Abstract
Cumulative evidence has proven that proliferation, differentiation and migration of cardiac stem cells (CSCs) dominate early heart development and contribute to the later occurrence of heart disease. Among other mechanisms, microRNAs work as the ‘fine-tuning’ to modulate the levels of target genes in a specific cell type. The distinct microRNA signatures in CSCs reveal the stages and functions of CSCs. The focus of this review is to summarize recent knowledge advances in CSC proliferation, differentiation and migration and to discuss how microRNAs regulate these processes during heart development and in heart disease. Better understanding of microRNA regulation on CSCs under different situations will enable the unveiling of the mechanisms of heart disease and open new avenues in the therapeutic potentials of microRNA modulation to treat heart disease.
Keywords: Cardiac stem cell, proliferation, differentiation, migration, microRNA regulation, therapeutic potentials
Introduction
Heart disease is the leading cause of death in the USA. Over 1.2 million adults have a myocardial infarction (MI) each year [1]. New insights into how human hearts can be regenerated following injury suggest that endogenous cardiac stem cells (CSCs) in the adult mammalian heart can be rapidly activated and can differentiate into the major functional cell lineages, including cardiomyocytes (CMs), smooth muscle cells (SMCs) and endothelial cells (ECs) for myocardial regeneration [2–4]. These findings agree that CSCs dominate early heart development and the later occurrence of heart disease, strengthening the substantial clinical interest in CSCs in myocardial regeneration [5,6]. Although resident CSCs only occupy 0.005–2% of all adult cardiac cells [7], the regenerative capacity of rare CSCs for cardiac homeostasis and myocardial repair has led to broader studies on them [7,8]. microRNAs (miRNAs) are small noncoding RNAs that modulate gene expression by binding to the targeted mRNAs, and play a crucial part in regulating cardiac development, proliferation, differentiation and migration of CSCs [9–12], which have been linked to the initiation and development of heart disease. Here, we review the recent advances in our knowledge of proliferation, differentiation and migration of CSCs. In particular, we summarize how microRNAs regulate these processes during the development of the heart and in heart disease, as well as their therapeutic potentials.
Cardiac stem cells
Subsets of cardiac stem cells
Growing interest in the implementation of CSCs in heart development and disease has aroused superfine studies on CSC subsets. Markers, such as CD45 [2] and CD133 [13], can discriminate the subpopulations of c-kit+ cells for myocardial regeneration. Compared with CD45+/c-kit+ CSCs, CD45−/c-kit+ CSCs are more committed to CMs in adult mouse or rat hearts [2]. In addition, CD133+/c-kit+ cells exhibit phenotypic properties of endothelial progenitor cells [13]. These studies have indicated that c-kit alone is not sufficient for screening a CSC population for clinical interests. LIM-homeodomain transcription factor (Isl1) has regained attention because lineage-tracing analyses have demonstrated the major contributions of Isl1+ CSCs to CMs during heart development and their commitment to cardiac tissue formation after injury [14]. A recent clinical trial suggests that stage-specific embryonic antigen (SSEA)-1+/Isl1+ CSCs have weak immunogenicity and express natural-killer-cell-activating receptor ligands, which reduce the immune rejection and inflammatory responses [15]. These findings point to the important potential of Isl1+ CSCs for reparative therapy. Additionally, cardiosphere-derived cells (CDCs) have been reported as heterogeneous stem cell populations expressing multiple markers including c-kit, CD105 and Sca-1 [16]. Exosomes are small vesicles with a diameter ranging from 30 to 100 nm and are secreted by CDCs. They have attracted more attention because of their capabilities of suppressing fibrosis and inflammation [17,18] and promoting angiogenesis [8,18]. Of note, exosomes are filled with various miRNAs that have been reported as powerful regulators of cellular effects and therapeutic targets [16,19–21]. Hence, it is of interest to identify myocardium-specific markers to isolate CSCs and study the possible mechanisms of their therapeutic efficacy.
Proliferation, differentiation and migration of cardiac stem cells
Rare endogenous CSCs in adult hearts are not capable of restoring normal tissue homeostasis when extensive and irreversible damage occurs. To ensure sufficient CSCs for either transplantation or decent engrafted population, numerous studies have isolated different CSCs using specific markers and expanded them through different patterns. Rat and mouse CD45−/c-kit+ CSCs have been cultured on a gelatin-coated surface using cytokines such as fibroblast growth factor (FGF)2, epidermal growth factor (EGF), leukemia inhibitory factor (LIF) and erythropoietin (EPO) [2]. These CSCs displayed high commitment to CMs and robust regenerative potentials after being injected into the infarcted myocardium [2]. A recent study incorporates microparticles that can continuously deliver hepatocyte growth factor and insulin-like growth factor-1 into collagen scaffolds to enhance the proliferative potentials, as well as migration, of c-kit+ CSCs [22]. Preconditioning of CSCs with hypoxia [19] or mesenchymal stem cells (MSCs) [3,23] has been shown to enhance CSC proliferation. One of the potential mechanisms is through the secretion of exosomes that transport various paracrine factors such as functional proteins and miRNAs. Stem cell factor (SCF) secreted by MSCs has been reported to promote CSC proliferation through the SCF/c-kit signaling axis [3].
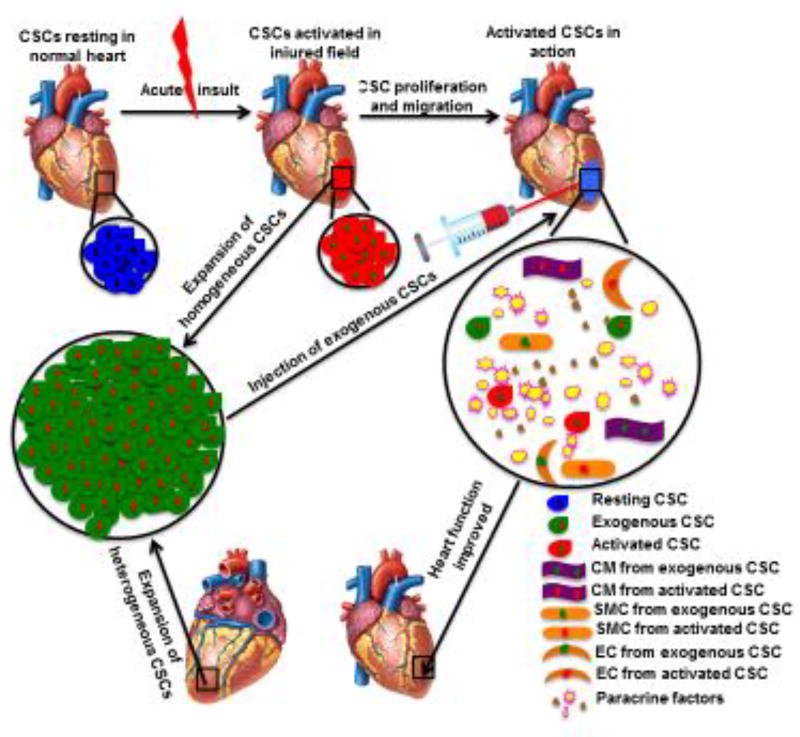
Numerous studies have provided evidence that CSCs can be rapidly recruited to the infarct border zone and activated to differentiate into the major functional cell lineages, especially CMs, after ischemic myocardial injury [3,19]. However, the exact mechanisms of CSC migration and differentiation remain elusive. In a rat MI model, c-kit+ CSCs and stromal-cell-derived factor (SDF)1 expression are dramatically increased in the injury area, and increased migration of c-kit+ CSCs is positively correlated with SDF1 expression [4]. Transplantation of c-kit+ CSCs preconditioned by MSC exosomes enhances neovascularization, reduces fibrosis and preserves cardiac function after MI [23]. In addition, long-term outcomes of injecting c-kit+ CSCs into rat hearts after MI have illustrated that c-kit+ CSCs activate endogenous cells to proliferate, migrate and differentiate into multiple cell lineages to complete cardiac repair [24]. After recording 602 soluble proteins and 20 high-confidence expressed miRNAs in extracellular vesicles secreted by human W8B2+ CSCs, investigators have further validated the biological activities of 284 proteins and three microRNA precursors and disclosed the paracrine effects of human W8B2+ CSCs on cardiac function and regeneration through promoting differentiation of endogenous cells [25]. Thus, it is believed that endogenous CSCs are capable of not only differentiating into the functional cells after migrating into the injured site but also being activated through paracrine effects rather than de novo myogenesis to carry out cardiac regeneration after myocardial injury (Figure 1).
Figure 1.
CSCs) in cardiac regeneration after myocardial injury. Resting endogenous CSCs are rapidly activated after myocardial injury. These activated CSCs proliferate speedily and migrate to the injured field to directly differentiate into functional cell lineages, including CMs, ECs and SMCs. Meanwhile, exogenous factors could further enhance the regenerative capacity of endogenous CSCs. For example, engrafted CSCs that are injected into the injured border zone promote differentiation of activated CSCs into trilineage cells via paracrine effects. All these processes contribute to the improvement of the heart function. Abbreviations: CSCs, cardiac stem cells; CMs, cardiomyocytes; SMCs, smooth muscle cells; ECs, endothelial cells.
Potentials of cardiac stem cells in heart development and disease
Emerging evidence has uncovered the fact that cardiac development follows a stem cell paradigm where a limited number of CSCs are able to generate various cell types for de novo myogenesis [7,26]. Any error in the step-wise processes of CSC commitment to differentiated progeny can cause cardiac malformation and congenital heart disease (CHD) [5,6]. As one of the earliest CSC markers, Mesp1 expression plays an essential part in CSC specification and differentiation. Inducible gain-of-function experiments and the single-cell RNA sequence have illustrated that Mesp1 is indispensable to CSC migration, early lineage restriction and regional segregation in vivo and in vitro [6,26]. Isl1+ CSCs are mainly found in the second heart field and contribute to the major population of CMs in all heart chambers during cardiac development [14]. Isl1+ CSCs are crucial to maintaining the proliferative and differentiation capacity for normal heart development in utero. Fetal hypoxia suppresses Isl1 expression and induces cardiac malformation and CHD [5]. Thus, CSCs play a dominant part in early heart development and the later occurrence of heart disease.
Many strides have provided evidence that CSCs have potential in the prevention and treatment of heart disease. Repeated administration of c-kit+ CSCs has markedly improved the left ventricular (LV) function in a rat MI model [27,28]. The long-term outcomes of administration of c-kit+ CSCs in a rat MI model have confirmed the beneficial effects of these cells on LV remodeling after a 1-year follow-up [24]. These studies have confirmed the regenerative capacity of c-kit+ CSCs. By contrast, a review has included the studies that refuted the myocardial capacity of c-kit+ cells [29]. The inconsistent findings and mixed results suggest that c-kit+ cells are not ready for clinical interest even though the future therapeutic potential is feasible. Mounting evidence has demonstrated the promising potentials of CDCs in cardiac repair. A pig MI model has revealed that CDCs and CDC-derived exosomes reduce scars and minimize adverse remodeling to improve LV ejection fraction [18]. More importantly, Phase I/II clinical trials have evidenced that intracoronary delivery of CDCs to patients with heart failure significantly improves ventricular function and reduces the late complications after a 1- or 2-year follow-up [8,30]. These preclinical and clinical studies have enlightened the safety and therapeutic potentials of CDCs in heart disease.
miRNA signature and function in cardiac stem cells
miRNAs and cardiac stem cells in heart development
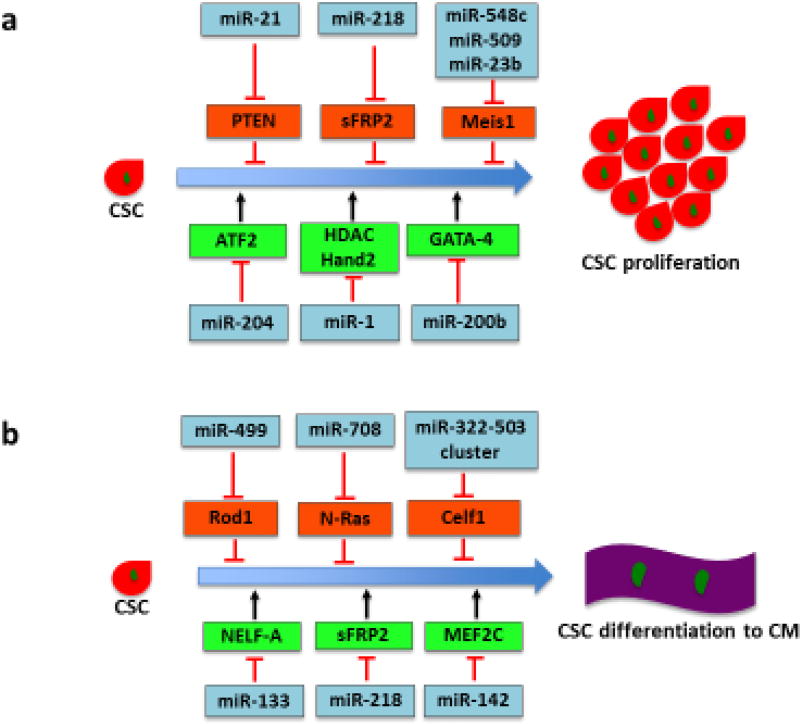
miRNAs are small (~22 nucleotide) noncoding RNAs that most commonly regulate gene expression by binding to complementary sites found within the 3′ untranslated regions (3′UTRs) of the targeted mRNAs. miRNAs are commonly located in intergenic regions and introns of protein-coding genes and are transcribed either in clusters or individually. The majority of miRNAs are transcribed by RNA polymerase II which generates the primary transcript with a stem-loop structure – called pri-miRNA. pri-miRNAs are subsequently processed by protein complexes containing the endonuclease Drosha into the precursor miRNAs (pre-miRNAs), which are ~70 nucleotides and are subsequently exported to the cytoplasm. The endonuclease Dicer further cleaves the pre-miRNAs, resulting in the generation of the ~22 bp miRNA duplexes, which are incorporated in the RNA-induced silencing complex. One strand is then retained in the complex and becomes the mature miRNAs that silence the targeted genes by translational repression, mRNA destabilization and degradation. Each miRNA targets several hundred mRNAs and each mRNA is regulated by multiple miRNAs, which leads to a complex regulatory network. miRNAs are involved in a wide range of biological processes, such as development, homeostasis, cell differentiation and apoptosis, and disease condition, within different tissues including heart. Heart development is a dynamic and complex process that requires the interactions of multiple lineages of cardiac cells. The differentiation of CSCs into these lineages needs to be tightly regulated to enable effective coordinated interaction. miRNAs participate in various aspects of CSC biology, such as CSC proliferation, lineage commitment and migration, by modulating cardiac gene expression (Figure 2).
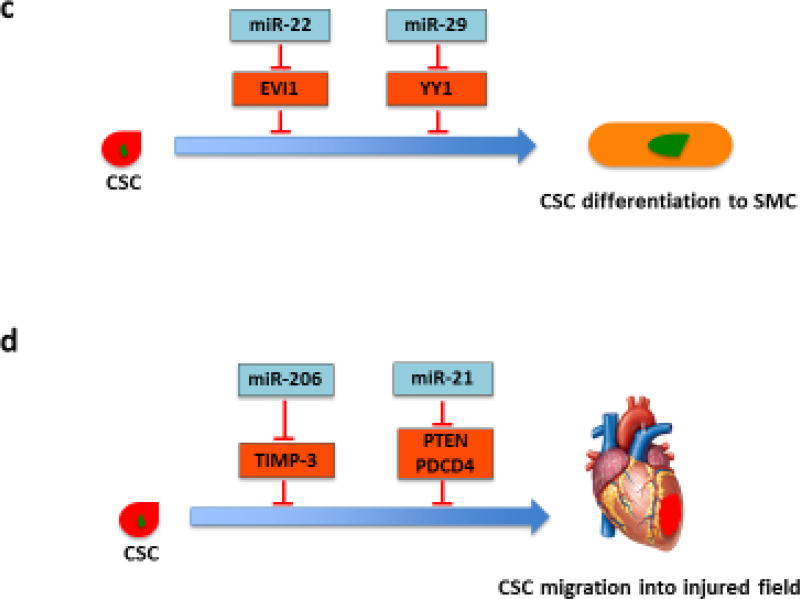
Figure 2.
Role of miRNAs in regulating CSC proliferation, differentiation and migration. (a) Various miRNAs regulate proliferation-related signaling pathways in CSCs. Several miRNAs, such as miR-21, miR-218, miR-548c, miR-509 and miR-23b, induce CSC proliferation by targeting negative regulators of cell proliferation, whereas miR-1, miR-200b and miR-204 inhibit CSC proliferation by modulating proliferation-related transcription factors. (b) Several miRNAs regulate CSC differentiation into CMs. miRNAs from the miR-322/-503 cluster (miR-499 as well as miR-708) promote CM commitment by suppressing the factors that inhibit cardiac differentiation. By contrast, miR-133 targets NELF-A, a nuclear factor promoting cardiogenesis. miR-218 and miR-142 inhibit CSC differentiation by modulating sFRP2, a negative regulator of proliferation, and cardiac transcription factor MEF2C, respectively. (c) miR-22 and miR-29 promote SMC commitment. Their targets, EVI1 and YY1, suppress the SMC marker gene expression and negatively regulate SMC transcription factors, respectively. (d) miR-206 and miR-21 have been shown to directly control migration of CSCs. Induction of CSC migration to injured heart could enhance cardiac regeneration. Abbreviations: ATF2, activating transcription factor 2; miRNAs, microRNAs; CSCs, cardiac stem cells; CMs, cardiomyocytes; SMC, smooth muscle cell; Celf1, CUG-binding protein Elav-like family member 1; EVI1, ecotropic virus integration site 1 protein homolog; GATA-4, GATA-binding protein 4; Hand 2, hand transcription factor 2; HDAC, histone deacetylase 4; MEF2C, myocyte enhancer factor 2C; NELF-A, negative elongation factor-A; PDCD4, programmed cell death 4; PTEN, phosphatase and tensin homolog; Rod1, regulator of differentiation 1; sFRP2, secreted frizzled-related protein 2; TIMP-3, tissue inhibitor of metalloproteinases-3; YY1, transcription factor Yin Yang 1.
miRNAs and proliferation of cardiac stem cells. The computational biology approach has found that increased expression of miRNAs in neonatal CSCs participates in cell cycle and proliferation pathways [31]. miR-1 is one of the most studied miRNAs that show a specific expression pattern in the heart. miR-1 targets a number of genes that are important for cardiac development or function, such as histone deacetylase (HDAC)4, hand transcription factor (Hand)2, connexin 43 (GJA1), and K+ channel subunit Kir2.1 (KCNJ2) [32]. Overexpression of miR-1 reduces the CSC proliferation rate by 25% and induces their differentiation into CMs, suggesting that miR-1 represses CSC proliferation and modulates CM homeostasis [33]. In addition, inhibition of miR-204 enhances the proliferation of human CSCs and impairs the differentiation potential. A possible target gene, activating transcription factor (ATF)2, mediates miR-204 regulation of CSC proliferation [34]. Similarly, miR-200b overexpression inhibits CSC growth possibly by targeting GATA-binding protein (GATA)-4 to downregulate the expression of cyclin D1 and myosin heavy chain (MHC) [35]. By contrast, recent studies have revealed that gain-of-function of miR-21 or miR-218 accelerates CSC proliferation [12,36,37]. miR-21 is shown to activate phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling by targeting the phosphatase and tensin homolog (PTEN) which inhibits phosphorylation of Akt [36]. PTEN-dependent PI3K/Akt signaling is known to protect cells from oxidation-induced apoptosis and promote cell proliferation. Similarly, miR-218 directly targets secreted frizzled-related protein (sFRP)2, a negative regulator of Wnt signaling [37]. Canonical Wnt signaling induces the expression of a series of growth factors regulating various cellular processes, such as gene transcription, cell proliferation, migration and polarity. It has been demonstrated that Wnt signaling is positively involved in cardiogenesis and CSC proliferation [37]. miR-548c, miR-509 and miR-23b have been reported to significantly enhance CSC proliferation via inhibition of Meis1 [38]. Therefore, these findings provide important insights into the molecular network that regulates CSC proliferation.
miRNAs and lineage commitment of cardiac stem cells. Although several cardiac-specific miRNAs, such as miR-1, miR-133 and miR-499, have been shown to drive the CM commitment of CSCs [33,39], the list of miRNAs has expanded recently. Compared with non-progenitors, c-kit+ CSCs display lower levels of miR-708, the expression of which is markedly upregulated upon CSC differentiation. Overexpression of miR-708 specifically promotes differentiation of CSCs to CMs [40]. In addition, the expression of miR-218 is upregulated during CSC differentiation into CMs. miR-218 activates Wnt signaling and then suppresses myocardial differentiation of CSCs in vitro through a positive feedback regulation of the Wnt inhibitor sFRP2 [37]. A recent study has identified that the miR-322/-503 cluster is specifically enriched in early CSCs characterized by Mesp1 expression [10]. Compared with other ‘myomiRs’ with late onset, the miR-322/-503 cluster acts early to initiate the cardiac program by inhibiting other lineages. CUG-binding protein Elav-like family member (Celf)1, an important factor in determining neural fate, has been identified as a possible target of the miR-322/-503 cluster [10]. Ectopic expression of miR-142 suppresses CSC differentiation into CMs via targeting cardiac transcription factor myocyte enhancer factor (MEF)2C [41]. Thus, these findings uncover molecular pathways that mediate the regulation of miRNAs at different stages of cardiac commitment of CSCs. Furthermore, ecotropic virus integration site 1 protein homolog (EVI1), a transcriptional regulator that binds the promoter region of target genes and blocks gene expression, has been identified as a novel target gene of miR-22 in SMCs. EVI1 suppression in SMCs simulates SMC marker gene expression, suggesting that the miR-22/EVI1 signaling axis is also important for SMC differentiation [42]. Another intriguing study has revealed the regulatory role of miR-29a in SMC differentiation from mouse ESCs. miR-29a targets transcription factor Yin Yang (YY)1, a negative regulator of SMC-related transcription factors [i.e., serum response factor (SRF), myocardin and MEF2C], which results in the muscle-specific gene expression and SMC commitment [9]. These interesting findings are significant because they provide further insights into the whole picture of this crucial regulatory network of miRNAs in promoting the differentiation of SMCs from CSCs.
miRNAs and migration of cardiac stem cells. miRNAs play a key part not only in regulating the proliferation and differentiation of CSCs but also in CSC migration. miRNAs are more involved in CSC homing in neonatal CSCs than in infant or child CSCs [31], suggesting that miRNAs play an important part in CSC migration. The treatment of high-mobility group box (HMGB)1 protein increases the number of c-kit+ CSCs within the infarcted region and induces myocardial regeneration, which is linked to the enhanced matrix metalloproteinase (MMP)-2 and MMP-9 activity and the upregulated expression of miR-206 [43]. In-line with these data, tissue inhibitor of metalloproteinases (TIMP)-3 is a potential target of miR-206, suggesting that miR-206 might promote the migration of CSCs into the scar by enhancing the activity of MMPs [43]. More recently, a line of evidence shows that overexpression of miR-21 promotes migration of Sca-1+ CSCs, thereby enhancing the capacity of Sca-1+ CSCs to repair damaged myocardium [12]. However, the precise mechanism that mediates the miR-21 function still needs to be further investigated. As for the study on CSC preconditioning, MSC-Exo is reported to improve the homing of CSCs in the rat infarcted myocardium. Importantly, the expression of a cohort of 22 miRNAs is significantly changed in CSCs after MSC exosome treatment [23]. Thus, in light of these findings, further studies are needed to define the exact roles of miRNAs in regulating the migration of CSCs in the next few years, which could provide the evidence on the feasibility of using miRNAs for human CSC therapeutic implications.
miRNAs and cardiac stem cells in heart disease
Congenital heart disease. CHDs are the most prevalent congenital malformations in infants, which contribute to >40% of infant deaths worldwide. Ventricular septal defect (VSD) is the most common form and represents 20–40% of CHDs. In animal models or human cardiac tissues, the reduced expression of miR-1 or miR-133 is associated with an increased risk of VSD [44]. miR-1 can directly regulate the levels of several targets, such as Hand2, SOX9 and GJA1, whereas miR-133 can regulate SRF in cardiac progenitor cells in VSD [44]. Another miRNA involved in the development of VSD is miR-30c, the expression of which is highly induced in the heart tissues of aborted embryos with VSD. Liu et al. have detected that miR-30c overexpression not only negatively regulates CM differentiation from P19 cells but also results in imbalance between P19 cell proliferation and apoptosis [45]. Interestingly, miR-30c can regulate Ptch1 and the downstream Shh signaling pathway which is essential for normal embryonic development by targeting transcriptional factor Gli2 [45]. This finding provides new insight into the complex regulatory mechanisms of miRNAs in the pathogenesis of VSD. More recently, miR-34a has been implicated in affecting the function of embryonic endocardial cells (ECCs) by modulating the Notch signaling in the CHD patients. miR-34a decreases proliferation rate and increases rate of apoptosis by targeting Notch1 [11]. Therefore, dysregulation of Notch signaling by miRNAs in CSCs might have a central role in CHD development, which could lead to the novel therapeutic strategies for treating CHDs.
Congestive heart failure. Ventricular remodeling that results in structural and functional impairment of the heart gives rise to human congestive heart failure (CHF). Loss of cardiac muscle cells contributes to decreased cardiac function and development of CHF. In a rodent model, the improved cardiac function of CHF rats treated with Yiqifumai is significantly associated with reversible regulation of seven miRNAs [46]. The pathway analysis of predicted target genes has shown these miRNAs are involved in myocardial hypertrophy and cell apoptosis. Several groups have reported miR-21 as one of the most upregulated miRNAs in rats with CHF. miR-21 is expressed in the majority of the cardiovascular cells, especially cardiac fibroblasts and CMs. CSC-derived exosomal miR-21 plays an inhibiting part in the apoptosis pathway through downregulating programmed cell death (PDCD)4 [47]. A restored miR-21/PDCD4 pathway using CPC-derived exosomes has been indicated to protect myocardial cells against oxidative stress-related apoptosis [47]. A second line of investigation by Shi et al. has demonstrated exosomal miR-21 derived from H2O2-treated MSCs can be transported to CSCs to inhibit PTEN expression and downstream PI3K/AKT signaling, which prevents CSCs from oxidative-stress-triggered cell death [48]. Moreover, miR-199 has been implicated in CHF, because the expression of miR-199 is significantly decreased in the myocardium during heart failure. The downregulation of miR-199 leads to upregulation of target gene CDK5 and ABL1 enzyme substrate (CABLES)1, thereby inducing the proapoptotic factor p53 activity and promoting cardiac c-kit+ cell apoptosis [49].
Acute myocardial infarction. Acute myocardial infarction (AMI) is pathologically characterized by cardiac cell death and impaired cardiac contractility owing to prolonged ischemia. Recently, considerable attention has been paid to understanding the role of miRNAs in ischemia-induced apoptosis of CSCs, which points to the possibility of the miRNAs becoming new therapeutic targets for treating AMI. Overexpression of miR-21 reduces myocardial infarct size by 36.9% and enhances heart function at 2 weeks after AMI, which is associated with reduced cardiac cell apoptosis in the MI areas of mouse hearts [50]. As discussed above, exogenous miR-21 protects c-kit+ CSCs from oxidative-stress-induced apoptosis and increases cell proliferation by inhibiting PTEN and activating the PI3K/AKT signaling pathway [12,36,48]. miR-146a has been implicated in MI, and injection of CDCs or a miR-146a mimic at the time of AMI has shown a cardioprotective role [51]. Likewise, by repressing snail1 expression in infarcted heart, constitutive activation of miR-133 in vivo dramatically improved cardiac function after MI [52]. By contrast, the adult heart has a limited regenerative capacity after ischemic injury owing to ineffective reactivation of endogenous CSC proliferation and differentiation. miRNA regulation has been linked to these processes. miR-29a is upregulated at an early phase during CSC differentiation. It decreases Wif1 gene methylation by targeting Dnmt3a expression, which inhibits Wnt signaling and stimulates differentiation of Sca-1+ CSCs. Injection of CSCs with downregulated Dnmt3a in mouse hearts enhances CSC differentiation in situ and improves functional recovery of infarcted hearts [21]. Thus, miR-29a overexpression in infarcted myocardium has a great potential for increasing the differentiation capacity of endogenous CSCs, which could be used as a therapeutic opportunity.
Therapeutic potential of miRNAs in heart disease
Dysregulation of miRNAs greatly impairs the function and repair efficacy of CSCs in heart disease. Identifying the miRNAs and the targeting gene pathways responsible for specific CSC-mediated cardiovascular effects raises the possibilities for developing novel CSC-based miRNA therapeutics. Indeed, emerging evidence supports that the therapeutic manipulation of miRNA-regulated processes improves CSC function and efficacy for the treatment of certain heart diseases. CSC transplants to the infarcted heart generate a large number of CMs with immature phenotypes, failing to restore myocardial mass and heart function. As discussed, miRNAs can promote preferential differentiation of human CSCs to functional CMs with adult properties. For example, miR-133 has been shown to improve the regenerative properties and survival of CSCs. Transplantation of CSCs infected with miR-133a viral vector into the rat infarcted hearts significantly recovers the heart function by inhibiting hypertrophy, as well as increasing angiogenesis and the number of cardiomyocytes [53]. It has been documented that miR-499 and miR-133 act in a synergic manner to induce progenitor cell differentiation into cardiomyocytes [39]. Thus, it will be interesting to test whether a combination of miRNAs can be used for therapeutically inducing cardiac regeneration in vivo.
The long-term expression of miRNAs driven by the viral vectors can cause uncontrolled proliferation and differentiation of the infected CSCs, which might not be desirable in clinical settings. The synthetic miRNA mimics or locked nucleic acid (LNA)-modified anti-miRNAs have been developed as an alternative to augment the functions of CSCs for cardiac repair and regeneration. The combined administration of modified miR-21 and miR-146a mimics reduces infarct size and improves cardiac function in a mouse model of AMI. The protective effects are greater compared with those induced by an individual miRNA [54]. miR-21 mimics can increase integrity of CSCs and improve their proliferation and migration via the target PTEN [12]. Moreover, miR-146a is suggested to enhance migration, proliferation and angiogenesis ability of endothelial progenitor cells [55]. Notably, a single injection of miR-199a and miR-590 mimics immediately after MI in mice is sufficient to induce cardiomyocyte proliferation and promote stable recovery of cardiac function [20]. miR-199a and miR-590 play important parts in regulating cardiac differentiation, and cell death and the metabolic process. Further studies could determine the potential functions of these two miRNAs in other cellular compartments that contribute to the beneficial effects, for example the resident CSCs. In human CSCs, miR-34 has an important role in maintaining an undifferentiated status and a low proliferation rate [56]. Inhibition of miR-34 through LNA promotes CSC proliferation and induces a cascade essential for cardiac repair [56], which provides further evidence for clinical intervention to enhance human heart repair.
Concluding remarks
A large body of evidence has suggested the fundamental role of miRNAs in regulating a variety of key cellular processes in CSCs during heart development, including CSC proliferation, differentiation and migration. Inappropriate expression of miRNAs in CSCs is involved in different pathophysiological conditions of the heart. Several miRNAs thus far have emerged as the attractive targets for enhancing stem cell therapy or endogenous repair processes. The overexpression or acute inhibition of miRNAs by adeno-associated virus (AAV) or LNAs, respectively, show great potential to enhance the survival of CSCs and drive the proliferation and differentiation of CSCs in vivo, as well as to increase the CSC therapeutic capacities, which sheds light on novel therapeutic opportunities for heart regeneration. Recently, the CRISPR/Cas9 genome-editing system began to show therapeutic potential for gene manipulation in cardiovascular diseases [57]. Compared with the traditional technologies including antisense inhibitors, it has higher robustness, specificity and stability for knocking-down miRNAs [58,59]. CRISPR/Cas9 ablation of individual miRNAs from the miR-17 family enables identification of the function of each miRNA in regulating cardiac differentiation of ESCs [60]. Therefore, it is encouraging to investigate the potential application of CRISPR/Cas9 technology for the elucidation of miRNA function in CSCs and miRNA-based therapeutic intervention in heart disease.
Despite these exciting findings, several questions need to be addressed in developing CSC-based miRNA therapeutics. For example, the complex regulatory networks of miRNAs in CSCs are just starting to be revealed. More work is needed to investigate the novel miRNAs and their underlying mechanisms of regulating CSC biology. It is also necessary to define the patterns of target genes of individual miRNAs with CSCs in various pathological settings. Another challenge we have to face is the off-target effect-induced toxicity in vivo. To get the highest efficiency with minimal side-effects, identifying appropriate dosages and developing new technologies for CSC-specific delivery might be needed in some cases. Finally, the therapeutic potential of single miRNAs has been implicated in experimental small animal models. More studies are still required to test the efficacy of this therapeutic strategy in large animals. Whether or not the use of a combination of miRNAs can induce robust cardiac regeneration by regulating different pathways within CSCs in clinical settings must also be further investigated.
Highlights.
CSCs dominate heart development and myocardial repair
miRNAs are essential for CSC proliferation, differentiation and migration
CSC based-miRNA therapeutics hold great promise for heart regeneration
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL083966 (L.Z.), HL118861 (L.Z.) and NS103017 (L.Z.). We apologize to those authors whose excellent studies covered by the scope of this review were unable to be cited because of space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: This review summarizes recent progress in miRNA regulation in CSCs and highlights their great potential as therapeutic targets for heart regeneration.
Conflicts of interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
- 1.Benjamin EJ, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vicinanza C, et al. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017;24:2101–2116. doi: 10.1038/cdd.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatzistergos KE, et al. Stimulatory effects of mesenchymal stem cells on cKit+ cardiac stem cells are mediated by SDF1/CXCR4 and SCF/cKit signaling pathways. Circ. Res. 2016;119:921–930. doi: 10.1161/CIRCRESAHA.116.309281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renko O, et al. SDF1 gradient associates with the distribution of c-Kit+ cardiac cells in the heart. Sci. Rep. 2018;8:1160. doi: 10.1038/s41598-018-19417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan X, et al. Disruption of spatiotemporal hypoxic signaling causes congenital heart disease in mice. J. Clin. Invest. 2017;127:2235–2248. doi: 10.1172/JCI88725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiapparo G, et al. Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration. J. Cell Biol. 2016;213:463–477. doi: 10.1083/jcb.201505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santini MP, et al. Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development. 2016;143:1242–1258. doi: 10.1242/dev.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishigami S, et al. Intracoronary cardiac progenitor cells in single ventricle physiology: The PERSEUS (Cardiac Progenitor Cell Infusion to Treat Univentricular Heart Disease) randomized Phase 2 trial. Circ. Res. 2017;120:1162–1173. doi: 10.1161/CIRCRESAHA.116.310253. [DOI] [PubMed] [Google Scholar]
- 9.Jin M, et al. MicroRNA-29a promotes smooth muscle cell differentiation from stem cells by targeting YY1. Stem Cell Res. 2016;17:277–284. doi: 10.1016/j.scr.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Shen X, et al. miR-322/-503 cluster is expressed in the earliest cardiac progenitor cells and drives cardiomyocyte specification. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9551–9556. doi: 10.1073/pnas.1608256113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu KH, et al. MicroRNA-34a modulates the Notch signaling pathway in mice with congenital heart disease and its role in heart development. J. Mol. Cell. Cardiol. 2018;114:300–308. doi: 10.1016/j.yjmcc.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, et al. Up-regulation of miRNA-21 expression promotes migration and proliferation of Sca-1+ cardiac stem cells in mice. Med. Sci. Monit. 2016;22:1724–1732. doi: 10.12659/MSM.895753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monsanto MM, et al. Concurrent isolation of 3 distinct cardiac stem cell populations from a single human heart biopsy. Circ. Res. 2017;121:113–124. doi: 10.1161/CIRCRESAHA.116.310494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartulos O, et al. ISL1 cardiovascular progenitor cells for cardiac repair after myocardial infarction. JCI Insight. 2016;1:e80920. doi: 10.1172/jci.insight.80920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menasche P, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Redgrave RE, et al. Cardiosphere-derived cells require endoglin for paracrine-mediated angiogenesis. Stem Cell Rep. 2017;8:1287–1298. doi: 10.1016/j.stemcr.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallet R, et al. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl. Sci. 2016;1:14–28. doi: 10.1016/j.jacbts.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallet R, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namazi H, et al. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J. Cell Biochem. 2017 doi: 10.1002/jcb.26621. [DOI] [PubMed] [Google Scholar]
- 20.Lesizza P, et al. Single-dose intracardiac injection of pro-regenerative microRNAs improves cardiac function after myocardial infarction. Circ. Res. 2017;120:1298–1304. doi: 10.1161/CIRCRESAHA.116.309589. [DOI] [PubMed] [Google Scholar]
- 21.De Pauw A, et al. Dnmt3a-mediated inhibition of Wnt in cardiac progenitor cells improves differentiation and remote remodeling after infarction. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill HS, et al. A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J. Tissue Eng. Regen. Med. 2016 doi: 10.1002/term.2392. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, et al. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J. Am. Heart Assoc. 2016;5:e002856. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang XL, et al. Long-term outcome of administration of c-kit(POS) cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ. Res. 2016;118:1091–1105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie S, et al. Biologically active constituents of the secretome of human W8B2(+) cardiac stem cells. Sci. Rep. 2018;8:1579. doi: 10.1038/s41598-018-19855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lescroart F, et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science. 2018;359:4174. doi: 10.1126/science.aao4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang XL, et al. Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J. Am. Heart Assoc. 2018;7:e007400. doi: 10.1161/JAHA.117.007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokita Y, et al. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ. Res. 2016;119:635–651. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Berlo JH, Molkentin JD. Most of the dust has settled: cKit+ progenitor cells are an irrelevant source of cardiac myocytes in vivo. Circ. Res. 2016;118:17–19. doi: 10.1161/CIRCRESAHA.115.307934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sano T, et al. Impact of cardiac progenitor cells on heart failure and survival in single ventricle congenital heart disease. Circ. Res. 2018;122:994–1005. doi: 10.1161/CIRCRESAHA.117.312311. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal U, et al. Age-dependent effect of pediatric cardiac progenitor cells after juvenile heart failure. Stem Cells Transl. Med. 2016;5:883–892. doi: 10.5966/sctm.2015-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian J, et al. Role of microRNAs in cardiac development and disease. Exp. Ther. Med. 2017;13:3–8. doi: 10.3892/etm.2016.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purvis N, et al. The role of microRNAs in cardiac stem cells. Stem Cells Int. 2015;2015:194894. doi: 10.1155/2015/194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao J, et al. MicroRNA-204 is required for differentiation of human-derived cardiomyocyte progenitor cells. J. Mol. Cell. Cardiol. 2012;53:751–759. doi: 10.1016/j.yjmcc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Li G, et al. Cardiac repair in a mouse model of acute myocardial infarction with trophoblast stem cells. Sci. Rep. 2017;7:44376. doi: 10.1038/srep44376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi B, et al. miR-21 increases c-kit(+) cardiac stem cell proliferation in vitro through PTEN/PI3K/Akt signaling. Peer J. 2017;5:e2859. doi: 10.7717/peerj.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, et al. miR218 modulates Wnt signaling in mouse cardiac stem cells by promoting proliferation and inhibiting differentiation through a positive feedback loop. Sci. Rep. 2016;6:20968. doi: 10.1038/srep20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castellan RFP, Meloni M. Mechanisms and therapeutic targets of cardiac regeneration: closing the age gap. Front. Cardiovasc. Med. 2018;5:7. doi: 10.3389/fcvm.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisano F, et al. Combination of miRNA499 and miRNA133 exerts a synergic effect on cardiac differentiation. Stem Cells. 2015;33:1187–1199. doi: 10.1002/stem.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng S, et al. Neonatal heart-enriched miR-708 promotes differentiation of cardiac progenitor cells in rats. Int. J. Mol. Sci. 2016;17:E875. doi: 10.3390/ijms17060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZY, et al. miR-142-3p contributes to early cardiac fate decision of embryonic stem cells. Stem Cells Int. 2017;2017:1769298. doi: 10.1155/2017/1769298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang F, et al. miRNA-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.027799. [DOI] [PMC free article] [PubMed]
- 43.Limana F, et al. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS One. 2011;6:e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoelscher SC, et al. MicroRNAs: pleiotropic players in congenital heart disease and regeneration. J. Thorac. Dis. 2017;9(suppl. 1):S64–81. doi: 10.21037/jtd.2017.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, et al. miR-30c regulates proliferation, apoptosis and differentiation via the Shh signaling pathway in P19 cells. Exp. Mol. Med. 2016;48:e248. doi: 10.1038/emm.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, et al. Analysis of microRNA expression profiles induced by Yiqifumai injection in rats with chronic heart failure. Front. Physiol. 2018;9:48. doi: 10.3389/fphys.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao J, et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7:e2277. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi B, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One. 2018;13:e0191616. doi: 10.1371/journal.pone.0191616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, et al. miR199a-3p regulates P53 by targeting CABLES1 in mouse cardiac c-kit(+) cells to promote proliferation and inhibit apoptosis through a negative feedback loop. Stem Cell Res. Ther. 2017;8:127. doi: 10.1186/s13287-017-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu GL, et al. Cardioprotective effect of microRNA-21 in murine myocardial infarction. Cardiovasc. Ther. 2015;33:109–117. doi: 10.1111/1755-5922.12118. [DOI] [PubMed] [Google Scholar]
- 51.Barile L, et al. Beneficial effects of exosomes secreted by cardiac-derived progenitor cells and other cell types in myocardial ischemia. Stem Cell Investig. 2017;4:93. doi: 10.21037/sci.2017.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017;8:268. doi: 10.1186/s13287-017-0722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izarra A, et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep. 2014;3:1029–1042. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang W, et al. Combination of microRNA-21 and microRNA-146a attenuates cardiac dysfunction and apoptosis during acute myocardial infarction in mice. Mol. Ther. Nucleic Acids. 2016;5:e296. doi: 10.1038/mtna.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su ZF, et al. Regulatory effects of miR-146a/b on the function of endothelial progenitor cells in acute ischemic stroke in mice. Kaohsiung J. Med. Sci. 2017;33:369–378. doi: 10.1016/j.kjms.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iannolo G, et al. MiR34 inhibition induces human heart progenitor proliferation. Cell Death Dis. 2018;9:368. doi: 10.1038/s41419-018-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strong A, Musunuru K. Genome editing in cardiovascular diseases. Nat. Rev. Cardiol. 2017;14:11–20. doi: 10.1038/nrcardio.2016.139. [DOI] [PubMed] [Google Scholar]
- 58.Chang H, et al. CRISPR/cas9, a novel genomic tool to knock down microRNA in vitro and in vivo. Sci. Rep. 2016;6:22312. doi: 10.1038/srep22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aquino-Jarquin G. Emerging role of CRISPR/Cas9 technology for microRNAs editing in cancer research. Cancer Res. 2017;77:6812–6817. doi: 10.1158/0008-5472.CAN-17-2142. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, et al. CRISPR/CAS9 ablation of individual miRNAs from a miRNA family reveals their individual efficacies for regulating cardiac differentiation. Mech. Dev. 2018;150:10–20. doi: 10.1016/j.mod.2018.02.002. [DOI] [PubMed] [Google Scholar]