Abstract
Superior capsular reconstruction has emerged as a promising technique in the treatment of massive irreparable rotator cuff tears. The technical aspects of the surgery continue to evolve, and scientific studies to evaluate these techniques are emerging. One such area of interest concerns the appropriate thickness of the graft and its role as a spacer. The original description of the graft was an autograft tensor fascia lata, which was folded to achieve a thickness of around 8 mm. It has been demonstrated that an 8-mm graft is superior biomechanically to a 4-mm graft, which exceeds the typical human dermal allograft thickness. Herein we describe a technique in which an acellular human dermal allograft was used to perform a superior capsular reconstruction and the remainder of the graft was used to resurface the undersurface of the acromion. This modification of the technique allows for arthroscopic acromial resurfacing, as well as effectively doubling the thickness of the spacer function of the graft. The technique and justification are described in detail, and this has become the senior author's standard approach to the massive irreparable rotator cuff tear in patients with Hamada stages 2 and 3.
Chronic massive rotator cuff tears, specifically those that are irreparable, persist as challenging problems for both patients and shoulder surgeons. These cuff tears remain difficult to treat owing to the chronicity of the tear leading to tendon retraction and inelasticity, which limits excursion during the repair.1, 2 In addition, with time, the rotator cuff muscles atrophy and are infiltrated with fat.1, 2, 3, 4, 5, 6 These factors contribute to the decreased healing and functional potential seen after primary repair of these injuries. In the search for a solution to this problem, many treatment options have been advocated. Depending on both patient and tear characteristics, previous options have included debridement, partial rotator cuff repair, use of a patch to either augment or bridge a repair, or muscle transfers.7, 8, 9 These approaches have reported variable results, and this has made the irreparable rotator cuff tear one of the most active areas of alternative techniques in the shoulder.
Evidence has shown that in addition to the rotator cuff tear found in these injuries, the superior capsule is often also torn owing to its intimate relationship with the cuff tissue.8 On the basis of this knowledge, evolution in treatment has focused not only on the rotator cuff tear itself but also on the important role the shoulder capsule plays in the joint. The shoulder capsule has been shown to be a key contributor to the static stability of the glenohumeral joint, with the anterior and posterior capsule providing for anterior and posterior stability, respectively.10 Through biomechanical analysis, it has been shown that glenohumeral translation is increased in all directions when there is a defect in the superior capsule.10 Additional early biomechanical cadaveric work by Mihata et al.11 determined that this superior translation could be completely restored by using a patch graft to reconstruct the superior capsule. In their work, the patch was placed from the superior glenoid to the greater tuberosity, as opposed to using the patch to bridge the gap between the remaining rotator cuff and its insertion.11
Subsequently, Mihata et al.12 proposed superior capsular reconstruction (SCR) with the use of a fascia lata autograft for massive, irreparable rotator cuff tears. This technique has shown improved clinical outcomes in patients with these difficult problems. At a minimum follow-up of 2 years, all 23 patients who underwent SCR with a fascia lata autograft had improved outcome scores, with patients' American Shoulder and Elbow Surgeons (ASES) scores improving from 23.5 preoperatively to 92.9 postoperatively.8 The acromiohumeral distance (AHD) was also found to increase an average of 4.1 mm after surgery.8 Although these early results are promising, the use of a fascia lata autograft in this technique requires a relatively large graft harvest site, leading to both increased operating room time and donor site morbidity.
In an effort to reduce donor site morbidity, the use of dermal allografts for SCR has been proposed.13 Theoretical advantages of the use of these grafts when compared with fascia lata autografts include reduced donor site morbidity and operating room time, ease of preparation, thickness, and strength of the graft. A recent multicenter study determined that SCR using a dermal allograft provided a successful outcome in approximately 70% of patients.14 The ASES scores of patients were found to improve from 43.6 to 77.5 after SCR, and AHD initially improved 1 mm from baseline at 2 weeks but subsequently decreased to only 0.1 mm of improvement at final follow-up.14
Unfortunately, although the use of dermal allografts for SCR has proven to be successful, outcomes have not been able to match those seen with the fascia lata autograft. One reason for this discordance may be owing to the difference in thickness of the grafts used. The fascia lata graft presented by Mihata et al.12 is folded over 2 to 3 times to obtain a graft thickness of 6 to 8 mm. This is in stark contrast to the 3-mm thickness provided by a dermal allograft.14 To further illustrate the importance of thickness, a biomechanical study comparing graft thickness in SCR determined that the use of an 8 mm thick graft provided greater stability compared with a 4-mm thick graft.14 One reason that improved results are seen with the autograft may be that perhaps the thicker graft, in addition to providing stability by restraint, also provides humeral stability by acting as a spacer in the subacromial space. The thicker graft better fills up the subacromial space, eliminating a potential space for the head to rise, and thus restoring the AHD to a more anatomic size as demonstrated in the autograft study. To address this potential limitation of thickness of the human dermal allografts, we propose using the leftover allograft as a spacer on the undersurface of the acromion, effectively increasing the thickness of the construct. The following presents a technique for SCR using a dermal allograft in which the effective spacer effect of the graft is doubled with the addition of an acromial acellular dermal allograft resurfacing.
Surgical Technique
Step 1: Preoperative Workup
Patients who present to our clinic with shoulder pain undergo a routine history and physical as well as a standard radiographic imaging series. In patients with symptomatic massive tears of the rotator cuff, the tear is assessed as to its chronicity, fatty infiltration, and ability to heal if repaired. The patient is then counseled concerning both nonoperative and operative options. If the patient is physiologically young and the tear is deemed at risk to be irreparable at the time of surgery, SCR is considered among other surgical options. As with any surgical decision, the patient is counseled on the risks and benefits and expectations of surgery. We consider this option in young patients who have massive rotator cuff tears. We obtain consent from all patients for repair versus reconstruction because the decision on reparability is generally made intraoperatively after releases and attempts at cuff mobilization have been exhausted.
Step 2: Surgical Positioning and Diagnostic Arthroscopy
The patient is brought to the operating room, and after the induction of general anesthesia, he or she is examined under anesthesia, which is standard for all arthroscopic shoulder procedures. The patient is positioned in the lateral decubitus position on a beanbag, with the use of a padded arm sleeve (STAR sleeve; Arthrex, Naples, FL), in balanced lateral suspension. A posterior portal (8-mm cannula; Arthrex) is established approximately 1 cm medial and 2 cm distal to the posterolateral acromial border. The arthroscope is introduced, and additional portals are established by an outside-in technique under direct visualization with the use of a switching stick. The anterior portal (8-mm cannula) is established first, approximately 1 cm inferior to the clavicle and lateral to the coracoid. A standard diagnostic arthroscopy is performed within the glenohumeral joint, and any pathology is treated appropriately. The subacromial space is then entered through the posterior portal, and posterolateral (8-mm cannula) and anterolateral (12-mm PassPort cannula; Arthrex) portals are created. A Nevasier portal (7-mm cannula; Arthrex) is also created to aid in anchor placement and graft passage. If indicated, subacromial decompression and acromioplasty are performed. Capsular and subacromial releases, as well as anterior rotator interval releases, are performed to attempt primary repair. If the cuff can be mobilized with these techniques, a primary or revision repair is accomplished. If the cuff tear is too retracted and cannot be mobilized without undue tension, we proceed with SCR. In such cases, the rotator cuff remnant is still mobilized for later incorporation into the SCR.
Step 3: Superior Glenoid Neck Preparation
To secure the medial edge of the graft to the glenoid, the superior glenoid neck is addressed and prepared. The medial edge of the graft should be secured to the glenoid neck adjacent to any residual rotator cuff tissue. The superior glenoid neck immediately medial to the superior labrum is biologically prepared to create a vascular bed for securing the medial aspect of the graft. This is achieved by removing all soft tissue on the superior glenoid neck with an ablation device (ArthroCare; Smith & Nephew, Andover, MD) or arthroscopic shaver and creating bleeding bone with a rasp (Glenoid Rasp; Arthrex) or burr. The prepared area corresponds to the medial attachment of the graft and should extend anteriorly to the edge of the rotator interval (or edge of the subscapularis) and posteriorly to the edge of the posterior cuff remnant. Normally, 3 anchors are placed medially, unless the defect is small, in which case only 2 anchors are used. Specifically, the location of the 3 medial anchors (3.0-mm Bio-SutureTak; Arthrex) is usually at the base of the coracoid anteriorly, at approximately the 2-o'clock position; at the posterior base of the glenoid, generally at the 12-o'clock position; and adjacent to the remnant of the infraspinatus tear, often at the 9- to 10-o'clock position posteriorly.
Step 4: Placement of Medial Anchors
The posterior anchor (SutureTak Bio Composite; Arthrex) is placed on the glenoid neck through the posterior portal. Once this anchor is drilled and placed, the suture tails are grasped and brought out of the posterior portal. Of note, this anchor is the most difficult to place as the bone is thinnest here and care must be taken to not drill into the glenoid face. Therefore, the urge to drill this anchor through the Nevasier portal, which increases the risk for penetration, should be resisted. Instead the anchor should be drilled through the safer posterior portal. The anterior portal is then used to place a second medial anchor (SutureTak Bio Composite) even with the base of the coracoid process, which usually corresponds to the 2 o'clock position. Once placed, the suture ends are also brought out of the anterior portal. The central anchor (SutureTak Bio Composite) is placed through Nevasier's portal, and care should be taken to aim slightly medially to avoid glenoid cartilage penetration.
Step 5: Graft Sizing and Preparation
The dimensions of the rotator cuff defect are measured using a calibrated arthroscopic probe (Arthrex). The anteroposterior distance is measured at the neck of the glenoid and at the lateral rotator cuff footprint. This can be performed through either the anterior or posterior portal. In addition, the surgeon measures the mediolateral distance through the anterolateral portal, keeping in mind to add 15 mm to this distance to accommodate double-row fixation over the greater tuberosity. These measurements are then used to size and prepare the acellular dermal graft (Arthroflex 301; Arthrex). The graft is prepared on the back table, sized, and trimmed accordingly. A marking pen is used to mark the position of suture insertion of the graft for later arthroscopic identification. It is important to place all sutures at least 5 mm from the edge of the graft to avoid losing fixation by the sutures pulling through the graft. The medial row of the humeral stitches should be placed 15 mm from the edge of the tendon, to allow a lateral double-row repair. Because of the graft thickness and durability, it is difficult to consistently and efficiently penetrate the graft with a free needle. Therefore, we use an arthroscopic suture passer (Scorpion; Arthrex) to pass sutures both arthroscopically and during graft preparation. In addition, using the Scorpion suture passer during graft preparation helps confirm that the graft can be easily penetrated before attempting it arthroscopically.
Step 6: Graft Insertion and Medial Fixation
The graft is brought just outside the shoulder near the anterolateral portal. A single limb of suture from the central anchor is retrieved out of the anterolateral portal. The suture limb is then passed through the graft in a simple fashion using the Scorpion device. Once passed, the suture limb is placed back through the anterolateral portal and both suture limbs from the central anchor are retrieved out of the Nevasier portal using a suture grasper. This creates a simple loop through the graft with the central anchor sutures. The graft is then rolled and placed in the jaws of an Allis clamp. The clamp is then used to push the graft through the cannula, whereas slack is taken up by pulling on both limbs of the central anchor. Once inside, the graft can be brought down to the central anchor with progressive tensioning of the 2 suture limbs. The clamp is removed, allowing for the graft to expand into position. A standard arthroscopic knot is then tied with the central anchor suture strands, thus securing the graft at 1 point and preventing malposition. Of note, care should be taken to keep the camera above the graft for the remainder of the case to make passage and fixation easier and to prevent disorientation. The posterior glenoid anchor is then secured to the graft through the posterior portal. With the Scorpion device, 1 suture limb of the posterior anchor is passed through the posterior medial portion of the graft, about 5 mm from both the posterior and medial borders of the graft. The second suture limb can also be passed in a similar manner if a mattress suture is desired. An arthroscopic knot is then tied over the graft and backed up with 3 reversed half hitches on alternating posts. The anterior glenoid anchor is then secured to the graft in a similar fashion through the anterior portal. Once all 3 anchors are secure, excess suture is cut and attention is turned to lateral fixation.
Step 7: Lateral Graft Fixation to Humerus
Securing the lateral graft is accomplished using a double-row transosseous-equivalent technique with the arm at 45° of abduction. Two single-loaded anchors (Corkscrew FT; Arthrex) are placed just lateral to the articular margin of the humeral head. A spinal needle can be used to determine the best trajectory for placement of the medial row anchors. Typically, this trajectory exits the shoulder just lateral to the acromion. Internal and external rotation of the arm can assist in anchor placement. One anchor should be placed anteriorly near the superior aspect of the biceps groove and another anchor secured posteriorly near the posteromedial edge of the greater tuberosity, adjacent to the remnant cuff attachment. The sutures from each anchor are passed through their respective area of the graft with an arthroscopic suture-passing device (Scorpion) and tied arthroscopically. Two lateral row anchors (4.75 SwiveLock; Arthrex) are then placed both anteriorly and posteriorly along the lateral rotator cuff footprint on the greater tuberosity, through the anterior and posterior portals, respectively. As with the medial anchors, internal and external rotation of the humerus can assist in creating the optimal trajectory for the lateral row anchors. The suture ends are then secured to the lateral row in a transosseous-equivalent double-row fashion using knotless lateral row anchors (SwiveLock).
Step 8: Anterior and Posterior Edge Side-to-Side Fixation
Once the medial and lateral rows are secured, side-to-side simple sutures are placed using the arthroscopic suture passer, securing the posterior edge of the graft to the free edge of the teres minor or, if partially intact, the infraspinatus tendon. While the surgeon is viewing from the anterior portal, the posterolateral portal can be used to pass a free suture through the anterior edge of the intact posterior cuff near the musculotendinous junction, which is usually located just lateral to the glenoid. Once passed, the sutures can be brought out of the posterior portal and an arthroscopic suture passer can be used to pass the inferior strand of the suture through the posterior edge of the graft. This suture is then tied arthroscopically. The technique can then be repeated to pass additional simple sutures, securing the posterior graft to the posterior rotator cuff tendon as it extends laterally.
Anteriorly, side-to-side sutures are used to secure the anterolateral edge of the graft to the superolateral subscapularis tendon. This is accomplished by passing a free suture through the superolateral subscapularis tendon using the arthroscopic suture passer with access through the anterolateral portal. The suture ends are brought out of the anterior portal, and the inferior suture strand is passed through the anterolateral graft using the arthroscopic suture passer. The suture is then tied arthroscopically. Alternatively, as in the case of a small partial upper subscapularis tear, an anchor can be used to secure the anterior edge of the lateral part of the graft to the anterolateral humeral head near the biceps groove. This can be accomplished by passing a free suture through the graft and securing it with a knotless anchor near the superior edge of the subscapularis tendon. This closure is accounted for in sizing the graft, and thus the interval is not closed with tension. This does not produce the tightness seen when the rotator interval is closed with native tissue and prevents anterior superior escape.
Step 9: Acromial Graft Sizing and Preparation
Once the SCR graft is in place, attention is turned to preparation of the acromial allograft. Similar to the size measurements made for the SCR graft, a calibrated arthroscopic probe (Arthrex) is used to measure the dimensions of the acromion from both anterior to posterior and medial to lateral (Fig 1). Using the remaining excess dermal allograft, a rectangular acromial allograft is sized, cut, and trimmed according to the arthroscopic measurements. As in the SCR graft, a marking pen is used to mark the position of suture insertion into the graft. Again, it is important to place all sutures at least 5 mm from the edge of the graft to avoid suture pull-through and loss of fixation. An arthroscopic suture passer (Scorpion) is then used to pass a No. 2 FiberTape suture (Arthrex) in a horizontal mattress fashion on one short side of the rectangular graft (Fig 2). This step is then repeated on the opposite short side of the graft. The suture tails should be exiting on the same side of the graft (Fig 3). These short sides of the rectangular graft correspond to the anterior and posterior portions of the graft once attached to the acromion.
Fig 1.

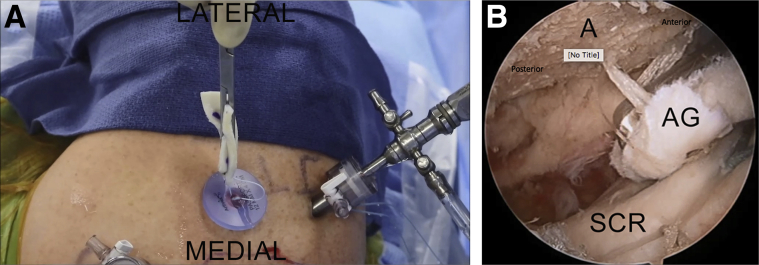
Right shoulder in lateral decubitus position, arm 45° abducted, posterior subacromial viewing portal, probe placed through lateral portal. A calibrated arthroscopic probe is used to measure the dimensions of the acromion for appropriate sizing of the acromial graft. Here the acromion is being measured in the medial to lateral (labeled) direction. (A, acromion.)
Fig 2.
An arthroscopic suture passer is used to pass No. 2 FiberTape suture in a horizontal mattress fashion on 1 short side of the rectangular graft. This is then repeated on the opposite short side of the graft.
Fig 3.
Finalized acromial graft demonstrating horizontal mattress sutures on each short side (anteroposterior acromial portion) of the graft with suture tails all exiting the same side.
Step 10: Anterior Acromial Bony Tunnel Preparation and Suture Passage
After preparation of the graft, the acromial tunnels are created beginning with the anterior medial bony tunnel. An anterior cruciate ligament tunnel guide is inserted through the anterolateral portal and positioned on the acromion approximately 5 mm from the anterior edge of the acromion and 5 mm from the medial border to allow for bony fixation but to avoid the acromioclavicular joint. The tunnel is then drilled with a 1.4-mm drill bit from the outside in (Fig 4). Once drilled, a Hewson suture passer is advanced through the tunnel and a suture grasper is used to retrieve it out of the anterolateral portal. The medial limb of the suture from the anterior side of the acromial graft is then passed through the created acromial tunnel via the Hewson suture passer (Fig 5).
Fig 4.
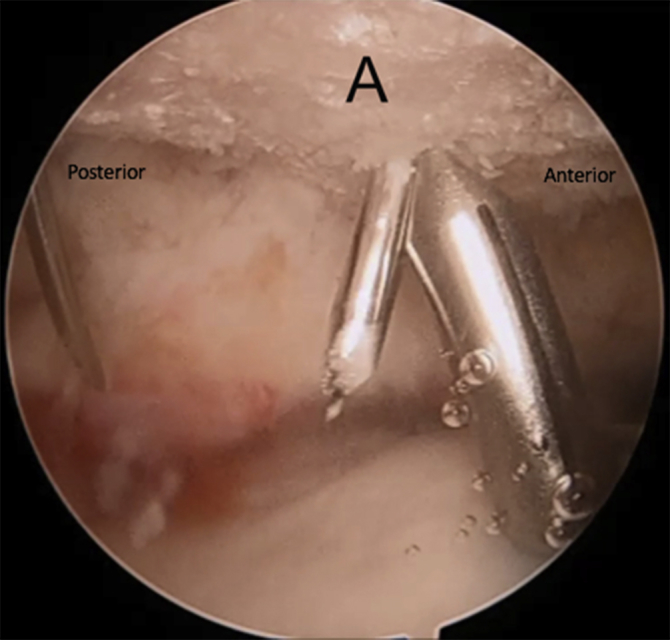
Right shoulder in lateral decubitus position, arm 45° abducted, lateral subacromial viewing portal, anterolateral working portal. Creation of the anterior medial bony tunnel using an anterior cruciate ligament tunnel guide inserted through the anterolateral portal. The tunnel is then drilled from outside in using a 1.4 mm drill bit. (A, acromion.)
Fig 5.
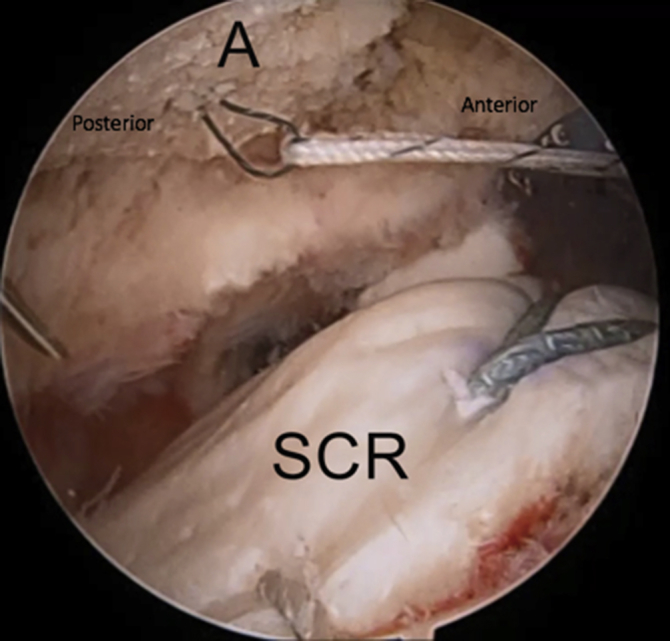
Right shoulder in lateral decubitus position, arm 45° abducted, lateral subacromial viewing portal, anterolateral working portal. The medial limb of suture from the anterior side of the acromial graft is passed through the anterior medial bony tunnel with the use of a previously passed Hewson suture passer. (A, acromion; SCR, superior capsular reconstruction graft.)
Step 11: Anterior Acromial Soft Tissue Tunnel Preparation and Suture Passage
A spinal needle is introduced through the soft tissues just lateral to the anterior acromion, in line with the bony tunnel. A 0 PDS suture is then introduced through the spinal needle and shuttled out of the anterolateral portal using a suture grasper (Fig 6). The PDS suture is tied to the free limb of the previously passed suture. The PDS suture is retrieved, pulling the free edge of the suture through the soft tissue tunnel (Fig 7). Using a technique similar to that of SCR graft insertion, the acromial graft is then passed through the anterolateral passport by pushing the graft through the passport (Fig 8A), whereas the anterior medial and anterior lateral suture limbs are sequentially tightened (Fig 8B).
Fig 6.
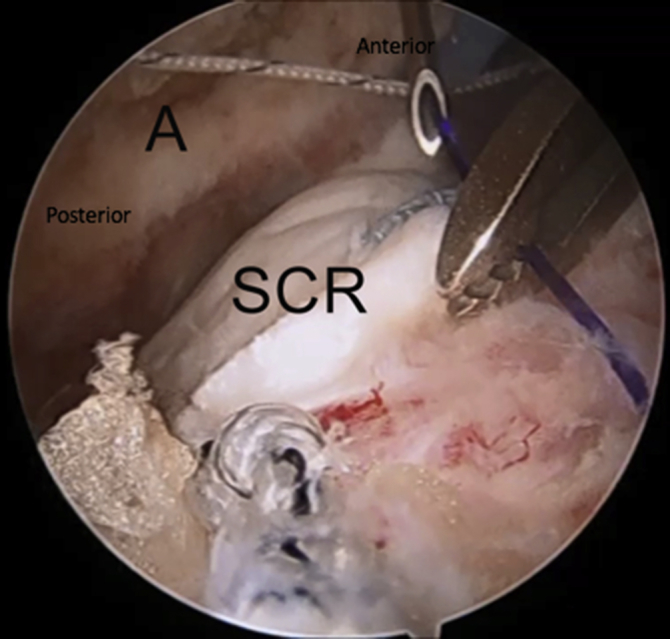
Right shoulder in lateral decubitus position, arm 45° abducted, lateral subacromial viewing portal, anterolateral working portal. A 0 PDS suture is introduced through the spinal needle used to create the anterior acromial soft tissue tunnel. Once introduced, the suture is then shuttled out of the anterolateral portal using a suture grasper. (A, acromion; SCR, superior capsular reconstruction graft.)
Fig 7.
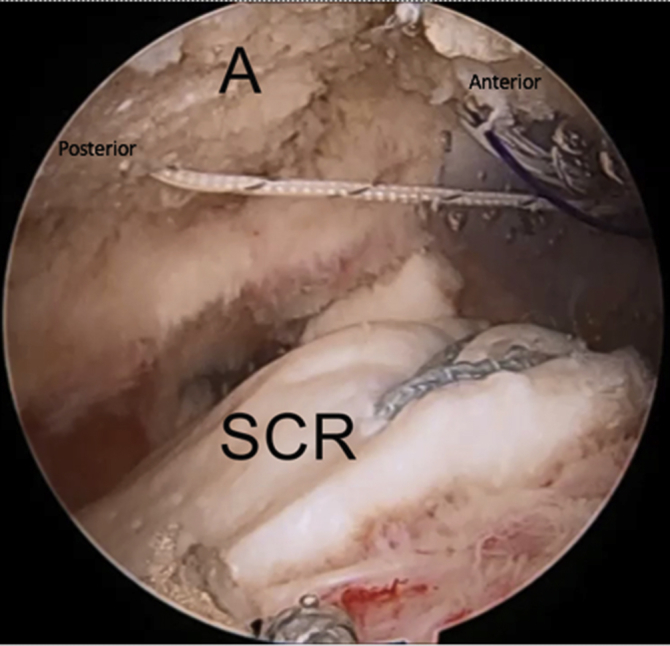
Right shoulder in lateral decubitus position, arm 45° abducted, lateral subacromial viewing portal, anterolateral and anterior working portal. The lateral limb of suture from the anterior side of the acromial graft is tied to the PDS suture. With retrieval of the PDS suture, the lateral limb is passed through the anterior soft tissue tunnel. (A, acromion; SCR, superior capsular reconstruction graft.)
Fig 8.
Right shoulder in lateral decubitus position, arm 45° abducted, lateral subacromial viewing portal, anterolateral portal. The acromial graft is passed through the anterolateral passport using a “push/pull” technique. The graft is passed by pushing it through the passport using a grasper (A) while simultaneously tightening the anterior medial and anterior lateral suture limbs sequentially (B). (A, acromion; AG, acromial graft; SCR, superior capsular reconstruction graft.)
Step 12: Posterior Acromial Bony Tunnel Preparation and Suture Passage
The posterior bony tunnel is created similarly to the anterior tunnel using the anterior cruciate ligament guide approximately 5 mm from the posterior edge of the acromion and as far medial as possible, ensuring enough bone for a tunnel. The Hewson suture passer is advanced through the tunnel, and a suture grasper is used to retrieve it out of the posterolateral portal. A suture grasper is then used to grasp the medial limb of the suture from the posterior side of the graft and bring it out of the same posterolateral portal. The lateral suture limb can also be brought out of the posterolateral portal at the same time, however, it is important to ensure that the limbs are kept separated. Using the suture passer, the medial suture limb is passed through the created posterior acromial tunnel. Care should be taken to prevent twisting of the graft. This is accomplished by grasping the suture from the same surface as done on the anterior side.
Step 13: Posterior Acromial Soft Tissue Tunnel Preparation and Suture Passage
The posterior soft tissue tunnel is then created using a spinal needle and PDS suture in the same manner as the anterior soft tissue tunnel. The PDS suture is passed through the spinal needle and brought out of the posterolateral portal using a suture grasper. The PDS is tied to the free limb of the previously passed suture and retrieved, pulling the free edge of the suture through the soft tissue tunnel. A hemostat is used through the exiting soft tissue tunnel to locate the suture exiting from the bony tunnel. The 2 free suture ends of the anterior graft are then tied over the acromion through the single skin stab wound. This is followed by the 2 free suture ends of the posterior graft, securing the graft to the acromion (Fig 9). Table 1 presents the pearls and pitfalls of this technique, and Video 1 presents a demonstration of this technique.
Fig 9.
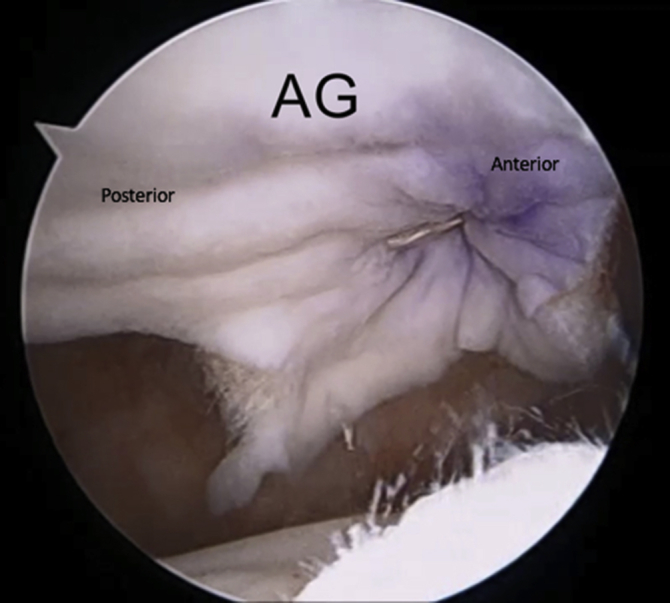
Right shoulder in lateral decubitus position, arm 45° abducted, lateral subacromial viewing portal, anterolateral portal. The 2 free suture ends of the anterior graft are tied over the acromion, followed by the 2 free suture ends of the posterior graft. This secures the acromial graft to the underside of the acromion. (AG, acromial graft.)
Table 1.
Technical Pearls and Pitfalls of Superior Capsular Reconstruction Using Doubled Acellular Dermal Allograft
| Pearls | Pitfalls |
|---|---|
| Short sides of rectangular acromial graft correspond to anteroposterior of acromion. | Care must be taken in patients with previous subacromial decompression (adequate edges with drilling of acromion). |
| Ensure 5 mm from each edge when passing suture. | Sutures should exit same side of the graft. |
| No. 2 FiberWire suture. | |
| Ensure 5 mm from each edge of acromion when drilling. |
Step 14: Final Inspection, Closure, and Postoperative Rehabilitation
Once the graft is secured, the humerus can be gently moved through its range of motion to test the tension on the SCR graft and its response to motion while being arthroscopically visualized. All portals are then closed in a standard manner. Postoperatively, the patient is treated by a massive repair protocol. This includes placement in a shoulder immobilizer with an abduction pillow. The patient is allowed to perform elbow, wrist, and hand exercises, along with gentle passive glenohumeral motion, for 6 weeks. Progressive motion is begun at 6 weeks postoperatively, and strengthening is begun at 12 weeks. The patient gradually returns to activity when motion, strength, and confidence return over a 6-month postoperative period.
Discussion
Various treatment options have been developed for massive, irreparable rotator cuff tears. Although some success has been seen, they remain a difficult problem in a younger, active population. Patients in this population not only desire pain relief but also often need more function from their shoulder; considering this, successful options in older patients are not as reproducible in the younger group. Although reverse shoulder arthroplasty has been shown to restore function and improve pain in an older population, similar clinical results have not been demonstrated in patients younger than 65 years.15, 16 In addition, complications seen with this procedure increase when used in younger patients.17 Other treatment options have not found success either, with high rates of recurrent tears. A retear rate of up to 52% has been found when performing a partial repair for these massive, irreparable tears.18 The use of patch augmentation has not provided the same benefit seen in primary repair and its use as a bridge between remaining cuff tissue and the greater tuberosity has been found to result in a retear rate of up to 100%.18, 19
SCR may offer advantages over other treatment options, in that it may restore and rebalance the force couples of the shoulder joint. Burkhart et al.20 originally proposed that the rotator cable complex aids in stability of the glenohumeral joint and keeps it reduced by keeping the humeral head from subluxing superiorly. Although this is true, it is the superior capsule that acts as a static stabilizer to superior translation, with the rotator cuff aiding in dynamic reinforcement of the capsule and providing strength.13 This loss of superior stabilization resulting in humeral head malposition and improper muscle fulcrum contributes to some of the pain seen in these patients.13 To achieve restoration of complete strength, the rotator cuff must be repaired, but pain reduction and functional improvement can be achieved with reconstruction of the superior capsule.
Reconstruction of the superior capsule has emerged as a valid treatment of massive, irreparable rotator cuff tears. Mihata et al.12 demonstrated early successful results of SCR when using a fascia lata autograft. After harvesting the fascia lata graft through an incision on the lateral thigh, it was folded over 2 to 3 times to achieve a graft thickness of 6 to 8 mm.8 Patients were found to have improvements in pain, AHD, and motion and clinical outcomes that were comparable to outcomes of complete rotator cuff repairs. Dermal allografts, which had previously been used in rotator cuff repair augmentation, were introduced as an alternative to decrease donor site morbidity.13 Patients undergoing SCR with a dermal allograft were found to have a successful outcome approximately 70% of the time, with motion, AHD, and clinical outcomes improving postoperatively.14
Although the use of dermal allografts for SCR has had success, it has not reached the levels seen in SCR when using a fascia lata autograft. Some of the discordance in results may be attributed to the differences in graft used. The characteristics of the graft are important to outcomes, as higher demand is placed on the graft owing to lack of rotator cuff protection. Recent biomechanical cadaveric research has compared fascia lata autografts with human dermal allografts in their use in SCR. It was determined that both the autograft and allograft restored subacromial contact pressure and superior glenohumeral joint force, but although superior translation was completely restored with the autograft, it was only partially restored with the allograft and remained greater than that of the intact condition.21 Dermal allografts were also found to elongate 15% during the testing, whereas the length of the autografts were unchanged.21
The thickness of the graft may also play a major role in the outcomes seen after the procedure. The native capsule has been found to be about 4.4 to 9.1 mm thick, and the thickness of the autografts used in the SCR procedures of Mihata et al. fell into this range.12, 22 In contrast, the maximal thickness of commercially available dermal allografts are 3 to 4 mm.13 Some of the grafts used for the reconstructions of Denard et al14 were even thinner, ranging from 1 to 3 mm thick. The importance of graft thickness is even more important when stratifying their results based on thickness of the graft used. The overall rate of success seen in their reconstructions was 67.8%, but only 40% in 1-mm thick grafts.14 When they excluded the reconstructions done with 1-mm grafts, their success rate rose to 75.5% in Hamada grade 1 or 2 shoulders.14 Graft thickness was also shown to play a role in the rate of graft healing, as a greater percentage of those that healed on postoperative magnetic resonance imaging were 3 mm thick.13 In addition, 100% of those grafts that healed had a successful outcome, whereas success was seen in only 45.5% of grafts that did not heal.14 The healed group also demonstrated decreased postoperative pain and higher ASES scores.14 The argument to use thicker grafts in SCR has also been made in biomechanical research. Mihata et al.23 investigated the subacromial peak contact pressure and glenohumeral superior translation in SCR using both a 4- and 8-mm–thick fascia lata graft. They noted that although subacromial peak contact pressure was decreased with both grafts, only the 8-mm graft reduced superior translation.
As demonstrated, increasing the thickness of the construct to that of the native superior capsule may lead to better outcomes. Although dermal allografts reduce morbidity, they are significantly more expensive than autografts. Thus, the use of a second graft to achieve the desired thickness is not financially feasible. The allografts are usually large enough in size that excess graft is left after determining the desired dimensions needed for SCR. Folding this excess over to increase the thickness, as seen with autografts, potentially decreases the ability of the graft to heal. Depending on the way the graft is folded, either the graft will have 2 dermal sides on its outside or 2 dermal sides touching each other, not an ideal healing situation either way. As demonstrated in this technique, the excess graft is usually large enough that it can be used as a second allograft. The first SCR graft is attached to the glenoid and greater tuberosity as in previously described SCR techniques using an allograft.24 The second graft made from the excess allograft is then attached to the underside of the acromion.
At this time, there are no studies that demonstrate superiority to a traditional SCR with dermal allograft, or noninferiority to use of a fascia lata autograft. Potential risks of this technique include acromial fracture owing to drilling into the acromion. This risk can be increased in patients with previous aggressive subacromial decompression. Aside from a potential increase in acromial fracture risk, the risks of this procedure are similar to those for SCR. Table 2 presents advantages and disadvantages to this technique.
Table 2.
Advantages and Disadvantages of Superior Capsular Reconstruction Using Doubled Acellular Dermal Allograft
| Advantages | Disadvantages |
|---|---|
| Increased graft thickness. | Increased operative time. |
| Potential correction of acromiohumeral distance. | Risk of acromial fracture. |
| No increased cost. | Clinically unproven at this time. |
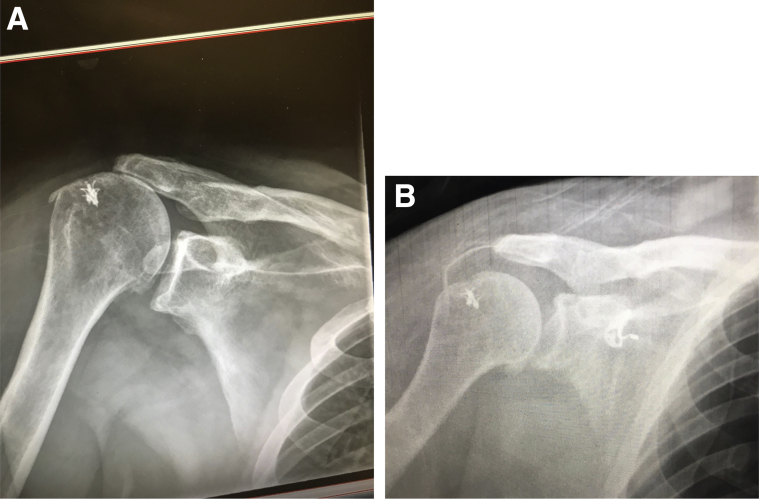
This technique provides various advantages over traditional SCR with dermal allograft. When the acromial graft thickness is combined with that of the SCR graft, the potential spacer effect is increased to 8 mm, similar to the thickness seen with the use of autografts. This may lead to better restoration of superior translation than that seen with just an allograft SCR by itself. Early radiographic findings have demonstrated this improvement in AHD in patients who have undergone SCR with the addition of an acromial allograft spacer (Fig 10). In this technique, the acromion is resurfaced and in addition to preparation for the graft, this may also play a role in pain relief. Additionally, the acromial graft is inexpensive as it is fashioned from the excess graft used for SCR, and this technique remains minimally invasive as it can be accomplished arthroscopically. The use of this technique at our institution has led to promising short-term results, but longer-term follow-up is needed to evaluate its full potential for success in this challenging problem.
Fig 10.
Radiographs of a patient who underwent allograft superior capsular reconstruction with the addition of an acromial allograft spacer. Preoperative (A) and postoperative (B) radiographs demonstrate the improvement in acromiohumeral distance.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: J.M.T. is a consultant for Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Technique video demonstrating superior capsular reconstruction with acellular dermal allograft in an irreparable massive rotator cuff tear with the addition of a further acromial acellular dermal allograft to increase graft thickness. The video is recorded with the patient in the lateral decubitus position and the patient's right arm abducted 45°.
References
- 1.Bedi A., Dines J., Warren R.F., Dines D.M. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 2.Oh J.H., Kim S.H., Kang J.Y., Oh C.H., Gong H.S. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38:672–678. doi: 10.1177/0363546509352460. [DOI] [PubMed] [Google Scholar]
- 3.Goutallier D., Postel J.M., Bernageau J., Lavau L., Voisin M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 4.Melis B., Wall B., Walch G. Natural history of infraspinatus fatty infiltration in rotator cuff tears. J Shoulder Elbow Surg. 2010;19:757–763. doi: 10.1016/j.jse.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Melis B., Nemoz C., Walch G. Muscle fatty infiltration in rotator cuff tears: Descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009;95:319–324. doi: 10.1016/j.otsr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Oh J.H., Kim S.H., Choi J.-A., Kim Y., Oh C.H. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468:1558–1564. doi: 10.1007/s11999-009-0818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.-J., Kim S.-H., Lee S.-K., Seo J.-W., Chun Y.-M. Arthroscopic repair of massive contracted rotator cuff tears: Aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2013;95:1482–1488. doi: 10.2106/JBJS.L.01193. [DOI] [PubMed] [Google Scholar]
- 8.Ciampi P., Scotti C., Nonis A. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: A 3-year follow-up study. Am J Sports Med. 2014;42:1169–1175. doi: 10.1177/0363546514525592. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A.K., Hug K., Boggess B., Gavigan M., Toth A.P. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: Clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41:872–879. doi: 10.1177/0363546512475204. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara Y., Mihata T., Tamboli M. Role of the superior shoulder capsule in passive stability of the glenohumeral joint. J Shoulder Elbow Surg. 2014;23:642–648. doi: 10.1016/j.jse.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Mihata T., McGarry M.H., Pirolo J.M., Kinoshita M., Lee T.Q. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: A biomechanical cadaveric study. Am J Sports Med. 2012;40:2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 12.Mihata T., Lee T.Q., Watanabe C. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Hirahara A.M., Adams C.R. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4:e637–e641. doi: 10.1016/j.eats.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denard P.J., Brady P.C., Adams C.R., Tokish J.M., Burkhart S.S. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy. 2018;34:93–99. doi: 10.1016/j.arthro.2017.08.265. [DOI] [PubMed] [Google Scholar]
- 15.Ek ETH. Neukom L., Catanzaro S., Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199–1208. doi: 10.1016/j.jse.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Boileau P., Gonzalez J.-F., Chuinard C., Bicknell R., Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600–606. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Mulieri P., Dunning P., Klein S., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 18.Berth A., Neumann W., Awiszus F., Pap G. Massive rotator cuff tears: Functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010;11:13–20. doi: 10.1007/s10195-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore D.R., Cain E.L., Schwartz M.L., Clancy W.G. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34:392–396. doi: 10.1177/0363546505281237. [DOI] [PubMed] [Google Scholar]
- 20.Burkhart S.S., Esch J.C., Jolson R.S. The rotator crescent and rotator cable: An anatomic description of the shoulder’s “suspension bridge”. Arthroscopy. 1993;9:611–616. doi: 10.1016/s0749-8063(05)80496-7. [DOI] [PubMed] [Google Scholar]
- 21.Mihata T., Bui C.N.H., Akeda M. A biomechanical cadaveric study comparing superior capsule reconstruction using fascia lata allograft with human dermal allograft for irreparable rotator cuff tear. J Shoulder Elbow Surg. 2017;26:2158–2166. doi: 10.1016/j.jse.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Nimura A., Kato A., Yamaguchi K. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21:867–872. doi: 10.1016/j.jse.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Mihata T., McGarry M.H., Kahn T., Goldberg I., Neo M., Lee T.Q. Biomechanical effect of thickness and tension of fascia lata graft on glenohumeral stability for superior capsule reconstruction in irreparable supraspinatus tears. Arthroscopy. 2016;32(3):418–426. doi: 10.1016/j.arthro.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Tokish J.M., Beicker C. Superior capsule reconstruction technique using an acellular dermal allograft. Arthrosc Tech. 2015;4:e833–e839. doi: 10.1016/j.eats.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technique video demonstrating superior capsular reconstruction with acellular dermal allograft in an irreparable massive rotator cuff tear with the addition of a further acromial acellular dermal allograft to increase graft thickness. The video is recorded with the patient in the lateral decubitus position and the patient's right arm abducted 45°.