Abstract
Programmed cell death 4 (Pdcd4), a tumor suppressor, is frequently down-regulated in various types of cancer. Pdcd4 has been demonstrated to efficiently suppress tumor promotion, progression, and proliferation. The biochemical function of Pdcd4 is a protein translation inhibitor. Although the fact that Pdcd4 inhibits protein translation has been known for more than a decade, the mechanism by which Pdcd4 controls tumorigenesis through translational regulation of its target genes is still not fully understood. Recent studies show that Pdcd4 inhibits translation of stress-activated-protein kinase interacting protein 1 to suppress tumor invasion, depicting a picture of how Pdcd4 inhibits tumorigenesis through translational inhibition. Thus, understanding the mechanism of how Pdcd4 attenuates tumorigenesis by translational control should provide a new strategy for combating cancer.
Keywords: eIF4A, tumor promotion, tumor invasion, proliferation
Graphical Abstract
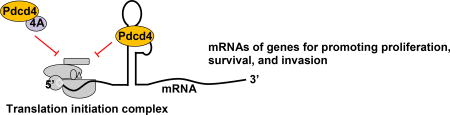
Programmed cell death 4 (Pdcd4) functions as a tumor suppressor and an inhibitor of protein translation. Pdcd4 is the binding partner of eIF4A. By binding with eIF4A, Pdcd4 inhibits eIF4A’s helicase activity to attenuate translation of mRNAs with structured 5’UTR. In addition, Pdcd4 may directly bind with mRNA to interfere the translation process. The translational targets of Pdcd4 are involved in cell proliferation, survival, and invasion. Thus, understanding the mechanism by which Pdcd4 suppresses translation may provide a new strategy for cancer intervention.
Introduction
The cDNA of Programmed cell death 4 (Pdcd4) was initially cloned from mouse cells when Pdcd4 expression was up-regulated by treatment with dexamethasone (Shibahara et al. 1995). Subsequently, this gene was identified in different species including human, rat, and chicken (Azzoni et al. 1998, Goke et al. 2002, Schlichter et al. 2001); and the deduced amino acid sequences are highly conserved among these species. For example, the amino acid sequence of Pdcd4 from human and mouse shares about 92% identity and 96% homology. The expression of Pdcd4 is frequently down-regulated in numerous types of cancers including breast, colon, liver, lung, pancreas, and skin cancers. It has been demonstrated that Pdcd4 functions as a tumor suppressor, inhibiting tumor promotion, invasion/metastasis, and proliferation. The established biochemical function of Pdcd4 is that Pdcd4 inhibits eIF4A’s helicase activity and thereby suppresses protein translation. Thus, suppression of tumorigenesis by Pdcd4 is believed to occur through translation inhibition of a set of genes involved in tumor cell proliferation, migration, invasion, and metastasis. In this review, we will discuss current understanding of the mechanisms by which Pdcd4 regulates tumorigenesis through translational control of its target gene expression.
Structural motifs of Pdcd4
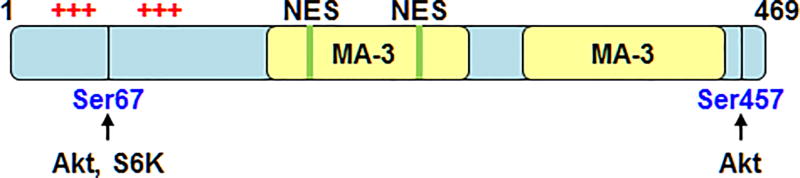
Pdcd4 protein composed of 469 amino acids contains two so-called “MA-3” domains, two phosphorylation sites, two clusters of positively charged residues, and two nuclear export signals (NES) (Figure 1). Amino acid sequence alignment and protein secondary structure analysis indicate that the two MA-3 domains on Pdcd4 reside at residues 164 to 275 and 327 to 440. The MA-3 domain typically spans about 120 amino acid residues and contains 7 or 8 α-helices, which is also found in other proteins including translation initiation factor eIF4G, nonsense-mediated mRNA decay 2 protein, and the nuclear cap-binding protein CBP80 (Aravind and Koonin 2000). Crystal structure and mutation analyses demonstrate that Pdcd4 uses MA-3 domains to interact with eukaryotic initiation factor 4A (eIF4A) (Chang et al. 2009, Loh et al. 2009, Yang et al. 2004). In addition, Pdcd4 protein can be phosphorylated by Akt or p70S6K at Ser67, and phosphorylation at Ser67 is recognized by β transducin repeats-containing proteins (β-TRCP), an E3 ligase, resulting in proteasomal degradation of Pdcd4 (Dorrello et al. 2006, Palamarchuk et al. 2005). Palamarchuk et al. also suggested that phosphorylation of Pdcd4 at Ser67 and Ser457 leads to nuclear localization (Palamarchuk et al. 2005). Pdcd4 was also reported to bind with RNA through two clusters of positively charged amino acids (Bohm et al. 2003, Wedeken et al. 2010), and these two clusters are required for Pdcd4 to bind with A-MYB and C-MYB mRNAs and suppress their translation (Biyanee et al. 2015, Singh et al. 2011). Since Pdcd4 contains two NES signals, it is speculated to shuttle between nucleus and cytoplasm (Bohm et al. 2003). Pdcd4 suppresses protein translation in cytoplasm via binding with eIF4A, but the function of Pdcd4 in nucleus is unknown.
Figure 1.
Schematic diagram of the functional motifs and sites of Pdcd4. Arrows indicate the phosphorylation sites by Akt or S6K1. Green boxes designate the potential nuclear translocation signals (NES). The +++ indicates the cluster of positively charged residues. The MA-3 domains are in yellow.
Regulation of Pdcd4 expression
Pdcd4 expression can be regulated at levels of epigenetics, transcription, post-transcription, and post-translation, which have been previously discussed (Yang et al. 2014). Here, we only discuss two essential and well-known mechanisms: ubiquitin-mediated degradation and micro RNA-mediated degradation.
As aforementioned, Pdcd4 can be phosphorylated at Ser67 by p70S6K and Akt. Upon phosphorylation by p70S6K or Akt, Pdcd4 is recognized and bound by an ubiquitin E3 ligase, β-TRCP (Dorrello et al. 2006). The β-TRCP belongs to the F-box family, the substrate recognition component of Skp1-Cul1-F-box protein (SCF) ubiquitin ligase complex, which transfers ubiquitin molecules to target proteins for proteasomal degradation (Cardozo and Pagano 2004). Thus, after binding with β-TRCP, Pdcd4 is ubiquitinated and consequently degraded by proteasome. The other important mechanism to regulate Pdcd4 expression is through microRNA (miRNA). miRNA is a RNA molecule containing 20–25 nucleotides that binds with target RNA at 3’ untranslated region (3’UTR) and negatively regulates gene expression. The 3’UTR of Pdcd4 contains a perfectly matched target sequence for miR-21 (Asangani et al. 2008, Lu et al. 2008). miRNA-21 binds with pdcd4 mRNA to attenuate the translation of pdcd4 mRNA. Bioinformatics analyses predict that more than 80 micro RNAs potentially target Pdcd4, suggesting an important role of microRNA in regulating Pdcd4 expression (Dweep et al. 2011).
Pdcd4 inhibits tumor promotion
Using RNA differential display assay, Pdcd4 was found to be highly expressed in JB6 transformation-resistant cells comparing to the JB6 transformation susceptible cells (Cmarik et al. 1999). The mouse epidermal JB6 cells have 3 genetic variants, namely transformation-resistant, transformation-susceptible, and transformed cells. Exposure of transformation-susceptible JB6 cells to tumor promoter such as 12-O-tetradecanoylphorbol-13-acetate (TPA) or tumor necrosis factor α (TNFα) results in neoplastic transformation. On the other hand, the transformation-resistant JB6 cells resist TPA- or TNFα-induced transformation. Thus, this cell culture system is an ideal tool for studying the biochemical events during tumor promotion. To demonstrate the inhibitory role of Pdcd4 on tumor promotion, expression of PDCD4 antisense in transformation-resistant JB6 cells to lower the Pdcd4 protein level results in a gain of transformation-susceptible phenotype (Cmarik et al. 1999). In consistence with this finding, ectopic expression of PDCD4 cDNA in transformation-susceptible JB6 cells render cells insensitive to TPA-induced neoplastic transformation (Yang et al. 2003b). In addition to these cultured cell studies, transgenic mice with over-expression of Pdcd4 in skin efficiently reduces 7,12-dimethylbenz(a)anthrance (DMBA)/TPA induced papilloma formation (Jansen et al. 2005), while knockout of Pdcd4 expression in mouse skin increases the number of papilloma after DMBA/TPA treatment (Schmid et al. 2008). Collectively, these results indicate that Pdcd4 inhibits tumor promotion.
Pdcd4 inhibits tumor invasion and metastasis
Beyond inhibiting tumor promotion, over-expression of PDCD4 cDNA also suppresses tumor invasion or intravasation in many types of cancer cells including breast, colon, liver, gastric, and ovarian cancer (Gonzalez-Villasana et al. 2012, Leupold et al. 2007, Nieves-Alicea et al. 2009, Wei et al. 2012, Yang et al. 2006, Yu et al. 2014, Zhang et al. 2009). On the other hand, lowering Pdcd4 expression by PDCD4 siRNA or shRNA stimulates invasion in breast, colon, lung, and nasopharyngeal cancer cells (Santhanam et al. 2010, Wang et al. 2008, Wang et al. 2010, Yang et al. 2015, Zhen et al. 2017). In animal studies, knockdown of Pdcd4 in colon cancer cells promotes metastasis to lymph node and liver when these cells are injected into cecum of nude mice (Wang et al. 2013). Mice with Pdcd4 knockdown develops tumors with frequent metastasis to liver and kidney (Hilliard et al. 2006). Moreover, knockdown of Pdcd4 also causes a decrease in expression of several epithelial markers including E-cadherin, α-catenin, and γ-catenin as well as an increase in expression of mesenchymal markers like β-catenin, N-cadherin, and fibronectin in both cultured cells and mice (Wang et al. 2008, Wang et al. 2010, Wang et al. 2013), suggesting that Pdcd4 knockdown induces epithelial to mesenchymal transition (EMT). EMT is the process for cells to acquire a fibroblast-like phenotype, lose epithelial polarity, and boost migration to penetrate the surrounding extracellular matrix (Grunert et al. 2003), which is a key step for tumor cells to gain invasive and metastatic ability. All of the above evidence indicates that Pdcd4 functions as an invasion/metastasis inhibitor.
Pdcd4 inhibits cell proliferation
Immunohistochemical staining of human intraductal papillary mucinous neoplasm of pancreas tissues showed that Pdcd4 expression is inversely correlated with the level of proliferation marker Ki-67 (Hayashi et al. 2010), suggesting that Pdcd4 may regulate tumor cell proliferation. In line with the animal studies, over-expression of Pdcd4 suppresses proliferation in several cancer cell lines including breast carcinoma (Cuesta and Holz 2016), hepatocellular carcinoma (Zhang et al. 2009), nasopharyngeal carcinoma (Zhen et al. 2013), glioma (Chen et al. 2016), and mesenchymal stem cells (Liu et al. 2017). Conversely, knockdown of Pdcd4 stimulates proliferation by increasing cyclin D1 expression in colon carcinoma cells (Guo et al. 2011). Similarly, Pdcd4 null (Pdcd4−/−) mouse embryonic fibroblast (MEF) cells appear to proliferate faster than wild-type (Pdcd4+/+) MEF cells (Wang et al. 2017). These findings indicate that Pdcd4 suppresses cell proliferation. In contrast to the aforementioned results, Ullman and colleagues showed that Pdcd4 can be methylated at Arg110 and the methylated Pdcd4 interacts more robustly with eIF4A resulting in enhanced viability of cells during nutrient and growth factor depletion (Fay et al. 2014, Powers et al. 2011). It is believed that Pdcd4 binds with eIF4A to inhibit translation of mRNAs with structured 5’ untranslated region (5’UTR) (see below). Usually, the mRNAs of growth factors, growth promotion genes, and oncogenes carry structured 5’UTR (De Benedetti and Harris 1999). It is unknown how Pdcd4 improves cell viability during nutrient and growth factor depletion by binding with eIF4A. Thus, it is necessary to identify the translational targets of Pdcd4 under such conditions.
Pdcd4 is the binding partner of eIF4A
In the yeast two-hybrid assay, using Pdcd4 cDNA as bait to screen the mouse cDNA library, Pdcd4 was identified to interact with eIF4A and this interaction was further confirmed by glutathione S-transferase pull-down, immunoprecipitation, immunolocolization, and mammalian two-hybrid assays (Goke et al. 2002, Yang et al. 2003a, Zakowicz et al. 2005). The Pdcd4 crystal structure showed that each MA-3 domain consists of eight or nine α-helices (Chang et al. 2009, Loh et al. 2009). The analyses of Pdcd4-eIF4A co-crystal structure further revealed that Pdcd4 uses these two MA-3 domains to interact with eIF4A (Chang et al. 2009, Loh et al. 2009), in which one Pdcd4 molecule is sandwiched between two molecules of eIF4A (Chang et al. 2009, Loh et al. 2009). Pdcd4 binds to eIF4A in an approximately vertical position with each MA3 domain contacting both N- and C-terminal domains of eIF4A (Chang et al. 2009, Loh et al. 2009). The interactions between Pdcd4 and eIF4A are ionic interactions or hydrogen bonds mediated by several conserved residues on both Pdcd4 and eIF4A. For example, Glu249 and Asp253 in one MA-3 domain of Pdcd4 form salt bridges with Arg161 and Arg110 in the first eIF4A molecule and the Asp414 and Asp418 of the other MA3 domain interact similarly with the Arg161 and Arg110 in the second eIF4A molecule. Consistent with these observations, mutation of Glu249 to Lys, Asp253 to Ala, Asp414 to Lys, or Asp418 to Ala in Pdcd4 forfeits the binding ability to eIF4A in the mammalian two-hybrid assay (Yang et al. 2004). In contrast to the crystal structure analyses, Waters et al. used NMR analyses to reveal that Pdcd4 and eIF4A forms Pdcd4-eIF4A complex in a 1:1 ratio in solution and only one MA3 domain of Pdcd4 interacts with one eIF4A molecule (Waters et al. 2011). In support of this notion, they found that only double mutation of Glu249 to Ala/Asp253 to Ala but Asp414 to Ala or Asp 418 to Ala abolish Pdcd4’s binding ability to eIF4A in immunoprecipitation assays. It is possible that the interactions between Pdcd4 and eIF4A are different in crystal and solution conditions. However, it is unclear whether the discrepancy of the result of mutation analyses is due to the different experimental methods, i.e. mammalian two hybrid vs. immunoprecipitation.
Pdcd4 is an inhibitor of translation
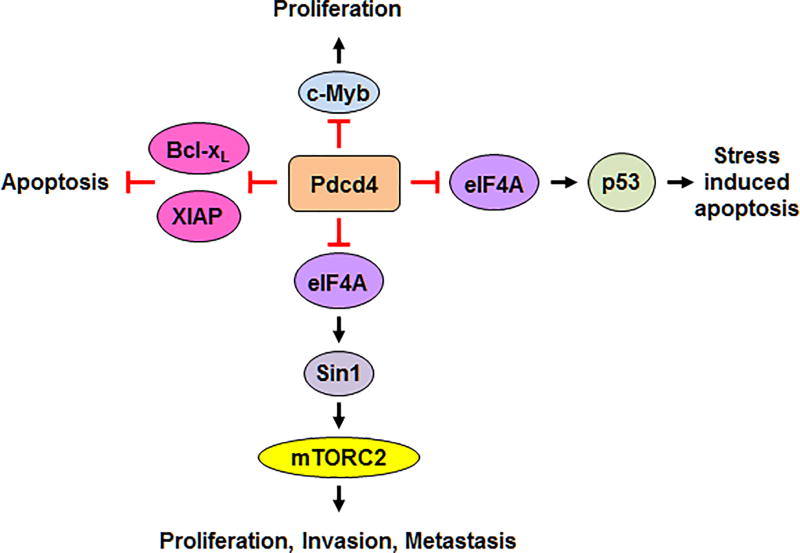
By binding with eIF4A, an ATP dependent RNA helicase, Pdcd4 inhibits eIF4A’s helicase activity (Yang et al. 2003a). The eIF4A is critical for unwinding structured mRNA, allowing translation initiation complex to move along the mRNA and locate the translation initiation codon during translation initiation. Thus, inhibition of eIF4A’s helicase activity is expected to curb translation of mRNAs with structured 5’UTR more efficiently than those without. This model was supported by the finding that Pdcd4 suppresses translation of the luciferase with a synthetic stem-loop structure at 5’UTR greater than the one without it (Yang et al. 2004). In agreement with this finding, Wedeken et al., reported that P53 5’UTR mRNA can form a stable secondary structure and Pdcd4 efficiently inhibits translation of luciferase fused with P53 5’UTR (Wedeken et al. 2011). Additionally, knockdown of Pdcd4 increases the distribution of P53 mRNA to polysomal fractions in a polysomal fractionation assays, suggesting that p53 is a genuine target of Pdcd4 (Wedeken et al. 2011). Under stress conditions such as UV radiation, Pdcd4 expression is downregulated and thereby p53 translation increases in response to stress induced apoptosis (Figure 2). Recently, this model was further confirmed by identification of another Pdcd4 target, stress-activated-protein kinase interacting protein 1 (Sin1), whose mRNA also forms a stable secondary structure at 5’UTR (Wang et al. 2017). Pdcd4 inhibits translation of luciferase fused with SIN1 5’UTR (Sin1-5’UTR-Luc) in a concentration dependent manner but not the luciferase without SIN1-5’UTR. However, Pdcd4 mutant defective in eIF4A binding is not able to inhibit translation of Sin1-5’UTR-Luc. In agreement with above observations, inhibition of eIF4A activity by chemical inhibitor, silvestrol, sufficiently inhibits Sin1-5’UTR-Luc translation and Sin1 expression, suggesting that eIF4A activity plays a role in Pdcd4 inhibited Sin1 translation. Sin1 is an exclusive component of mammalian target of rapamycin complex 2 (mTORC2). Inhibition of Sin1 translation by Pdcd4 results in repression of mTORC2 activity and thereby suppresses Akt activation to attenuate tumor proliferation and invasion (Figure 2). Taken together, these results reveal that Pdcd4 inhibits eIF4A activity to suppress translation of mRNAs with structured 5’UTR.
Figure 2.
Pdcd4 translational targets are involved in regulation of proliferation, apoptosis, invasion, and metastasis. Pdcd4 inhibits eIF4A activity to suppress translation of p53 and Sin1. Inhibition of p53 translation results in repression of stress induced apoptosis, while inhibition of Sin1 translation attenuates cell proliferation, invasion, and metastasis. Besides partners with eIF4A, Pdcd4 may directly bind with mRNAs of c-Myb, Bcl-xL, and XIAP to inhibit their translations, leading to suppression of proliferation and anti-apoptosis.
Apart from the eIF4A dependent mechanism, Pdcd4 may directly bind with mRNA to suppress its translation. It was reported that Pdcd4 inhibits mRNA translation of antiapoptotic proteins, Bcl-xL and X chromosome-linked inhibitor of apoptosis (XIAP), through directly binding to the internal ribosome entry site element and blocking the formation of translation initiation complex (Liwak et al. 2012). In response to survival signaling, p70S6K2 phosphorylates Pdcd4 resulting in Pdcd4 degradation, which leads to enhanced translation of XIAP and Bcl-xL (Liwak et al. 2012) (Figure 2). Moreover, Fehler et al. suggested that by association with poly(A) binding protein, Pdcd4 may bind with C-MYB mRNA and impede its translation elongation (Fehler et al. 2014). c-Myb is a transcription factor, whose target genes are involved in proliferation and survival (Ramsay and Gonda 2008) (Figure 2). Whether Pdcd4 binds with mRNA through a specific sequence, motif, or tertiary structure remains unknown, requiring further investigation.
Mechanisms of regulating tumorigenesis by Pdcd4
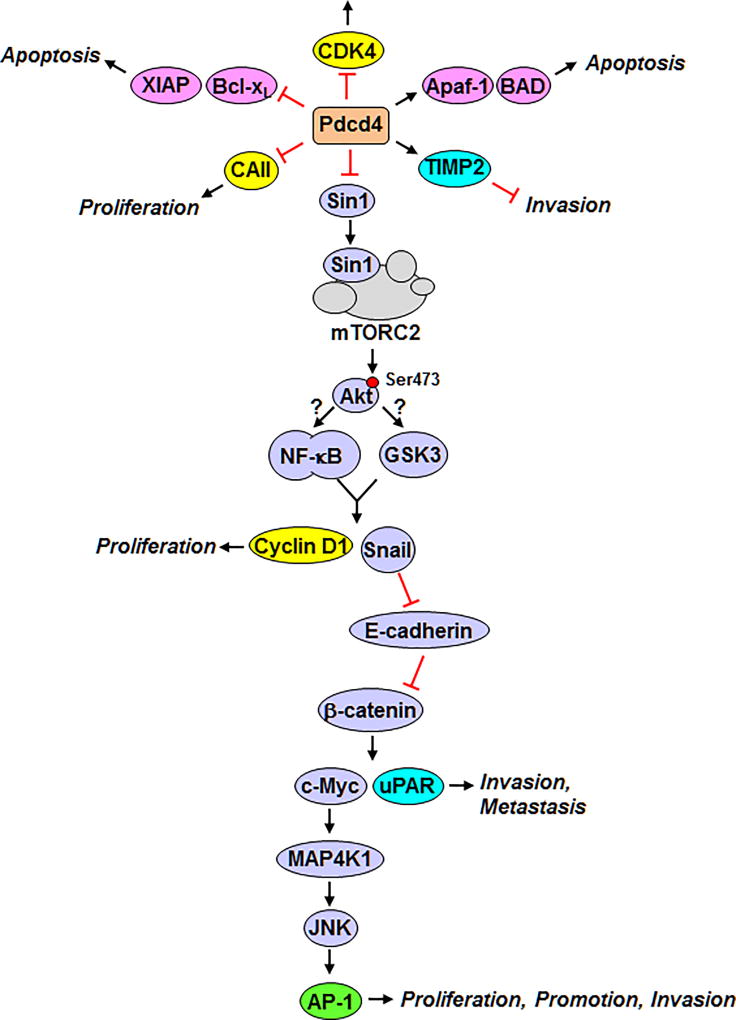
Over-expression of Pdcd4 in JB6 transformation-susceptible cells inhibits both basal and TPA-induced AP-1 transactivation (Yang et al. 2001). Transcription factor AP-1 is a homodimer or heterodimer of Jun and Fos proteins, which plays a crucial role in regulation of tumor promotion. For instance, repression of AP-1 transactivation by dominant negative c-Jun or AP-1 inhibitor, SR11302, leads to inhibition of TPA-induced neoplastic transformation in JB6 cells (Dong et al. 1994, Li et al. 1997). Several downstream targets of AP-1 dependent transcription are involved in cell proliferation, transformation, and invasion (Ozanne et al. 2000). Therefore, inhibition of AP-1 activation by Pdcd4 is a key event for suppression of tumorigenesis. Using transactivation assays, Pdcd4 was further shown to inactivate AP-1 transactivation through inhibition of c-Jun activation in mouse JB6 cells (Yang et al. 2003b). Furthermore, Bitomsty et al. discovered that Pdcd4 interferes activation and phosphorylation of c-Jun by suppression of Jun N-terminal kinase (JNK) activation in avian QT6 cells (Bitomsky et al. 2004). How does Pdcd4 inhibit JNK and c-Jun activation? Pdcd4 was found to regulate the expression of a JNK upstream kinase, mitogen-activated protein kinase kinase kinase kinase 1 (MAP4K1), to control the activation of JNK, c-Jun, and AP-1 in colon HT29 cells (Wang et al. 2012) (Figure 3). Mutation of the c-Myc binding site, located within the −536 bp of MAP4K1 promoter, dramatically reduces MAP4K1 promoter activity (Wang et al. 2012). In addition, lower c-Myc expression by C-MYC siRNA inhibits MAP4K1 expression (Wang et al. 2012). These findings indicate that c-Myc regulates MAP4K1 expression. Moreover, knockdown of Pdcd4 suppresses E-cadherin expression inducing activation of β-catenin dependent transcription and thereby stimulating expression of its downstream targets, c-Myc and urokinase-type plasminogen activator (u-PAR) (Wang et al. 2010). The uPAR, a 55–60 kDa glycosylated receptor, binds to its ligand and leads to degradation of extracellular matrix components allowing tumor cells to penetrate basal membrane during invasion (Blasi 1993). Collectively, when Pdcd4 is knocked down, elevates c-Myc and u-PAR expression. Both contribute to the promotion of tumor invasion (Kariko et al. 1993) (Figure 3).
Figure 3.
Pdcd4 suppresses tumorigenesis through inhibition of multiple pathways. Pdcd4 inhibits Sin1 translation to inactivate mTORC2. Suppression of mTORC2 activity attenuates activation and phosphorylation at Ser473 of Akt resulting in decreased expression of cyclin D1 and Snail through inactivation of NF-κB or GSK3. Reduction of cyclin D1 expression leads to decreased cell proliferation. The transcription repressor Snail negatively regulates E-cadherin expression resulting in activation of β-catenin dependent transcription and increased c-Myc and u-PAR expression. u-PAR has been demonstrated to promote tumor cell invasion; c-Myc subsequently stimulates MAP4K1 expression and thereby activates AP-1 dependent transcription to regulate tumor proliferation, promotion, and invasion. Inhibition of TIMP expression by Pdcd4 was also reported to suppress invasion. Pdcd4 may repress cell proliferation through suppression of anti-apoptotic XIAP and Bcl-xL or stimulation of apoptotic Apaf-1 and BAD expression. Attenuation of cell proliferation by Pdcd4 also can be achieved by inhibition of CDK4 and CAII expressions. The color of yellow, blue, and pink indicates the proteins involved in proliferation, invasion, and apoptosis, respectively. The protein with multiple functions is in green.
Loss of E-cadherin is a distinctive feature for EMT to gain the ability of migration and invasion. E-cadherin expression can be regulated by various mechanisms including repression by zinc-finger transcription factors, Snail (Wang et al. 2010). Snail, a transcription repressor, binds to E-boxes on the E-cadherin promoter and suppresses its transcription (Cano et al. 2000). Knockdown of Snail in the Pdcd4 knockdown cells restores the E-cadherin promoter activity, showing that Snail does contribute to the inhibition of E-cadherin expression by Pdcd4 knockdown. How does Pdcd4 regulate Snail expression? Recently, we found that knockdown of Akt abolishes Pdcd4 knockdown-induced Snail expression (Wang et al. 2017). As the consequence of Snail downregulation, E-cadherin expression increases in Akt knockdown cells. One potential pathway for Akt up-regulating Snail expression is through activation of NF-κB (Julien et al. 2007). Akt phosphorylates IKKβ resulting in phosphorylation of the NF-κB inhibitor, IκB, and the phosphorylated IκB is subsequently ubiquitinated and degraded leading to NF-κB activation. The other possible mechanism is that Akt phosphorylates glycogen synthase kinase 3 (GSK3) to inactivate it, preventing proteasomal degradation of Snail (Zhou et al. 2004). Further investigation is needed to determine through which pathway Akt regulates Snail expression.
Pdcd4 has been demonstrated to regulate Akt activation (Guo et al. 2011, Lankat-Buttgereit et al. 2008, Wei et al. 2012). Akt mediates numerous cellular functions including proliferation, invasion, and metastasis, and is frequently activated in many types of human cancers (Vivanco and Sawyers 2002). Akt activity is mainly regulated by 3-phosphoinositide-dependent kinase 1 (PDK1) and mTORC2. Phosphorylation of Thr308 by PDK1 increases Akt kinase activity, but its maximal activity requires phosphorylation of Ser473 by mTORC2 (Guertin and Sabatini 2007). Recent studies demonstrate that Pdcd4 inhibits mTORC2 activation via suppression of Sin1 translation to attenuate Akt activity (Wang et al. 2017). Inhibition of Sin1 translation by Pdcd4 sufficiently attenuates invasion in colon carcinoma (Wang et al. 2017), suggesting that Pdcd4 inhibits the mTORC2-Akt axis to suppress invasion (Figure 3). In addition, regulation of Akt activation by Pdcd4 also contributes, at least in part, to the promotion of cell proliferation by controlling cyclin D1 expression (Guo et al. 2011). Collectively, Pdcd4 suppresses Sin1 translation to attenuate the mTORC2 activity resulting in inhibition of Akt activation and expression of its downstream targets, cyclin D1 and Snail. It is known that Snail negatively regulates E-cadherin expression to suppress β-catenin and AP-1 dependent transcriptions.
As mentioned above, Pdcd4 may directly inhibit translation of anti-apoptotic proteins, XIAP and Bcl-xL to promote apoptosis. In addition, Pdcd4 has been reported to increase apoptosis by up-regulating pro-apoptotic proteins. Up-regulation of Pdcd4 expression in the lung of K-Ras-null mice via aerosol delivery of PDCD4 cDNA significantly increases the expression of pro-apoptotic proteins BAD and Apaf-1 (Jin et al. 2006). In the same study, the authors also found that up-regulation of Pdcd4 suppresses the expression of cell cycle regulatory molecule, cyclin-dependent kinase 4 (CDK4) (Jin et al. 2006). While down-regulation of Pdcd4 by delivery of PDCD4 shRNA into the lung of A/J mice enhances CDK4 expression (Hwang et al. 2010), indicating that Pdcd4 inhibits cell proliferation and induces apoptosis in mouse lung. In addition, it has shown that Pdcd4 inhibits invasion in breast cancer cells by enhancing the expression of tissue inhibitor of metalloproteinase 2 (TIMP2) (Gonzalez-Villasana et al. 2012, Nieves-Alicea et al. 2009). The TIMP2 is a natural inhibitor of the matrix metalloproteinases (MMPs), which suppresses cell migration and invasion by preventing penetration of extracellular matrix. Lankat-Buttgereit et al. found that Pdcd4 inhibits carbonic anhydrase type II (CAII) expression (Lankat-Buttgereit et al. 2004). CAII hydrates CO2 to bicarbonate, which is an important substrate for synthesis of lipids, amino acids, and pyrimidine for fast growth tumor cells.
Conclusions and future directions
Pdcd4 is well positioned to be an integrator of inhibiting protein translation and suppressing tumorigenesis. It appears that Pdcd4 inhibits translation of a set of genes including Sin1, p53, c-Myb, Bcl-XL, and XIAP to suppress tumorigenesis (Fehler et al. 2014, Liwak et al. 2012, Wang et al. 2017, Wedeken et al. 2011). Thus, identification of more Pdcd4 translational targets will allow us to understand in depth how Pdcd4 inhibits tumorigenesis. Pdcd4 is known to inhibit the activation of Akt, one of the most frequently activated kinases in numerous cancers, which regulates cell survival, proliferation, apoptosis, and invasion. In addition, recent studies indicates that Pdcd4 inhibits mTORC2 activation, which also plays a fundamental role in regulating tumor cell migration, invasion, and metastasis (Zhou and Huang 2011). Since Pdcd4 expression is frequently down-regulated in cancerous cells, restoration or stimulation of Pdcd4 expression is expected to inhibit the mTORC2-Akt axis, which should be a promising strategy for cancer prevention and treatment. Thus, compounds that efficiently elevate Pdcd4 expression should have great potential for cancer therapeutics.
Acknowledgments
We apologize to our colleagues for any publications not being cited which is due to space limitations and the cross-disciplinary nature of this review article. We would like to thank the Markey Cancer Center’s Research Communications Office for assistance with manuscript preparation and edition.
Funding
This work was supported by the National Institute of Health/ National Cancer Institute grant (CA187839).
Abbreviations
- CAII
carbonic anhydrase type II
- 5’UTR
5’untranslated region
- eIF4A
eukaryotic initiation factor 4A
- β-TRCP
β-transducin repeats-containing proteins
- EMT
epithelial to mesenchymal transition
- GSK3
Glycogen synthase kinase 3
- JNK
Jun N-terminal kinase
- MAP4K1
mitogen-activated protein kinase kinase kinase kinase 1
- mTORC2
mammalian target of rapamycin complex 2
- p70S6K
70 kDa ribosomal S6 kinase
- Pdcd4
programmed cell death 4
- Sin1
stress-activated-protein kinase interacting protein 1
- TIMP2
tissue inhibitor of metalloproteinase 2
- TPA
12-O-tetradecanoylphorbol-13-acetate
- XIAP
X chromosome-linked inhibitor of apoptosisp
Footnotes
Conflict of interest statement
The authors have declared no conflict of interest.
References
- Aravind L, Koonin EV. Eukaryote-specific domains in translation initiation factors: implications for translation regulation and evolution of the translation system. Genome Res. 2000;10:1172–1184. doi: 10.1101/gr.10.8.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- Azzoni L, Zatsepina O, Abebe B, Bennett IM, Kanakaraj P, Perussia B. Differential transcriptional regulation of CD161 and a novel gene, 197/15a, by IL-2, IL-15, and IL-12 in NK and T cells. J Immunol. 1998;161:3493–3500. [PubMed] [Google Scholar]
- Bitomsky N, Bohm M, Klempnauer KH. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene. 2004;23:7484–7493. doi: 10.1038/sj.onc.1208064. [DOI] [PubMed] [Google Scholar]
- Biyanee A, Ohnheiser J, Singh P, Klempnauer KH. A novel mechanism for the control of translation of specific mRNAs by tumor suppressor protein Pdcd4: inhibition of translation elongation. Oncogene. 2015;34:1384–1392. doi: 10.1038/onc.2014.83. [DOI] [PubMed] [Google Scholar]
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993;15:105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- Bohm M, Sawicka K, Siebrasse JP, Brehmer-Fastnacht A, Peters R, Klempnauer KH. The transformation suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene. 2003;22:4905–4910. doi: 10.1038/sj.onc.1206710. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Chang JH, Cho YH, Sohn SY, Choi JM, Kim A, Kim YC, Jang SK, Cho Y. Crystal structure of the eIF4A-PDCD4 complex. Proc Natl Acad Sci U S A. 2009;106:3148–3153. doi: 10.1073/pnas.0808275106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bian Y, Zhao S, Kong F, Li X. Suppression of PDCD4 mediated by the long non-coding RNA HOTAIR inhibits the proliferation and invasion of glioma cells. Oncol Lett. 2016;12:5170–5176. doi: 10.3892/ol.2016.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, Matsuhashi S, Colburn NH. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci U S A. 1999;96:14037–14042. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta R, Holz MK. RSK-mediated down-regulation of PDCD4 is required for proliferation, survival, and migration in a model of triple-negative breast cancer. Oncotarget. 2016;7:27567–27583. doi: 10.18632/oncotarget.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A, Harris AL. eIF4E expression in tumors: its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. doi: 10.1016/s1357-2725(98)00132-0. [DOI] [PubMed] [Google Scholar]
- Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci U S A. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by "walking" the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Fay MM, Clegg JM, Uchida KA, Powers MA, Ullman KS. Enhanced arginine methylation of programmed cell death 4 protein during nutrient deprivation promotes tumor cell viability. J Biol Chem. 2014;289:17541–17552. doi: 10.1074/jbc.M113.541300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehler O, Singh P, Haas A, Ulrich D, Muller JP, Ohnheiser J, Klempnauer KH. An evolutionarily conserved interaction of tumor suppressor protein Pdcd4 with the poly(A)-binding protein contributes to translation suppression by Pdcd4. Nucleic Acids Res. 2014;42:11107–11118. doi: 10.1093/nar/gku800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goke A, Goke R, Knolle A, Trusheim H, Schmidt H, Wilmen A, Carmody R, Goke B, Chen YH. DUG is a novel homologue of translation initiation factor 4G that binds eIF4A. Biochem Biophys Res Commun. 2002;297:78–82. doi: 10.1016/s0006-291x(02)02129-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Villasana V, Nieves-Alicea R, McMurtry V, Gutierrez-Puente Y, Tari AM. Programmed cell death 4 inhibits leptin-induced breast cancer cell invasion. Oncol Rep. 2012;27:861–866. doi: 10.3892/or.2011.1600. [DOI] [PubMed] [Google Scholar]
- Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–665. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Guo X, Li W, Wang Q, Yang HS. AKT Activation by Pdcd4 Knockdown Up-Regulates Cyclin D1 Expression and Promotes Cell Proliferation. Genes Cancer. 2011;2:818–828. doi: 10.1177/1947601911431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Aishima S, Miyasaka Y, Nakata K, Morimatsu K, Oda Y, Nagai E, Oda Y, Tanaka M, Tsuneyoshi M. Pdcd4 expression in intraductal papillary mucinous neoplasm of the pancreas: its association with tumor progression and proliferation. Hum Pathol. 2010;41:1507–1515. doi: 10.1016/j.humpath.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Hilliard A, Hilliard B, Zheng SJ, Sun H, Miwa T, Song W, Goke R, Chen YH. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol. 2006;177:8095–8102. doi: 10.4049/jimmunol.177.11.8095. [DOI] [PubMed] [Google Scholar]
- Hwang SK, Minai-Tehrani A, Lim HT, Shin JY, An GH, Lee KH, Park KR, Kim YS, Beck GR, Jr, Yang HS, Cho MH. Decreased level of PDCD4 (programmed cell death 4) protein activated cell proliferation in the lung of A/J mouse. J Aerosol Med Pulm Drug Deliv. 2010;23:285–293. doi: 10.1089/jamp.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res. 2005;65:6034–6041. doi: 10.1158/0008-5472.CAN-04-2119. [DOI] [PubMed] [Google Scholar]
- Jin H, Kim TH, Hwang SK, Chang SH, Kim HW, Anderson HK, Lee HW, Lee KH, Colburn NH, Yang HS, Cho MH, Cho CS. Aerosol delivery of urocanic acid-modified chitosan/programmed cell death 4 complex regulated apoptosis, cell cycle, and angiogenesis in lungs of K-ras null mice. Mol Cancer Ther. 2006;5:1041–1049. doi: 10.1158/1535-7163.MCT-05-0433. [DOI] [PubMed] [Google Scholar]
- Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- Kariko K, Kuo A, Boyd D, Okada SS, Cines DB, Barnathan ES. Overexpression of urokinase receptor increases matrix invasion without altering cell migration in a human osteosarcoma cell line. Cancer Res. 1993;53:3109–3117. [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Gregel C, Knolle A, Hasilik A, Arnold R, Goke R. Pdcd4 inhibits growth of tumor cells by suppression of carbonic anhydrase type II. Mol Cell Endocrinol. 2004;214:149–153. doi: 10.1016/j.mce.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit B, Muller S, Schmidt H, Parhofer KG, Gress TM, Goke R. Knockdown of Pdcd4 results in induction of proprotein convertase 1/3 and potent secretion of chromogranin A and secretogranin II in a neuroendocrine cell line. Biol Cell. 2008;100:703–715. doi: 10.1042/BC20080052. [DOI] [PubMed] [Google Scholar]
- Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550–4562. doi: 10.1038/sj.onc.1210234. [DOI] [PubMed] [Google Scholar]
- Li JJ, Westergaard C, Ghosh P, Colburn NH. Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res. 1997;57:3569–3576. [PubMed] [Google Scholar]
- Liu Y, Su D, Song T. Programmed cell death 4 inhibits proliferation and differentiation and induces apoptosis of human mesenchymal stem cells through suppressing the Wnt/β-catenin pathway. RCS Adv. 2017;7:26566–26573. [Google Scholar]
- Liwak U, Thakor N, Jordan LE, Roy R, Lewis SM, Pardo OE, Seckl M, Holcik M. Tumor suppressor PDCD4 represses internal ribosome entry site-mediated translation of antiapoptotic proteins and is regulated by S6 kinase 2. Mol Cell Biol. 2012;32:1818–1829. doi: 10.1128/MCB.06317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh PG, Yang HS, Walsh MA, Wang Q, Wang X, Cheng Z, Liu D, Song H. Structural basis for translational inhibition by the tumour suppressor Pdcd4. EMBO J. 2009;28:274–285. doi: 10.1038/emboj.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- Nieves-Alicea R, Colburn NH, Simeone AM, Tari AM. Programmed cell death 4 inhibits breast cancer cell invasion by increasing tissue inhibitor of metalloproteinases-2 expression. Breast Cancer Res Treat. 2009;114:203–209. doi: 10.1007/s10549-008-9993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne BW, McGarry L, Spence HJ, Johnston I, Winnie J, Meagher L, Stapleton G. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur J Cancer. 2000;36:1640–1648. doi: 10.1016/s0959-8049(00)00175-1. [DOI] [PubMed] [Google Scholar]
- Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res. 2005;65:11282–11286. doi: 10.1158/0008-5472.CAN-05-3469. [DOI] [PubMed] [Google Scholar]
- Powers MA, Fay MM, Factor RE, Welm AL, Ullman KS. Protein arginine methyltransferase 5 accelerates tumor growth by arginine methylation of the tumor suppressor programmed cell death 4. Cancer Res. 2011;71:5579–5587. doi: 10.1158/0008-5472.CAN-11-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- Santhanam AN, Baker AR, Hegamyer G, Kirschmann DA, Colburn NH. Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene. 2010;29:3921–3932. doi: 10.1038/onc.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter U, Burk O, Worpenberg S, Klempnauer KH. The chicken Pdcd4 gene is regulated by v-Myb. Oncogene. 2001;20:231–239. doi: 10.1038/sj.onc.1204071. [DOI] [PubMed] [Google Scholar]
- Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res. 2008;68:1254–1260. doi: 10.1158/0008-5472.CAN-07-1719. [DOI] [PubMed] [Google Scholar]
- Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene. 1995;166:297–301. doi: 10.1016/0378-1119(95)00607-9. [DOI] [PubMed] [Google Scholar]
- Singh P, Wedeken L, Waters LC, Carr MD, Klempnauer KH. Pdcd4 directly binds the coding region of c-myb mRNA and suppresses its translation. Oncogene. 2011;30:4864–4873. doi: 10.1038/onc.2011.202. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene. 2008;27:1527–1535. doi: 10.1038/sj.onc.1210793. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun ZX, Allgayer H, Yang HS. Downregulation of E-cadherin is an essential event in activating beta-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene. 2010;29:128–138. doi: 10.1038/onc.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Yang HS. Pdcd4 knockdown up-regulates MAP4K1 expression and activation of AP-1 dependent transcription through c-Myc. Biochim Biophys Acta. 2012;1823:1807–1814. doi: 10.1016/j.bbamcr.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhu J, Wang Y-W, D Y, Wang C, Liu J, Baker A, Colbun NH, Yang H-S. Tumor suppressor Pdcd4 attenuates Sin1 translation to inhibit invasion in colon carcinoma. Oncogene. 2017 doi: 10.1038/onc.2017.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhu J, Zhang Y, Sun Z, Guo X, Wang X, Lee E, Bakthavatchalu V, Yang Q, Yang HS. Down-regulation of programmed cell death 4 leads to epithelial to mesenchymal transition and promotes metastasis in mice. Eur J Cancer. 2013:1761–1770. doi: 10.1016/j.ejca.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters LC, Strong SL, Ferlemann E, Oka O, Muskett FW, Veverka V, Banerjee S, Schmedt T, Henry AJ, Klempnauer KH, Carr MD. Structure of the tandem MA-3 region of Pdcd4 protein and characterization of its interactions with eIF4A and eIF4G: molecular mechanisms of a tumor suppressor. J Biol Chem. 2011;286:17270–17280. doi: 10.1074/jbc.M110.166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeken L, Ohnheiser J, Hirschi B, Wethkamp N, Klempnauer KH. Association of Tumor Suppressor Protein Pdcd4 With Ribosomes Is Mediated by Protein-Protein and Protein-RNA Interactions. Genes Cancer. 2010;1:293–301. doi: 10.1177/1947601910364227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeken L, Singh P, Klempnauer KH. Tumor suppressor protein Pdcd4 inhibits translation of p53 mRNA. J Biol Chem. 2011;286:42855–42862. doi: 10.1074/jbc.M111.269456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N, Liu SS, Chan KK, Ngan HY. Tumour suppressive function and modulation of programmed cell death 4 (PDCD4) in ovarian cancer. PLoS One. 2012;7:e30311. doi: 10.1371/journal.pone.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H-S, Wang Q, Bajer MM, Schmid T. Pdcd4. In: Parsyan A, editor. Translation and Its Regulation in Cancer Biology and Medicine. Springer; New York: 2014. pp. 135–161. [Google Scholar]
- Yang HS, Cho MH, Zakowicz H, Hegamyer G, Sonenberg N, Colburn NH. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol. 2004;24:3894–3906. doi: 10.1128/MCB.24.9.3894-3906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003a;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, Colburn NH. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–676. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene. 2003b;22:3712–3720. doi: 10.1038/sj.onc.1206433. [DOI] [PubMed] [Google Scholar]
- Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, Tan TH, Colburn NH. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Meng H, Peng Q, Yang X, Gan R, Zhao L, Chen Z, Lu J, Meng QH. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Therapy. 2015;22:23–29. doi: 10.1038/cgt.2014.66. [DOI] [PubMed] [Google Scholar]
- Yu H, Zeng J, Liang X, Wang W, Zhou Y, Sun Y, Liu S, Li W, Chen C, Jia J. Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer by downregulating programmed cell death protein 4 (PDCD4) PLoS One. 2014;9:e105306. doi: 10.1371/journal.pone.0105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowicz H, Yang HS, Stark C, Wlodawer A, Laronde-Leblanc N, Colburn NH. Mutational analysis of the DEAD-box RNA helicase eIF4AII characterizes its interaction with transformation suppressor Pdcd4 and eIF4GI. RNA. 2005;11:261–274. doi: 10.1261/rna.7191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li J, Jiang Y, Xu Y, Qin C. Programmed cell death 4 (PDCD4) suppresses metastastic potential of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2009;28:71. doi: 10.1186/1756-9966-28-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Fang W, Zhao M, Luo R, Liu Y, Fu Q, Chen Y, Cheng C, Zhang Y, Liu Z. miR-374a-CCND1-pPI3K/AKT-c-JUN feedback loop modulated by PDCD4 suppresses cell growth, metastasis, and sensitizes nasopharyngeal carcinoma to cisplatin. Oncogene. 2017;36:275–285. doi: 10.1038/onc.2016.201. [DOI] [PubMed] [Google Scholar]
- Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, Long X, Jiang Q, Song Y, Cheng C, Wang H, Zhao M, Fu Q, Lyu X, Chen Y, Fan Y, Liu Y, Li X, Fang W. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e872. doi: 10.1038/cddis.2013.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- Zhou H, Huang S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr Protein Pept Sci. 2011;12:30–42. doi: 10.2174/138920311795659407. [DOI] [PMC free article] [PubMed] [Google Scholar]