Abstract
The photophysical properties of a deep-red fluorescent squaraine dye can be improved by encapsulating it within a tetralactam macrocycle. Three new tetralactams are described with different substituents (methyl, methoxy, methylenedioxy) on the macrocycle aromatic sidewalls. The capability of each tetralactam to encapsulate a squaraine dye in chloroform solution was determined experimentally using absorption, fluorescence, and NMR spectroscopy. Two of the tetralactams were found to thread a squaraine dye with association constants on the order of 106 M−1, while a third macrocycle exhibited no squaraine affinity. An X-ray crystal structure of the third tetralactam showed that the substituents sterically blocked squaraine association. Of the two tetralactams that encapsulate a squaraine, one induces an increase in squaraine fluorescence quantum yield, while the other quenches the squaraine fluorescence. The results suggest that these new squaraine binding systems will be useful for biological imaging and diagnostics applications.
Graphical Abstract
Tetralactam macrocycles with similar substituents (methyl, methoxy, methylenedioxy) on the macrocycle aromatic sidewalls have very different squaraine recognition properties.
Introduction
The supramolecular chemistry of tetralactam macrocycles has been an active research topic for several decades, and researchers have demonstrated host association with a wide range of neutral and charged guests.1–8 Our group has focused on tetralactam hosts that encapsulate deep-red fluorescent squaraine dyes. Our early work developed Leigh-type “clipping reactions” that assembled the macrocycle around a dumbbell-shaped dye to create a permanently interlocked squaraine rotaxane.9 We have also prepared squaraine rotaxanes by conducting “capping reactions” that threaded a macrocycle with a suitable dye precursor and then covalently attached stopper groups to each end of the encapsulated dye.10 More recently, we expanded the structural scope of the macrocycle threading process by quantifying the thermodynamic and kinetics for a wide range of different squaraine derivatives.11–12 We have demonstrated that macrocycle/squaraine complexes can be pre-assembled with sufficiently high stability for deployment as fluorescent probes for biological imaging.13 In other cases, we have shown that the change in fluorescence properties produced by the squaraine threading process can be exploited as a signaling mechanism for diagnostic applications.14
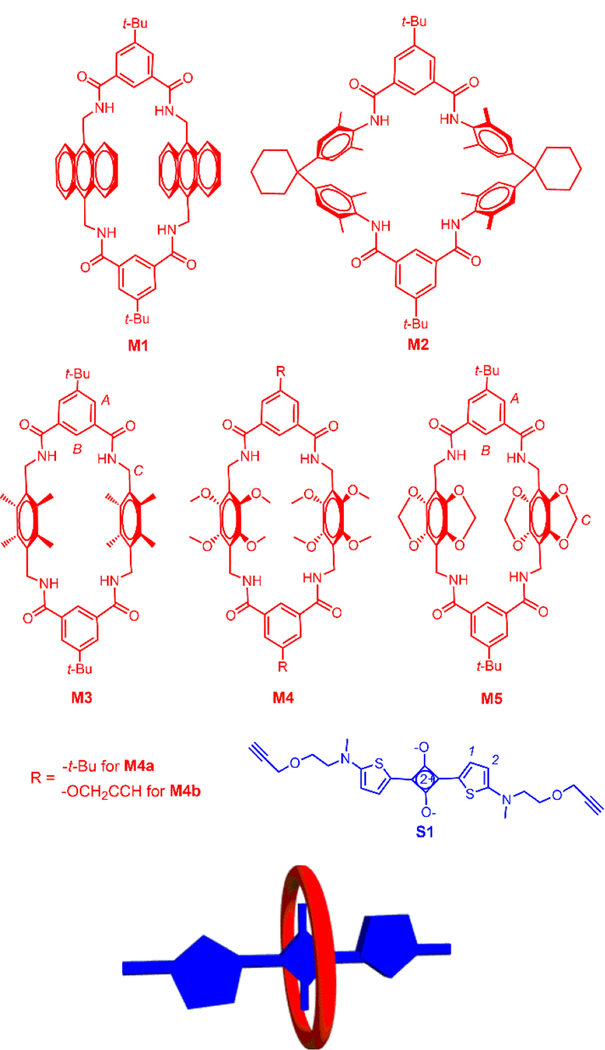
To date, most of our macrocycle threading work in organic solvents has involved tetralactam M1 (Scheme 1), which is a highly preorganized macrocycle with four convergent amide NH bonds and two parallel anthracene sidewalls. Encapsulation of a squaraine dye such as S1 is highly favored by the simultaneous formation of strong hydrogen bonds between the macrocycle NH resides and the squaraine oxygens and stacking interactions between the dye and macrocycle aromatic surfaces. Upon encapsulation, the absorption and emission maxima bands of the squaraine are red-shifted by 20–30 nm, a phenomenon that has been attributed to attenuated deformation of the amine substituents at each end of the squaraine structure.15 While M1 and its derivatives remain a valuable macrocycle family for further development as supramolecular hosts, there are some drawbacks in certain applications. For example, the anthracene sidewalls are susceptible to photooxidation, and several derivatives have been found to have relatively poor solubility. Thus, there is a need for analogous tetralactams with structurally different sidewalls. A few years ago we investigated the squaraine threading ability of the well-known tetralactam M2 which has two angular and electron-rich diphenylmethane sidewalls.1–2, 10 Compared to M1, the squaraine affinity in organic solvent was 40-fold lower, and there was a significance decrease in squaraine fluorescence quantum yield.10 A major reason for the decreased squaraine affinity is the reduced amount of aromatic stacking due to the angular sidewalls of M2. Therefore, we decided to design a new set of preorganized tetralactam macrocycles with parallel sidewalls.
Scheme 1.
Structures of tetralactam macrocycles M1-M5 and squaraine S1 with relevant atom labels. Also shown is a cartoon of a threaded macrocycle/squaraine complex.
In this current report, we describe the squaraine encapsulation properties of tetralactams M3, M4, and M5 in organic solvent. Recently, we prepared M3 and found that it can encapsulate several different anionic square planar metal complexes.16 An X-ray crystal structure of the empty macrocycle showed that it adopts a highly preorganized structure with four convergent amide NH bonds and two parallel 2,3,5,6-tetramethylbenzene sidewalls; thus, we expected the internal cavity to be highly complementary for squaraine guests. Tetralactams M4 and M5 are new structures that have four oxygen substituents on each aromatic sidewall. We expected the oxygen substituents to increase the π-electron density on the sidewalls and perhaps enhance electrostatic stacking interactions with the electron-deficient C4O2 core of an encapsulated squaraine dye. In the case of M4, it was not clear how the four methoxy groups on each sidewall would sterically arrange themselves and if they would block access of a squaraine guest to the macrocycle cavity. In the case of M5, these putative steric effects are removed by incorporating the oxygens atoms into benzodioxo rings which produces sidewalls that are essentially flat and parallel. In the following sections, we describe the synthesis and structure of the new tetralactams. In addition, we have conducted a series of spectroscopic studies that mixed each macrocycle with squaraine S1 in chloroform solvent and determined if a macrocycle/squaraine complex was formed. When a complex was formed we measured the association constant by conducting titration experiments and quantified the photophysical properties of the encapsulated squaraine.
Results and discussion
Synthesis
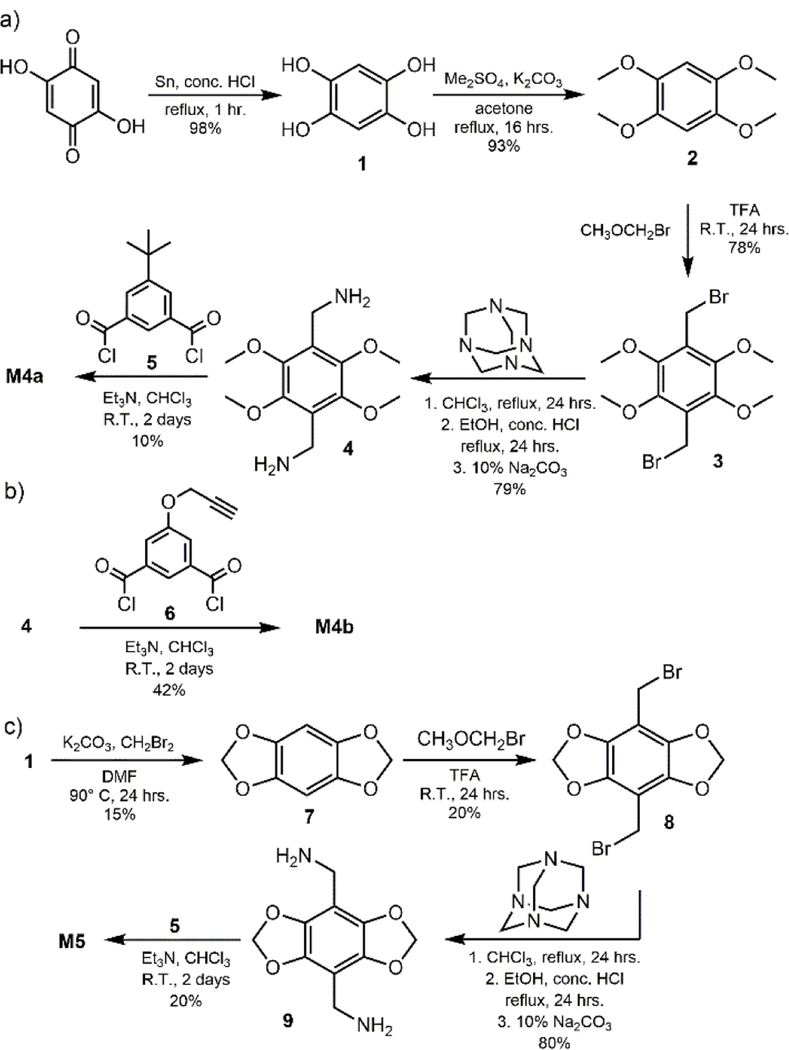
The syntheses of macrocycles M1, M2, and M3 and squaraine S1 have been previously reported.12, 16–17 Shown in Scheme 2 is the preparation of new tetralactams M4a, M4b, and M5. Starting material 1 was obtained in nearly quantitative yield following tin-mediated reduction of commercially available 2,5-dihydroxy-1,4-benzoquinone.19 Alkylation of 1 with dimethyl sulfate produced 2, which was reacted with bromomethyl methyl ether to yield 3.20–21 Bis(bromomethyl) 3 was converted to bis-amine 417, which was reacted separately with acid chlorides 5 or 6 under diluted conditions to yield macrocycles M4a and M4b, respectively. The starting material 7 was prepared by reacting 1 with dibromomethane under basic conditions. The same sequence as above was followed to give macrocycle M5.
Scheme 2.
Syntheses of a) M4a, b) M4b, and c) M5.
Squaraine Threading of M3
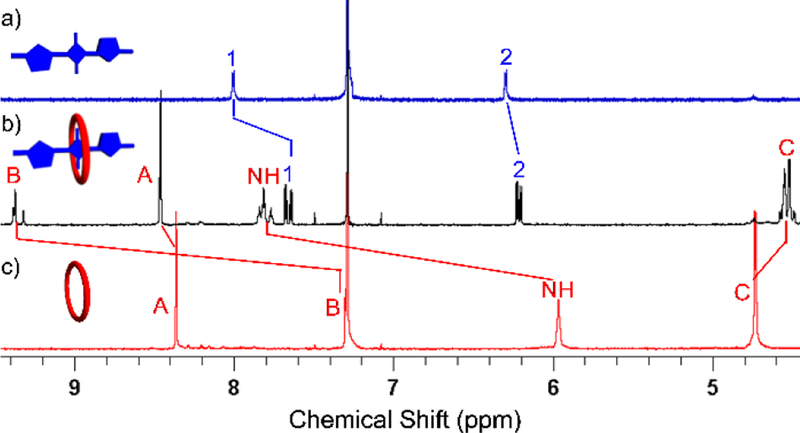
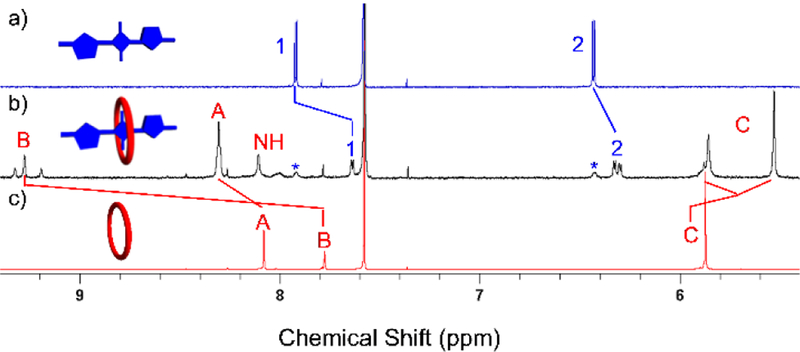
Shown in Figure 1 are the important 1H NMR spectral changes that occurred when macrocycle M3 and squaraine S1 were mixed at a 1:1 molar ratio in CDCl3. The spectra show several large changes in chemical shift that are diagnostic of macrocycle threading by the dye to produce M3⊃S1. Most notably, the signals for squaraine protons 1 and 2 sharpen and are shifted upfield, indicating increased dye rigidity and magnetic shielding by the surrounding aromatic sidewalls of M3. The signals for the macrocycle NH and protons B are strongly shifted downfield indicating hydrogen bonding with the oxygen atoms of the encapsulated squaraine guest. Furthermore, there are two signals for macrocycle protons B in a ratio of about 1:1, a phenomenon that also occurs when S1 is encapsulated by M1.11–12 The two different signals (one is a single peak and the other is a pair of single peaks) correspond to the two conformational isomers that can be adopted by encapsulated S1. The relative orientation of the two thiophene units in S1 can be either cis or trans, and when the conformation of the encapsulated squaraine is cis the two macrocycle protons B are chemically inequivalent. The amide protons of M3 are also split into a single peak and two sets of single peaks and protons C are split into two sets of signals due to the two conformations of encapsulated S1 (Figure S15).
Fig. 1.
Partial 1H NMR (500 MHz, CDCl3) of a) S1 (2.0 mM), b) M3⊃S1 (2.0 mM), and c) M3 (2.0 mM). Atom labels are provided in Scheme 1. The cartoons show S1 (colored blue) with the two thiophene units in a trans orientation.
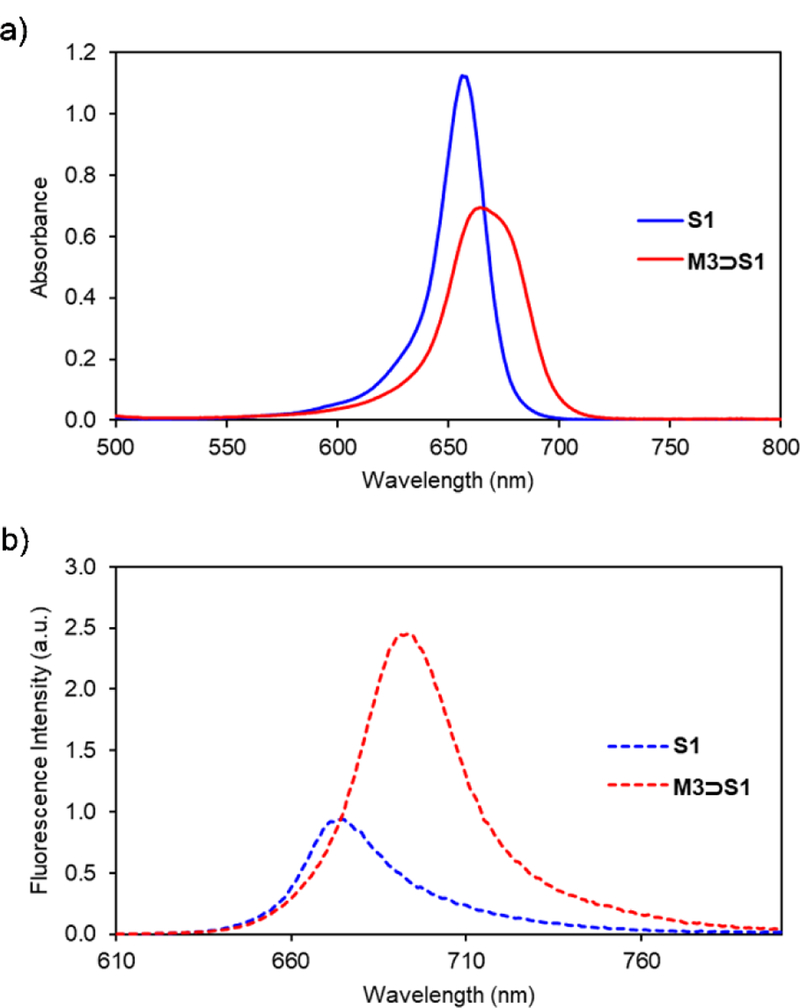
Threading of M3 by S1 was confirmed by absorption and fluorescence spectroscopy. Addition of one molar equivalent of M3 to a 3.0 μM solution of S1 in CHCl3 produced an 8 nm red shift in the squaraine absorption band and a 19 nm red shift in the squaraine emission band. These red shift effects are indicative of squaraine encapsulation, although the changes are smaller than those observed when S1 is encapsulated by M1.11–12 As stated above, the red shift effect is due to attenuated deformation of the amine substituents at each end of the squaraine structure.15 Thus, the relative difference is attributed to the narrower width of the cavity sidewalls in M3, which means the amine groups at each end of the encapsulated squaraine structure are not as constrained.15 The spectra in Figure 2 show that encapsulation of S1 inside M3 produces a slight decrease in molar absorptivity but a substantial increase in fluorescence intensity. Indeed, the fluorescence quantum yield for free S1 in CHCl3 is increased five-fold when it is converted into M3⊃S1 (Table 1).
Fig. 2.
a) Absorption and b) emission maxima for solutions of S1 or M3⊃S1 in CHCl3 (3.0 μM, ex. 600 nm, slit width: 2 nm at 25 °C).
Table 1.
Photophysical data in CHCl3 (3.0 μM) at 22 °C.
| Compound | λabs (nm) | λem (nm) | log ε | Φf a |
|---|---|---|---|---|
| S1 | 656 | 675 | 5.575 | 0.09 |
| M3⊃S1 | 664 | 694 | 5.365 | 0.45 |
relative to bis[4-(N,N-dimethylamino)phenyl]squaraine (Φf = 0.70 in chloroform).
The large enhancements in fluorescence emission induced by squaraine encapsulation made it straightforward to conduct fluorescence titration experiments which provided the association data in Table 2. Shown in Figure S16 is a titration isotherm that was produced by adding aliquots of M3 to S1 in CHCl3. Using a standard non-linear computer algorithm, the curve was fitted to a 1:1 binding model and a value of Ka = 5.1 × 106 M−1 was determined, which is an order of magnitude lower than the Ka determined previously for threading of M1 by S1.12 This decrease in affinity is attributed to the lower aromatic surface area of the macrocycle sidewalls which weakens stacking interactions with the encapsulated dye. The rate constant for threading of M3 by S1 was measured by monitoring the fluorescence increase due to appearance of the complex over time. The kinetic profile (Figure S17) was fitted to second-order kinetic model to give a rate constant (kon) of 9.1 × 103 M−1 s−1, which is an order of magnitude slower than the threading of M1 by S1.12 This decrease in kon is attributed to steric hindrance by the sidewall methyl groups on M3 that inhibit entry of S1 into the macrocyclic cavity.
Table 2.
Association constant (Ka) and rate constant (kon) for complex formation in CHCl3 (3.0 μM) at 22 °C.
| Complex | Ka (M−1) | kon (M−1s−1) |
|---|---|---|
| M3⊃S1 | (5.1 ± 2.9) × 106 | (9.1 ± 1.1) × 103 |
| M1⊃S1a | (7.7 ± 0.8) × 107 | (8.0 ± 0.3) × 104 |
Data taken from reference 12.
No Squaraine Threading of M4a
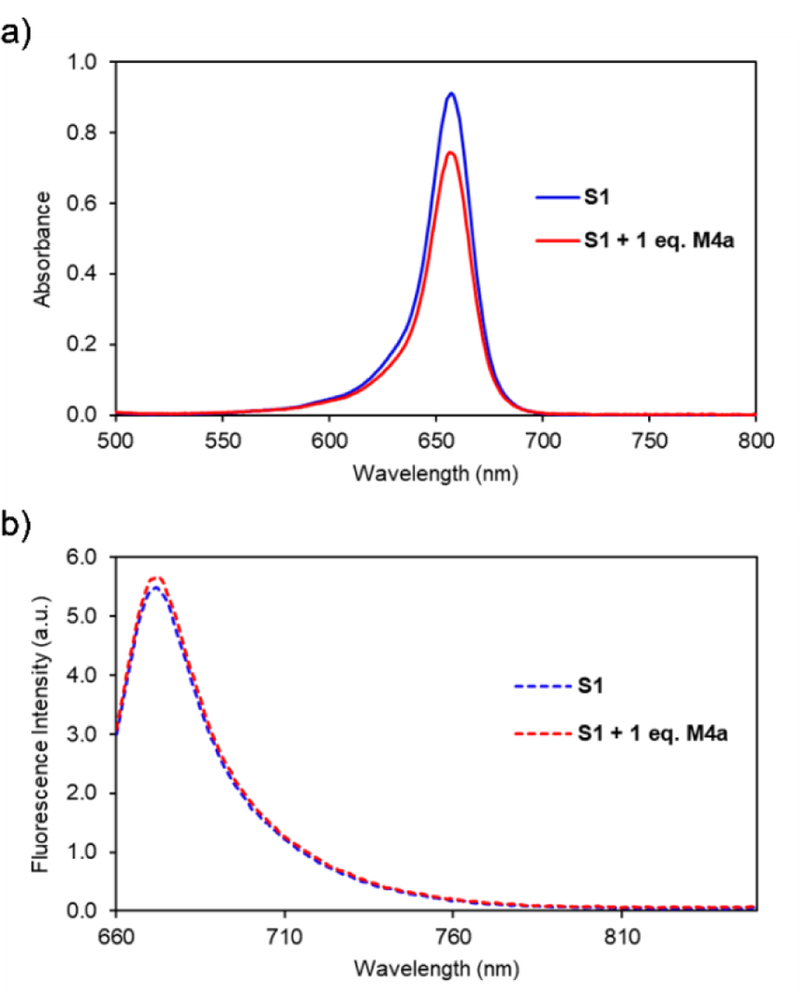
Macrocycle M4a is an analogue of M3 with methoxy groups instead of methyl groups on the two macrocyclic sidewalls. A collection of different spectroscopic experiments all suggested that S1 was unable to thread M4a in chloroform, even after standing for 24 hours. The 1H NMR spectra in Figure S18 show no change in chemical shift for either the macrocycle or dye protons when the compounds were mixed in CDCl3. The absorption and fluorescence spectra in Figure 3 show that addition of M4a to S1 produced no change in squaraine absorption maxima wavelength and no change in squaraine emission spectra. A lack of interaction was also observed when M4a or the closely related macrocycle M4b were mixed in solution with two other structurally related squaraine dyes (Figures S19 and S20).
Fig. 3.
a) Absorption and b) fluorescence spectra of S1 or S1 with one equivalent of M4a in CHCl3 (3.0 μM, ex. 650 nm, slit width: 2 nm at 25 °C).
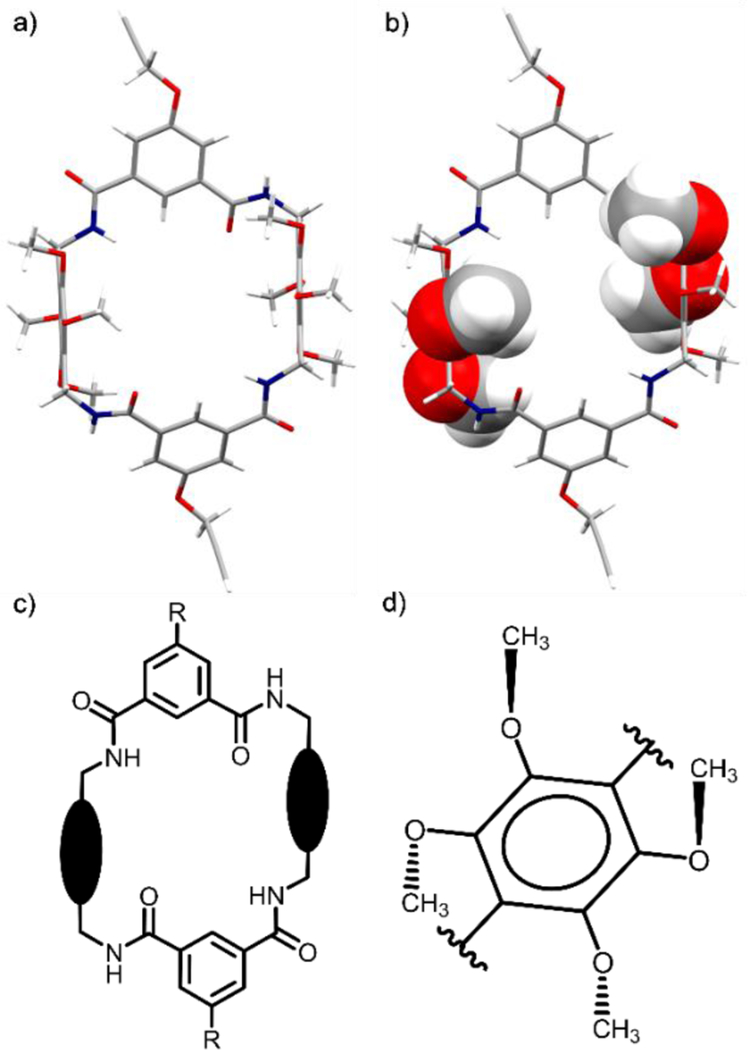
An explanation for the lack of squaraine encapsulation was gained by analyzing an X-ray crystal structure of M4b. The molecular pictures in Figure 4 highlight some important structural features that are induced by the four methoxy groups on each macrocycle sidewall. To relieve steric congestion, each pair of adjacent methoxy groups adopts a trans orientation relative to the plane of a phenyl sidewall (Figure 4d). Thus, with each macrocycle sidewall two methoxy groups are directed towards the macrocyclic cavity and two point away. The inward facing methoxy groups inhibit macrocycle threading in two ways: (a) they sterically block front and rear access of a squaraine guest to the macrocycle cavity (Figure 4b), (b) they force two of the four macrocycle amide NH residues to be directed out of the macrocyclic cavity creating a C2-symmetric host conformation that does not have a complementary shape for a squaraine guest (Figure 4c).22
Fig. 4.
X-ray crystal structure of M4b illustrated as a) capped-sticks model and b) mixed model that emphasizes how the methoxy groups block front and rear access to the macrocyclic cavity. c) Schematic representation of the macrocyclic conformation of M4b with two of the four amide NH residues directed out of the cavity. d) Schematic representation illustrating how each pair of adjacent methoxy groups adopt a trans orientation relative to the plane of a phenyl sidewall in M4b.
Squaraine Threading of M5
To obviate the steric congestion created by the methoxy groups in M4 we designed macrocycle M5, which incorporates the adjacent sidewall oxygens into benzodioxole rings and creates macrocycle sidewalls that are essentially planar and parallel. The limited solubility of macrocycle M5 in chloroform required the use of a CDCl3/CD3OD solvent system for NMR studies. Figure 5 shows the important 1H NMR spectral changes that occurred when macrocycle M5 and squaraine S1 were mixed at a 1:1.1 molar ratio. As in the case of M3⊃S1 in Figure 1 above, there are several large changes in chemical shift indicating the formation of M5⊃S1. The signals for squaraine protons 1 and 2 are shifted upfield, and the macrocycle protons B are shifted far downfield. Moreover, there are two signals for protons B (a single peak and a pair of single peaks) corresponding to the cis and trans conformations that can be adopted by the encapsulated S1. It is worth noting that there are no NH signals in the 1H NMR spectrum of free M5 due to proton-deuterium exchange with the CD3OD, but NH signals are present in the spectrum of M5⊃S1. The difference in proton-deuterium exchange is because the amide protons in free M5 are much more exposed to the solvent than the amide protons in M5⊃S1. The same phenomenon is also observed with permanently interlocked squaraine rotaxanes.22 As with M3⊃S1, there are multiple peaks for the amide protons and protons C of M5, corresponding to the two conformations of encapsulated S1 (Figure S21).
Fig. 5.
Partial 1H NMR (500 MHz, 1:1 CDCl3:CD3OD) of a) S1 (1.0 mM), b) M5⊃S1 (1.0 mM, asterisks indicate signals for free S1), and c) M5 (1.0 mM). Atom labels are provided in Scheme 1. The cartoons show S1 (colored blue) with its two thiophene units in a trans orientation.
The threading of M3 was also studied by absorption and fluorescence methods. Interestingly, free M5 exhibits a moderate absorption band at 325 nm, with broad emission at 480 nm (Figure S14). While this optical property was not directly relevant to the present study which focused on the changes in squaraine fluorescence induced by the macrocycle, it does raise the possibility that suitable derivatives of M5 may have future value as a fluorescent host for non-fluorescent guests such as biomedically important saccharides.23–24
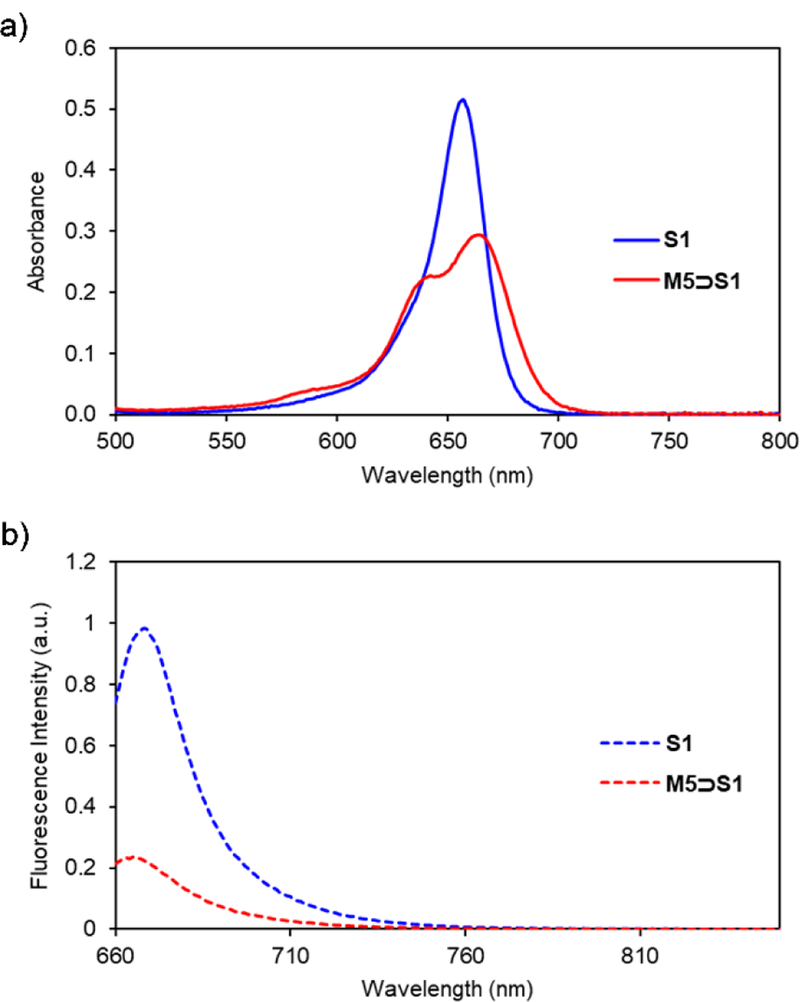
As expected, S1 was able to thread M5, a process that produced a 7 nm red shift in squaraine absorption (Figure 6a). Interestingly, a blue-shifted shoulder appeared in the squaraine absorption spectrum indicating self-aggregation of the threaded complex, M5⊃S1, a phenomenon that is in line with the relatively low solubility of the complex (Figure S22). Fluorescence spectra of the samples showed that the emission of S1 is strongly quenched when it is encapsulated inside M5 (Figure 6b). This is in contrast to the fluorescence enhancement that is observed when S1 is encapsulated by M1 or M3. There are two contributing factors for the squaraine quenching by M5. One factor is the highly electron-rich aromatic sidewalls in M5 which can deactivate the excited state of encapsulated S1 by photoinduced electron transfer. This explanation is supported by the literature knowledge that squaraines are quenched when covalently linked to electron-rich aromatic rings,25 and also when they are encapsulated by macrocycle M2 which also has electron-rich sidewalls.10 The other reason for the diminished fluorescence of M5⊃S1 is the self-aggregation which is known to promote excited state quenching due to energy transfer between multiple chromophores within an aggregate.26 A fluorescence titration experiment that added aliquots of M5 to S1 in CHCl3 produced a curve (Figure S23) that was fitted to a 1:1 binding model, and produced an association constant of 8.6 × 105 M−1, approximately six times lower than the S1 affinity displayed by M3. Thus, we did not confirm our hypothesis that tetralactam affinity for squaraine dyes would be improved by using macrocycles with increased π-electron density on the macrocycle sidewalls. On the other hand, new tetralactam M5 has higher squaraine affinity than M2, which is the only other tetralactam known to quench the fluorescence of an encapsulated squaraine. As we have shown previously with M2, it should be possible to develop molecular shuttles or binding assays that “turn on” fluorescence when a squaraine is displaced from the quenching confines of M5.10, 27
Fig. 6.
Changes in a) absorption and b) emission of S1 and M5⊃S1 in CHCl3 (3.0 μM, ex. 650 nm, slit width: 2 nm at 25 °C).
Conclusion
Macrocycles M3 and M5 are two new tetralactams with highly preorganized structures and open cavities that can encapsulate squaraine dyes with high affinity in chloroform solution. Squaraine threading of M3 leads to enhancement of squaraine fluorescence, whereas threading of M5 leads to quenching of squaraine fluorescence. A third macrocycle, M4, has no squaraine affinity due to the steric effects imposed by the methoxy groups on the macrocyclic sidewalls. The next step in the project is to determine if water-soluble versions of M3 can be threaded by squaraine dyes to create pre-assembled fluorescent probes for biological imaging. It should also be possible to exploit the squaraine quenching ability of macrocycle M5 and create threaded molecular shuttles or squaraine displacement systems that indicate the presence of analytes with large changes in deep-red fluorescence intensity.
Experimental
Materials
1H and 13C NMR spectra were recorded on Bruker AVANCE III HD 400 and 500 MHz spectrometers. Chemical shift is presented in ppm and referenced by residual solvent peak. Mass spectrometry (MS) was performed using a Bruker microTOF II spectrometer. Reactions were monitored by TLC plate (precoated with 60 Å silica gel, F254) purchased from SILICYCLE and visualized by UV light (254, 365 nm). Flash column chromatography was performed using silica gel (silicaFlash P60 from SILICYCLE) as the stationary phase. Absorption spectra were collected using an Evolution 201 UV-Vis Spectrometer with ThermoInsight software. Fluorescence spectra were collected using a Horiba Fluoromax-4 Fluorometer with FluorEssence software. Tetralactam macrocycles M1,17 M2,18 and M3;16 squaraine S1;12 acid chlorides 528 and 6;29 and hydroquinone derivatives 1,19 2,20 and 321 were synthesized according to previously reported procedures.
Synthesis of Bisamine 4
1,4-Bis(bromomethyl)-2,3,5,6-tetramethoxybenzene 3 (2.0 g, 5.24 mmol) and hexamethylenetetramine (2.2 g, 15.71 mmol) were dissolved in chloroform (120 mL) and refluxed for 24 hours. The white precipitate was collected by filtration and washed with cold chloroform. The solid was then dispersed into a solution of ethanol (100 mL) and concentrated hydrochloric acid (20 mL) and refluxed for 24 hours. The flask was then cooled to 0° C and the solid was collected by filtration, washed with cold ethanol, and allowed to dry in open air. The solid was then stirred in 10% sodium carbonate solution (200 mL), chloroform (80 mL) was added while stirring, and the biphasic solution was separated. The aqueous layer was further extracted with chloroform (3 × 50 mL) and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was removed and the product dried in vacuo to give 4 as a white solid (1.06 g, 79%). 1H NMR (400 MHz, CD3CN, 25 °C): δ 3.83 (s, 12H), 3.75 (s, 4H). 13C NMR (100 MHz, CD3CN, 25 °C): δ 148.68, 131.59, 61.82, 36.81. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C12H21N2O4+ 257.1496; Found 257.1506.
Synthesis of Macrocycle M4a
A solution of acid chloride 5 (100 mg, 0.39 mmol) in dry chloroform (100 mL) was added by syringe pump to a solution of bisamine 4 (100 mg, 0.39 mmol) and triethylamine (500 μL) in dry chloroform (200 mL) over 24 hours. After complete addition, the mixture was stirred for an additional 24 hours. The solvent was then removed and the residue purified by column chromatography (1:3 CHCl3:ethyl acetate) to give M4a as a white solid (35 mg, 10%). 1H NMR (500 MHz, CDCl3, 25 °C): δ 7.92 (s, 4H), 7.82 (s, 2H), 6.80 (t, J = 5.9 Hz, 4H), 4.70 (d, J = 5.8 Hz, 8H), 3.88 (s, 24H), 1.32 (s, 18H). 13C NMR (100 MHz, CDCl3, 25 °C): δ 166.46, 152.64, 147.94, 134.93, 127.41, 125.63, 122.24, 61.17, 35.23, 34.22, 31.37. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C48H61N4O12+ 885.4280; Found 885.4303.
Synthesis of Macrocycle M4b
A solution of acid chloride 6 (500 mg, 1.95 mmol) in dry chloroform (50 mL) was added by syringe pump to a solution of bis-amine 4 (500 mg, 1.95 mmol) and triethylamine (2.7 mL) in dry chloroform (500 mL) over 24 hours. When the addition was completed, the mixture was stirred for an additional 24 hours. The solvent was removed and the residue was purified by column chromatography (1:3 CHCl3:ethyl acetate) to give M4b as a white solid (361 mg, 42%). 1H NMR (400 MHz, CDCl3, 25 °C): δ 7.50 (s, 4H), 7.45 (s, 2H), 6.71 (s, 4H), 4.77 (s, 4H), 4.65 (d, J = 5.9 Hz, 8H), 3.83 (s, 24H), 2.55 (s, 2H). 13C NMR (100 MHz, CDCl3, 25 °C): δ 166.03, 158.01, 147.70, 137.10, 125.94, 118.33, 116.53, 77.81, 76.50, 60.97, 56.46, 34.03. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C46H49N4O14+ 881.3240; Found 881.3234.
Synthesis of Benzodioxole 7
1,2,4,5-Tetrahydroxybenzene 1 (17 g, 120 mmol), dibromomethane (41 g, 239 mmol) and K2CO3 (166 g, 1200 mmol) were stirred in DMF (200 mL) at 90 °C for 24 hours. After cooling to room temperature, the solid was removed by filtration. The filtrate was concentrated and the residue purified by column chromatography (5:1 hexane:CH2Cl2) to give 7 as a white solid (2.98 g, 15%). 1H NMR (400 MHz, CDCl3, 25 °C): δ 6.49 (s, 2H), 5.87 (s, 4H). 13C NMR (100 MHz, CDCl3, 25 °C): δ 141.47, 101.41, 93.38. HRMS (ESI-TOF) m/z: [M+H]+ Calcd for C8H7O4+ 167.0339; Found 167.0311.
Synthesis of Bisbromo 8
To a stirred solution of compound 7 (2.0 g, 12.04 mmol) in 20 mL trifluoroacetic acid was added bromomethyl methyl ether (3.73 g, 1.8 mL, 30.12 mmol) at 0 °C. The solution was stirred at room temperature for 24 hours, then neutralized by sodium hydroxide and extracted with ethyl acetate and sodium bicarbonate solution. The combined organic fractions were dried over anhydrous sodium sulfate, then the solvent was removed by rotary evaporation and dried further in vacuo. The residue was purified by column chromatography (5:1 hexane:CH2Cl2) to obtain 8 as a pure yellow solid (850 mg, 20%). 1H NMR (500 MHz, CDCl3, 25 °C): δ 6.02 (s, 4H), 4.41 (s, 4H). 13C NMR (100 MHz, CDCl3, 25 °C): δ 139.88, 105.31, 102.52, 20.58. LC-MS (ESI-TOF) m/z: [M]+ Calcd for C10H8Br2O4+ 349.8789; Found 349.8784.
Synthesis of Bisamine 9
Compound 8 (200 mg, 0.57 mmol) and hexamethylenetetramine (240 mg, 1.71 mmol) were dissolved in chloroform (10 mL) and refluxed for 24 hours. The yellow precipitate was collected by filtration and washed with cold chloroform. The solid was then dispersed into 15 mL ethanol and 2 mL concentrated hydrochloric acid and refluxed for an additional 24 hours. The flask was cooled to 0 °C and the solid was collected by filtration, washed with cold ethanol, and allowed to dry in open air. The solid was dispersed into 10% sodium carbonate solution (20 mL) and stirred, to which chloroform (20 mL) was then added. The biphasic solution was separated and the aqueous layer was further extracted with chloroform (3 × 20 mL). The combined organic phases were dried over anhydrous sodium sulfate and the solvent removed to yield 9 as a pale yellow solid (102 mg, 80%). 1H NMR (500 MHz, CD3CN, 25 °C): δ 5.87 (s, 4H), 3.68 (s, 4H). 13C NMR (100 MHz, DMSO-d6, 25 °C): δ 138.70, 108.47, 100.83, 35.56. LRMS (ESI-TOF) m/z: [M+H-NH2]+ Calcd for C10H11NO4+ 209; Found 209.
Synthesis of Macrocycle M5
To a solution of bis-amine 9 (100 mg, 0.45 mmol) and triethylamine (500 μL) in dry chloroform (500 mL) was added acid chloride 5 (115 mg, 0.45 mmol) in dry chloroform (50 mL) by syringe pump over 24 hours. After complete addition, the mixture was stirred for an additional 24 hours, after which the solvent was removed and the residue purified by column chromatography (1:3 CHCl3:ethyl acetate) to yield M5 as a white solid (38 mg, 21%). 1H NMR (400 MHz, CDCl3, 25 °C): δ 8.05 (s, 4H), 7.67 (s, 2H), 6.58 (t, J = 5.1 Hz, 4H), 5.85 (s, 8 H), 4.59 (d, J = 5.4 Hz, 8H), 1.36 (s, 18H). 13C NMR: Limited solubility prevented 13C NMR analysis. HRMS (ESI-TOF) m/z: [M+Na]+ Calcd for C44H44N4NaO12+ 843.2848; Found 843.2853.
Crystal Structure of Macrocycle M4b
An arbitrary sphere of data was collected on a colorless tablet-like crystal grown from a THF/hexanes solution. The specimen had approximate dimensions of 0.218 × 0.183 × 0.080 mm. Diffraction data were recorded on a Bruker APEX-II diffractometer using a combination of ω- and φ-scans of 0.5°.30 Data were corrected for absorption and polarization effects and analyzed for space group determination.31 The structure was solved by dual-space methods and expanded routinely.32 The model was refined by full-matrix least-squares analysis of F2 against all reflections.33 All non-hydrogen atoms were refined with anisotropic atomic displacement parameters. The asymmetric unit consists of one molecule of the macrocycle M4b and several diffuse, disordered solvent molecules. The solvent molecules appeared to be THF, but in the final analysis, solvent contribution was accounted for using the SQUEEZE routine in PLATON.34 Two voids, each of 891 Å3, were located. Electron density totaling 239 e- in each void was accounted for. Crystal data for C46H48N4O14: Mr = 880.88; Monoclinic; space group P21/n; a = 16.2510(17) Å; b = 20.991(2) Å; c = 16.6756(17) Å; α = 90°; β = 96.331(2)°; γ = 90°; V = 5653.8(10) Å3; Z = 4; T = 120(2) K; λ(Mo-Kα) = 0.71073 Å; μ(Mo-Kα) = 0.077 mm−1; dcalc = 1.035 g.cm−3; 76278 reflections collected; 11743 unique (Rint = 0.0624); giving R1 = 0.0471, wR2 = 0.1061 for 7850 data with [I>2σ(I)] and R1 = 0.0812, wR2 = 0.1189 for all 11743 data. Residual electron density (e–.Å−3) max/min: 0.256/−0.261. Atomic coordinates, bond lengths and angles, and displacement parameters have been deposited at the Cambridge Crystallographic Data Centre (CCDC number 1872795).
Association Measurements
A solution of 3.0 μM squaraine S1 in chloroform was placed in a 1 mL quartz cuvette and titrated with aliquots from a macrocycle stock solution (300 μM macrocycle and 3.0 μM squaraine dye in chloroform). Following each addition of macrocycle, a fluorescence spectrum was acquired, and the squaraine emission at a single point was plotted to produce an isotherm that was fitted to a 1:1 binding model using Origin 8.1 software.
Kinetic Measurements
An aliquot of macrocycle solution (1.5 molar equivalents) in chloroform was added to a solution of 3.0 μM squaraine S1 in chloroform and the increase in fluorescence intensity at a single wavelength (due to formation of the threaded complex) was monitored over time. The kinetic curve was fitted to second-order kinetic model using Origin 8.1 software.
Supplementary Material
Acknowledgements
This work was supported by the University of Notre Dame and a grant from the National Institutes of Health (GMR01059078).
Footnotes
Electronic Supplementary Information (ESI) available: [NMR and absorption/emission spectra, titration data]. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Schalley CA, Weilandt T, Bruggemann J, and Vögtle F Top. Curr. Chem, 2004, 248, 141–200. [Google Scholar]
- 2.Hunter CA Chem. Commun, 1991, 749–751.
- 3.Eckelmann J, Saggiomo V, Sönnichsen FD, and Lüning U New J. Chem, 2010, 34, 1247–1250. [Google Scholar]
- 4.Howe ENW, Bhadbhade M, and Thordarson P J. Am. Chem. Soc, 2014, 136, 7505–7516. [DOI] [PubMed] [Google Scholar]
- 5.Xue M, Yang Y, Xiaodong C, Xuzhou Y, and Huang F Chem. Rev, 2015, 115, 7398–7501. [DOI] [PubMed] [Google Scholar]
- 6.Johnston AG, Leigh DA, Pritchard RJ, and Deegan MD Angew. Chemie Int. Ed, 1995, 34, 1209–1212. [Google Scholar]
- 7.Chang SY, Kim HS, Chang KJ, and Jeong KS Org. Lett, 2004, 6, 181–184. [DOI] [PubMed] [Google Scholar]
- 8.Destecroix H, Renney CM, Mooibroek TJ, Carter TS, Stewart PFN, Crump MP, and Davis AP Angew. Chem. Int. Ed, 2015, 54, 2057–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassensmith JJ, Baumes JM, and Smith BD Chem. Commun, 2009, 6329–6338. [DOI] [PMC free article] [PubMed]
- 10.Gassensmith JJ, Barr L, Baumes JM, Paek A, Nguyen A, and Smith BD Org. Lett, 2008, 10, 3343–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peck EM, Liu W, Spence GT, Shaw SK, Davis AP, Destecroix H, and Smith BD J. Am. Chem. Soc, 2015, 137, 8668–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Peck EM, Hendzel KD, and Smith BD Org. Lett, 2015, 17, 5268–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peck EM, Battles PM, Rice DR, Roland FM, Norquest KA, and Smith BD Bioconjug. Chem, 2016, 27, 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Gómez-Durán CFA, and Smith BD J. Am. Chem. Soc, 2017, 139, 6390–6395. [DOI] [PubMed] [Google Scholar]
- 15.Jacquemin D, Perpete EA, Laurent AD, Assfeld X, and Adamo C Phys. Chem. Chem. Phys, 2009, 11, 1258–1262. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Oliver AG, and Smith BD J. Am. Chem. Soc, 2018, 140, 6810–6813. [DOI] [PubMed] [Google Scholar]
- 17.Gassensmith JJ, Arunkumar E, Barr L, Baumes JM, DiVittorio KM, Johnson JR, Noll BC, and Smith BD J. Am. Chem. Soc, 2007, 129, 15054–15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer C, Nieger M, Mogck O, Böhmer V, Ungaro R, and Vögtle F Eur. J. Org. Chem, 1998, 155–161.
- 19.Weider PR, Hegedus LS, and Asada H J. Org. Chem, 1985, 50, 4276–4281. [Google Scholar]
- 20.Pirnat K, Gaberscek M, and Dominko R J. Power Sources, 2013, 235, 214–219. [Google Scholar]
- 21.Syper L, Meochowski J, and Kloc K Tetrahedron, 1982, 39, 781–792. [Google Scholar]
- 22.Murgu I, Baumes JM, Eberhard J, Gassensmith JJ, Arunkumar E, and Smith BD J. Org. Chem, 2010, 76, 688–691. [DOI] [PubMed] [Google Scholar]
- 23.Ke C, Destecroix H, Crump MP, and Davis AP Nat. Chem, 2012, 4, 718–723. [DOI] [PubMed] [Google Scholar]
- 24.Stewart P, Renney CM, Mooibroek TJ, Ferheen S, and Davis AP Chem. Commun, 2018, 54, 8649–8652. [DOI] [PubMed] [Google Scholar]
- 25.Arunkumar E, and Ajayaghosh A Chem. Commun, 2005, 599–601. [DOI] [PubMed]
- 26.Chen H, Farahat MS, Law K-Y, and Whitten DG J. Am. Chem. Soc, 1996, 118, 2584–2594. [Google Scholar]
- 27.Gassensmith JJ, Matthys S, Lee JJ, Wojcik A, Kamat PV, and Smith BD Chem. Eur. J, 2010, 16, 2916–2921. [DOI] [PubMed] [Google Scholar]
- 28.Al-Sayah MH, McDonald R, and Branda NR Eur. J. Org. Chem, 2004, 173–182.
- 29.Shaw S, Liu W, Gómez-Durán CFA, Schreiber C, de Lourdes Betancourt Mendiola M, Zhai C, Roland F, Padanilam S, and Smith BD Chem. Eur. J, 2018, 24, 13821–13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.APEX3 Bruker AXS, 2016. Madison, Wisconsin, USA. [Google Scholar]
- 31.Krause L, Herbst-Irmer R, Sheldrick GM, and Stalke DJ Appl. Crystallogr, 2015, 48, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheldrick GM Acta Crystallogr, 2015, A71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheldrick GM Acta Crystallogr, 2015, C71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spek AL Acta Crystallogr, 2009, D65, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.