.Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta Paediatr 2015;104(1):12–18. [DOI] [PubMed] [Google Scholar]
- 75.El Marroun H, Schmidt MN, Franken IH, et al. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology. 2014;39(4):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou S, Rosenthal DG, Sherman S, Zelikoff J, Gordon T, Weitzman M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr Probl Pediatr Adolesc Health Care. 2014;44(8):219–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rose-Jacobs R, Richardson MA, Buchanan-Howland K, et al. Intrauterine exposure to tobacco and executive functioning in high school. Drug Alcohol Depend 2017;176:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khoury JE, Milligan K, Girard TA. Executive Functioning in Children and Adolescents Prenatally Exposed to Alcohol: A Meta-Analytic Review. Neuropsychology review. 2015;25(2):149–170. [DOI] [PubMed] [Google Scholar]
- 79.Coriale G, Fiorentino D, Di Lauro F, et al. Fetal Alcohol Spectrum Disorder (FASD): neurobehavioral profile, indications for diagnosis and treatment. Rivista di psichiatria 2013;48(5):359–369. [DOI] [PubMed] [Google Scholar]
- 80.Clarke MJ. Individual patient data meta-analyses. Best Pract Res Clin Obstet Gynaecol 2005;19(1):47–55. [DOI] [PubMed] [Google Scholar]
- 81.Psychoyos D, Vinod KY. Marijuana, Spice ‘herbal high’, and early neural development: implications for rescheduling and legalization. Drug Test Anal. 2013;5(1):27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol Med 2015;45(15):3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am J Prev Med 2016;50(1):1–8. [DOI] [PubMed] [Google Scholar]
- 85.Lester BM, LaGasse LL, Seifer R. Cocaine Exposure and Children: The Meaning of Subtle Effects. Science. 1998;282(5389):633–634. [DOI] [PubMed] [Google Scholar]
- 86.Goldschmidt L, Richardson GA, Larkby C, Day NL. Early marijuana initiation: The link between prenatal marijuana exposure, early childhood behavior, and negative adult roles. Neurotoxicol Teratol 2016;58:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McGue M. Classical and molecular genetic research on general cognitive ability. The Hastings Center Report. 2015;45(S1):S25–S31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


