Abstract
Objective
Systemic immunosuppressive treatment of pediatric chronic anterior uveitis (CAU), both juvenile idiopathic arthritis (JIA)-associated and idiopathic varies, making it difficult to identify best treatments. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed consensus treatment plans (CTPs) for CAU for the purpose of reducing practice variability and allowing future comparison of treatments by comparative effectiveness analysis techniques.
Methods
A core group of pediatric rheumatologists, ophthalmologists with uveitis expertise, and a lay advisor comprised the CARRA uveitis workgroup who performed literature review on pharmacologic treatments, held teleconferences, and developed a case-based survey administered to the CARRA membership to delineate treatment practices. We utilized 3 face-to-face consensus meetings using nominal group technique to develop CTPs.
Results
The survey identified areas of treatment practice variability. We developed 2 CTPs for the treatment of CAU, case definitions, and monitoring parameters. The first CTP is directed at children naïve to steroid-sparing medication, and the second at children initiating biologic therapy with options for methotrexate, adalimumab and infliximab. We defined a core dataset and outcome measures with data collection at 3 and 6 months after therapy initiation. The CARRA membership voted acceptance of the CTPs with a >95% (N = 233) approval.
Conclusion
Using consensus methodology, two standardized CTPs were developed for systemic immunosuppressive treatment of CAU. These CTPs are not meant as treatment guidelines, but are designed for further pragmatic research within the CARRA research network. Use of these CTPs in a prospective comparison effectiveness study should improve outcomes by identifying best practice options.
Pediatric chronic anterior uveitis (CAU) is an inflammatory ocular disease that can lead to vision loss and ocular complications in up to 60% of affected children (1–15). Idiopathic uveitis, i.e. without associated systemic illness, constitutes approximately 50% of pediatric CAU (1, 2, 16–19). Juvenile idiopathic arthritis (JIA) is the most common systemic disease associated with pediatric CAU, wherein 10-15% of these children will develop CAU (20, 21). Early detection and appropriate timely treatment may prevent sight-threatening complications such as cataracts, glaucoma, and synechiae (22).
There are presently no widely accepted approaches to the treatment of CAU. There are few pediatric randomized controlled trials, except for adalimumab in JIA-associated uveitis (JIA-U) (23, 24). Topical steroids are typical initial therapy, but prolonged use can lead to complications such as cataracts and increased intraocular pressure. Inadequate response to, and/or toxic effects from steroids necessitate the addition of steroid-sparing immunosuppressive therapy. However, evidence for specific agents is lacking.
Best practice guidelines for management of pediatric CAU have been developed by multiple groups but are not widely adopted in North America (25–27). Examination of JIA-associated patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry, a large registry of North American pediatric rheumatology patients, demonstrated that a broad range of biologic and non-biologic medications are prescribed (28). Additionally, the lack of pediatric standardized outcome measures for CAU limits the comparison of treatment strategies. Management is further complicated by the need for close collaboration between ophthalmologists and rheumatologists, with disease assessment by ophthalmologists, while steroid sparing systemic treatment typically prescribed by rheumatologists.
Through CARRA, we developed standardized treatment approaches, i.e. consensus treatment plans (CTPs), for children with typical JIA-associated and idiopathic CAU. These CTPs are meant for use in pragmatic research within the CARRA network, and not intended as standard treatment guidelines. We chose to include idiopathic CAU as systemic treatment approaches are the same as JIA-U, ocular complications similar, and this is an underserved population for research. In addition, the antinuclear antibody (ANA) + CAU may represent a forme fruste of JIA. These CTPs were developed through a robust consensus process and represent current clinical practice of North American pediatric rheumatologists, with expert input from ophthalmologists specializing in uveitis care. These CTPs, as with other CTPs developed by CARRA, differ from expert guidelines in that they are treatment strategies developed by consensus methods amongst CARRA members with the primary goal of streamlining care and reducing practice variability (29). Ultimately, formal implementation of these CTPs in the treatment of patients enrolled in the CARRA registry will facilitate comparative effectiveness studies of different treatment approaches (29–33). We developed two CTPs with multiple treatment options intended for common CAU scenarios: 1) initiation of MTX therapy, and 2) initiation of biologic therapy.
II. Materials and methods
Core Workgroup
A core workgroup of 10 board-certified pediatric rheumatologists with special interest in CAU, two ophthalmologists with expertise in uveitis, and a parent of a child with JIA-U was formed. Tasks of the workgroup included: 1) defining a target population, 2) identifying similarities and disparities among treatment approaches, 3) reviewing the literature on comparative efficacies of treatment approaches, and 4) achieving consensus on criteria to assess inflammation and treatment response. To identify relevant literature on uveitis treatment strategies, we performed a search of the PubMed database using the terms “juvenile arthritis”, “uveitis”, “treatment”, “subcutaneous”, “oral”, “dose”, “methotrexate”, “TNF inhibitor”, “etanercept”, “adalimumab”, and “infliximab” through April 1, 2014, and updated in July 2016.
Besides face-to-face meetings, workgroup interactions occurred via teleconferences, surveys and email discussions between April 2012 and June 2016 (Figure 1).
Figure 1.
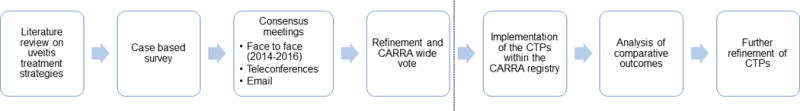
Delphi Survey
To better understand existing practice patterns in the treatment of CAU by the pediatric rheumatology community, we administered an anonymous web-based survey to CARRA voting members who actively treat children with CAU (trainees ineligible). We presented clinical scenarios to identify common approaches for selection of: 1) an initial steroid-sparing agent in CAU, 2) a second-choice steroid-sparing agent in the event of initial treatment failure in patients with and without complications from CAU, and 3) a second-choice steroid-sparing agent in the event of intolerance to initial therapy (Supplementary Appendix).
Consensus Meetings
The first face-to-face meeting of the workgroup was held in April 2014. One ophthalmologist participated via conference call (NS). A syllabus consisting of a summary of the pre-consensus survey, prior phone discussions, literature review, and existing guidelines was presented. Two CTPs were drafted for two scenarios of uncontrolled CAU: 1) initiation of MTX in children naïve to steroid-sparing agents, and 2) initiation of biologic therapy in children with inadequate response or intolerant to MTX.
Modified nominal group technique (NGT) was used to seek consensus (defined as ≥75% agreement) on the two draft CTPs (30–33). The NGT discussion was facilitated by an experienced moderator (HB), and responses were tabulated by a non-voting CARRA member (LH).These post-consensus CTPs were further refined by the uveitis workgroup during follow-up calls, and via NGT in face-to-face discussion in April 2015. An ophthalmologist (GNH) specializing in uveitis was present for these discussions.
The CTP strategies on the use of MTX and TNFi for CAU were presented to the CARRA JIA research committee in April 2016. Approval was obtained after members reviewed patient characteristics, data collection items, collection time points, primary and secondary outcomes, and the final CTP strategies. Consensus was based on show of hands or anonymous formal voting when needed. It is important to note that the number of voting members varied at each session, hence the variance in our numbers.
We disseminated final CTPs to the CARRA-wide membership as an anonymous online survey to confirm willingness to use at least one of the treatment plans to support comparative effectiveness research.
III. Results
Delphi Survey Results
Our case-based survey was sent to all CARRA members (Supplementary Appendix). We received 129 responses (50% response rate). Five respondents indicated that they did not provide care for children with uveitis and were excluded from analysis.
For a child with CAU not controlled by topical corticosteroids, MTX was most frequently selected as the initial systemic drug. Respondents could select more than one response; half of the respondents (N = 60/120, 50%) selected oral dosing as the initial mode of administration, while subcutaneous dosing was selected more frequently (79/120, 66%). Therapy with a TNFi, with or without concomitant use of MTX, was less frequently selected (1-6%).
For patients with continued uncontrolled uveitis despite MTX, most (114/120, 95%) would add, instead of substitute, a systemic immunosuppressive agent. Adalimumab (73/117, 62%) was favored over infliximab (40/117, 34%). In contrast, infliximab (63/120, 53%) was favored over adalimumab (52/120, 43%) in a child with uncontrolled uveitis and uveitis-related complications despite MTX.
In the case of MTX intolerance requiring drug discontinuation in a child with inactive uveitis, adalimumab was the most frequently selected alternative (66/112, 59%) followed by mycophenolate mofetil (29/112, 26%) and infliximab (13/112, 11%). Less frequently selected systemic therapies included abatacept (2/112, 2%), azathioprine (1/112, 1%), and etanercept (1/112, 1%).
Key questions considered important to address through a CTP were: 1) timing of and criteria for initiation of a systemic agent, 2) selection of first-line and second-line therapies, and 3) criteria for assessing response to therapy.
Face-to-face Consensus Meetings
Based on the practice variability noted in the preconsensus survey, the workgroup agreed to develop CTPs that would determine: 1) the preferred form of MTX administration and 2) preferred biologic therapy for CAU. Accordingly, we developed two CTPs for children with uncontrolled CAU: one for initiation of MTX in patients who have failed topical steroids, and one for initiation of TNF inhibitor (TNFi) therapy. Consensus on these CTPs was achieved by the JIA research committee at the 2016 CARRA annual meeting (27/28, 96%; 25/25, 100%).
Target Population
Table 1 defines the patient population targeted for these CTPs. There was consensus among the uveitis workgroup at the 2014 meeting and the CARRA JIA research committee in 2016 that these CTPs are appropriate for CAU that is: 1) idiopathic, or 2) JIA-associated, the most common categories of pediatric non-infectious uveitis. Although enrolling two distinct patient populations may introduce heterogeneity in the observed response to therapy, both are treated similarly, and a diagnosis of JIA does not influence outcomes (21). Two distinct target populations emerged from the consensus discussions, each with their own CTPs. The first CTP is for children naïve to steroid-sparing therapy and compares oral versus subcutaneous MTX administration. The second CTP is for a heterogeneous population of children initiating a TNFi: 1) failed MTX, 2) intolerant of MTX, and 3) naïve to MTX but with need for urgent treatment as determined by the clinician (e.g., patients presenting with acute uveitis and ocular complications from either uveitis or steroid therapy). This CTP compares adalimumab weekly, adalimumab every other week, and infliximab. Based on expert opinion, the more severely affected eye will dictate treatment in bilateral disease.
Table 1.
Characteristics of patients for use of the CTPs
Patients should have:
|
Patients should not have:
|
Complications include: increased intraocular pressure, hypotony, cataracts, posterior synechiae, band keratopathy, and cystoid macular edema.
Both CTPs are suitable for the treatment of children who fulfill any of the following criteria: 1) ongoing uveitis activity despite the use of topical steroids, 2) worsening uveitis activity while on topical steroids, 3) recurrent uncontrolled disease (≥1+ anterior chamber (AC) cells) with taper of topical steroids to twice daily or less, 4) development of new ocular complications attributable to either inflammation or treatment during topical steroid therapy, and 5) intolerant or unable to adhere to therapy with topical corticosteroid drops. Examples of complications include increased intraocular pressure, hypotony, cataracts, posterior synechiae, band keratopathy, and cystoid macular edema. While twice-daily topical steroids is not preferred for long term management, based on expert opinion, it is acceptable, as this dose is accepted by the ophthalmology community for patients who do not have corticosteroid-induced ocular hypertension(34). There was consensus that these CTPs could also be applied to children on systemic steroids or with a history of unsuccessful subtenon steroid injections.
Experts agreed that the CTPs were not designed for the treatment of children with other uveitis categories, i.e. intermediate and posterior uveitis, symptomatic acute unilateral anterior uveitis (AAU), uveitis attributable to other inflammatory conditions (e.g., sarcoidosis, Behcet’s disease), the presence of ocular co-morbidities that could affect interpretation of outcomes, and contraindications to therapy (Table 1). There was ≥80% consensus on all points. The CTPs were restricted to CAU because it is most common in children along with the lack of generally accepted criteria to assess disease activity in intermediate, posterior, or panuveitic uveitis. Patients with previous exposure to a biologic agent within 3 months prior to enrollment are also not appropriate for these CTPs.
Categorization of Uveitis Disease Activity
Consensus was achieved to adopt the Standardization of Uveitis Nomenclature (SUN) Working Group methods of reporting clinical data (Table 2) (35). These include a grading scheme for AC cells and flare, uveitis activity, ocular complications, and outcomes. There was consensus that the course of uveitis can be categorized as: 1) inactive, 2) worsened, 3) improved or 4) controlled based on the degree of AC cells (35).
Table 2.
Adapted Standardization of Uveitis Nomenclature (SUN) Definitions22.
| Grading Scheme for Anterior Chamber Cells: | |
|---|---|
| Grade | Cells in Field* |
| 0 | 0 |
| 0.5+ | 1-5 |
| 1+ | 6-15 |
| 2+ | 16-25 |
| 3+ | 26-50 |
| 4+ | >50 |
| Definitions of Disease Activity: | |
|---|---|
| Inactive | Grade 0 cells in anterior chamber |
| Worsening activity | Two step increase in inflammation by anterior chamber cells, or 3+ to 4+ |
| Improved activity | Two step decrease in level of inflammation by anterior chamber cells, or decrease to 0 |
| Remission | Inactive disease for ≥3 months after discontinuing all treatments for eye disease |
Field size is 1 mm × 1 mm slit beam
There was consensus to define adequately controlled CAU as follows: 1) not on systemic steroids; 2) no more than 0.5+ AC cells; 3) on topical steroids ≤2 drops/day; and 4) no new ocular complications for at least 3 months. We agreed with consensus that although 0.5+ AC cells is considered “active” by SUN criteria, we would not necessarily escalate therapy based on the presence of 0.5+ cells. For the purposes of these CTPs, the presence of AC cells ≥1+ (6-15 cells/hpf) constitutes uncontrolled uveitis.
MTX Therapy Consensus Treatment Plan
Patients with CAU as defined, naive to steroid-sparing therapy, are appropriate for the MTX CTP. Although the majority of the uveitis workgroup agreed that subcutaneous MTX has higher bioavailability and is preferred, data for superior efficacy of subcutaneous administration is lacking. In addition, a survey of pediatric rheumatologists indicated both routes are used equally (28). Therefore, both oral and subcutaneous MTX are treatment options. Dosing for MTX is 0.5-1 mg/kg/week, with a maximum of 30 mg per week; doses closer to 1 mg/kg/week are preferred (Fig. 2).
Figure 2.
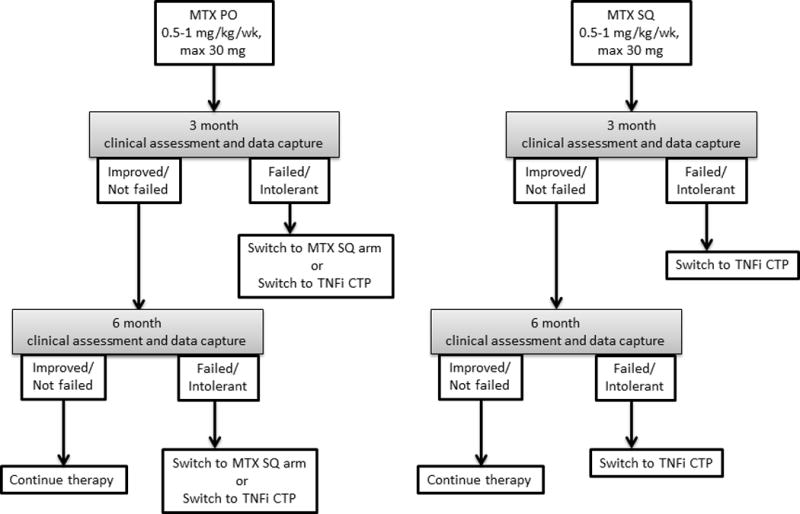
Consensus was reached that 3 months of treatment is necessary before assessing MTX efficacy. After 3 months, patients who failed MTX are recommended to change to the TNFi CTP. For children on oral MTX, an alternative is to enter the subcutaneous MTX arm. In addition, JIA patients who develop new uveitis while on MTX for arthritis would be considered to have failed MTX; the TNFi CTP should be considered.
TNFi Consensus Treatment Plan
Patients who fail MTX should be considered for the TNFi CTP using monoclonal antibody TNFi. For patients who are not intolerant of MTX, TNFi should be added to, rather than replace, MTX. The TNFi CTP can also be considered for MTX-naïve patients with uncontrolled (≥1+ AC cells) uveitis and severe disease (e.g. ocular structural complications due to uveitis, or complications of topical steroid therapy on presentation). MTX should be started simultaneously, utilizing either the subcutaneous or oral MTX options from the MTX CTP. Consensus was achieved at the 2015 meeting that we would not specifically define “severe disease” at this time, but that the TNFi CTP should be considered at the provider’s discretion. Although inclusion of patients who are MTX naïve and failed MTX may confound analysis of outcomes, we should be able to correct for this in analysis, and do not want to limit therapy in children in whom the clinician has determined that TNFi initiation is necessary.
There was unanimous agreement that etanercept has no role in the treatment of pediatric uveitis, and that there is insufficient data to recommend either adalimumab or infliximab as the preferred agent. Selection is left open to the treating provider, acknowledging that there may be individual factors influencing this decision, such as patient preference for medication route, insurance coverage, and compliance concerns.
The TNFi CTP includes three treatment options: 1) adalimumab SQ injections weekly, 2) adalimumab SQ injections every other week, and 3) infliximab infusions every 4 weeks after loading. Dosing for adalimumab parallels those for polyarticular JIA: 10 mg for patients 10 kg to <15 kg; 20 mg for patients 15 kg to <30 kg; 40 mg for patients ≥30 kg (Fig. 3). The workgroup agreed that adalimumab dose can be escalated 8 weeks after initiation if uveitis remains uncontrolled (≥1+ AC) or if unable to begin tapering steroids after 4 weeks due to persistent CAU. Dose escalation through either doubling the every-other-week dose (if on 10 or 20 mg), or increasing the frequency to weekly, are equally acceptable.
Figure 3.
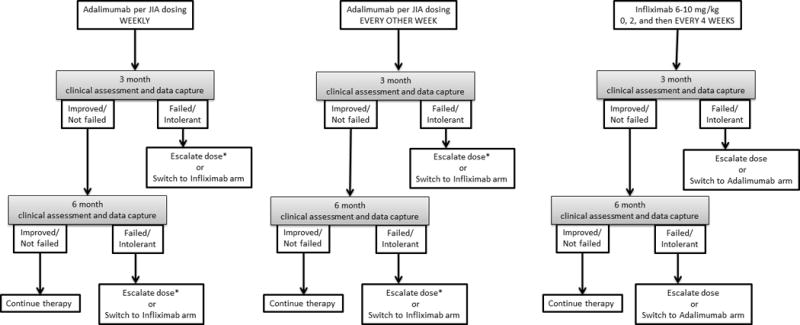
Dosing for infliximab starts at 6-10 mg/kg (Fig. 3). A loading regimen is recommended, giving infusions at 0, and 2 weeks, followed by every 4 weeks thereafter. Dose escalation is permitted based on exam after 8 weeks, up to a maximum dose of 20 mg/kg. MTX dose can be lowered while on a TNFi.
The CARRA JIA research committee agreed with infliximab dosing range of 6-10 mg/kg (27/28, 96%), and both a weekly and every other week adalimumab dosing arm (27/28, 96%).
Core Documentation for Children with CAU
We defined the data collection items, time points and outcome measures for data collection through consensus discussions at the 2015 meeting (10/13, 77%), and obtained approval by the JIA committee (26/26, 100%). Data will be collected at enrollment, 3 months and 6 months. An eye exam should occur within 6 weeks after starting therapy but will not be considered a separate study visit. All eye exam records in between study visits should be reviewed. Data points include demographics, uveitis clinical data (duration, age at uveitis onset, JIA-associated or idiopathic uveitis, anatomical location, laterality, AC cells by SUN criteria, visual acuity, ocular complications, ocular surgeries), reason not adherent to topical steroids if applicable, current and maximum daily steroid use (topical and systemic), start and stop dates of medications, and patient reported outcome measures (PROs) (Table 3). PROs will include the Patient-Reported Outcome Measurement Information System (PROMIS) global health scale, and the Effects of Youngsters’ Eyesight on Quality of Life (EYE-Q) (36, 37). Adverse effects of therapy, such as leukopenia and hepatorenal toxicity from MTX, will be recorded. Monitoring toxicity of medications schedule is deferred to the prescribing physician. Definitions of disease activity, recurrence, flare, and complications will be based on SUN criteria (35) (Table 2). Ophthalmology exam results will be included.
Table 3.
Data collection points
| Data collection points at 0, 3, 6 months or end of study | |||
|---|---|---|---|
| Variables | 0 mos | 3, 6 mos and/or end of study | |
| Baseline | Disease duration | X | |
| Age at disease onset | X | ||
| JIA subtype or idiopathic uveitis | X | ||
| Uveitis | Anatomical Location | X | X |
| Laterality | X | X | |
| Anterior chamber cells by SUN criteria | X | X | |
| Visual acuity | X | X | |
| Ocular complications | X | X | |
| Ocular surgeries | X | X | |
| PROs | VAS | X | X |
| Overall QOL: PROMIS global health score | X | X | |
| Uveitis related QOL: EYE-Q | X | X | |
Interpretation of Patient Response
Primary outcome is defined as improvement or worsening of AC cells at 6 months as defined by SUN criteria. Secondary outcome measures include proportion with inflammation <1+ cells, visual acuity, eye complications, eye surgeries, PROs, adverse events, and corticosteroid use. It is the goal of most uveitis specialists to reduce AC cells to below a threshold of 1+ cells.
Treatment Failure is defined as ongoing uncontrolled uveitis, development of damage/eye complications, or intolerance/non-adherence to treatment. Another CTP treatment arm can be considered for patients who fail initial treatment. If treatment changes for arthritis but not uveitis, or if the patient chooses not to continue in the CTP, this will be captured by the CTP.
Methotrexate Intolerance
Suggestions for management of MTX intolerance were considered beyond the scope of these CTPs. The workgroup emphasizes that MTX intolerance can often be managed through the use of anti-emetics, folic acid and/or leucovorin and dose adjustment, but children experiencing MTX intolerance can also be considered for the TNFi CTP.
Systemic Steroids
The workgroup acknowledged that provision and dosing of systemic and topical corticosteroids are typically made by the treating ophthalmologist, rather than the rheumatologist. Therefore, this CTP does not include corticosteroid recommendations. However, based on expert opinion, systemic steroids should be avoided in the treatment of CAU. Systemic steroids should be used only as a temporizing measure while awaiting efficacy of steroid-sparing therapy, and steroid taper should begin no longer than 2 weeks after initiation of a steroid-sparing agent. This was unanimously agreed upon by the CARRA JIA committee (27/27, 100%).
Ophthalmology Screening
Although the AAP has guidelines for ophthalmology screening of JIA children, no guidelines exist for children with a history of CAU. Expert consensus was reached that children with uncontrolled uveitis should be monitored at least every 2-6 weeks (27/28, 96%).
Third-line Therapy
There are insufficient data to recommend treatment of uveitis refractory to MTX and TNFi. Although consensus was not achieved, members considered one or more of these medications: mycophenolate mofetil (13/18, 72%), abatacept (10/18, 56%), cyclosporine (7/18, 39%), tocilizumab (6/18, 33%), golimumab (1/18, 5%) azathioprine (1/18, 5%), leflunomide (1/18, 5%), and rituximab (1/18, 5%). These preferences may change as experience with these agents grows.
Post-consensus survey
The workgroup sought approval from the CARRA-wide membership through an online survey. Response rate was 81% (N = 247/306); among these, 10% (24/247) reported that they did not manage uveitis, and their responses were excluded. Consensus was achieved on the target population (216/223, 97%) and on the criteria for application of the TNFi CTP (215/223, 97%). Ninety-seven percent (215/233) reported willingness to use at least one arm of the methotrexate CTP and 99% (220/233) at least one arm of the TNFi CTP. There was broad consensus on the data collection measures outlined above.
IV. Discussion
Informed by the available medical evidence, expert consensus was achieved among pediatric rheumatologists and ophthalmologists participating in CARRA on treatment strategies for children with CAU. Hence, these CTPs may provide general guidance for the management of typical pediatric CAU, but are not meant as treatment guidelines. Rather, as with CTPs developed for other rheumatologic conditions, these CTPs are primarily intended to facilitate future comparative effectiveness research within the CARRA network (29).
Two CTPs were developed for use in patients enrolled in the CARRA registry. One for MTX in children without prior exposure to DMARDs, and the other for TNFi in children who failed MTX, are MTX intolerant, or in need of urgent treatment as determined by the clinician. These CTPs may not be relevant for cases that do not fit the most common scenarios described here. Active uveitis may be associated with active arthritis; these plans are intended to be used in situations where uveitis is guiding the choice of therapy.
In general, MTX is the first line agent for children with uveitis in need of systemic immunosuppression. In complicated or refractory disease, infliximab and adalimumab are equally preferred, and little data supports superiority of either TNFi (38–43). Small studies suggest that adalimumab may be as effective or superior to infliximab in achieving remission, but there were differences in the dose and frequency given (41, 44–46). Doses of infliximab greater than 7.5 mg/kg and as high as 20mg/kg/dose every 4 weeks may be necessary for recalcitrant disease (42, 47–49).
Our CTPs are intended to help standardize treatment approaches while also allowing future comparison of different treatment strategies in observational comparative effectiveness studies. Accordingly, our CTPs provide options that allow for comparatively higher dose regimens of TNFi and are intended for children with both JIA-associated and idiopathic CAU.
In addition to standardization of care, there is also a need for standardized outcome measures. The SUN criteria can be used for measuring treatment response. Heiligenhaus et. al. proposed outcome measures specific for children with JIA-U (50). We propose an expanded group of outcome measures through these CTPs (Table 3).
Regular monitoring by an ophthalmologist experienced in uveitis is crucial. Although guidelines for children with JIA exist, none exist for children with idiopathic uveitis, as this typically falls within the treating ophthalmologist’s purview. We suggest that children with uncontrolled uveitis or undergoing therapy changes be monitored at least every 2-6 weeks. In addition, if access to a uveitis specialist is available, all children should be evaluated at least once. We emphasize the importance of close communication between pediatric rheumatologists and ophthalmologists to ensure best visual outcomes. This can be done through shared medical records, combined subspecialty clinics, and/or standardized communication forms.
These CTPs address two important issues in CAU treatment. First, the preferred route of MTX administration is unknown. Subcutaneous administration has higher bioavailability and may have fewer gastrointestinal side effects (51, 52). Since the route of administration will be based on provider’s and patient’s preference, we may be able to optimize route through the conduct of observational studies of patients treated with these CTPs. This CTP will also enable comparative study of adalimumab and infliximab.
A limitation is the inability to recommend a tapering schedule for topical steroids, as this is purveyed by ophthalmologists. Collaboration between subspecialties is crucial. As with any analysis of JIA-U therapy, treatment may be guided by arthritis. This would be captured in data collection and not be used for comparison. Since data on treatment using other TNFi are lacking, they were not included in this CTP.
With implementation of these CTPs utilizing the CARRA registry in comparative effectiveness research, we can address factors associated with treatment success, including preferred duration of therapy, and the risk of relapse after medication discontinuation (49, 53, 54).
V. Conclusion
There is significant variability in current treatment strategies of CAU. We outline a consensus-based strategy to standardize the initial care of children with JIA-associated and idiopathic CAU. Standardizing care will enable comparative effectiveness studies and future clinical trials, identification of preferred treatment, and ultimately optimize visual outcomes for children with CAU.
Supplementary Material
Significance and Innovations.
Systemic immunosuppressive treatment of children with JIA-associated anterior uveitis and idiopathic chronic anterior uveitis varies significantly among pediatric rheumatologists.
Consensus treatment plans for pediatric chronic anterior uveitis were developed by the Childhood Arthritis and Rheumatology Research Alliance to standardize systemic therapies for children with chronic anterior uveitis, and enable comparison of treatments, with the goal of ultimately improving visual outcomes.
Acknowledgments
The authors wish to thank Carol Wallace, Karyl Barron, Yukiko Kimura, Daniel Kingsbury, Anjali Patwardhan, Jennifer Weiss, and Jay Mehta.
Grant support: Supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1RC1-AR-058605-01), the Childhood Arthritis and Rheumatology Research Alliance (CARRA), the Arthritis Foundation, the Waisie Foundation, and Friends of CARRA.
Other financial support: Dr. Angeles-Han is supported by Award Number K23EY021760 from the National Eye Institute, the Rheumatology Research Foundation, and the CCHMC Research Innovation and Pilot Fund. Dr. Sen is supported by the NEI Intramural Research Program. The Effects of Youngsters’ Eyesight on Quality of Life (EYE-Q) has a license through Emory University and was developed by Dr. Angeles-Han. No royalties have been received by any authors.
Footnotes
DR. SHEILA T ANGELES-HAN (Orcid ID : 0000-0002-9552-6464)
References
- 1.Kump LI, Cervantes-Castaneda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112(7):1287–92. doi: 10.1016/j.ophtha.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111(12):2299–306. doi: 10.1016/j.ophtha.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Kanski JJ. Juvenile arthritis and uveitis. Survey of ophthalmology. 1990;34(4):253–67. doi: 10.1016/0039-6257(90)90026-r. [DOI] [PubMed] [Google Scholar]
- 4.Kotaniemi K, Kautiainen H, Karma A, Aho K. Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: a prospective study. Ophthalmology. 2001;108(11):2071–5. doi: 10.1016/s0161-6420(01)00773-4. [DOI] [PubMed] [Google Scholar]
- 5.Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis-related uveitis. J Aapos. 2008;12(6):539–45. doi: 10.1016/j.jaapos.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 6.BenEzra D, Cohen E, Behar-Cohen F. Uveitis and juvenile idiopathic arthritis: A cohort study. Clin Ophthalmol. 2007;1(4):513–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997;104(2):236–44. doi: 10.1016/s0161-6420(97)30329-7. [DOI] [PubMed] [Google Scholar]
- 8.Angeles-Han ST, McCracken C, Yeh S, Jenkins K, Stryker D, Rouster-Stevens K, et al. Characteristics of a cohort of children with Juvenile Idiopathic Arthritis and JIA-associated Uveitis. Pediatric rheumatology online journal. 2015;13:19. doi: 10.1186/s12969-015-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angeles-Han ST, Pelajo CF, Vogler LB, Rouster-Stevens K, Kennedy C, Ponder L, et al. Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J Rheumatol. 2013;40(12):2088–96. doi: 10.3899/jrheum.130302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paroli MP, Speranza S, Marino M, Pirraglia MP, Pivetti-Pezzi P. Prognosis of juvenile rheumatoid arthritis-associated uveitis. European journal of ophthalmology. 2003;13(7):616–21. doi: 10.1177/112067210301300704. [DOI] [PubMed] [Google Scholar]
- 11.Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K, German Uveitis in Childhood Study G Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford) 2007;46(6):1015–9. doi: 10.1093/rheumatology/kem053. [DOI] [PubMed] [Google Scholar]
- 12.Thorne JE, Woreta F, Kedhar SR, Dunn JP, Jabs DA. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. American journal of ophthalmology. 2007;143(5):840–6. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Chen CS, Roberton D, Hammerton ME. Juvenile arthritis-associated uveitis: visual outcomes and prognosis. Can J Ophthalmol. 2004;39(6):614–20. doi: 10.1016/s0008-4182(04)80026-7. [DOI] [PubMed] [Google Scholar]
- 14.Bolt IB, Cannizzaro E, Seger R, Saurenmann RK. Risk factors and longterm outcome of juvenile idiopathic arthritis-associated uveitis in Switzerland. J Rheumatol. 2008;35(4):703–6. [PubMed] [Google Scholar]
- 15.Saurenmann RK, Levin AV, Feldman BM, Rose JB, Laxer RM, Schneider R, et al. Prevalence, risk factors, and outcome of uveitis in juvenile idiopathic arthritis: a long-term followup study. Arthritis and rheumatism. 2007;56(2):647–57. doi: 10.1002/art.22381. [DOI] [PubMed] [Google Scholar]
- 16.Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol. 2016;134(11):1237–45. doi: 10.1001/jamaophthalmol.2016.3229. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara M, Eggenschwiler L, Stephenson A, Montieth A, Nakhoul N, Araujo-Miranda R, et al. The Challenge of Pediatric Uveitis: Tertiary Referral Center Experience in the United States. Ocular immunology and inflammation. 2018:1–8. doi: 10.1080/09273948.2017.1420202. [DOI] [PubMed] [Google Scholar]
- 18.Stoffel PB, Sauvain MJ, von Vigier RO, Beretta-Piccoli BC, Ramelli GP, Bianchetti MG. Non-infectious causes of uveitis in 70 Swiss children. Acta Paediatr. 2000;89(8):955–8. doi: 10.1080/080352500750043422. [DOI] [PubMed] [Google Scholar]
- 19.BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89(4):444–8. doi: 10.1136/bjo.2004.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–78. doi: 10.1016/S0140-6736(07)60363-8. [DOI] [PubMed] [Google Scholar]
- 21.Holland GN, Denove CS, Yu F. Chronic anterior uveitis in children: clinical characteristics and complications. American journal of ophthalmology. 2009;147(4):667–78 e5. doi: 10.1016/j.ajo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. The British journal of ophthalmology. 2002;86(1):51–6. doi: 10.1136/bjo.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quartier P, Despert V, Poignant S, Elie C, Kone-Paut I, Belot A, et al. THU0235 Adjuvite: A Double-Blind, Randomized, Placebo-Controlled Trial of Adalimumab in Juvenile Idiopathic Arthritis Associated Uveitis. Annals of the rheumatic diseases. 2016;75(Suppl 2):273. doi: 10.1136/annrheumdis-2017-212089. [DOI] [PubMed] [Google Scholar]
- 24.Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med. 2017;376(17):1637–46. doi: 10.1056/NEJMoa1614160. [DOI] [PubMed] [Google Scholar]
- 25.Bou R, Adan A, Borras F, Bravo B, Calvo I, De Inocencio J, et al. Clinical management algorithm of uveitis associated with juvenile idiopathic arthritis: interdisciplinary panel consensus. Rheumatology international. 2015 doi: 10.1007/s00296-015-3231-3. [DOI] [PubMed] [Google Scholar]
- 26.Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–96 e3. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 27.Heiligenhaus A, Michels H, Schumacher C, Kopp I, Neudorf U, Niehues T, et al. Evidence-based, interdisciplinary guidelines for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Rheumatology international. 2012;32(5):1121–33. doi: 10.1007/s00296-011-2126-1. [DOI] [PubMed] [Google Scholar]
- 28.Henderson LA, Zurakowski D, Angeles-Han ST, Lasky A, Rabinovich CE, Lo MS, et al. Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance Registry. Pediatric rheumatology online journal. 2016;14(1):9. doi: 10.1186/s12969-016-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ringold S, Nigrovic PA, Feldman BM, Tomlinson GA, von Scheven E, Wallace CA, et al. The Childhood Arthritis & Rheumatology Research Alliance Consensus Treatment Plans: Towards Comparative Effectiveness in the Pediatric Rheumatic Diseases. Arthritis Rheumatol. 2018 doi: 10.1002/art.40395. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt EM, Kimura Y, Beukelman T, Nigrovic PA, Onel K, Prahalad S, et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis care & research. 2012;64(7):1001–10. doi: 10.1002/acr.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mina R, von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis care & research. 2012;64(3):375–83. doi: 10.1002/acr.21558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis care & research. 2012;64(8):1175–85. doi: 10.1002/acr.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringold S, Weiss PF, Colbert RA, DeWitt EM, Lee T, Onel K, et al. Childhood arthritis and rheumatology research alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis. Arthritis care & research. 2014;66(7):1063–72. doi: 10.1002/acr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117(7):1436–41. doi: 10.1016/j.ophtha.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angeles-Han ST, Yeh S, McCracken C, Jenkins K, Stryker D, Myoung E, et al. Using the Effects of Youngsters’ Eyesight on Quality of Life Questionnaire to Measure Visual Outcomes in Children With Uveitis. Arthritis care & research. 2015;67(11):1513–20. doi: 10.1002/acr.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin DE, Gross HE, Stucky BD, Thissen D, DeWitt EM, Lai JS, et al. Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes. 2012;10:22. doi: 10.1186/1477-7525-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-De-Vicuna C, Diaz-Llopis M, Salom D, Bou R, Diaz-Cascajosa J, Cordero-Coma M, et al. Usefulness of adalimumab in the treatment of refractory uveitis associated with juvenile idiopathic arthritis. Mediators of inflammation. 2013;2013:560632. doi: 10.1155/2013/560632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tynjala P, Kotaniemi K, Lindahl P, Latva K, Aalto K, Honkanen V, et al. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatology (Oxford) 2008;47(3):339–44. doi: 10.1093/rheumatology/kem356. [DOI] [PubMed] [Google Scholar]
- 40.Vazquez-Cobian LB, Flynn T, Lehman TJ. Adalimumab therapy for childhood uveitis. The Journal of pediatrics. 2006;149(4):572–5. doi: 10.1016/j.jpeds.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 41.Simonini G, Taddio A, Cattalini M, Caputo R, de Libero C, Parentin F, et al. Superior efficacy of Adalimumab in treating childhood refractory chronic uveitis when used as first biologic modifier drug: Adalimumab as starting anti-TNF-alpha therapy in childhood chronic uveitis. Pediatric rheumatology online journal. 2013;11:16. doi: 10.1186/1546-0096-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ardoin SP, Kredich D, Rabinovich E, Schanberg LE, Jaffe GJ. Infliximab to treat chronic noninfectious uveitis in children: retrospective case series with long-term follow-up. American journal of ophthalmology. 2007;144(6):844–9. doi: 10.1016/j.ajo.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saurenmann RK, Levin AV, Rose JB, Parker S, Rabinovitch T, Tyrrell PN, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford) 2006;45(8):982–9. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 44.Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis care & research. 2011;63(4):612–8. doi: 10.1002/acr.20404. [DOI] [PubMed] [Google Scholar]
- 45.Doycheva D, Zierhut M, Blumenstock G, Stuebiger N, Januschowski K, Voykov B, et al. Immunomodulatory therapy with tumour necrosis factor alpha inhibitors in children with antinuclear antibody-associated chronic anterior uveitis: long-term results. The British journal of ophthalmology. 2014;98(4):523–8. doi: 10.1136/bjophthalmol-2013-303935. [DOI] [PubMed] [Google Scholar]
- 46.Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year followup data from the Italian Registry. J Rheumatol. 2013;40(1):74–9. doi: 10.3899/jrheum.120583. [DOI] [PubMed] [Google Scholar]
- 47.Kahn P, Weiss M, Imundo LF, Levy DM. Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology. 2006;113(5):860–4 e2. doi: 10.1016/j.ophtha.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Richards JC, Tay-Kearney ML, Murray K, Manners P. Infliximab for juvenile idiopathic arthritis-associated uveitis. Clinical & experimental ophthalmology. 2005;33(5):461–8. doi: 10.1111/j.1442-9071.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- 49.Lerman MA, Burnham JM, Chang PY, Daniel E, Foster CS, Hennessy S, et al. Response of pediatric uveitis to tumor necrosis factor-alpha inhibitors. J Rheumatol. 2013;40(8):1394–403. doi: 10.3899/jrheum.121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heiligenhaus A, Foeldvari I, Edelsten C, Smith JR, Saurenmann RK, Bodaghi B, et al. Proposed outcome measures for prospective clinical trials in juvenile idiopathic arthritis-associated uveitis: a consensus effort from the multinational interdisciplinary working group for uveitis in childhood. Arthritis care & research. 2012;64(9):1365–72. doi: 10.1002/acr.21674. [DOI] [PubMed] [Google Scholar]
- 51.Braun J, Kastner P, Flaxenberg P, Wahrisch J, Hanke P, Demary W, et al. Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis and rheumatism. 2008;58(1):73–81. doi: 10.1002/art.23144. [DOI] [PubMed] [Google Scholar]
- 52.Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses >/=15 mg may be overcome with subcutaneous administration. Annals of the rheumatic diseases. 2014;73(8):1549–51. doi: 10.1136/annrheumdis-2014-205228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakoor A, Esterberg E, Acharya NR. Recurrence of uveitis after discontinuation of infliximab. Ocular immunology and inflammation. 2014;22(2):96–101. doi: 10.3109/09273948.2013.812222. [DOI] [PubMed] [Google Scholar]
- 54.Ayuso VK, van de Winkel EL, Rothova A, de Boer JH. Relapse Rate of Uveitis Post-Methotrexate Treatment in Juvenile Idiopathic Arthritis. American journal of ophthalmology. 2011;151(2):217–22. doi: 10.1016/j.ajo.2010.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


