Abstract
Purpose
Topical therapy (TT) for upper tract urothelial carcinoma (UTUC) has been explored as a kidney sparing approach to treat carcinoma in situ (CIS) and as adjuvant for endoscopically treated Ta/T1 tumors. In bladder cancer data supports use of salvage TT for repeat induction. We investigate the outcomes of salvage TT for UTUC in patients ineligible for or refusing nephroureterectomy.
Methods
A single center retrospective review on patients receiving salvage TT via percutaneous nephrostomy tube or cystoscopically placed ureteral catheters was performed. Primary outcome was response to therapy based on International Bladder Cancer Group criteria.
Results
51 patients with 58 renal units (RUs) received TT. Of these, 17 patients with 18 RUs received second line TT, with a median follow up of 36.5 months (IQR: 24.5–67 months). 44% (8/18) received salvage TT for refractory disease and 56% (10/18) as re-induction. 5 RUs with CIS were unresponsive to initial TT and went on to receive salvage TT, of which 20% (1/5) responded. 13 RUs recurred or relapsed following initial TT and received salvage TT for papillary tumors, with 62% (8/13) responding.
Conclusion
Our data provides preliminary clinical rationale for second line TT for refractory and recurrent, endoscopically-managed papillary UTUC in patients ineligible for or refusing nephroureterectomy. However, refractory upper tract CIS appears to have poor response to salvage TT.
Keywords: Upper Tract Urothelial Carcinoma, Chemotherapy, Ureteral Cancer, topical therapy, renal pelvis cancer
Introduction
Upper urinary tract urothelial carcinoma (UTUC) is a relatively rare genitourinary malignancy that accounts for approximately 5–10% of urothelial tumors[1]. Despite being an uncommon pathology, recent data suggests that UTUC incidence is increasing[2,3]. Radical nephroureterectomy (RNU) with bladder-cuff excision is considered the gold standard of care for UTUC[4]. Due to the risk of cardiovascular morbidity, chronic renal failure and contralateral tumors, nephron sparing approaches are desired in many patients. Nephron sparing approaches are especially critical for patients with bilateral tumors, tumors in a solitary kidney, chronic kidney disease (CKD), systemic diseases that place patients at risk for kidney disease, or those predisposed to recurrent tumors as in genetic syndromes[4,5]. Furthermore, patients with select small, low grade tumor may be amenable to safely having their tumor managed with a nephron sparing approach in an elective setting[6,7].
Endoscopic management of UTUC via a percutaneous or ureteroscopic approach is feasible and safe in selected patients[4]. Unfortunately, reported recurrence rates following this treatment modality range from 30% to 70% depending upon tumor stage and grade[6,8], although a recent study by Musi and colleagues using the Thulium laser has shown lower recurrence rates of 20–25% after a median follow up of 26.3 months [9]. In bladder cancer, intravesical instillation therapy has been established as a viable adjuvant therapy to reduce recurrence and progression of superficial disease[10]. Based on this success, adjuvant topical therapy has been explored in an effort to reduce recurrences following endoscopic UTUC management in patients ineligible for conventional surgery, and as a primary treatment for those with isolated upper tract carcinoma in situ (UTCIS). Most reports of chemotherapeutic and immunomodulatory agents given for UTUC have been given as induction therapy only, and it is unclear if recurrences are reduced[11]. One of the largest experiences in 55 patients with 64 renal units (RUs) has shown response rates of 40% (17/42) in RUs with UTCIS, and only 41% (13/22) response when induction therapy is given as an adjuvant [12]. Another recent study evaluating mitomycin-c as induction and maintenance as adjuvant for endoscopically-managed papillary tumors in 28 renal units showed 3-year recurrence-free, progression-free, and nephroureterectomy-free survival rates of 60%, 80%, and 76%[13].
Currently, nephroureterectomy is the established treatment for patients who develop recurrence or progression after topical therapy. However, many patients have contraindication to this and a nephron sparing approach is still desired. In a small select population, salvage endoscopic management and subsequent intracavitary instillation therapy is the only option given potential limitations due to patient concerns and/or significant comorbidities. In bladder cancer, data supports use of salvage topical therapy for repeat induction but to our knowledge this approach has yet to be investigated for UTUC[14–16].
This study evaluates outcomes, recurrence patterns and complication data regarding the use of salvage upper tract instillation of topical therapy in a select, elderly patient population ineligible for or refusing nephroureterectomy.
Materials and Methods
Patients
Demographic, clinical, pathological and outcome information was retrospectively reviewed following international research ethics board approval on all patients receiving topical therapy for UTUC from 3/2005–10/2016 at MD Anderson Cancer Center. Following initial overview, inclusion criteria comprised of patients diagnosed with cTx/cTa/cT1/cTis UTUC who received second line induction and maintenance topical therapy. The cTx classification was assigned when pathology could not conclusively assess invasion at time of endoscopic resection due to lack of lamina propria or muscle in the specimen. Patients not receiving further topical treatment following first round of induction and maintenance were excluded altogether from further analysis. Variables extracted during chart review included patient’s age, sex, race, smoking history, family history, Lynch syndrome status, cytology results, biopsy and endoscopy results, agent used, route of delivery, doses given, complications, and renal function.
Endoscopy
Endoscopic management consisted of retrograde pyelography and ureteroscopic biopsy. Staged ureteroscopic or percutaneous resection and/or laser ablation of all visible disease burden was conducted either in a single or staged fashion, but was completed prior to initiating topical therapy, and was repeated after any relapse of disease. A holmium laser was used for laser ablation, with settings at 5–10 Hertz and 1.0 Joules. Genitourinary fellowship trained pathologists reviewed all pathologic results. Patients were classified as being treated on an elective, imperative, or palliative basis. Elective patients were treated with curative intent and had a normal contralateral upper tract and normal renal function. Imperative patients were treated with curative intent and had either bilateral tumors, tumor in a solitary kidney, or CKD. Palliative patients were treated with the goal of local control for maintenance of renal function.
Topical Therapy
Patients were treated either via percutaneous nephrostomy tube (NT) or by cystoscopically placed weekly ureteral catheters. The method of delivery was determined by patient preference after being informed of the pros and cons of both modalities. Patients with NTs were given 2 weeks to have the tract mature before initiating infusion, with the NT changed every 3 months. Patients treated with a ureteral catheter had office-based flexible cystoscopy and placement of a multi-hole ureteral catheter (Beacon tip© or Royal Flush©, Cook Medical, Bloomington, IN) performed under fluoroscopic guidance using only intraurethral 2% lidocaine jelly for analgesia. A foley catheter tied to the ureteral catheter prevented dislodgment during treatment. These procedures have been previously detailed[13].
Patients were treated by nurses in the urology ambulatory office setting with topical treatments composed of either Mitomycin C (MMC), Bacillus Calmette-Guérin (BCG), Gemcitabine or Mitogel formulation. First line treatment was MMC for adjuvant therapy, and BCG for UTCIS, except during a nationwide shortage of both when Gemcitabine was used as first line. MMC treatments were composed of 40 mg of MMC mixed in 20 mL of physiologic saline. BCG treatments were composed of 1 ampule of BCG in 50 mL physiologic saline as per the concentration used for intravesical use. Gemcitabine treatments were formulated to a concentration of 1000 mg per 50 mL. Mitogel was formulated at a concentration of 60 mg per 15 mL. Treatments were delivered by slow drip for up to 2 hours and controlled by manometry pressures at or below 20mmHg. Pressures were allowed to rise to 30mmHg as long as patients remained asymptomatic. Patients were asked to assume different positions (left side, right side, prone and supine) every 15–20 minutes to ensure adequate contact with the entire upper tract system. After completion, the ureteral and Foley catheters were removed. Patients with NTs had the tube capped. Urinary cultures were performed at the initial visit and subsequently only in the presence of symptoms or signs of infection. Prophylactic oral antibiotics were prescribed for 1–2 doses at the time of each treatment.
Induction courses consisted of once weekly instillations for 6 weeks. Maintenance course consisted of either once monthly for at least 3 months, or once weekly instillations for 3 weeks, following previously published SWOG guidelines[17].
Follow-Up
Patients were followed up every 3 months in the first year, then every 6 months for at least 2 years. Evaluations consisted of ureteroscopy, urine cytology, chemistry panel, complete blood count, and triple-phase computed tomography (CT). Ureteroscopy, urine cytology, and laboratories were performed in the first 3 months after induction, then at a minimum every six months, with at least annual CT. In patients with contraindication to contrasted scan, magnetic resonance urogram or retrograde studies were substituted for CT.
Outcome measures and statistical analyses
Primary outcomes measured were recurrence-free, progression-free, and nephroureterectomy-free rate on a per-renal unit (RU) basis, and cancer specific and overall survival on a per-patient basis. Patient outcomes were classified based upon recommendations outlined by the International Bladder Cancer Group[18], with response defined as no evidence of disease after 6 months, refractory cases classified as recurrence within 6 months, and relapse as recurrence after 6 months, after start of topical treatment. Salvage topical treatment was defined as re-initiation of therapy for refractory or relapsing RU’s following primary topical therapy. Sub-analyses were performed to evaluate recurrence-free, progression-free, and nephroureterectomy-free rates based on delivery method. A secondary outcome measure was treatment tolerability. Adverse events were recorded and classified based on the Clavien-Dindo scale.
Results
Baseline Characteristics of Topical Therapy Patients
Between March 2005 and October 2016, we identified 51 patients with 58 renal units who received topical therapy. 55% (32/58) of RUs had low grade UTUC, 22% (13/58) had high grade UTUC, 17% (10/58) had UTCIS, and 5% (3/58) had unknown disease grade due to insufficient biopsy tissue. For RUs with adjuvant indications 79% (38/48) were responsive, 19% (9/48) refractory, 2% (1/48) were intolerant to TT and 25.0% (12/48) had a relapse. After TT for UTCIS, 60% (6/10) of RUs were responsive, 40% (4/10) refractory and 10% (1/10) relapsed.
Baseline Characteristics of Second Line Topical Therapy Patients
Seventeen patients received second line topical therapy in 18 renal units as 1 patient had bilateral disease (Table 1). The median time from initial diagnosis to initiation of second line treatment was 11 months (IQR: 7.25–15 months). The median follow up time following initial diagnosis was 36.5 months (IQR: 24.5–67 months). 41.2% (7/17) of patients had solitary kidneys and 58.8% (10/17) had bilateral functioning kidneys. 41.2% (7/17) of patients were classified as having renal insufficiency, and median GFR at diagnosis was 62.5 (IQR: 53–73.5). 27.8% (5/18) of renal units were classified as undergoing elective treatment, 66.7% (12/18) imperative (7 solitary kidneys and 5 renal insufficiency) and 5.6% (1/18) palliative. 44.4% (8/18) RUs received treatment instillations via ureteral catheter while 55.6% (10/18) via NT.
Table 1.
Baseline Demographics for 2nd Line Topical Therapy (TT)
| Variable | n1 or median2 | %1 or IQR2 |
|
|---|---|---|---|
| Number of Patients | 17 | ||
| Number of Renal Units | 18 | ||
| Age | 78.35 | 68–82.6 | |
| Sex | Male | 14 | 82.4% |
| Female | 3 | 17.6% | |
| Race | White | 16 | 94.1% |
| Hispanic | 0 | 0.0% | |
| Black | 1 | 5.9% | |
| Asian | 0 | 0.0% | |
| Smoking History | Y | 9 | 52.9% |
| N | 8 | 47.1% | |
| Lynch Syndrome Status | Y | 1 | 5.9% |
| N | 16 | 94.1% | |
| Prior or Concurrent Bladder Cancer | Y | 8 | 47.1% |
| N | 9 | 52.9% | |
| Solitary Kidney | Y | 7 | 41.2% |
| N | 10 | 58.8% | |
| Renal Insufficiency | eGFR>60 | 7 | 41.2% |
| eGFR<60 | 10 | 58.8% | |
| GFR | Median | 62.5 | 53–73.5 |
| Indication for Topical Therapy | Elective | 5 | 27.8% |
| Imperative | 12 | 66.7% | |
| Palliative | 1 | 5.6% | |
| Baseline Grade | High | 11 | 61.1% |
| Low | 7 | 38.9% | |
| Baseline Stage | Tx | 5 | 27.8% |
| Ta | 10 | 55.6% | |
| T1 | 1 | 5.6% | |
| CIS | 5 | 27.8% | |
| Delivery Method | Ureteral Catheter | 8 | 44.4% |
| Nephrostomy Tube | 10 | 55.6% | |
| First Line Treatment Delivered | BCG | 10 | 55.6% |
| Mitomycin | 8 | 44.4% | |
| Indication for 2nd Line Topical Therapy | Reinduction | 3 | 16.7% |
| Failure | 15 | 83.3% | |
| Relapse | 6 | 33.3% | |
| Refractory | 8 | 44.4% | |
| Intolerant | 1 | 5.6% | |
| Time from diagnosis to initiation of 2nd line treatment (Months) | 11 | 7.25–15 | |
| Time from last Topical Therapy to initiation of 2nd line treatment (Months) | 3 5 | 3–5.75 | |
| Follow Up- from Initiation of 2nd Line Treatment (Months) | 24 | 13.75–44 | |
| Follow Up- Time From Initial Diagnosis (Months) | 36.5 | 24.5–67 |
Baseline data for patients receiving second line therapy. 1-For categorical variables 2 -For continuous variables
Salvage Topical Therapy Results
5 renal units with UTCIS received second line topical therapy. Amongst these, 20% (1/5) of renal units received treatment with MMC while 80% (4/5) received BCG instillations (Table 2). 1 renal unit receiving MMC experienced treatment failure. Of 4 BCG treated renal units, 1 (25%) was responsive, and 3 (75%) were failures.
Table 2.
Summary of indications, treatments, and responses of salvage topical therapy.18 patients received first line therapy based on initial indication of upper tract carcinoma in situ (UTCIS) or Papillary (Adjuvant) Tumors.
| INDICATION | 2nd Line Therapy |
Renal units N=18 |
Rounds of Induction Median (Min- Max) |
Rounds of Maintenance Median (Min- Max) |
Response n (%) |
Refractory n (%) |
Late Relapse n (%) |
|---|---|---|---|---|---|---|---|
| UTCIS | All | 5 | 6 (3–6) | 1.5 (1–2) | 1 (20%) | 4 (80%) | 0% |
| MMC | 1 | 0% | 1 (100%) | 0% | |||
| BCG | 4 | 1 (25%) | 3 (75%) | 0% | |||
| ADJUVANT | All | 13 | (6,3–6) | 2 (1–9) | 8 (62%) | 4 (31%) | 1* (8%) |
| MMC | 5 | 3 (60%) | 1 (20%) | 1 (20%) | |||
| BCG | 5 | 3 (60%) | 2 (40%) | 0% | |||
| GEM | 2 | 1 (50%) | 1 (50%) | 0% | |||
| Mitogel | 1 | 1 (100%) | 0% | 0% |
One patient was disease free at 3 months and then had a late recurrence 7 months after discontinuation of TT.
Legend: MMC-Mitomycin C, BCG- Bacillus Calmette-Guerin, GEM- Gemcitabine
Of the 13 renal units treated with adjuvant salvage TT, 38% (5/13) received MMC, 38% (5/13) received BCG, 15% (2/13) Gemcitabine and 8% (1/13) Mitogel. Among these RUs, 62% (8/13) were responsive, 31% (4/13) were refractory, and 8% (1/13) had recurrence following completion of induction and maintenance.
Of the overall 18 renal units receiving salvage topical therapy, 50% (9/18) responded to treatment, 44% (8/18) had refractory disease and 6% (1/18) had a relapse (Table 3). 22% (4/18) of renal units went on to receive third line topical therapy while a majority of renal units, 78% (14/18), did not undergo further TT. Of the 9 patients experiencing refractory disease or recurrence following second line TT, 55.6% (5/9) of patients required a nephroureterectomy, 3 of whom had pT3 disease (Table 3). 11.1% (1/9) of patients died due to causes unrelated to UTUC. The remaining 88.9% (8/9) of patients are alive with no evidence of disease at a median follow up of 31 months (IQR:23–67 months).
Table 3.
Detailed recurrence and follow up status of 17 patients and 18 renal units receiving salvage topical therapy.
| Variables | N | % | |
|---|---|---|---|
| Total Number of Patients | 17 | ||
| Total Number of Renal Units | 18 | ||
| Responders | 9 | 50% | |
| Recurrence | Failure | 9 | 50% |
| Late Relapse | 1 | 6% | |
| Refractory | 8 | 44% | |
| Intolerant | 0 | ||
| Received Third Agent | Yes | 4 | 22% |
| No | 14 | 78% | |
| Progressed | Yes | 3 | 17% |
| No | 15 | 83% | |
| Nephroureterectomy | Yes | 5 | 28% |
| pTa N0 low grade | 1 | 6% | |
| pTis N0 high grade | 1 | 6% | |
| mpT3 N0 high grade | 3 | 16% | |
| No | 13 | 72% | |
| Upper Tract Recurrence | Yes | 9 | 50% |
| No | 9 | 50% | |
| Bladder Recurrence | Yes | 6 | 33% |
| No | 12 | 67% | |
| Distant Recurrence | Yes | 1 | 13% |
| No | 17 | 94% | |
| Patient Status | Alive, NED | 15 | 88% |
| Alive with disease | 0 | ||
| Dead of disease | 0 | ||
| Dead, other cause | 2 | 12% | |
| Follow up in months –median (IQR) | 36.5 (24.5–67) | ||
NED=No Evidence of Disease
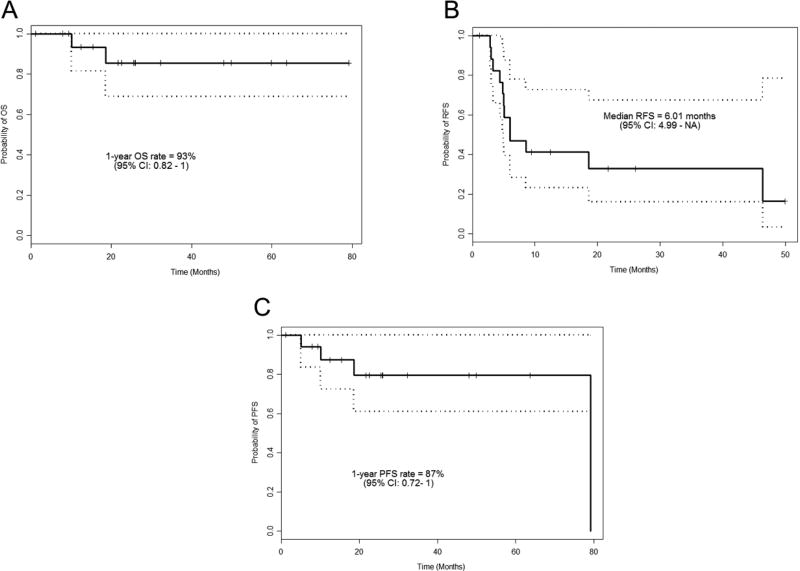
Two of the 17 patient receiving second line therapy died of other causes (at 18.7 and 10.1 months after the initiation of salvage therapy). The estimated median follow up time from the start of second line therapy for the 17 patients was 25.9 months (95% Confidence Interval: 21.6–59.8 months). The estimated median recurrence-free survival time was 6.01 months (95% Confidence Interval 4.99 months – not reached). The estimated 1 year progression-free survival rate was 87% (95% Confidence Interval 0.71–1). Figure 1 shows Kaplan Meier estimates for overall survival, RFS and PFS.
Figure 1.
Kaplan–Meier estimates for overall survival (A), recurrence-free (B), and progression-free (C) survival.
Adverse Events
4/17 (24%) patients experienced an adverse event (AEs) during the course of second line treatment. These AEs consisted of 1/17 (5.9%) grade 1 UTI, 2/17 (11.8%) grade 3 ureteral strictures and 1/17 (5.9%) grade 1 lower urinary tract symptoms. No events were identified related to topical treatment absorption such as bone marrow depression, anemia or leukopenia.
Discussion
Second line salvage topical therapy as induction and maintenance for UTUC appears to be a well-tolerated, feasible and potentially beneficial approach to conservatively managing refractory or relapsing, endoscopically-managed cTa-T1 tumors, whereby a 62% response rate was seen, compared to 79% in the first line setting; but not for refractory or relapsing UTCIS whereby a 20% response rate was seen, compared to 60% in the first line setting.
48 renal units initially presented with papillary tumors that were endoscopically managed. Of these 48 renal units that received adjuvant topical therapy, 71% (34/48) responded to first line topical therapy and 2% (1/48) were intolerant to topical treatment. Of the 13 renal units that had a recurrence and received second line treatment, 62% (8/13) had a response to salvage topical therapy at a median follow up time of 36.5 months (IQR:24.5–67).
10 renal units initially presented with UTCIS and received topical therapy. Of these, 60% (6/10) initially responded, 40% (4/10) had refractory disease and 1% (1/10) experienced recurrence. For these 5 renal units with UTCIS and did not respond to initial therapy, only 20% (1/5) responded to second line treatment.
Our results are comparable to other published series that investigated topical therapy in the first line setting. In our study, 50% (9/18) overall responded to second line treatment. In a study conducted by Palou et al., the investigators employed a percutaneous technique on 19 patients followed by administration of adjuvant treatment (14 BCG and 5 MMC)[19]. In that study 60% of patients receiving topical therapy experienced recurrence. In another study conducted by Martinez Pineiro et al., 26 patients received adjuvant topical therapy regimens for UTUC, and 19 recurrencess occurred in those receiving BCG (12.5%), thiotepa (60%), and MMC (14%)[20]. These varying outcome comparisons between the Palou and Martinez Pineiro studies underscore the variability associated with providing topical therapy amongst small patient subpopulations with rare conditions. We also recognize the difference between endoscopic approaches of UTUC versus bladder cancer can be significant due to issues such as achieving complete resection, dwell time of topical therapy, and anatomical differences. The result from our study of a 50% response to salvage therapy is similar to the expected 50–60% seen in bladder cancer[21–23]. Hence while our study is encouraging, a larger effort is required to draw definitive conclusions regarding the efficacy of second line topical therapy.
Adverse events (AEs) were noted in 4/17 (24%) patients. No patients discontinued therapy due to AEs. In the report by Musi and colleagues, Grade 1, 2, and 3 AEs were reported in 38%, 47.6%, and 2.4%, respectively [9]. An important consideration is that we cannot definitively identify topical therapy directly caused these local adverse events. For example the cause of the strictures could have been due to prior ureteroscopy and instrumentation. However, because of this uncertainty and for the sake of transparency, it was included as a complication. Stricture formation is a known complication of both ureteroscopy as well topical therapy and has been previously described; its cause may be magnified in these situations where there are multiple manipulations of the upper tract with additive effect [13,24]. New paradigms are needed for better delivery of topical therapy to improve responses and lower AEs.
Limitations of our study include biases inherent to retrospective analyses. Given the low incidence of UTUC, the efficacy and tolerability of second line treatment is difficult to evaluate via randomized trials. Consequently, retrospective studies such as ours offer the next best option towards providing some evidence regarding disease management strategies for this rare condition and challenging patient presentations. Another limitation is that the number of patients in this series is small and therefore not adequately powered to draw definitive conclusions regarding the efficacy of second line therapy, requiring additional validation. A third limitation is that our patient population received a heterogeneous array of treatment, but data regarding the optimal agent is still lacking. Going forward more standardization is required in terms of administered topical treatments.
Conclusion
Within the limitations of small subgroups, our data suggests that in properly selected patients with refractory or recurrent papillary UTUC after endoscopic management and first line adjuvant topical therapy, salvage topical therapy may be associated with likelihood of response to a second line agent. However, refractory or recurrent UTCIS was much less responsive to second line topical therapy. Improved paradigms for conservative management of UTUC are needed.
Acknowledgments
We would like to recognize support from the Monteleone Family Foundation Endowment for Research in Kidney and Bladder Cancer, and the Eleanor and Scott Petty Fund for Upper Tract Urothelial Cancer Research.
Conflict of interest: A. Kamat is a consultant to the following companies; Photocure, Telesta Therapeutics, Sanofi, Merck, Abbott Molecular, Theralase, Heat Biologics, Spectrum Pharmaceuticals and Oncogenix and has received research funding from FKD Industries; In addition, A. Kamat has a patent CyPRIT-Cytokine Panel for Response to Intravesical Immunotherapy pending. J. Karam is a consultant to the following companies; Pfizer, EMD Serono and Novartis. C. Dinney is a paid consultant to FKD Therapies. The corresponding author is a consultant to the following companies; Taris, Urogen, and Peloton Therapeutics and has received research funding from AT&T Foundation and Specialized Program in Oncology Research (SPORE).
Footnotes
Statements:
Ethical standards: This review does not involve human subjects and meets Helsinki declaration for protection of human subjects.
| Balasubramanian: | Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing |
| Metcalfe: | Data collection or management, Data analysis, Manuscript writing/editing |
| Wagenheim: | Data collection or management, Data analysis, Manuscript writing/editing |
| Xiao: | Data collection or management, Data analysis, Manuscript writing/editing |
| Papadopoulos: | Data collection or management, Manuscript writing/editing |
| Navai: | Data collection or management, Manuscript writing/editing |
| Davis: | Data collection or management, Manuscript writing/editing |
| Karam: | Data collection or management, Manuscript writing/editing |
| Kamat: | Data collection or management, Manuscript writing/editing |
| Wood: | Data collection or management, Manuscript writing/editing |
| Dinney: | Data collection or management, Manuscript writing/editing |
| Matin: | Protocol/project development, Data collection or management, Data analysis, Manuscript writing/editing |
References
- 1.Soria F, Shariat SF, Lerner SP, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC) World J Urol. 2017;35(3):379–387. doi: 10.1007/s00345-016-1928-x. [DOI] [PubMed] [Google Scholar]
- 2.Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nature Clinical Practice Urology. 2007;4(8):432–443. doi: 10.1038/ncpuro0875. [DOI] [PubMed] [Google Scholar]
- 3.Zigeuner R, Pummer K. Urothelial carcinoma of the upper urinary tract: Surgical approach and prognostic factors. European urology. 2008;53(4):720–731. doi: 10.1016/j.eururo.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Roupret M, Babjuk M, Comperat E, et al. European Guidelines on Upper Tract Urothelial Carcinomas: 2013 Update. European urology. 2013;63(6):1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Mork M, Hubosky SG, Roupret M, et al. Lynch Syndrome: A Primer for Urologists and Panel Recommendations. Journal of Urology. 2015;194(1):21–29. doi: 10.1016/j.juro.2015.02.081. [DOI] [PubMed] [Google Scholar]
- 6.Johnson GB, Fraiman M, Grasso M. Broadening experience with the retrograde endoscopic management of upper urinary tract urothelial malignancies. BJU Int. 2005;95:110–113. doi: 10.1111/j.1464-410x.2005.05210.x. [DOI] [PubMed] [Google Scholar]
- 7.Roupret M, Babjuk M, Comperat E, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. European urology. 2015;68(5):868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Keeley FX, Bibbo M, Bagley DH. Ureteroscopic treatment and surveillance of upper urinary tract transitional cell carcinoma. Journal of Urology. 1997;157(5):1560–1565. [PubMed] [Google Scholar]
- 9.Musi G, Mistretta FA, Marenghi C, et al. Thulium Laser Treatment of Upper Urinary Tract Carcinoma: A Multi-Institutional Analysis of Surgical and Oncological Outcomes. J Endourol. 2018;32(3):257–263. doi: 10.1089/end.2017.0915. [DOI] [PubMed] [Google Scholar]
- 10.Veeratterapillay R, Heer R, Johnson MI, Persad R, Bach C. High-Risk Non-Muscle-Invasive Bladder Cancer-Therapy Options During Intravesical BCG Shortage. Curr Urol Rep. 2016;17(9) doi: 10.1007/s11934-016-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shariat SFMS, Stenzl A. Upper Tract Urothelial Carcinoma (UTUC) A Joint SIU-ICUD International Consultation. Published by the Société Internationale d’Urologie (SIU) wwwsiu-urologyorg. 2013 [Google Scholar]
- 12.Giannarini G, Kessler TM, Birkhauser FD, Thalmann GN, Studer UE. Antegrade perfusion with bacillus Calmette-Guerin in patients with non-muscle-invasive urothelial carcinoma of the upper urinary tract: who may benefit? European urology. 2011;60(5):955–960. doi: 10.1016/j.eururo.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe M, Wagenheim G, Xiao LC, et al. Induction and Maintenance Adjuvant Mitomycin C Topical Therapy for Upper Tract Urothelial Carcinoma: Tolerability and Intermediate Term Outcomes. J Endourol. 2017;31(9):946–953. doi: 10.1089/end.2016.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinney CPN, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guerin. Urol Oncol-Semin Ori. 2013;31(8):1635–1642. doi: 10.1016/j.urolonc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Tang DH, Chang SS. Management of carcinoma in situ of the bladder: best practice and recent developments. Ther Adv Urol. 2015;7(6):351–364. doi: 10.1177/1756287215599694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Rundstedt FC, Lerner SP. Bacille-Calmette-Guerin non-responders: how to manage. Transl Androl Urol. 2015;4(3):244–253. doi: 10.3978/j.issn.2223-4683.2015.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamm DL. Preventing progression and improving survival with BCG maintenance. European urology. 2000;37:9–15. doi: 10.1159/000052376. [DOI] [PubMed] [Google Scholar]
- 18.Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016;34(16):1935-+. doi: 10.1200/JCO.2015.64.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palou J, Piovesan LF, Huguet J, Salvador J, Vicente J, Villavicencio H. Percutaneous nephroscopic management of upper urinary tract transitional cell carcinoma: Recurrence and long-term followup. Journal of Urology. 2004;172(1):66–69. doi: 10.1097/01.ju.0000132128.79974.db. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Pineiro JA, Garcia Matres MJ, Martinez-Pineiro L. Endourological treatment of upper tract urothelial carcinomas: analysis of a series of 59 tumors. The Journal of urology. 1996;156(2 Pt 1):377–385. doi: 10.1097/00005392-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Luciani LG, Neulander E, Murphy WM, Wajsman Z. Risk of continued intravesical therapy and delayed cystectomy in BCG-refractory superficial bladder cancer: an investigational approach. Urology. 2001;58(3):376–379. doi: 10.1016/s0090-4295(01)01187-6. [DOI] [PubMed] [Google Scholar]
- 22.Lam JS, Benson MC, O'Donnell MA, et al. Bacillus Calmete-Guerin plus interferon-alpha2B intravesical therapy maintains an extended treatment plan for superficial bladder cancer with minimal toxicity. Urologic oncology. 2003;21(5):354–360. doi: 10.1016/s1078-1439(03)00012-7. [DOI] [PubMed] [Google Scholar]
- 23.Punnen SP, Chin JL, Jewett MA. Management of bacillus Calmette-Guerin (BCG) refractory superficial bladder cancer: results with intravesical BCG and Interferon combination therapy. Can J Urol. 2003;10(2):1790–1795. [PubMed] [Google Scholar]
- 24.Aboumarzouk OM, Somani B, Ahmad S, Nabi G, Townell N, Kata SG. Mitomycin C instillation following ureterorenoscopic laser ablation of upper urinary tract carcinoma. Urol Ann. 2013;5(3):184–189. doi: 10.4103/0974-7796.115746. [DOI] [PMC free article] [PubMed] [Google Scholar]