Abstract
Advances in biomaterials for drug delivery are enabling significant progressin biology and medicine. Multidisciplinary collaborations between physical scientists, engineers, biologists, and clinicians generate innovative strategies and materials to treat a range of diseases. Specifically, recent advances include major breakthroughs in materials for cancer immunotherapy, autoimmune diseases, and genome editing. Here, strategies for the design and implementation of biomaterials for drug delivery are reviewed. A brief history of the biomaterials field is first established, and then commentary on RNA delivery, responsive materials development, and immunomodulation are provided. Current challenges associated with these areas as well as opportunities to address long-standing problems in biology and medicine are discussed throughout.
Keywords: biomaterials, drug delivery, immune therapy, nanomedicine, polymers
1. Introduction
A major focus of drug-related research has long been the synthesis and discovery of potent, pharmacologically active agents to manage, treat, or cure disease.[1] Globally, the market for pharmaceutical spending is expected to surpass $1.3 trillion by 2018.[2] However, it is now apparent that the therapeutic benefit and potency of a drug are not directly correlated; rather it is linked to the method of drug formulation and delivery within the body. The mode of delivery affects numerous factors that contribute to therapeutic efficacy, including pharmacokinetics, distribution, cellular uptake and metabolism, excretion and clearance, as well as toxicity.[3] Furthermore, drugs can lose their pharmacological activity due to changes in environmental factors such as moisture, temperature, and pH, which can occur in the body or during storage. As the biotechnology industry continues to develop new classes of biopharmaceuticals, improved fundamental understanding of how drug delivery affects safety and efficacy, along with new delivery technologies, are needed.[4] However, drug delivery remains a prominent challenge, including our limited understanding of biological barriers that limit drug delivery. These unmet needs and limitations have given rise to considerable research efforts focused on the design, implementation, and translation of biomaterials for drug delivery.
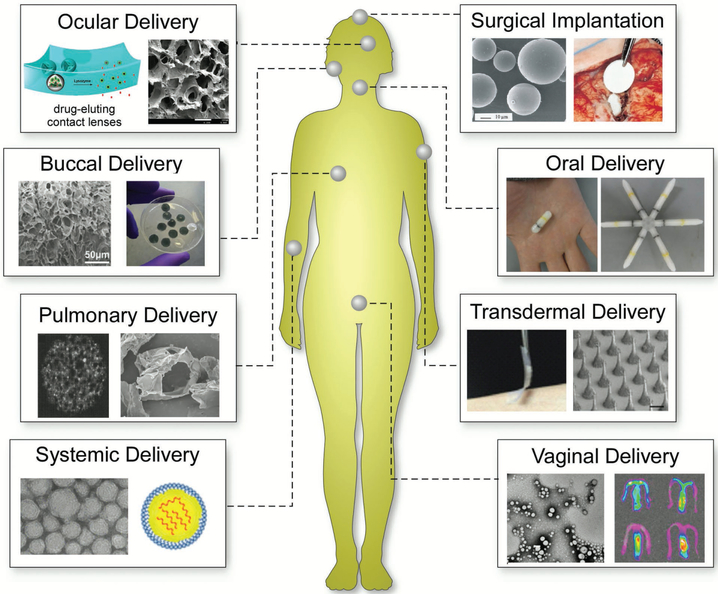
Biomaterials, in a collaborative effort by engineers, chemists, physicists, biologists, and clinicians, have been designed for use in advanced drug delivery systems for over 60 years.[5] Biomaterials have improved the delivery and efficacy of a range of pharmaceutical compounds including antibodies, peptides, vaccines, drugs and enzymes, among others.[6] In particular, polymer and lipid-based materials[7] for drug delivery have been driven by advances in organic and synthetic chemistry, materials science, genetic engineering, and biotechnology.[8] Many of these materials have been designed to release therapeutics for extended periods of time and can be further modified to target specific locations within the body, thereby reducing the amount of drug to achieve the desired therapeutic effect along with reduced toxicity to the patient.[9] The physicochemical properties of biomaterials and their intended route of administration can be systematically tailored to maximize therapeutic benefits. Biomaterials have enhanced oral and injectable drug delivery,[10] the most common modes of drug administration,[11] while also creating new avenues for drug delivery including via pulmonary, transdermal, ocular, and nasal routes (Figure 1).[12] Each route has its own advantages and limitations (Table 1), requiring the design of biomaterials to be uniquely suited for drug delivery to the intended administration route.
Figure 1.
Examples of biomaterials and their routes of administration for in vivo use. In addition to pills and injections, biomaterials have been developed to successfully administer drugs in a variety of other ways. Images for ocular delivery: left: Reproduced with permission.[150b] Copyright 2014, American Chemical Society; right: reproduced with permission.[237a] Copyright 2014, Elsevier. Images for buccal delivery: reproduced with permission.[237e] Copyright 2015, Elsevier. Images for pulmonary delivery: left: reproduced with permission.[12e] Copyright 1997, American Association for the Advancement of Science; right: reproduced with permission.[237f] Copyright 2009, Springer Science. Images for systemic delivery: reproduced with permission.[237d] Copyright 2016, National Academy of Sciences, USA. Images for surgical implantation: left: reproduced with permission.[237b] copyright 2002, Adis International; right: reproduced with permission.[237c] Copyright 1998, Elsevier. Images for oral delivery: reproduced with permission.[237g] Copyright 2016, American Association for the Advancement of Science. Images for transdermal delivery: reproduced with permission.[170j] Copyright 2015, National Academy of Sciences, USA. Images for vaginal delivery: reproduced with permission.[237h] Copyright 2017, Elsevier.
Table 1.
Representative advantages and disadvantages of different routes for drug delivery, as well as tissue targets and examples of therapies and delivery systems.
| Drug delivery route | Advantages | Disadvantages | Targets | Examples |
|---|---|---|---|---|
| Injections: intravenous (IV), intramuscular (IM), subcutaneous (SQ), depot | Applied to a large number of drugs | Rapidly cleared from body (IV) | Tissues with blood access (IV) | Chemotherapy (IV) |
| Rapid onset (IV) | Frequent injections required (IV) | Systemic | Vaccines (IM) | |
| Controlled release (IM, SQ) | Difficult to administer (IV) | Muscle (IM) | Insulin (SQ) | |
| As much as 100% bioavailability | Large gauge needles required (depot implant) | Hormones (Depot) | ||
| Lower burst release (depot implants) | Immunotoxicity (IV) | Hydrogels | ||
| Avoids reconstitution and/or suspension (depot implants) | Liver toxicity (IV) | Nanoparticles | ||
| Oral | High patient compliance | Low bioavailability | Systemic | Liquid medications |
| Ease of use | Variable absorption | Capsules | ||
| Lack of targeted systems | Pills | |||
| Degradation of drug in stomach and liver | Hydrogels | |||
| Variable adsorption in presence of food | Nanoparticles | |||
| Not amenable for macromolecule delivery | Microparticles | |||
| Transdermal | Painless administration | Low bioavailability | Systemic | Patches |
| Sustained and controlled release | Expensive | Skin | Microneedles | |
| Active control of continuing and discontinuing administration | Materials can be large, bulky | Creams | ||
| Reduced side effects | Variable absorption | Nanoparticles | ||
| High patient compliance | Incorrect dosages can be applied for some materials (creams) | Hydrogels | ||
| Pulmonary (i.e., inhalation) | Ease of use | Administration devices are large, bulky | Lungs | Aerosols |
| High bioavailability | Inconsistent delivery due to variation in patient technique | Systemic | Dry powders | |
| Rapid absoprtion and systemic uptake | Local lung toxicity and immunogenicity | Brain | Nanoparticles | |
| Direct access to lungs | Microparticles | |||
| Surgical implantation | Direct access to a range of diseased tissues | Potential infection due to surgery | Local, to a range of diseased tissues | Polymer implants |
| Reduced off target toxicity | Foreign body response and rejection | Microparticles | ||
| Requires surgical intervention | Materials can be large, bulky | Hydrogels | ||
| Potentially requires anaesthetics | Potentially requires immunosuppressing drugs | |||
| Time, cost, labor burden of procedure | ||||
| Mucosal routes: vaginal, nasal, buccal | Ease of use | Low bioavailability | Systemic | Films |
| Noninvasive | Variable absorption | Brain (nasal) | Sprays | |
| Self administerable | Local | Gels | ||
| Nervous system (nasal) | ||||
| Ocular: topical and injections | High patient compliance (topical) | High tear dilution and turnover rate (topical) | Eye | Eye drops |
| Noninvasive (topical) | Cornea acts as significant barrier (topical) | Injections | ||
| Self administerable (topical) | Toxicity due to high dosages (topical, injection) | Hydrogels | ||
| Direct delivery to retina (injection) | Retinal detachment, hemorrage, cataract (injection) | |||
| Sustained drug levels (injection) |
Despite the advances, challenges remain in emerging areas that require new classes of materials for drug delivery. Indeed, advances in genetic engineering and biotechnology have led to the development of new classes of nucleic acid, antibody, and protein-based therapeutics that will require a new wave of bio-materials capable of therapeutic protection, specificity, and controlled release. As biologists and clinicians continue to unravel biological responsive mechanisms within the body,[13] new “smart” or responsive biomaterials which have the potential to exploit and respond to these mechanisms are in demand for the development of next-generation precision medications. Immunologists continue to better understand the immune and foreign body responses (FBRs),[14] and thus the development of high-performance biocompatible materials will be crucial for the development of implantable devices for long-term controlled drug release, cell-based therapies, implantable sensors, as well as tissue engineering and regenerative medicine. Here, we provide a historical perspective of biomaterials research for drug delivery, along with the challenges and opportunities for biomaterials in three emerging areas of drug delivery: (i) nucleic acid delivery, (ii) “smart” bioresponsive materials for controlled drug delivery, and (iii) biomaterials to improve biocompatibility in drug delivery. We highlight the challenges currently presented across the field of drug delivery, breakthroughs in biomaterials research to overcome these hurdles, as well as future considerations and opportunities for biomaterials translation to the clinic.
2. Biomaterials: A Historical Background
2.1. Clinical Need for Controlled Drug Delivery
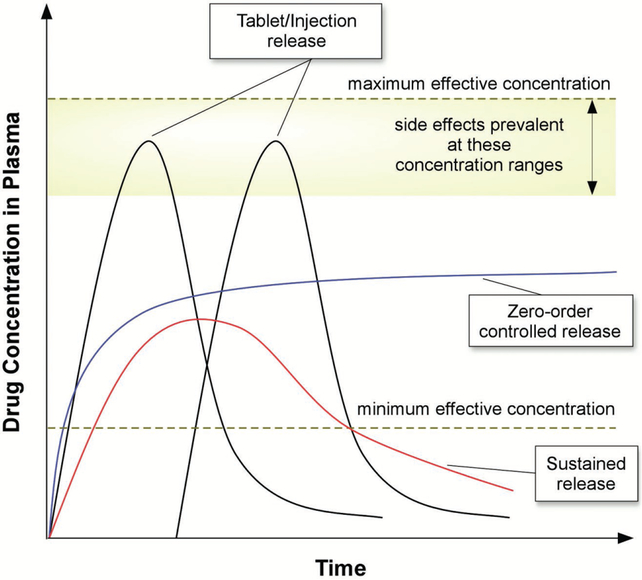
The need for materials for controlled drug release arose from the general problems associated with conventional dose delivery methods. Generally, drug administration required frequent, repeated doses that result in high variability of circulating drug concentrations throughout the treatment period (Figure 2). Upon administration, drug levels increase to therapeutic concentrations, but in some cases toxic side effects arise when the concentration rises above the maximum safe levels.[15] These methods also result in rapid drug level decreases to concentrations that are no longer therapeutic, which can be a result of metabolism, degradation, and transport away from the therapeutic target.[15] Collectively, this results in both wasted drug and material, and increased risk to patients due to reduced therapeutic efficacy as well as potential toxic side effects.[16] To address these issues, approaches for slowing the rate of release were developed.[17] These “sustained release” technologies contained the desired therapeutic in the form of capsules which were generally administered orally, and in some cases formulated for parenteral administration.[5,16] Drug release was dampened through the use of slowly dissolving cellulose coatings, the addition of drug-complexing substances to decrease drug solubility, the use of compressed tablets, as well as the employment of emulsion and suspensions[16] all housed within capsules. Sustained release formulations, however, still were influenced strongly by patient-to-patient variability, environmental effects, and required repeated dosages.[16]
Figure 2.
Schematic representation of drug plasma levels after various dosing regimens.
As an alternative to sustained release, the ideal controlled drug release system offers several advantages. Such delivery materials release drugs at rates that do not change with time (i.e. zero-order release), maintaining release within the therapeutic window and avoiding the inefficiencies of the drug concentration peaks and valleys of conventional formulations (Figure 2). By avoiding “peaks and valleys” and remaining within the therapeutic window, controlled release materials provide the benefit of reducing the total amount of drug required to achieve therapeutic efficacy. By decreasing the number of required doses these materials would also improve patient adherence, which is only 50% in developed nations.[18] By controlling drug release over longer therapeutic windows (i.e., days to years), such materials can also be injected and/or implanted directly within a specific diseased tissue, thereby limiting off-target side effects and increasing potency. In addition to avoiding “peaks and valleys,” controlled release systems must enhance the targeting of drugs to specific tissues and cells within the body to avoid off target effects.[7,19] To enhance tissue specificity, active targeting strategies utilizing affinity ligands on the surface of biomaterials have been employed for specific retention and uptake by diseased tissues and cells.[20] In this approach, ligands that bind to surface molecules or receptors overexpressed in diseased cells and tissues are selected for and conjugated to delivery materials.[21] Materials designed for controlled release should ideally also protect drugs from rapid clearance and/or degradation within the body.
Developing such biomaterials for controlled release is challenging and requires a multidisciplinary approach, incorporating engineers, physical scientists, biologists, and clinicians.[22] Design parameters include: (i) the incorporation of adequate drug within the host material for prolonged release profiles that are required to achieve therapeutic efficacy, (ii) protection of therapeutics from breakdown in vivo while also maintaining biological activity, and (iii) predictable release over the course of the therapeutic regimen, ranging from days to years. Additionally, the materials themselves and their degradation products should be nontoxic and biocompatible within the body, avoiding patient discomfort prior to and following administration. The expense of a particular material-drug formulation, due to the cost of material synthesis and/or fabrication, must also be taken into account during the design phase.
2.2. Biomaterials for Controlled Release of Small Molecules
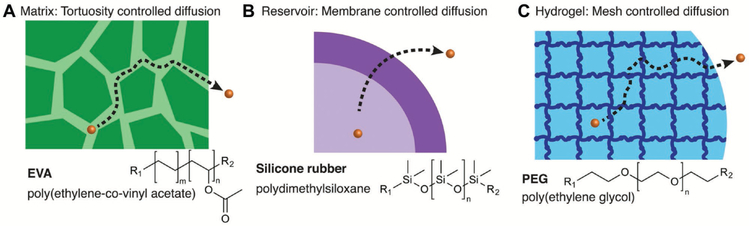
Initial studies describing the incorporation of bioactive molecules into solid polymeric materials for achieving a sustained release profile were conducted in the 1950s and 1960s for agricultural applications.[16] Soon thereafter, polymeric biomaterials as controlled drug release systems for medical applications were pioneered in the 1960–70s.[23] The first reported biomaterial for controlled molecule release was silicon rubber when it was observed that hydrophobic, lipophilic small-molecule (molecular weight < 300 g mol−1) dyes diffused through the wall of silicon tubing (Figure 3).[23d] Given that medical grade silicones are biocompatible and used for implantation for a range of medical applications, this discovery led to the use of silicone rubbers for the controlled release of drugs, including atropine, histamine, anesthetics, steroids, and antimalarial and antischistosomal agents.[23e,f,24] Notably, implanted silicone rubber released drugs over the course of days to months in dogs, rats, and sheep,[23e,24a,b] demonstrating that biomaterials induce controlled release of biologically active agents in the body. These reports suggested that modulating pharmacological actions by controlling drug release from biomaterials could be achieved, ultimately leading to the formation of ALZA in 1968 for the commercialization of some technologies.[23d] This work further led to the development of an early drug delivery system approved by the U.S. Food and Drug Administration (FDA) in 1990, Norplant (now Jadelle), a contraceptive composed of silicone rubber tubes implanted in the forearm that releases levonorgestrel for up to 5 years with pregnancy rates of less than 1% per year.[25] Research within the field of biomaterials, drug delivery, and controlled release accelerated during this period, giving rise to the development of osmotic pumps for oral drug delivery in dogs,[26] drug-loaded hydrogels for ophthalmic drug delivery,[27] polymeric and albumin microsphere-based encapsulation for sustained release of drugs in rats, rabbits, and humans,[28] as well as new mathematical models to quantify drug release from biomaterials.[29] Hydrogels, 3D networks of polymer chains crosslinked to form matrices with high water content, are now widely used in drug delivery and tissue engineering due to their tunable physical, chemical, and biological properties.[30] Broadly speaking, hydrogels demonstrate application in areas such as regenerative medicine.[31] In drug delivery, PEG has been utilized as a “stealth material” that enhances the circulation half-life of drugs, reduces drug accumulation in clearance organs such as the liver, while also enhancing the surface biocompatability of materials.[32] More comprehensive overviews on hydrogels[30,33] as well as the history of bio-materials for drug delivery and controlled release are detailed elsewhere.[23d,34]
Figure 3.
Examples of controlled release platforms. A) The controlled release of macromolecules can be controlled via matrix tortuosity-controlled diffusion. B) Membrane controlled diffusion can be used to control the release of small molecules from materials including silicone rubbers. C) Hydro-gels can also be used for the controlled release of drugs via mesh size and network swelling. Adapted with permission.[3] Copyright 2016, American Chemical Society
2.3. Biomaterials for Controlled Release of Macromolecules
With the emergence of genetic engineering in the 1970s, large-scale production of proteins and other complex macromolecules became a reality. Similar to small-molecule delivery, controlled release of proteins and other macromolecules (i.e., insulin, heparin, enzymes) required the development of new biomaterials or new biomaterial designs. Synthetic materials were required that could ensure the delivery of proteins and macromolecules in unaltered forms to preserve their biological function, while simultaneously providing protection from degradation in vivo. However, silicone and other polymers used by ALZA for small-molecule release, such as ethylene vinyl acetate (EVA) copolymer and poly(hydroxyethlmethacrylate) (p(HEMA)), were impermeable to proteins and other macromolecules.[23d,35] Furthermore, it was largely thought within the controlled release community that proteins and other macromolecules could not be encapsulated and released at controlled rates from polymers.[36] Pioneering work first published in 1976 changed this perspective.[23a] By making solutions of polymer and its solvent (e.g., methylene chloride for EVA) mixed with lyophilized protein, and then evaporating the solvent to induce phase separation of protein from polymer, tortuous networks of interconnected pores were formed within the polymer matrix and thus were freely permeable to water.[23a] When the polymer was exposed to aqueous conditions, proteins and other macromolecules (MW > 1 000 000 g mol−1) embedded within the polymer diffused out of these pores as fluid entered. The narrow constrictions slowed macromolecule release to enable diffusion out of the polymer over a 100 day period (Figure 3).[23a] Biological activity of proteins were largely retained within these polymers, as EVA containing tumor angiogenesis factor and implanted into rabbit corneas induced vessel sprouting from the corneal edge, and grew towards the polymer in every case.[23a] These pioneering technologies led to rapid progress in the fields of biomaterials and drug delivery, with the development of a new generation of polymers which release macromolecules in a controlled manner (Figure 3).
2.4. Evolution of Biomaterials for Drug Delivery
In the following decades came a dramatic expansion of biomaterials development for the controlled release of macromolecules, exploiting diffusion, chemical, swelling, and magnetic-based mechanisms, among others, for controlling the release rates of the incorporated drug (Figure 4).[16] Additionally, observations in the 1960s that phospholipids in aqueous systems can form bilayered structures led to the development of liposomes as the first nanoscale drug carriers in the 1970s.[37] The field then expanded to include dendrimers, micelles, polymeric nanospheres, and inorganic nanomaterials (e.g., gold, silicon, metal, iron oxide) in the burgeoning field of nanotechnology-based drug delivery in subsequent decades.[38] As an alternative to pills and injections, transdermal delivery systems have utilized biomaterials science and microfabrication technology to create drug-containing, biodegradable microneedle patches that painlessly pierce the skin to increase drug permeability, which dissolve and leave no sharp waste after use.[12a,39] More recently, stimuli-responsive, “smart” (also known as “intelligent”) bio-materials have been designed that respond to a range of environmental stimuli (e.g., temperature, pressure, pH, enzymes, glucose), biological signals, or pathological abnormalities for actuating drug release.[13,40] Similarly, new biomaterials have been developed that are remotely trigged by stimuli including visible light, near-infrared (NIR) light, ultrasound, electric currents, and magnetic fields for on demand and pulsatile drug delivery.[41]
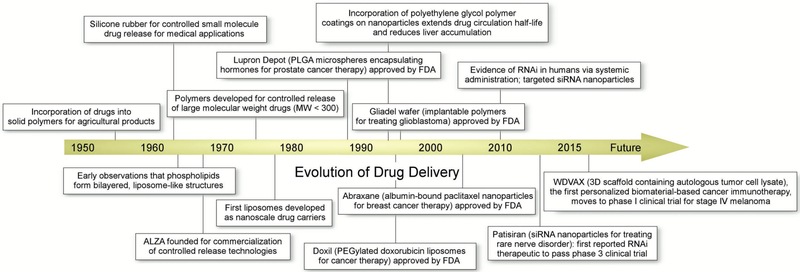
Figure 4.
Timeline representing key moments in the history of biomaterials research.
2.5. Clinical and Commercial Impact of Drug Delivery Materials
Many of these materials have translated into drug delivery systems used in the clinic, and are being commercialized for a range of disease therapies (Table 2).[11] Lupron Depot, a poly(lactic-co-glycolic) acid (PLGA) microsphere formulation encapsulating the hormone leuprolide, was originally approved by the FDA in 1989 for the treatment of advanced prostate cancer, and has since been approved for endometriosis.[42] Lupron Depot has been considered a commercial success, with over $1 billion in annual sales.[43] PLGA, poly(lactic acid) (PLA), and polyglycolic acid (PGA) materials have been utilized in several subsequent FDA approved microparticle depot systems developed by Genentech and Alkermes (Table 2), due to their versatility in tuning material biodegradation time as well as their high biocompatibility arising from their natural degradation products, lactic acid and glycolic acid. Clinically relevant nanoparticles include Doxil, the first FDA approved cancer nanomedicine for the treatment of Kaposi’s sarcoma (approved 1995) and for recurrent ovarian cancer (approved 1998).[6,44] Doxil, a poly(ethylene glycol) (PEG) coated (i.e., PEGylated) liposomal formulation encapsulating the chemo-therapeutic doxorubicin,[45] enhanced circulation half-life and tumor uptake of the drug, while reducing its toxicological profile in patients compared to free drug.[46,96,97] More recently approved nanoparticle formulations include Marqibo, a liposomal formulation encapsulating vincristine FDA approved in 2012 for the treatment of a rare leukemia,[47] and Abraxane an albumin-bound paclitaxel nanoparticle formulation originally approved by the FDA in 2005 for the treatment of breast cancer.[48] An example of a transdermal drug delivery system is Duragesic, a patch containing the opioid fentanyl embedded within an acrylate polymer matrix, which was developed by ALZA and FDA approved in 1990 for chronic pain treatment.[12a] OROS, an osmotically controlled oral drug delivery technology, was also developed by ALZA and has been incorporated into several oral delivery products including Concerta, which has generated over $1 billion in annual sales.[49] Implantable biomaterials used in the clinic include the Gliadel wafer, which consists of dime sized wafers comprised of the chemotherapeutic agent carmustine and a polymer matrix made of poly(carboxyphenoxy-propane/sebacic acid), which are surgically inserted into the brain post-tumor resection.[50] Gliadel wafer was FDA approved in 1996 for use as an adjunct to surgery in patients with recurrent glioblastoma multiforme, and in 2003 was approved for use as a first time treatment, increasing patient survival up to six months in some cases.[51] Collectively, the estimated market for advanced drug delivery systems is anticipated to grow from roughly $178.8 billion in 2015 to nearly $227.3 billion by 2020.[52]
Table 2.
| Type of drug delivery system | Clinically approved drugs |
|---|---|
| Nanoparticles | Abraxane (Paclitaxel), Doxil (Doxorubicin), DaunoXome (Daunorubicin), Marqibo (Vincristine), MEPACT (Mifamurtide), Onivyde MM-398 (Irinotecan), ADYNOVATE (antihemophilic factor (recombinant) PEGylated), Estrasorb (estradiol), AmBisome (amphotericin B), Depocyte (cytarabine), Visudyne (Verteporfin) |
| Microparticle-based depots | Zmax (Azithromycin), Decapeptyl/Trelstar (Triptorelin), Vivitrol (Naltrexone), Arestin (Minocycline), Risperdal/Consta (Risperidone), Sandostatin LAR Depot (Octreotide), Nutropin Depot (Somatropin), Lupron Depot (Leuprolide), DepoCyt (Cytarabine), DepoDur (Morphine), Bydureon (Exenatide), Somatuline LA (Lanreotide), Zoladex (Goselerin), Suprefact Depot (Buselerin), Signifor (Pasireotide) |
| Transdermal materials and devices | Transderm-Scop (Scopolamine), Nitro-Dur (Nitroglycerin), Catapres-TTS (Clonidine), Estraderm (Estradiol), Duragesic (Fentanyl), Androderm (Testosterone), Combipatch (Estradiol with norethindrone), Lidoderm (Lidocaine), Climara Pro (Estradiol with levonorgestrel), Oxytrol (Oxybutynin), Synera (Lidocaine and tetracaine), Daytrana (Methylphenidate), Emsam (Selegiline), Neupro (Rotigotine), Exelon (Rivastigmine), Sancuso (Granisetron), Butrans (Buprenorphine), Ortho Evra (Estradiol and norelgestromin), Qutenza (Capsaicin), Flector (Diclofenac epolamine), NicoDerm/Habitrol/ProStep (Nicotine), Retin-A (Tretinoin), IONSYS (Fentanyl), SonoPrep (Lidocaine via ultrasound), Iontocaine (Lidocaine and epinephrine via iontophoresis), LidoSite (Lidocaine and epinephrine via iontophoresis) |
| Oral | Concerta (Methylphenidate), Ditropan XL (Oxybutynin), Teczem (Enalapril Diltiazem), Dilacor XR (Diltiazem), Covera-HS (Verapamil), DynaCirc CR (Isradipine), Minipress XL (Prazosin), Procardia XL (Nifedipine), Fortamet (Metformin), Altoprev (Lovastatin), Glucotrol XL (Glipizide), Invega (Paliperidone), Tegretol-XL (Carbamazepine), Allegra D (Pseudoephedrine and Fexofenadine), Efidac/24 (Pseudoephedrine and Brompheniramine or Chlorphenir-amine), Volmax (Albuterol), Orenitram (Treprostinil), Sudafed 24 h (Pseudoephedrine), Exalgo (Hydromorphone), Vesanoid (Tretinoin), Syndros (Dronabinol), Venclexta (venetoclax), Farydak (panobinostat), Renagel (Sevelamer) |
| Pulmonary | Tudorza/Pressair (Aclidinium), Proventil HFA (Albuterol), Ventolin HFA (Albuterol), ProAir HFA (Albuterol), Combivent Respimat (Albuterol and ipratropium), DuoNeb (Albuterol and ipratropium), Brovana (Arformoterol), QVAR (Beclomethasone), Pulmicort Flexhaler (Budesonide), Symbicort (Budesonide and Formoterol), Alvesco (Ciclesonide), Breo/Ellipta (Fluticasone and vilanterol), Flovent HFA (Fluticasone), Flovent/Diskus (Fluticasone), Foradil/Aerolizer (Formoterol), Perforomist (Formoterol), Arcapta Neohaler (Indacaterol), Atrovent HFA (Ipratropium), Xopenex HFA (Levalbuterol), Asmanex/Twisthaler (Mometasone), Dulera (Mometasone and Formoterol), Serevent/Diskus (Salmeterol), ADVAIR Diskus (Salmeterol Fluticasone), ADVAIR HFA (Salmeterol Fluticasone), Spiriva/Handihaler (Tiotropium), Cayston (Aztreonam), Ventavis (Iloprost), Tyvaso (Treprostinil), TOBI Podhaler (Tobramycin), Afrezza (human insulin) |
| Implants | Vitrasert (Ganciclovir), Retisert (Fluocinolone), Ozurdex (Dexamethasone), Zoladex (Goserelin), Gliadel (Prolifeprosan and Carmustine), Vantas/Supprelin LA (Histrelin), Viadur (Leuprolide), Nexplanon (Etonogestrel), NuvaRing (Etonogestrel and ethinyl estradiol), Mirena/Norplant (Levonorgestrel), Paragard (Copper) |
3. Strategies, Modifications, and Materials for RNA Delivery In Vitro and In Vivo
3.1. Introduction
Every year, thousands of patients are diagnosed with diseases caused by the misregulation of both intracellular and secreted proteins.[53] Many cancers, for example, are caused by the overexpression of specific oncogenes which results in rapid and uncontrolled cell proliferation.[54] Alternatively, diseases including type I and type II diabetes are characterized by insufficient insulin levels in the bloodstream as a result of cellular resistance and/or the autoimmune response.[55] Other diseases, including cystic fibrosis, are characterized by the production of proteins of incorrect structure, a problem that originates at the genetic level in affected patients.[56] In short, aberrant protein production is a hallmark of many diseases found in medical cases around the globe.
Given this commonality, scientists and medical professionals alike often treat disease by administering therapeutic molecules (i.e., drugs) into the body that can regulate gene expression. In the most traditional sense, this process has been achieved by administering either small-molecule or protein-based drugs.[57] Small-molecule-based drugs can enter target cells and often act by inhibiting specific proteins through competitive binding; however, small molecules can readily accumulate in off-target tissues and are often poorly soluble. Moreover, only an estimated 2–5% of proteins in the body can be inhibited utilizing this mechanism of action; this implies that the majority of the human genome is “undruggable.”[58] Protein therapeutics, by contrast, offer increased specificity for their molecular targets or replace defective and/or missing proteins. However, it can be difficult to deliver exogenous proteins into the cytoplasm of target cells, and stability as well as size concerns with protein therapeutics can limit their application.[57]
To overcome these limitations, ribonucleic acids (RNAs) have been proposed as an alternative class of therapeutic molecules. RNAs are a promising class of drug candidates because they can endogenously regulate protein concentrations within target cells in vivo.[59] Short interfering RNAs (siRNAs), antisense oliognucleotides (ASOs), and microRNAs (miRNAs), for example, can silence specific genes to decrease protein concentrations;[60] messenger RNAs (mRNAs), by contrast, can be translated by ribosomes to upregulate protein concentrations within target cells (which, in turn, can also be secreted into the bloodstream);[61] finally, combinations of hybrid RNAs (such as sgRNA with the CRISPR/Cas9 gene editing system) can alter DNA at the molecular level to correct defective genes.[62] In short, RNAs can target both the druggable and undruggble parts of the human genome, ultimately serving as a new therapeutic paradigm inspired by the central dogma of biology.[63]
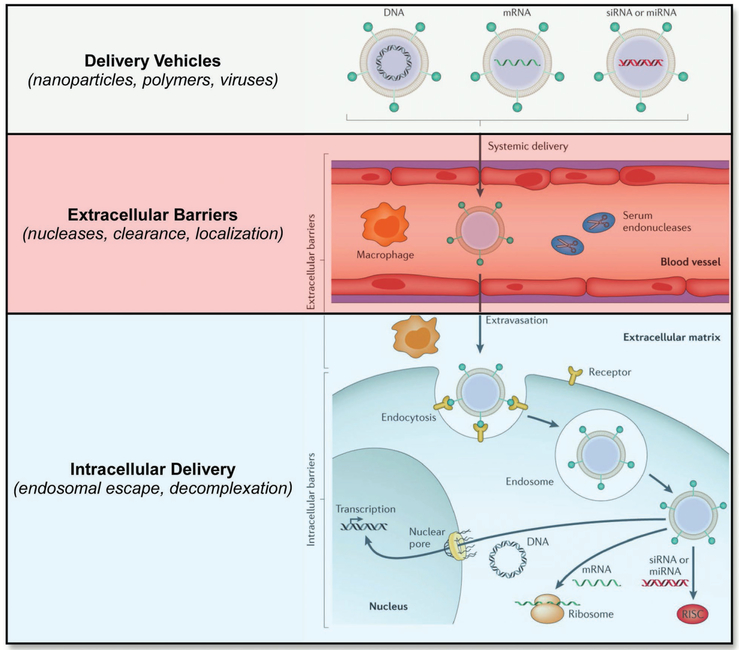
But if RNAs boast so much therapeutic potential, why are there still so few RNA-based drugs currently available on the market? Indeed, it has been known since 1990 that exogenously administered RNAs can alter protein expression in vivo, yet the number of small-molecule and protein-based drugs vastly outnumbers those of RNA origin.[59b] The answer to this question lies at least in part due to extracellular and intracellular barriers associated with therapeutic RNA administration (Figure 5). When administered systemically, for example, RNAs trigger a similar immune response to that of invading pathogens given their similarity in molecular structure.[64] Additionally, RNAs are prone to degradation in the bloodstream due to chemical instability as well as the presence of circulating nucleases.[62] Upon exiting the bloodstream, RNAs must then navigate a complex extracellular matrix (ECM) and localize to target cells. Once there, the RNAs must enter into the cytoplasm or nucleus, a problem that is made challenging due to the fact that large, anionic biomolecules do not readily traverse the cellular membrane and can instead become entrapped within endosomal compartments.[65] It is also important to note that these same intracellular barriers apply to the local delivery of RNAs given that they must access the cytoplasm or nucleus of target cells for therapeutic benefit. The combination of these physiological barriers, in addition to the difficultly in sequence selection of therapeutic RNAs, has thus far limited their clinical translation and demands our attention.
Figure 5.
Delivery barriers to RNA delivery. Adapted with permission.[62] Copyright 2014, Macmillan Publishers Limited, part of Springer Nature.
In this section, we will delineate some of the emerging strategies and materials that aim to address the challenges associated with RNA delivery in vivo. We will begin by highlighting approaches that improve both RNA stability and cellular internalization. We will then focus our conversation on strategies to entrap and protect RNAs, paying particular attention to the material classes that improve the potency and biodistribution of RNA therapeutics. Our aim with this section, therefore, is to not only highlight some of the challenges of RNA delivery in vivo, but also to further establish RNA therapeutics as an emerging platform for the treatment of human disease.
3.2. RNA Modification Strategies
In using RNAs therapeutically, one approach is to transfect target cell populations in vitro with naked, unmodified RNAs. This process is inefficient because the charge density, size, and hydrophilicity of nucleic acids prevent efficient translocation of RNAs across cellular membranes.[60] To combat these inherent limitations, advances in electroporation,[66] microinjection,[67] sonoporation, laser irradiation, and hydrostatic pressure transfection have improved RNA transfection.[68] These advances have enabled scientists to explore the role that individual RNAs have in altering cell behaviors in vitro by silencing genes and upregulating the concentration of encoded proteins in a dose dependent and time controlled manner.
However, the instability and immunogenicity of naked, unmodified RNAs limits their efficacy when therapeutically administered in vivo. The human body is replete with mechanisms to prevent exogenous RNAs from entering target cells—circulating nucleases in the blood stream, for example, can degrade systemically administered RNAs.[69] Additionally, pattern recognition mechanisms, including toll like receptors, associate exogenous RNA with pathogens thereby inducing an immune response. Another underlying issue is that nonspecific tissue accumulation can limit the targeting of specific organs (and in turn, specific cell populations).[62]
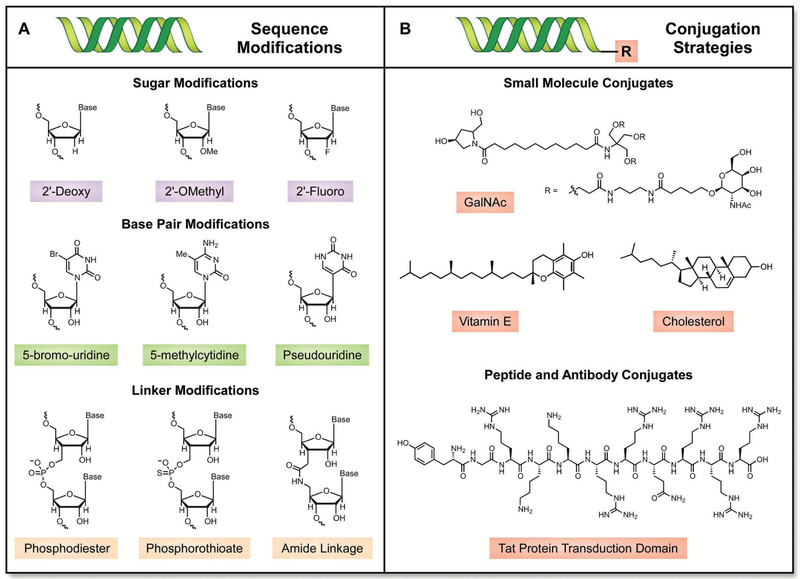
One strategy to overcome these physiological barriers is to alter RNA sequences with chemically modified sugars and linkers (Figure 6A).[70] Sugar and linker modification strategies are best suited for short RNAs that can be synthesized via established oligonucleotide synthesis techniques; longer RNAs (such as mRNA) are traditionally produced using in vitro transcription and are, accordingly, more difficult to modify in this fashion. The most common sugar modifications employed in RNA therapeutics involve substituting the endogenous 2′-hydroxy group with a 2′-fluoro, 2′-O-methyl, or 2′-deoxy substituent;[71] common linker modifications replace endogenous phosphodiester bonds with phosphorothioate or amide-based linkages.[72] By contrast, base pair modification strategies can be used for both short and long RNAs; 5-bromo-uridine, 5-methylcytidine, and pseudouridine have all been incorporated into potential RNA therapeutics.[73] It should be noted, however, that base pair modifications within siRNAs and ASOs are generally better tolerated than those found in mRNAs.[64b,74] This is because even slight modifications in mRNA structure can alter ribosomal translation, whereas chemically modified siRNAs and ASOs remain active.[75] Instead, variations in the untranslated region, 5′ caps, and polyadenylated 3′ tails are more commonly employed in potential mRNA therapeutics.[61a,76] To date, the examples that best exemplify the power of RNA modification involve four ASOs that have been clinically approved. These ASOs, which include mipomersen for hypercholesterolemia,[77] eteplirsen for Duchenne muscular dystrophy,[78] nusinersen for spinal muscular atrophy,[79] and fomivirsen for ocular cytomegalovirus,[80] are all clinically approved and contain some form of chemical modification within their RNA backbone.
Figure 6.
A) Common sugar, base pair, and linker modifications used in RNA delivery. B) Representative chemical ligands used for direct conjugation strategies to RNAs.
The direct conjugation of RNAs with molecular ligands represents yet another strategy to improve nucleic acid delivery in vivo (Figure 6B). While direct conjugation strategies may improve the pharmacokinetic properties of a given RNA sequence, they can also have a pronounced effect on therapeutic targeting of specific organs (due in part to receptor-mediated endocytosis). For example, an array of molecular targeting ligands including vitamin E,[81] GalNAc,[82] cholesterol,[83] cell-penetrating peptides,[84] and antibodies[85] have been directly appended to RNAs for therapeutic investigation. Although they hold promise for all RNA therapeutics, direct conjugation approaches are frequently explored for applications involving siRNAs. Unlike mRNAs and ASOs, siRNAs are duplexed, and only the antisense strand binds to the RISC complex and induces RNA interference.[65a] Accordingly, the sense strand can be readily modified with a targeting ligand without significantly interfering with the silencing potential of the siRNA. To date, siRNAs modified with GalNAc, a complex galactose derivative, are one of the most pronounced success stories of RNA conjugation—following subcutaneous administration, siRNA GalNAc conjugates can induce silencing in the liver without the need for a delivery vector with a median effective dose of ≈1 mg kg−1 in mice.[86]
As a concluding thought, it should be noted that RNA modification strategies are specific to both sequence and application. Given that RNAs vary in size, molecular architecture, and their routes of synthesis, RNA modification strategies are inherently difficult to generalize—in short, what works for siRNAs may not work for ASOs nor mRNAs, with the same holding true in reverse. Nevertheless, RNA modifications have to date yielded the highest number of clinically validated drugs, and ongoing efforts will continue to utilize this strategy to inspire new solutions to delivery barriers associated with RNA therapeutics.
3.3. RNA Complexation Strategies
Whereas the success of RNA modification strategies is heavily dependent on RNA identity, RNA complexation strategies are more generalizable in nature.[60,62,87] While RNAs are structurally dissimilar in many ways, they share at least one common parameter—anionicity. Electrostatic complexation, the process by which cationic delivery materials can condense anionic RNAs, can therefore serve as a general mechanistic paradigm for protecting RNAs from degradation while simultaneously improving circulation time, stability, and cellular uptake (Figure 7).[61a,65a] Although viruses (such as adeno-associated viruses) have also been used to deliver RNAs via complexation strategies, their use has been extensively reviewed elsewhere and will not be of focus here.[88] Instead, we will highlight major subclasses of nonviral delivery vectors that have been developed over the years.
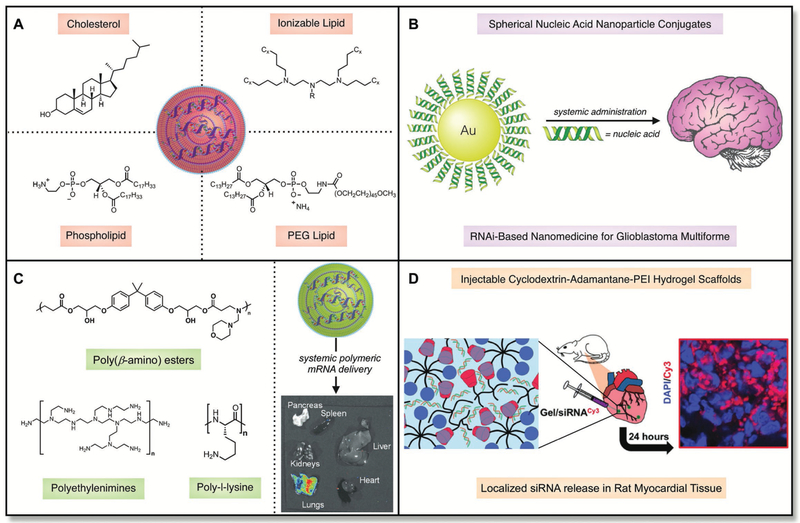
Figure 7.
A) In addition to RNA, lipid nanoparticles consist of four primary components—cholesterol, a phospholipid, a lipid anchored poly(ethylene glycol) derivatie, and an ionizable lipid. B) Spherical nucleic acids have been developed that can deliver RNA therapeutically to the brain following systemic administration. C) Polymer nanoparticles have been developed that can deliver RNAs to the lungs. Adapted with permission.[108b] Copyright 2016, WILEY-VCH. D) Injectable hydrogels have been used to localize siRNAs to the myocardium in mice. Adapted with permission.[109] Copyright 2017, American Chemical Society.
Cationic lipids are small-molecule-based systems that were originally employed for DNA delivery and have since been explored for RNA administration.[89] From a structural standpoint, cationic lipids consist of polar amine cores that have been covalently modified with nonpolar hydrophobic tails.[65a,90] The amine cores can either contain permanently cationic centers (quaternary ammonium salts) or amine cores that can be reversibly protonated (ionizable amines). In general, ionizable amines demonstrate improved toxicity profiles relative to quaternary ammonium salts. Several commercially available and proprietary cationic lipids including Lipofectamine, MegaFectin, and TransIT are widely used for the delivery of RNAs via the formation of lipoplexes;[91] other lipids including 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) also complex into lipoplexes and have been used to deliver RNAs.[92] Although the potency of these materials can be limited in vivo, their ease of access to the general scientific community makes them attractive RNA delivery materials.
To improve the potency of lipoplexes, additional excipients can be coformulated alongside the cationic lipid to form lipid nano-particles (LNPs) (Figure 7A).[93] LNPs are composite supramolecular materials consisting of four primary components in addition to the nucleic acid: i) cholesterol (fluidizes the membrane),[46a,94] ii) lipid anchored poly(ethylene glycol) (decreases nonspecific uptake and aggregation),[95] iii) a phospholipid (modifies bilayer structure),[65a,96] and iv) an ionizable/cationic lipid (complexes the RNA and improves endosomal escape).[97] LNP efficacy and bio-distribution can be tailored in vivo by either modulating the ratio of these four components or by designing and synthesizing new ionizable/cationic lipids.[93a,b,98] Current advances have created thousands of ionizable lipid materials by employing both rational design and combinatorial strategies.[99] The most potent ionizable lipid materials discovered thus far for the in vivo delivery of nucleic acids include DLinDMA,[97b] C12–200,[100] 503O13,[93c] OF-02,[93b] and OF-Deg-Lin.[98] These materials traditionally incite biological responses in the liver or the spleen of mice when administering siRNA or mRNA cargoes. Nonlipid-based nanoparticles have also been explored as therapeutic delivery options, including those derived from gold (Figure 7B).[101] These particles have demonstrated potency in the brain and can also reverse impaired wound healing. Interestingly, these gold siRNA nanoparticles can also be administered topically for gene regulation.[102]
Polymeric materials also serve as versatile foundations for RNA delivery (Figure 7C).[62,103] Natural and naturally derived polymers including chitosan (consists of repeating units of N-acetyl-d-glucosamine and d-glucosamine subunits), polyaspartamide (consists of repeating units of aspartamide), and poly-l-lysine (consists of repeating units of lysine) can condense and deliver RNAs.[104] It is important to note that these materials all consist of subunits that can be protonated under acidic conditions. This protonation serves as the driving force for electrostatic complexation and may also aid in endosomal escape.[105] Synthetic materials, including those derived from polyethylenimine (a water-soluble polymer that can exist in linear, branched, and dendritic forms), have also been synthesized.[106] JetPEI is a commercially available version of PEI that has been used for the in vivo delivery of nucleic acids; one drawback, however, is that JetPEI does have toxicity and repeat dosing concerns due to its nondegradable chemical structure.[92] PEIs and dendrimers have also been synthetically modified with aliphatic tails to improve their potency and deliver nucleic acid cargos to the lungs;[107] however, they still remain nondegradable, which could be a concern for long term use. As a degradable alternative, poly(beta-amino esters) have been developed—these materials are traditionally synthesized via the condensation of polyamine small molecules with diacrylates, both of which are commercially available.[108] Finally, polymer-based hydrogel scaffolds have also been explored for the controlled delivery of nucleic acids. Burdick and co-workers, for example, have recently demonstrated that a polyethylenimine/poly(ethylene glycol) host–guest hydrogel can be used for local siRNA delivery (Figure 7D).[109] Artzi and co-workers have also shown that RNA-triple-helix hydrogels can be used to locally modulate endogenous miRNA expression in cancer models.[110] Moreover, Forbes and Peppas have also demonstrated delivery of RNA to murine macrophages, and interest still resides in the development of oral delivery systems for RNAs.[111]
To summarize, it should be noted that hybridized approaches, that is, approaches that use delivery materials to complex modified RNAs, are extremely common. In ongoing clinical trials for RNA therapeutics, for example, this hybridized strategy has been employed to mitigate immunogenicity, increase stability, promote cellular uptake, and improve the potency of the therapeutic. Advances in these areas will continue to shape RNA delivery and help further establish the therapeutic potential of this field.
3.4. Remaining Questions, Emerging Leads, and Future Perspectives
The field of RNA delivery is replete with detailed studies, emerging leads, and innovative materials designs. Unique chemical modifications and delivery vectors have ushered in an age where we can affect biological processes in vivo using exogenously delivered RNAs, and these advances are impacting the pharmaceutical market in real time. Indeed, Alynlam pharmaceuticals recently announced that their drug Patisiran, an RNAi-based therapy for the treatment of ATTR amyloidosis with polyneuropathy, successfully passed phase III clinical trials. Importantly, this result should help pave the way for additional RNAi based therapeutics as this is the first drug of its class to successfully reach this endpoint.
Nevertheless, as researchers in the field answer ever more questions, new areas of interest continue to emerge. For example, many recent efforts to codeliver RNAs for CRISPRCas9 have been undertaken—these approaches are challenging because multiple types of RNAs must be entrapped within the same particle, complicating formulation strategies.[61b] More-over, other work in this area has demonstrated the potential benefit of using viral and nonviral delivery vectors in tandem to induce gene editing in vivo.[112] Recent advances in structure guided chemical modifications of guide RNAs has also enabled gene editing using exclusively nonviral vectors.[113] Still others are focused on answering questions surrounding both mechanism of action of RNA based drugs as well as how these molecules interact with the immune system.
In short, the early pioneering work in RNA therapy serves as a tremendous platform for current research. Breakthroughs from chemists, physicists, biologists, engineers, and medical professionals alike have helped lay the foundation for both current and future studies. With continued effort and interest, therefore, breakthroughs in targeting the genome with RNA therapeutics will continue, helping to establish this field as a new therapeutic paradigm for the treatment of human disease.
4. Bioresponsive Polymers: From Design to Implementation
4.1. Introduction
From a drug delivery standpoint, an ideal therapeutic would treat or cure a disease without causing any side effects.[8] Despite advancements within medicine and science, however, we are still far from realizing this goal. Many chemotherapeutics, for example, kill both cancerous and healthy cell populations.[114] This is because these medications are preferentially taken up into rapidly dividing cells, a physiology that exists in both diseased and healthy tissues.[115] As a result, patients suffer from nausea, hair loss, fatigue, and in almost all cases, a temporary reduction in quality of life.[116]
To address these issues, scientists and medical professionals alike aim to improve upon the precision of therapeutics.[18] In an ideal world, a completely “precise” medication would be one that can control the amount of administered drug, in both space and time, exclusively to diseased cell populations. Although many strategies to achieve therapeutic precision exist, a major area of biomaterials research involves entrapping drugs within “triggerable” materials.[117] Under physiological conditions, a triggerable material might simply act as a noneluting drug reservoir. Yet, upon exposure to altered physiological conditions within the body, such materials can respond to physiological cues and ultimately release their drug cargo into the surrounding environment to treat disease.[118] These materials can therefore serve as a general platform for improving the precision of therapeutics, independent of the target of interest.
In this section, we will delineate select advances that have helped to establish “triggerable” systems as biomaterials. We discuss how to best design these systems, covering areas ranging from synthesis to formulation, as well as how to make these materials function properly within living organisms. After a brief discussion surrounding what makes polymers ideal platform materials for responsive applications, we will then transition to specific “triggers” that have been exploited within the body. We will then conclude with further thoughts to address the future of bioresponsive materials.
4.2. Polymers—an Ideal Platform for Responsive Biomaterials
For biomedical applications, several classes of materials are regularly employed due to their overarching material properties. Metals, for example, exhibit high conductivity, malleability, and excellent wear properties.[119] As a result, metals are used in wide array of medical devices ranging from pace makers to joint replacements. By contrast, ceramics are less conductive and have high strength.[120] This set of properties makes ceramics ideal base materials for applications in dental restoration, ranging from veneers to crowns to onlays.[121] While metals and ceramics are well suited for many applications, they are perhaps not an ideal choice to create “responsive” materials. This is because the fundamental chemistry of metals and ceramics can be difficult to tune; as a result, it can be difficult to incorporate specific “triggers” into metal and ceramic-based materials that will respond to physiological cues in their immediate environment.
Polymers are one class of materials suitable for addressing the limitations posed by metals and ceramics in creating “responsive” materials.[122] Broadly defined, polymers are molecules consisting of repeat units of individual monomers.[123] Interestingly, polymers are found in both living systems and nonbiological areas. Proteins, for example, consist of repeat units of amino acids;[124] alternatively, plastic bags consist of polyethylene, a hydrophobic and readily processable material that also finds use in pipes, electrical wires, and joint replacements.[125] This broad applicability of polymers stems from the fact that they are, generally speaking, readily tunable from a chemical standpoint. For example, the molecular weight of polymers can be controlled via monomer stoichiometry using controlled polymerization strategies including ATRP,[126] RAFT,[127] NMO,[128] and ROMP;[129] their melting temperature, by contrast, can be modified by incorporating one or more exogenous monomers into the polymerization mixture;[123] finally, postpolymerization modifications can transform functional groups on the surface of reactive polymers into different molecular structures.[130] In short, polymers are a versatile class of materials that are ubiquitous in the modern world.
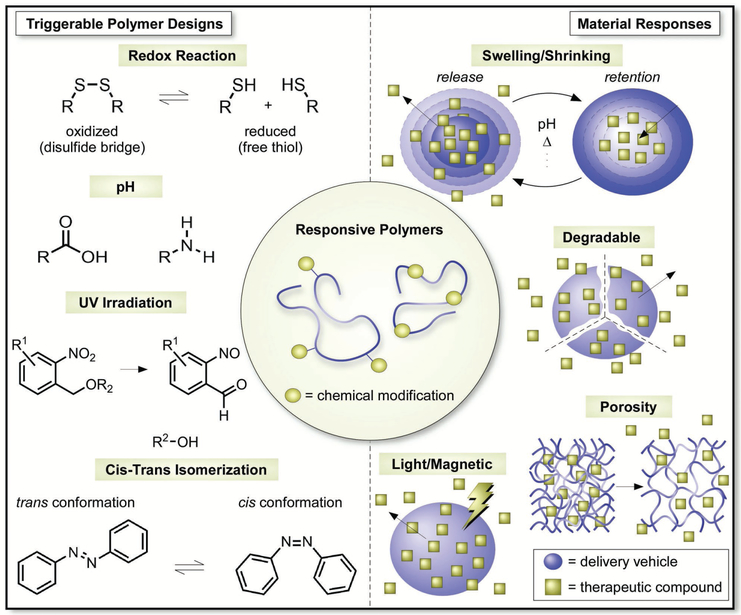
Beyond their chemical tunability, an additional parameter that makes polymers a strong candidate for responsive materials is that they can be formulated with drugs to control release.[13] Since the 1960s, polymers have been used for controlled release applications involving small and large molecular therapeutic cargos.[34b,131] From a mechanistic standpoint, these controlled release materials operate via one of several mechanisms (Figure 8).[3] In degradable systems, for example, the drug is released through pores; in erodible systems, by contrast, the drug elutes as the surface degrades. Osmotic pumps respond to changes in osmotic gradients and release their cargo through pre-existing holes. Finally, hydrogels, matrices, and reservoirs can control drug delivery via Fickian or non-Fickian diffusion, often times controlled by the mesh size of the base material. It should be noted here as well that many of these systems have been implemented in living systems, and accordingly, many polymeric materials have been developed that are fully biocompatible.
Figure 8.
The design of “triggerable” materials that respond to environmental stimuli for the temporally and spatially controlled delivery of therapeutics.
Building upon this strong foundational platform, great interest now resides in creating versions of these materials that are bioresponsive.[34a] For the purposes of this discussion, a bioresponsive material will be defined as one that can respond to a specific “trigger” inside or outside of the human body. Given that the body is replete with unique pathologies (pH gradients, temperatures, enzymes, small molecules, etc.) scientists and medical professionals are now creating materials that will respond to physiological alterations in both space and time. Here, we aim to highlight this work by identifying specific classes of trigger-responsive polymers. Of note, we pay particular attention to functional group combinations that impart these responsive properties, and we also delineate select applications for which each of these polymer classes have been explored. In doing so, we hope to highlight select work that has been conducted thus far and inspire future discussion surrounding the milestone area of biomaterials research.
4.3. Triggerable Classes of Polymers for Biomaterials Applications
To date, polymers that can respond to a number of different triggers have been developed and explored for biomaterial applications.[8,131a,132] It is important to note that these triggers include chemical, biological, and physical stimuli.[13] Whereas many chemical and biological stimuli often occur within the body, those of physical origin are often external to the body and can be used to prompt drug delivery remotely. The aim for each of these systems is to improve the precision of drugs, as well as to improve patient quality of life. Below, we frame our discussion by identifying specific classes of responsive polymers and subsequently describe their use for biomaterials applications.
4.3.1. Redox-Sensitive Polymers
The human body consists of compartmentalized regions of differing redox potential.[13] The reducing agent glutathione, for example, is found at a concentration two to three orders of magnitude larger within cells than outside of them.[133] Contrastingly, oxidizing agents that include hydrogen peroxide are associated with tissue inflammation and injury.[134] These differences in redox potential between a local tissue/cellular environment and their surroundings present an opportunity to create bioresponsive materials that are triggered via oxidation or reduction within the body (Figure 9A).
Figure 9.
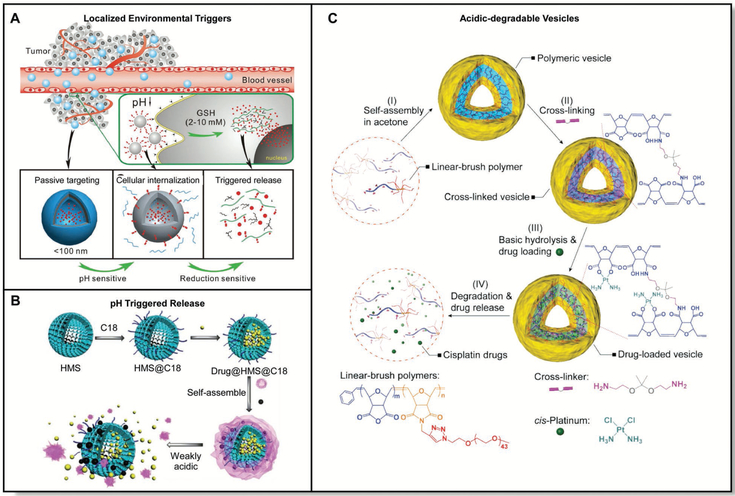
A) Localized regions throughout diseased tissue can be exploited for selective uptake of polymer vesicles and triggers for drug delivery. Adapted with permission.[148] Copyright 2014, Royal Society of Chemistry. B) Controlled release of anticancer therapeutics from nanoparticles due to localized weakly acidic pH conditions. Adapted with permission.[146] Copyright 2012, Royal Society of Chemistry. C) Acid-degradable polymers for the release of anticancer drugs. Adapted with permission.[147] Copyright 2015, Royal Society of Chemistry.
In order to respond to reduction triggers within the body, materials derived from disulfides are commonly employed.[135] Disulfide-based materials are frequently used as bioresponsive materials because disulfide bridges can be reduced under mild conditions to afford dithiol analogues. Within the cell, this process is most commonly mediated by glutathione, a tripeptide consisting of glycine, cysteine, and glutamic acid. To date, dilsufide based materials have been exploited for applications ranging from protein delivery to gene expression, among others.[136] Importantly, disulfide/dithiol interchange is a reversible chemical reaction which can be important for biomedical applications.
Interestingly, many sulfur-based materials have also been developed to respond to oxidation triggers. Sulfur is a unique atom in that it can exist in multiple oxidation states; accordingly, sulfur based materials including block copolymers have been prepared for applications in areas such as gene delivery.[137] Alternatively, materials derived from boronic acids/esters have also been developed to respond to oxidation triggers.[138] In the presence of oxidizing agents such as hydrogen peroxide, boronic acids/esters can be converted into the corresponding alcohol. This chemical process has been exploited for triggered protein release applications using dextran as a base material, among others.
Finally, materials that can respond to both oxidation and reduction triggers have also been explored. One of the most common functional group motifs used for these dual activation materials are diselenides. Diselenides are similar in chemical structure to disulfides and have also been incorporated into responsive polymers.[139] Unlike disulfide materials, however, diselenides are sensitive to both oxidation and reduction, which allows for alternative triggers within nanobiotechnology applications.[140]
4.3.2. pH-Responsive Polymers
Many tissues, fluids, and organelles within the human body contain different pH values. For example, the stomach, the vagina, and lysosomes naturally exist at acidic pHs (<7). Alternatively, many others exist at neutral or near-neutral pHs including the ocular surface (7.1), the blood (≈7.4), and bile (7.8).[13] Moreover, pH gradients exist across many organ barriers, and many disease pathologies such as the tumor microenvironment exhibit different pHs relative to those in a healthy tissue.[141] Accordingly, one strategy to improve the efficacy and precision of therapeutic molecules involves the design of polymeric drug delivery systems that can respond to specific pHs.
As a general strategy to create pH-sensitive materials, it is common to incorporate chemical functional groups that can be protonated or deprotonated within polymeric matrices.[142] For example, amine containing polymers including those derived from dimethylaminoethylmethacrylate are protonated under acidic conditions to yield reversibly cationic materials.[143] By contrast, carboxylate containing polymers including poly(acrylic acid) are deprotonated under basic conditions to afford anionic matrices. Given that the charge of these polymers can be readily altered, materials derived from these polymers can respond to pH changes by swelling, degrading, shrinking, or dissociating.[131a] In doing so, these materials can release their drug cargo in a pH-responsive fashion within target tissues and organs in the body. To date, pH responsive materials have been used for a variety of applications including nucleic acid delivery, doxorubicin delivery, and taste masking, among others.[106,144]
One specific area where pH-responsive materials have improved therapeutic targeting is in the treatment of tumors. The tumor microenvironment often exists at a lower pH (≈5.7) than its surroundings (≈6.8–7) due to localized acidosis.[145] Given this difference, multifunctional acid sensitive nano-composites have been explored for the controlled release of anticancer drugs (Figure 9B).[146] Importantly, these materials were also functionalized with folic acid, improving the targeting of these materials to overexpressed folic acid receptors on the cancer cell surface. Moreover, a similar concept has been employed for materials incorporating acid-sensitive diaminoketal cross links, and drug-laden versions of these materials have demonstrated increased cellular uptake relative to that observed for the free drug alone (Figure 9C).[147] Finally, acid responsive poly(ethylene glycol) derivatives have also been designed for the controlled release of therapeutics using hydra-zine chemistry, and tumor targeting with pH-responsive materials continues to be an area of interest to the drug delivery community (Figure 10A).[148]
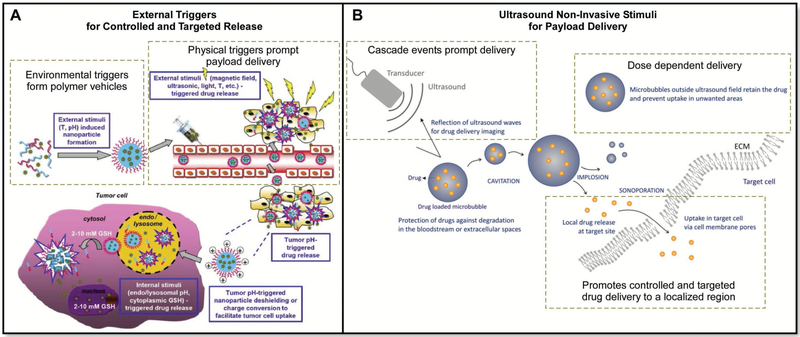
Figure 10.
A) Targeting tumor cells with pH responsive materials. Adapted with permission.[148] Copyright 2014, Royal Society of Chemistry. B) Delivering a payload to a localized area of the body using noninvasive ultrasound to trigger release from microbubbles or nanoparticles. Adapted with permission.[160a] Copyright 2012, Elsevier.
4.3.3. Hydrolysis and Enzymatically Responsive Polymers
Hydrolysis-sensitive polymeric materials have also been designed, synthesized, and implemented in vivo for drug delivery purposes. Hydrolysis prone materials by definition can be degraded by water, a trigger that is ubiquitous in the human body. This degradative process most commonly occurs through the nucleophilic addition of water into an electrophilic functional group on a polymer. Commonly employed electrophilic functional groups on polymers include esters and anhydrides, each of which have been employed in multiple types of responsive materials.[149] The Gliadel wafer is one example product on the market that demonstrates the power of hydrolysis-sensitive materials for drug delivery.[51] Consisting of the chemotherapeutic Carmustine impregnated within a polyanhydride material, the Gliadel wafer can be implanted into brain tumors for the controlled release of a chemotherapeutic to malignant gliomas. Of note, the Gliadel wafer improves the 6 month survival rate of patients diagnosed with glioblastoma multiforme.[51]
Enzyme-responsive polymers have also been developed for drug delivery. The concentrations of specific enzymes including matrix metalloproteins, hyaluronidases, phospholipases, and prostate specific antigen can deviate from normal values in association with specific disease pathologies.[13] Accordingly, many enzyme-responsive polymer systems have been developed, with applications ranging from tumor imaging, to doxorubicin delivery, and minimizing inflammation in the colon, among others.[150]
4.3.4. Temperature-Responsive Polymers
Temperature-sensitive polymers can also be used for drug delivery purposes.[151] The human body resides at a temperature of 37 °C; by contrast, ambient temperature is ≈25 °C. To take advantage of this difference, polymer systems that flow at room temperature but gel at body temperature have been developed—these materials are predominantly used for local delivery applications, capitalizing on the sol-gel transition of specific polymers. Many base materials have been used for temperature responsive polymer development including poloxamers, poly(N-alkylacrylamides), poly(N-vinylcaprolactams), cellulose, xyloglucan, and chitosan. Of note, the material properties of thermoresponsive polymers can be modulated by employing one or more of several different strategies.[152] These strategies include varying the ratio of monomers, end-group modifications, and postpolymerization modifications. Each of these strategies has afforded temperature-responsive polymers for varied biomaterials applications.[131a,142,152b,153]
4.3.5. Magnetic-Responsive Polymers
Magnetic pulsing techniques serve as yet another “trigger” for controlling the release of drugs from responsive materials.[41f,154] This concept has been extended to designing systems to release compounds to specific organs by pairing therapeutic treatment with drug-loaded polymers and magnetic resonance imaging (MRI) techniques.[41f] Select examples include: i) the systematic release of dopamine from alginates impregnated with magnetic beads; ii) targeted plasmid delivery to the lung using chitosan nanoparticles; and iii) insulin delivery, among others.[155] Magnetic “triggers” have also been combined with pH-responsive materials to afford dual responsive drug delivery systems.[156] The combination of two or more environmental responses in a single material can be highly advantageous. For example, if one were to include magnetic particles within a polymer that was designed to degrade in highly acidic conditions, then one could use MRI imaging to pinpoint the exact location that the drug was delivered upon dispersion of the particles within, for instance, the stomach.
An added benefit to incorporating magnetic material within a delivery nanoparticle is that it can double as a retrieval method. When designing any material or drug that will be implanted in a patient, it is important to establish a contingency plan. In case of an undesired immune response or rejection, for both molecular chemicals and living tissue alike, being able to remove the injected or implanted material is crucial. Having a magnetic system allows for the material to be more easily removed, especially in a self-circulating system (e.g., the blood stream or intraperitoneal spaces). Accounting for these factors into a drug–polymer design broadens the project scope and challenges interdisciplinary research in order to achieve a unified engineered material. It is also important to note that some magnetic responsive systems have been approved by the FDA.[157]
4.3.6. Acoustic-Responsive Polymers
Another way to stimulate the release of a material’s payload is with acoustics.[41g,h,158] Material properties have been altered and optimized to release growth promoting molecules from acoustically responsive scaffolds using a megahertz-range ultra-sound system responsive polymer.[159] These designs permit the release of a payload through noninvasive techniques, wherein fibrin scaffolds were impregnated with a payload. In order to control the release of the drug from the polymer scaffold, a double-layer emulsion was created using a microfluidic device for a tiered delivery system via a sonosensitive emulsion. Broader designs can also be used, including microbubbles with drug dissolved in the fluid and a range of nanoparticle designs (Figure 10B).[160]
4.3.7. Light-Responsive Polymers
An alternative method for external stimulation of drug delivery has been through the use of noninvasive and painless techniques including light-stimulated therapies.[161] The ease by which drugs can be delivered by light stimulation has been a major motivation for the design of systems to respond to this style of noninvasive trigger. Light stimulation drug delivery has been desirable due to the controlled spatial and temporal release of a therapeutic payload with both UV- and visible-wavelength irradiation. This technique provides a remote-activated approach that does not require direct patient contact.[162] Current challenges associated with light activated controlled drug release include the distance of the polymer vehicle from the light source, the density of native host tissue that the light has to penetrate to reach the delivery vehicle, and the potential for drug molecule degradation upon exposure to light.
One underlying mechanism of light-induced drug delivery involves a shift in molecular conformation including cis-trans isomerization and ring opening reactions.[163] This technology has been used to target melanoma cells through the release of drugs from a light-responsive azobenzene modified amphiphilic block copolymer.[164] Upon irraditaion, the conformation of the azobenzene switches, thereby altering the self-assembling structures and releasing the payload.
4.3.8. Electrically Responsive Polymers
Electrically responsive polymers represent yet another class of tunable materials for biomaterials applications.[165] The human body is replete with electrical stimuli; for example, neurons transmit information via electrical signals.[166] To directly interface with these cell populations and for other forms of orthogonal drug delivery in the body, different classes of electrically responsive polymers have been developed. From a chemical standpoint, electrically responsive materials tend to be highly conjugated aromatic systems.[167] Polypyrrole, for example, has been used extensively as a base material for electronic applications and the biocompatibility of polypyrrole nanoparticles has been studied in mice.[168] To date, electrically responsive polymers have been used for an array of biomaterials applications including controlled drug release, and have also been used in tandem with temperature responsive systems to form dual responsive materials, among others.[169]
4.3.9. Swelling and Contracting Polymers
Certain polymers have been designed to swell or shrink in response to an external stimuli.[10c,122c,170] Changes in porosity can result from leaching of ionic cross-linking molecules, which in turn alters the diffusion pathways for sensing molecules. Alginate is a commonly employed polymer that is isolated from seaweed and is relatively biocompatible. Tuning the spatial and temporal release of encapsulated materials is rather challenging, but has been successfully applied for a variety of applications using alginates. A recent example includes the sustained delivery of vascular endothelial growth factor (VEGF) and subsequent analogues from alginate to a localized region within the body. Using an injectable alginate design, the controlled release of VEGF was utilized to promote lymphatic vessel development through improved vascularization.[171] In general, these hybrid designs have the potential to create future generations of materials for the paralleled delivery of therapeutics, regional specific sensing, and secondary responses for noninvasive detection.
4.4. Concluding Thoughts and Future Directions
We are currently in the midst of a global acceleration within the field of drug delivery. The development, formulation, and engineering of next-generation therapeutics is already underway. Researchers are actively paralleling material design and synthesis to entrap novel drug discoveries, which are working to meet clinical demands. What remains challenging is the design of polymer libraries that will remain broadly applicable to chemical, biological, and physical stimuli. A prominent factor is the diversity of environmental conditions that a material will encounter within the human body. Patient heterogeneity creates a continual challenge for the design of living materials. Enhancing the biocompatibility of implantable or injectable materials is a continual challenge and, as we have seen from recent advances, a number of unmet challenges must still be addressed to further our understanding of this field. As we continue to elucidate the physiological factors that underlie normal and diseased conditions, we will be better suited to create responsive and adaptive materials for drug delivery. Equipped with the fundamental understanding of these biological environments and the advancement of molecular immunology, living material designs will continue to grow in sophisitication over time.
5. Immune Engineering: From Suppression to Weaponization
5.1. Introduction
The immune system has evolved to protect the host from invading pathogens by identifying and eliminating potential threats.[172] These same defense mechanisms serve as the largest barrier in the development of bioengineered treatment options. Biomaterials have enabled significant advances in drug delivery and immunotherapy, and have changed the landscape of tissue regeneration and wound healing.[173] Although implants such as pacemakers and drug-eluting stents are commonly used, their efficacy and half-life is shortened by their recognition by the immune system.[174] Over time, implanted devices trigger the accumulation of macrophages that impede function and structural integrity, and induce robust inflammatory responses that can lead to tissue damage, shock, or the need for lifelong immunosuppression. Similarly, injected polymeric micro- and nanoparticles for drug delivery can initiate inflammation at the site of injection and at target organs, and can be immunogenic, thereby complicating their approval for use in humans.[175]
As medical applications for solid implants, nanoparticles, and hydrogels grow rapidly, a better understanding of how bio-materials interact with the immune response is required.[175,176] Consequently, efforts have been undertaken to improve bio-compatibility through the development of new polymers, modulation of surface chemistry on existing delivery platforms, and incorporation of immunomodulators.[177] A plethora of studies examine the biological underpinnings of the immune response against biomaterials with the hopes of limiting the foreign body response and toxicity.[177] Although research on overcoming immunological barriers is of paramount importance, innate and adaptive immune responses can also be exploited to enhance killing of potential threats to the host. By harnessing the power of the body’s natural defenses against both biomaterials and antigens, the immune response can be programmed to target and eliminate tumors and infection. Therefore two paradigms emerge: the use of biomaterials 1) to minimize or suppress the immune response and 2) to weaponize the immune response against disease-causing agents (Figure 11).
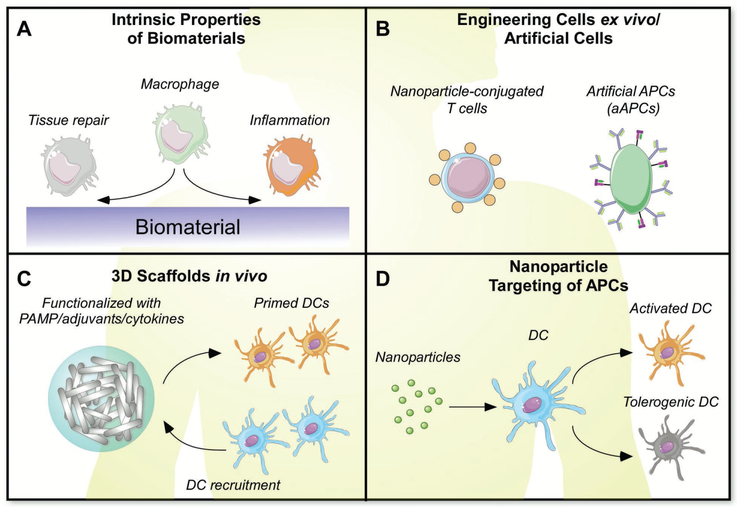
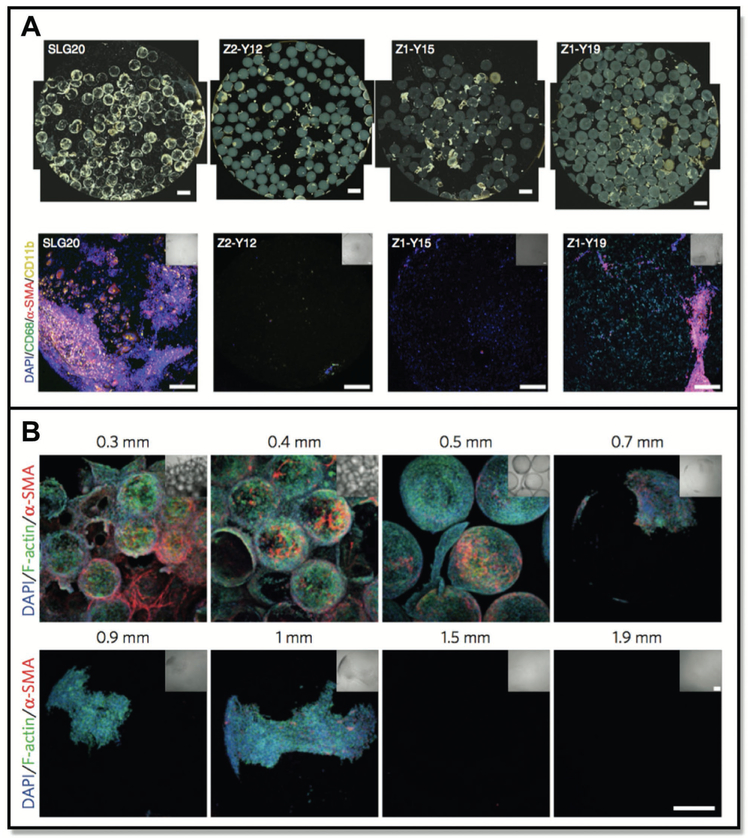
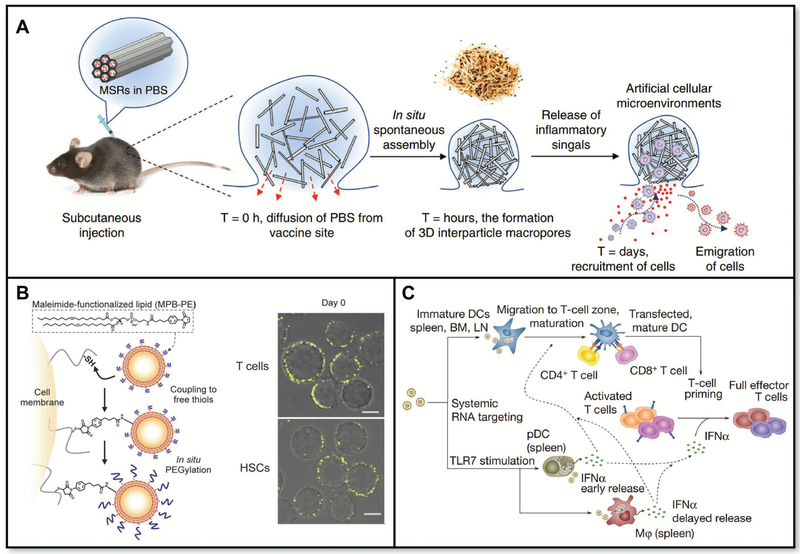
Figure 11.
A) Intrinsic properties of biomaterials can influence cellular response. B) Cellular engineering for therapeutic applications. C) 3D scaffolds can alter cell activation. D) Nanoparticles can be targeted to specific cell populations.
In this section, we will focus on biomaterial strategies that aim to suppress, stimulate, or shape the immune response, either directly or indirectly. We will begin with an overview of how the immune system recognizes invaders and initiates inflammation, and how the foreign body response is initiated. We will then discuss how inflammation can be suppressed or limited using recent biomaterial delivery approaches and bio-compatible materials. Finally we will describe how intrinsic properties of biomaterials and novel approaches to deliver to cargo can enhance immunity against vaccine and tumor antigens.
5.2. Activation of the Immune System by Pathogens and Biomaterials
A basic understanding of the mechanisms behind host defense is fundamental to efficiently design biomaterials that are compatible in local and systemic environments of the body. The immune system has evolved to rapidly detect invading pathogens and nonself patterns to protect against damage and disease.[178] The innate arm of the immune system has evolved from early eukaryotes and serves as the first line of defense against invading pathogens. Innate cells including macrophages, neutrophils, and dendritic cells are critical in controlling early stages of infection. These cells express pattern recognition receptors (PRRs) to recognize conserved pathogen-associated molecular patterns (PAMPs), such as viral nucleic acids and polysaccharides from bacterial cell walls.[172] One class of PRRs called toll-like receptors (TLRs) is present on the surface and in endosomal compartments of host cells.[179] Upon recognition of PAMPs, TLRs lead to the production of type I interferons, key mediators in the antiviral response, and proinflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and the inactive form of interleukin-1beta (pro-IL1β). These cytokines lead recruitment of leukocytes that participate in the inflammatory response. Adjuvants such as CpG oligodeoxynucleotides (CpG-ODN) and polyinosinic:polycytidylic acid (poly I:C) are detected by TLRs to bolster the response to vaccines. The inflammasome, another class of PRRs, forms a cytoplasmic complex of proteins that senses cellular damage, stress, viral and bacterial proteins, and commonly used vaccine adjuvants such as alum.[180] Upon activation of the inflammasome, recruited enzymes called caspases cleave and release IL-1β, resulting in a cascade of inflammatory events including neutrophil recruitment, and initiation of adaptive immunity days after infection.[181] The adaptive arm of the immune system, comprised of cellular and humoral responses, evolved about 500 million years ago and is only present in vertebrates. T cells and antibody-secreting B cells bear receptors that target specific antigenic sequences and establish immunological memory to prevent further reinfection.[182] Cytotoxic T cells are able to bind and kill host cells infected by pathogens. B cells release antigen-specific antibodies that can neutralize extracellular pathogens. Antigen-presenting cells (APCs) such as dendritic cells program the differentiation and function of T- and B-cell responses in lymphoid organs through a combination of cognate receptor engagement, costimulatory signaling, and cytokine production.[183] In addition to the direct elimination of infected cells and microbes, activated lymphocytes go on to release cytokines that act on diverse innate and adaptive cell types that perform effector functions including mucus secretion, antimicrobial peptide release, and tissue repair.[184]
After acute injury or infection, inflammatory responses are followed by an active phase of resolution to return the tissue to homeostasis. Endogenous resolution agonists such as ω−3-derived resolvins, protectins, and maresins, and ω−6-derived lipoxins promote the apoptosis of neutrophils, the influx of nonphlogistic macrophages to clear debris and dying cells, and the initiation of tissue repair while preventing the entry of additional inflammatory cells into the tissue. These specialized proresolving mediators exhibit potent effects in a number of chronic inflammatory disease models.[185] Resolvin D2 has been shown to resolve sepsis by clearing local and systemic bacterial burden and limiting excessive inflammation, thereby regulating the immune response without immuno-suppression.[186] Cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) are also involved in limiting the inflammatory response and maintaining homeostasis.[187] A state of chronic inflammation can ensue if resolution fails to occur.
The FBR to a biomaterial or implanted device is the consequence of chronic inflammatory and wound healing responses over time.[174] After implantation, plasma proteins, clotting factors, and extracellular matrix proteins begin to adhere to the surface of biomaterials, resulting in the recruitment of macrophages, their secretion of proinflammatory cytokines, and activation of the complement cascade. As adherent macrophages bind and attempt to engulf the biomaterial, these cells fuse leading to the formation of foreign body giant cells (FBGCs).[188] Cytokines and degradation products from FBGCc hasten the demise of the implant and diminish bacteriocidal activity of adherent cells, stimulation of lymphocytes, and extracellular matrix remodeling in the vicinity. The granulation tissue that arises consists of fibroblasts and neovasculature, which eventually forms the fibrous capsule that engulfs an implant.[189] The FBR is typically a nonspecific immune response involving innate immune cells and non-specific recruitment of lymphocytes.[190] In some cases, orthopedic and metal implants can initiate allergic hypersensitivity reactions involving antibodies and T cells against polymeric degradation products and metal salts.[191] Of note, the FBR is distinct from tissue rejection after organ transplantation, an event that takes place in an antigen-specific manner due to recognition of non-self molecules expressed by the donor tissue.[192]
The immune system has evolved mechanisms to ensure that adaptive immune cells do not target self-antigens. This concept, called immunological tolerance, is orchestrated in part by negative costimulatory signaling during antigen presentation, and is maintained by regulatory T cells (Tregs) and other immunosuppressive factors that avert immune responses against self-peptides and commensal microbes.[193] When tolerance is broken, autoimmunity and inflammatory diseases can arise. Tumor cells employ tolerance mechanisms to impede recognition and destruction by innate and adaptive immune cells, via induction negative costimulatory pathways and Tregs.[194]
5.3. Limiting Inflammation Using Biomaterials
The implantation of biomaterial devices has revolutionized the field of medicine. Just as organ transplantation can replace defective parts of the body, inorganic pacemakers, stents, and bone implants have been designed to compensate for dysfunctional tissues within the setting of disease. Biomaterial devices have been fabricated to conform to their target locations and mechanical needs; the viscoelasticity of hydrogels is compatible for soft tissue implantation while nanoparticles are able to travel through the circulation to target specific cells types or remain systemic to exact their function.[175]
Both natural and synthetic materials delivered to the human body face the same challenge of attack by the immune system. Although the mechanisms by which they are recognized by host cells differ, the quest to suppress ensuing innate and adaptive responses remains a significant challenge. Biomaterial implants employ the use of nonfouling surfaces that prevent protein adsorption, porosity that favors differentiation of anti-inflammatory macrophages, and incorporation of siRNA against IL-4 and mTOR, mediators implicated in driving FBR responses.[195] Studies of the in vivo efficacy of polymer-based therapeutics in immunotherapy and tissue engineering reveal that the physicochemical properties of biomaterials are sufficient to stimulate the immune system. Characteristics such as shape, size, charge, and hydrophobicity of biomaterials may influence how biomaterials interact with the immune system (Figure 12).[118] For example, dendritic cells undergo maturation in the presence of hydrophobic biomaterials such as PLGA and chitosan, as compared to alginate or hyaluronic acid.[196] Subcutaneous implantation of zwitterionic poly(carboxybetaine methacrylate) hydrogels in mice were ultralow fouling and stimulated blood vessel formation nearby, fostering the recruitment of macrophages with an anti-inflammatory phenotype and preventing capsule formation for 3 months.[197] Biomaterials composed of ECM can have diverse consequences on the phenotype of macrophages depending on the tissue source of ECM.[198] Peptide nanofibers expressing a net negative charge via anionic amino acids are less likely to stimulate uptake by APCs, or antibody and T-cell responses as compared to positively charged fibrillized peptide biomaterials.[199] Conversely, anionic bacterial polysaccharides where positively charged motifs have been introduced are able to activate monocytes and dendritic cells via TLR2, resulting in enhanced T cell activation and proliferation.[200]
Figure 12.
Size, shape, charge, and polarity may play a role in the immune response to biomaterials.
Recent studies have shed light on how size and surface chemistry on hydrogels could greatly improve treatment of type I diabetes (Figure 13). Spherical materials, 1.5 mm in diameter or larger were shown to reduce the fibrotic response compared to those with a smaller diameter or different shape.[118] This observation was made in rodents and nonhuman primates, and could have important implications for biocompatibility in humans. Triazole modifications on alginate hydrogels have been shown to mitigate fibrosis and the foreign body response against encapsulated human stem cell-derived beta cells in immune-competent diabetic mice.[201] Extrahepatic transplants of hydrogels functionalized with VEGF and containing islet grafts are able to improve the survival and function of encapsulated cells in mice.[202]
Figure 13.
A) Modified alginate hydrogels implanted in cynomolgus macaques mitigate the foreign body response. Adapted with permission.[201a] Copyright 2016, Nature Publishing Group. B) Increasing alginate sphere size results in reduced cellular deposition and firbrosis. Adapted with permission.[118] Copyright 2015, Nature Publishing Group.
Just as our immune system has evolved to protect us from invading pathogens and foreign bodies, cells and tissues employ mechanisms to prevent unwanted clearance or immunity against self-antigens. Knowledge of these signals could facilitate the design of biomaterials that can prevent excessive inflammatory responses and enhance the half-life of implanted devices. For example, “don’t eat me” signals such as CD47 expressed on the cell surface prevent clearance or phagocytosis of cells that should be left undisturbed.[203] Expression of this marker on cancer cells prevents targeting and killing by immune cells. Rodriguez et al. demonstrated how peptides designed from human CD47 and conjugated to nanoparticles can prevent clearance, and enhance circulation and delivery of therapeutics into tumors in vivo.[204]
Immunological tolerance is critical for preventing unwanted or exaggerated antigen-specific cellular and humoral responses.[205] When tolerance is broken, as in autoimmune diseases, food allergies, and hypersensitivity disorders, patients are routinely administered immunosuppressive drugs that can lead to opportunistic infections and cancer. Polymeric coencapsulation of the mTOR inhibitor rapamycin with an antigen of choice can induce a state of tolerance while minimizing systemic effects and promoting dose sparing.[206] This occurs by facilitating uptake by dendritic cells and initiation of tolerogenic T- and B-cell responses. Injection of PLGA nanoparticles containing either protein or peptide antigens and rapamycin leads to an increase in antigen-specific Tregs and a sustained reduction in antibody responses after challenge in models of experimental autoimmune encephalomyelitis (EAE), oral and airway allergies, and hemophilia.[206] In another study of EAE, the experimental model of multiple sclerosis, Tostanoski et al. used intralymph node injections of PLGA microparticles containing myelin oligodendrocyte glycoprotein (MOG) and rapamycin to reverse paralysis, and trigger regulatory T cell accumulation and lymph node reorganization in mice.[207]
The resolution of inflammation is an active process, in part orchestrated by ω−3 derived lipid mediators that promote clearance of pathogens, apoptosis of neutrophils, and initiation of tissue repair.[208] Encapsulation of such resolution agonists into biomaterials can prevent local inflammatory responses and extend the life of drug delivery devices. Resolvin D1 (RvD1) loaded in Pluronic gels or PLGA films can prevent neointimal hyperplasia and significantly decrease arterial inflammation after sterile injury.[209] Aspirin-triggered RvD1 encapsulated into a PLGA film is able to initiate vascular remodeling and tissue repair using controlled release.[210] Fredman et al. similarly loaded a synthetic peptide that binds to the receptor of RvD1 into collagen iv-targeted nanoparticles, which led to protection against atherosclerosis in hypercholesterolemic mice.[211] This strategy to incorporate endogenous lipid mediators into biomaterials for drug delivery eliminates potential concerns frequently associated with protein delivery, such as antidrug antibody production.
Studies into the molecular mechanisms of inflammation following implantation of biomaterials have led to promising drug targets to prevent fibrosis. Inhibition of CSF1R, a molecule involved in the foreign body response, prevents macrophage deposition and extends the life of implanted biomaterials.[212] Conversely, addition of CD200 to the surface of PLGA microparticles and films inhibited proinflammatory cytokine secretion and enhanced nonphlogistic phagocytosis by macrophages.[213]
Although a great deal of research has been focused on how intrinsic biomaterial characteristics can induce an inflammatory response, the exact mechanisms by which innate and adaptive immune responses are initiated are still largely unknown. As these details become evident and are combined with newly emerging immunotherapies, our flexibility in delivering implants, scaffold, and biomaterials will be expedited.
5.4. Biomaterial Design to Harness the Immune System
As we elucidate the fundamental mechanisms underlying innate and adaptive immunity, our ability to harness the power of the immune system to target tumors and infections has led to numerous breakthroughs. The FDA has recently approved Kymriah, (CAR) T-cell therapy for the treatment of B-cell acute lymphoblastic leukemia.[214] This form of adoptive cell therapy involves removing T cells from a patient and genetically engineering chimeric T-cell receptors to target an antigen of choice.[215] Although CAR T-cell therapy is revolutionizing the treatment of many forms of hematological malignancies, this success is currently impaired by systemic toxicity and cytokine release syndrome after infusion, and difficulties infiltrating solid tumors. Bioengineering approaches can overcome these obstacles by targeting small-molecule drugs, engineered cells, or vaccines to specific organs, cell types, or even tumors within the body, resulting in release with desired kinetics and biodistribution (Figure 14). A recent study employed a novel microfabrication method to develop a single injection platform for the pulsatile release of vaccines. Ovalbumin (OVA)-containing microparticles delivered by subcutaneous injection into mice release at desired time points and result in higher OVA-specific antibody titers as compared to bolus injections.[216] Moreover, Stephan et al. demonstrate how a macroporous scaffold comprised of polymerized alginate and functionalized with stimulatory and adhesion molecules can be implanted into tumor resection sites to deliver, expand, and disseminate tumor-targeting T cells to prevent relapse.[217] A similar biopolymer scaffold was employed to deliver CAR T cells and micro-particles containing a stimulator of IFN genes (STING) agonist thereby eradicating tumors and limiting antigen escape variants in mouse models of melanoma and pancreatic cancer.[218]
Figure 14.
A) Spontaneous assembly of mesoporous silica rods recruits host cells for maturation in vivo. Adapted with permission.[223] Copyright 2015, Nature Publishing Group. B) Stable conjugation of nanoparticles to the surfaces of T cells and hematopoietic stem cells via cell surface thiols. Adapted with permission.[234a] Copyright 2010, Nature Publishing Group. C) RNA-lipoplexes trigger interferon alpha release, maturation of antigen-presenting cells and effector T-cell differentiation. Adapted with permission.[232] Copyright 2016, Nature Publishing Group.
Biomaterials themselves can potentiate adjuvant responses from the immune system. PLGA has been demonstrated to activate the NLRP3 inflammasome leading to stronger adaptive immunity.[219] Titanium dioxide nanoparticles lead to proinflammatory cytokine secretion and maturation of dendritic cells, resulting in stimulation and proliferation of T-cell responses.[220] Strategies using biomaterials to boost vaccine and antitumor responses have been well investigated.[221]
During conventional vaccination methods, the ability to ensure that APCs effectively acquire soluble antigen and adjuvant without triggering systemic toxicity is a major challenge. This can be overcome by targeting vaccine formulations to lymphoid organs where immune responses are concentrated, or by recruiting APCs to the vaccine delivery site. The latter strategy, demonstrated in vivo by implantation of scaffolds able to modulate the phenotype of immune cells, has been greatly explored for cancer vaccines.[222] Spontaneously assembling scaffolds made of mesoporous silica rods (MSRs) have been developed that can recruit dendritic cells into its macroporous 3D micro-environment and prime them for antigen presentation via sustained release of antigen and adjuvant.[223] This leads to potent antigen-specific T cell and antibody responses against a target of choice. In addition to the adjuvants released by the rods, it is possible that degradation of the amorphous silica itself is stimulating the inflammasome, enhancing resultant adaptive immunity.
Another vaccine delivery strategy is based on a polylactide-co-glycolide (PLG) matrix containing immobilized CpG ODN, granulocyte-macrophage colony-stimulating factor (GM-CSF) to promote dendritic cell recruitment, and autologous tumor lysate that confers multiple antigens.[224] The scaffold leads to robust priming of CTLs and local and systemic anti-tumor responses in a B16 melanoma model. This study has led to the first personalized biomaterial-based cancer immunotherapy, called WDVAX, which has recently moved to a phase I clinical trial for stage IV melanoma.[225]
Lymph node-targeting vaccines have also been designed, informed by the capacity of albumin to capture and deliver dyes to the lymph node (LN) for cancer biopsies.[226] Amphiphilic vaccines synthesized with a lipophilic albumin-binding tail and conjugated to a heterobifunctional PEG polymer accumulate in the LN and generate a 30-fold increase in T cell priming, thereby enhancing tumor killing while limiting systemic toxicity.
Biomaterials can be used to mimic the APCs presentation of antigens to T cells to trigger immunity and memory responses. Artificial antigen presenting cells (aAPCs) made of spherical polymeric microparticles harboring surrogate major histocompatibility complexes (MHC) and anti-CD28 monoclonal antibodies bind the T-cell receptor (TCR) and CD28 on CD8+ T cells, leading to their activation. Such aAPCs can synergize with anti-PD1 mAb to increase antigen-specific killing by CD8+ T cells in tumor microenvironments.[227] A study of the effects of particle geometry on CD8+ T-cell activation revealed that ellipsoidal aAPCs had prolonged contact with CD8+ T cells, increasing T cell proliferation and leading to a significant reduction in tumor volume in a murine B16 melanoma model.[228] This highlights the importance of capturing the in vivo behavior of phagocytic cells and their ability to change shape to promote cell–cell interactions.
Similarly the shape and size of the particles that phagocytes ingest can determine cytokine production and T-cell skewing.[229] Rod-shaped gold nanoparticles (AuNP) coated with West Nile Virus Envelope protein were able rupture lysosomal compartments and activate the NLRP3 inflammasome leading to IL-1beta and IL-18 secretion, while spherical and cubic AuNPs led to increased TNF-α, IL-6, IL-12, and GM-CSF from bone-marrow-derived dendritic cells (BMDCs).[230] Silica nanoparticles coated with varying poly(amino acid)s (PAAs) reveal that increasing hydrophobicity results in increasing levels of IL-1β secretion from BMDCs and IFNγ released from T cells.[231] Collectively, these data can inform the design of polymeric particles with enhanced adjuvancy during vaccination.
As discussed earlier in this review, delivery of nucleic acids into cells using nanoparticles has not only improved our ability to express new proteins, but also to stimulate the innate immune system to target an antigen of choice. Lipid nanoparticles for mRNA delivery have been efficacious in the B16F10 melanoma model.[93d] Kranz et al. used commonly used lipids, N-[1-(2,3dioleyloxy)propyl]-N,N,N-trimethylammonium chlo-ride (DOTMA) and DOPE to formulate cationic RNA lipoplexes (RNA-LPX) that efficiently underwent phagocytosis by APCs while protecting encapsulated RNA from extracellular degradation.[232] Recognition of RNA by TLR7 in macrophages and plasmacytoid dendritic cells led to the production IFNα and priming of antigen-specific T-cell responses. To highlight its potential as a cancer immunotherapy, RNA-LPX encoding tumor antigens were injected into mice harboring either B-16 OVA or CT-26, both aggressively growing subcutaneous tumors. Rejection and clearance of tumors resulted in potent effector responses by CD8+ T cells. A phase I clinical trial is already underway for the treatment of patients with advanced malignant melanoma.[232]
Biomaterial design and dose-sparing abilities of nano- and microparticle vaccine formulations can facilitate optimal release kinetics that result in robust germinal center responses in the LN. One method to study such kinetics employed an osmotic minipump and computational modeling to demonstrate that continuous administration of increasing doses of HIV-1 env antigen, rather than bolus doses of varying concentrations, led to maximal antigen capture in the lymph node, plasma cell generation, and antigen-specific antibody titers. It would be interesting to see how such a dosing schedule also affects the quality and affinity of the antibody response.[233]
In addition to enabling antigen presentation, cytotoxic T lymphocyte (CTL) function can be directly exploited to deliver immunostimulants at the interface with target cells. Recent studies have demonstrated that drug-loaded lipid nanoparticles can be conjugated to the surface of CTLs.[234] Upon TCR binding of HIV-specific nanocapsule–CTL conjugates (NC-CTL) with cognate CD4+ T cells in vivo, granzyme secretion and lysis of NC containing an IL-15 superagonist resulted in enhanced killing of HIV-infected CD4+ T cells.[235] This strategy could have powerful implications for targeting latently infected cells in patients with HIV.
Significant efforts are being undertaken to improve adoptive cell therapy (ACT), the process of stimulating T cells ex vivo and reintroducing them into a patient to target cancers. Typically, the effector function of donor T cells is rapidly suppressed by tolerogenic signaling within the tumor microenvironment.[194] Zheng et al. targeted immunoliposomes loaded with a transforming growth factor-β (TGF-β) inhibitor to the internalizing receptor CD90 and the noninternalizing receptor CD45 expressed on donor T cells. This led to sustained activation and proliferation, which correlated with enhanced tumor infiltration by T cells and suppression of tumor growth.[236]
As we continue to elucidate the mechanisms by which bio-materials can stimulate immune responses, our progress in fine-tuning antiviral and tumor-killing responses will be catalyzed. Bioengineering strategies combined with our new knowledge of how the immune system responds to infectious disease and cancer will help us to change the lives of countless individuals.
5.5. Future Perspectives
As the field of immunoengineering continues to burgeon, a greater understanding of how and why biomaterials induce innate and adaptive immune responses will be revealed. Thus far, the exact mechanisms surrounding size, charge, hydrophobicity, and shape in activating immunity remain unclear. Collaborations between bioengineers and clinicians can advance the success of biomaterial-based immunotherapies, but the dearth of data on how novel biomaterials will behave in a human setting must be addressed. As many of the drug delivery systems and bioscaffolds discussed are easily tunable, there is ample room to use the same model system to incorporate a range of drugs and vaccines to treat a wide range of diseases.
6. Concluding Remarks
As a community, the biomaterials field strives to improve global health and well-being. Over the last 60 years, our ever-evolving biotechnology platform has impacted countless lives around the globe by enabling therapeutic paradigms that were once thought impossible. These tremendous advances are truly a testament to the power of collaboration within science. Engineers, for example, have modulated the rate at which we can administer drugs into the body; chemists and materials scientists have created systems that can respond to local and remote physiological stimuli; biologists have pinpointed the mechanistic routes of disease; physicists have modeled drug interactions with complex receptors; and medical professionals have conducted clinical trials and implemented next generation therapies for disease management. This multidisciplinary approach is a hallmark of the biomaterials field, and it is one that will continue to influence our research as we build further upon its diverse platform.
As we continue forward, many of the challenges that shaped the field 60 years ago remain the same today. How, for example, do we ensure that our research can have maximal impact on improving global health? The translation of biomaterials from bench top to bedside is a daunting process. However, advances in biomaterials continue to enable next generation strategies that are safe and effective in human patients. Nevertheless, as our knowledge progresses within this field, more questions arise, and we must continue to refine our work to address these issues. It is our hope, therefore, that with continuing effort within the fields of engineering, chemistry, biology, medicine, and physics, that we will create even greater progress in biomaterials, with the ultimate goal of improving societal health and well-being for all.
Acknowledgements
This article is part of the Advanced Materials Hall of Fame article series, which recognizes the excellent contributions of leading researchers to the field of materials science. M.J.M. acknowledges the Burroughs Wellcome Fund Career Award at the Scientific Interface, an NIH F32 Fellowship (award number CA200351), and a grant from the Burroughs Wellcome Fund (no. 1015145). P.S.P. acknowledges the Cancer Research Institute (CRI) Irvington Postdoctoral Fellowship.
Biographies

Owen S. Fenton is currently a postdoctoral associate in the laboratory of Professor Robert Langer. He received his Ph.D. in chemistry at MIT in the laboratory of Professor Daniel G. Anderson where he focused on the development of non-viral vectors for in vivo RNA delivery. He also received his M.Sc. in chemistry at MIT for his work on the total synthesis of alkaloid natural products under the tutelage of Professor Mohammad Movassaghi. His current work involves the development of responsive biomaterials using concepts and techniques from organic synthesis and chemical engineering.

Padmini S. Pillai is currently a postdoctoral fellow with Robert Langer at the Massachusetts Institute of Technology. She received her doctorate in immunobiology at Yale University and her B.A. in biochemistry from Regis College. Her research focuses on biomaterial strategies to deliver vaccines and small-molecule drugs to mucosal tissues. She previously identified the role of pattern recognition receptors in innate resistance to influenza A infection and strategies to boost disease tolerance.

Robert Langer is one of 13 Institute Professors (MIT’s highest honor). He grew up in Albany, New York, attending the public schools there. He then went to Cornell University where he received a B.S. in chemical engineering and MIT where he received an Sc.D. in the same field. He then did postdoctoral work at Boston’s children’s hospital before joining the MIT faculty in 1977. His research interests are in drug-delivery systems, biomaterials, nanotechnology, tissue engineering, and regenerative medicine.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adma.201705328
Contributor Information
Dr. Owen S. Fenton, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02142, USA Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA.
Dr. Katy N. Olafson, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02142, USA Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA.
Dr. Padmini S. Pillai, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02142, USA Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA.
Prof. Michael J. Mitchell, Department of Bioengineering, University of Pennsylvania, School of Engineering and Applied Science Philadelphia, PA 19104, USA
Prof. Robert Langer, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02142, USA Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA; Harvard-MIT Division of Health Sciences and Technology Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
References
- [1].Drews J, Science 2000, 287, 1960. [DOI] [PubMed] [Google Scholar]
- [2].Aitken M, Kleinrock M, Lyle J, Nass D, Caskey L, 2014 Medicines Use and Spending Shifts: A Review of the Use of Medicines in the U.S. in 2014, IMS Health Inc; 2015, available from: https://www.redaccionmedica.com/contenido/images/IIHI_Use_of_Medicines_Report_2015.pdf (accessed: April 2018). [Google Scholar]
- [3].Tibbitt MW, Dahlman JE, Langer R, J. Am. Chem. Soc 2016, 138, 704. [DOI] [PubMed] [Google Scholar]
- [4].Mitragotri S, Burke PA, Langer R, Nat. Rev. Drug Discovery 2014, 13, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yun YH, Lee BK, Park K, J. Controlled Release 2015, 219, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Allen TM, Cullis PR, Science 2004, 303, 1818. [DOI] [PubMed] [Google Scholar]
- [7].Langer R, Nature 1998, 392, 5. [PubMed] [Google Scholar]
- [8].Langer R, Science 1990, 249, 1527. [DOI] [PubMed] [Google Scholar]
- [9].Hoare TR, Kohane DS, Polymer 2008, 49, 1993. [Google Scholar]
- [10] a).Ensign LM, Cone R, Hanes J, Adv. Drug Delivery Rev 2012, 64, 557; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang S, Bellinger AM, Glettig DL, Barman R, Lee YA, Zhu J, Cleveland C, Montgomery VA, Gu L, Nash LD, Maitland DJ, Langer R, Traverso G, Nat. Mater 2015, 14, 1065; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA, J. Pharm. Sci 1999, 88, 933; [DOI] [PubMed] [Google Scholar]; d) Jeong B, Bae YH, Lee DS, Kim SW, Nature 1997, 388, 860; [DOI] [PubMed] [Google Scholar]; e) Guvendiren ML, Lu HD, Burdick JA, Soft Matter 2011, 8, 260. [Google Scholar]
- [11].Anselmo AC, Mitragotri S, J. Controlled Release 2014, 190, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12] a).Prausnitz MR, Langer R, Nat. Biotechnol 2008, 26, 1261; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Patton JS, Byron PR, Nat. Rev. Drug Discovery 2007, 6, 67; [DOI] [PubMed] [Google Scholar]; c) Illum L, Drug Discovery Today 2002, 7, 1184; [DOI] [PubMed] [Google Scholar]; d) Gaudana R, Ananthula HK, Parenky A, Mitra AK, AAPS J. 2010, 12, 348; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, Mintzes, Deaver D, Lotan N, Langer R, Science 1997, 276, 1868; [DOI] [PubMed] [Google Scholar]; f) Park JH, Allen MG, Prausnitz MR, J. Controlled Release 2005, 104, 51; [DOI] [PubMed] [Google Scholar]; g) Illum LJ, Jorgensen H, Bisgaard H, Krogsgaard O, Rossing N, Int. J. Pharm 1987, 39, 189; [Google Scholar]; h) Bourges JLG, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, BenEzra D, Behar-Cohen FF, Invest. Ophthalmol. Visual Sci 2003, 44, 3562. [DOI] [PubMed] [Google Scholar]
- [13].Lu Y, Aimetti AA, Langer R, Gu Z, Nat. Rev. Mater 2017, 1, 16075. [Google Scholar]
- [14] a).Hubbell JA, Thomas SN, Swartz MA, Nature 2009, 462, 449; [DOI] [PubMed] [Google Scholar]; b) Bridges AW, Garcia AJ, J. Diabetes Sci. Technol 2008, 2, 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schneider C, Langer R, Loveday D, Hair D, J. Controlled Release 2017, 262, 284. [DOI] [PubMed] [Google Scholar]
- [16].Langer RS, Peppas NA, Biomaterials 1981, 2, 201. [DOI] [PubMed] [Google Scholar]
- [17].Park K, J. Controlled Release 2014, 190, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Traverso G, Langer R, Sci. Transl. Med 2015, 7, 289ed286. [DOI] [PubMed] [Google Scholar]
- [19] a).Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC, Adv. Drug Delivery Rev 2014, 66, 2; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nat. Nanotechnol 2007, 2, 751. [DOI] [PubMed] [Google Scholar]
- [20] a).Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC, Chem. Soc. Rev 2012, 41, 2971; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brannon-Peppas LB, J. O., Adv. Drug Delivery Rev 2012, 64, 206. [Google Scholar]
- [21] a).Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A, Science 2012, 338, 903; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Steichen SD, Caldorera-Moore M, Peppas NA, Eur. J. Pharm. Sci 2013, 48, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mitchell MJ, Jain RK, Langer R, Nat. Rev. Cancer 2017, 17, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23] a).Langer R, Folkman J, Nature 1976, 263, 797; [DOI] [PubMed] [Google Scholar]; b) Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R, Science 1978, 202, 1290; [DOI] [PubMed] [Google Scholar]; c) Brownlee M, Cerami A, Science 1979, 206, 1190; [DOI] [PubMed] [Google Scholar]; d) Folkman J, Biomaterials 1990, 11, 615; [DOI] [PubMed] [Google Scholar]; e) Folkman J, Long DM Jr., Rosenbaum R, Science 1966, 154, 148; [DOI] [PubMed] [Google Scholar]; f) Folkman J, Reiling W, Williams G, Surgery 1969, 66, 194. [PubMed] [Google Scholar]
- [24] a).Dziuk PJ, Cook B, Endocrinology 1966, 78, 208; [DOI] [PubMed] [Google Scholar]; b) Bass PP, Purdon RA, Wiley JN, Nature 1965, 208, 591; [Google Scholar]; c) Powers KG, J. Parisitol 1965, 51, 53. [Google Scholar]
- [25] a).Diaz S, Pavez M, Miranda P, Robertson DN, Sivin I, Croxatto HB, Contraception 1982, 25, 447; [DOI] [PubMed] [Google Scholar]; b) Segal S, Am. J. Obstet. Gynecol 1987, 157, 1090. [DOI] [PubMed] [Google Scholar]
- [26] a).Theeuwes F, J. Pharm. Sci 1975, 64, 1987; [DOI] [PubMed] [Google Scholar]; b) Theeuwes F, Yum SI, Ann. Biomed. Eng 1976, 4, 343. [DOI] [PubMed] [Google Scholar]
- [27] a).Wichterle OL, Lim D, Nature 1960, 185, 117; [Google Scholar]; b) Sedlacek J, Cesk. Oftalmol 1965, 21, 509; [PubMed] [Google Scholar]; c) Armaly MF, Rao KR, Invest. Ophthalmol 1973, 12, 491. [PubMed] [Google Scholar]
- [28] a).Kramer PA, J. Pharm. Sci 1974, 63, 1646; [DOI] [PubMed] [Google Scholar]; b) Ekman B, Sjoholm I, Nature 1975, 257, 825; [DOI] [PubMed] [Google Scholar]; c) Kato T, Nemoto R, Mori H, Kumagai I, Lancet 1979, 2, 479; [DOI] [PubMed] [Google Scholar]; d) Lee TK, Sokoloski TD, Royer GP, Science 1981, 213, 233; [DOI] [PubMed] [Google Scholar]; e) Chang TM, Science 1964, 146, 524. [DOI] [PubMed] [Google Scholar]
- [29] a).Higuchi T, J. Pharm. Sci 1961, 50, 874; [DOI] [PubMed] [Google Scholar]; b) Higuchi T, J. Pharm. Sci 1963, 52, 1145; [DOI] [PubMed] [Google Scholar]; c) Higuchi WI, J. Pharm. Sci 1967, 56, 315; [DOI] [PubMed] [Google Scholar]; d) Singh P, Desai SJ, Simonelli AP, Higuchi WI, J. Pharm. Sci 1967, 56, 1548; [DOI] [PubMed] [Google Scholar]; e) Singh P, Desai SJ, Simonelli AP, Higuchi WI, J. Pharm. Sci 1967, 56, 1542; [DOI] [PubMed] [Google Scholar]; f) Ritger PLP, Peppas NA, J. Controlled Release 1987, 5, 23; [Google Scholar]; g) Ritger PLP, Peppas NA, J. Controlled Release 1987, 5, 37. [Google Scholar]
- [30] a).Zhang YS, Khademhosseini A, Science 2017, 356, 3627; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, Adv. Mater 2014, 26, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peppas NAH, Hilt JZ, Khademhosseini A, Langer R, Adv. Mater 2006, 18, 1345. [Google Scholar]
- [32] a).Gref R, Science 1994, 263, 1600; [DOI] [PubMed] [Google Scholar]; b) Gref R, Colloids Surf., B 2000, 18, 301; [DOI] [PubMed] [Google Scholar]; c) Ratner BD, J. Controlled Release 2002, 78, 211. [DOI] [PubMed] [Google Scholar]
- [33].Hoare TRK, Kohane DS, Polymer 2008, 49, 1993. [Google Scholar]
- [34] a).Langer RP, Peppas NA, AIChE J. 2003, 49, 2990; [Google Scholar]; b) Hoffman AS, J. Controlled Release 2008, 132, 153. [DOI] [PubMed] [Google Scholar]
- [35].Lee P, Shen Y, Eberle M, Invest. Ophthalmol 1975, 14, 43. [PubMed] [Google Scholar]
- [36].Langer R, Acc. Chem. Res 2000, 33, 94. [DOI] [PubMed] [Google Scholar]
- [37] a).Bangham AD, Horne RW, J. Mol. Biol 1964, 8, 660; [DOI] [PubMed] [Google Scholar]; b) Marty JJ, Oppenheim RC, Speiser P, Pharm. Acta Helv 1978, 53, 17; [PubMed] [Google Scholar]; c) Torchilin VP, Nat. Rev. Drug Discovery 2005, 4, 145. [DOI] [PubMed] [Google Scholar]
- [38] a).Farokhzad OC, Langer R, ACS Nano 2009, 3, 16; [DOI] [PubMed] [Google Scholar]; b) Deer PK, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nat. Nanotechnol 2007, 2, 751; [DOI] [PubMed] [Google Scholar]; c) Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R, Science 1994, 263, 1600; [DOI] [PubMed] [Google Scholar]; d) Malik NE, Evagorou EG, Duncan R, Anticancer Drugs 1999, 10, 767; [PubMed] [Google Scholar]; e) Harada AK, Kataoka K, Macrmolecules 1995, 28, 5294; [Google Scholar]; f) Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA, Science 2006, 312, 1027. [DOI] [PubMed] [Google Scholar]
- [39] a).Henry S, McAllister DV, Allen MG, Prausnitz MR, J. Pharm. Sci 1998, 87, 922; [DOI] [PubMed] [Google Scholar]; b) Lee JW, Park JH, Prausnitz MR, Biomaterials 2008, 29, 2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40] a).Yoshida RU, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T, Nature 1995, 374, 240; [Google Scholar]; b) Kopecek J, Eur. J. Pharm. Sci 2003, 20, 1; [DOI] [PubMed] [Google Scholar]; c) Peppas NAH, Hilt JZ, Khademhosseini A, Langer R, Adv. Mater 2006, 18, 1345; [Google Scholar]; d) Griset AP, Walpole J, Liu R, Gaffey A, Colson YL, Grinstaff MW, J. Am. Chem. Soc 2009, 131, 2469. [DOI] [PubMed] [Google Scholar]
- [41] a).Santini JT Jr., Cima MJ, Langer R, Nature 1999, 397, 335; [DOI] [PubMed] [Google Scholar]; b) Cima MJ, Lee H, Daniel K, Tanenbaum LM, Mantzavinou A, Spencer KC, Ong Q, Sy JC, Santini J Jr., Schoellhammer CM, Blankschtein D, Langer RS, J. Controlled Release 2014, 190, 157; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Farra R, Sheppard NF Jr., McCabe L, Neer RM, Anderson JM, Santini JT Jr., Cima MJ, Langer R, Sci. Transl. Med 2012, 4, 122ra121; [DOI] [PubMed] [Google Scholar]; d) Timko BP, Dvir T, Kohane DS, Adv. Mater 2010, 22, 4925; [DOI] [PubMed] [Google Scholar]; e) Derfus A. M. v. M., Maltzahn G, Harris TJ, Duza T, Vecchio KS, Ruoslahti E, Bhatia SN, Adv. Mater 2007, 19, 3932; [Google Scholar]; f) Hsieh DS, Langer R, Folkman J, Proc. Natl. Acad. Sci. USA 1981, 78, 1863; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Mitragotri S, Blankschtein D, Langer R, Science 1995, 269, 850; [DOI] [PubMed] [Google Scholar]; h) Huebsch N, Kearney CJ, Zhao X, Kim J, Cezar CA, Suo Z, Mooney DJ, Proc. Natl. Acad. Sci. USA 2014, 111, 9762; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Tong R, Hemmati HD, Langer R, Kohane DS, J. Am. Chem. Soc 2012, 134, 8848; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Kwon IC, Bae YH, Kim SW, Nature 1991, 354, 291. [DOI] [PubMed] [Google Scholar]
- [42] a).Okada H, Doken Y, Ogawa Y, Toguchi H, Pharm. Res 1994, 11, 1143; [DOI] [PubMed] [Google Scholar]; (b) Wright JC, Burgess DJ, Long Acting Injections and Implants, Springer; US, Boston, MA, USA: 2011, pp. 11–24. [Google Scholar]
- [43].Chaubal M, Drug Delivery Technol. 2002, 2, 34. [Google Scholar]
- [44].Barenholz Y, J. Controlled Release 2012, 160, 117. [DOI] [PubMed] [Google Scholar]
- [45] a).Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y, Cancer Res 1994, 54, 987; [PubMed] [Google Scholar]; b) Gabizon AA, Barenholz Y, Bialer M, Pharm. Res 1993, 10, 703. [DOI] [PubMed] [Google Scholar]
- [46] a).Allen TM, Cullis PR, Adv. Drug Delivery Rev 2013, 65, 36; [DOI] [PubMed] [Google Scholar]; b) Pillai G, SOJ Pharm. Pharm. Sci 2014, 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Silverman JA, Deitcher SR, Cancer Chemother. Pharmacol 2013, 71, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ferrari M, Nat. Rev. Cancer 2005, 5, 161. [DOI] [PubMed] [Google Scholar]
- [49].DeRuiter J, Holston PL, US Pharmacist 2012, 37, 12. [Google Scholar]
- [50] a).Brem H, Mahaley MS Jr., Vick NA, Black KL, Schold SC Jr., Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW, J. Neurosurg 1991, 74, 441; [DOI] [PubMed] [Google Scholar]; b) Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Lancet 1995, 345, 1008; [DOI] [PubMed] [Google Scholar]; c) Giese A, Kucinski T, Knopp U, Goldbrunner R, Hamel W, Mehdorn HM, Tonn JC, Hilt D, Westphal M, J. Neuro-Oncol 2004, 66, 351. [DOI] [PubMed] [Google Scholar]
- [51].Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H, Ann. Surg. Oncol 2008, 15, 2887. [DOI] [PubMed] [Google Scholar]
- [52].BBC Research, Global Markets and Technologies for Advanced Drug Delivery 2016, https://www.bccresearch.com/market-research/pharmaceuticals/advanced-drug-delivery-systems-tech-markets-report-phm006k.html (accessed: November 2017). [Google Scholar]
- [53] a).Le Quesne JP, Spriggs KA, Bushell M, Willis AE, J. Pathol 2010, 220, 140; [DOI] [PubMed] [Google Scholar]; b) Nguyen J, Szoka FC, Acc. Chem. Res 2012, 45, 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stratton MR, Science 2011, 331, 1553. [DOI] [PubMed] [Google Scholar]
- [55].World Health Organization Diabetes Fact Sheet, 2017, http://www.who.int.mediacentre/factsheets/fs312/en/ (accessed: November 2017).
- [56].O’Sullivan BP, Freedman SD, Lancet 2009, 373, 1891. [DOI] [PubMed] [Google Scholar]
- [57].Verdine GL, Walensky LD, Clin. Cancer Res 2007, 13, 7264. [DOI] [PubMed] [Google Scholar]
- [58].Hopkins AL, Groom CR, Nat. Rev. Drug Discovery 2002, 1, 727. [DOI] [PubMed] [Google Scholar]
- [59] a).Kaczmarek JC, Kowalski PS, Anderson DG, Genome Med 2017, 9, 60; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL, Science 1990, 247, 1465. [DOI] [PubMed] [Google Scholar]
- [60].Whitehead KA, Langer R, Anderson DG, Nat. Rev. Drug Discovery 2009, 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61] a).Kauffman KJ, Webber MJ, Anderson DG, J. Controlled Release 2016, 240, 227; [DOI] [PubMed] [Google Scholar]; b) Sahin U, Kariko K, Tureci O, Nat. Rev. Drug Discovery 2014, 13, 759. [DOI] [PubMed] [Google Scholar]
- [62].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Nat. Rev. Genet 2014, 15, 541. [DOI] [PubMed] [Google Scholar]
- [63].Auffray C, Chen Z, Hood L, Genome Med. 2009, 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64] a).Kariko K, Buckstein M, Ni H, Weissman D, Immunity 2005, 23, 165; [DOI] [PubMed] [Google Scholar]; b) Whitehead KA, Dahlman JE, Langer RS, Anderson DG, Ann. Rev. Chem. Biomol. Eng 2011, 2, 77. [DOI] [PubMed] [Google Scholar]
- [65] a).Kanasty R, Dorkin JR, Vegas A, Anderson D, Nat. Mater 2013, 12, 967; [DOI] [PubMed] [Google Scholar]; b) Patel S, Ashwanikumar N, Robinson E, DuRoss A, Sun C, Murphy-Benenato KE, Mihai C, Almarsson O, Sahay G, Nano Lett. 2017, 17, 5711; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, Buganim Y, Schroeder A, Langer R, Anderson DG, Nat. Biotechnol 2013, 31, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66] a).Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA, Mol. Therapy 2006, 13, 151; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Piggott JM, Sheahan BJ, Soden DM, O’Sullivan GC, Atkins GJ, Mol. Med. Rep 2009, 2, 753. [DOI] [PubMed] [Google Scholar]
- [67].Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH, J. Cell Biol 1993, 123, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mehier-Humbert S, Guy RH, Adv. Drug Delivery Rev 2005, 57, 733. [DOI] [PubMed] [Google Scholar]
- [69].Hajj KA, Whitehead KA, Nat. Rev. Mater 2017, 2, 17056. [Google Scholar]
- [70] a).Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP, Nature 2004, 432, 173; [DOI] [PubMed] [Google Scholar]; b) Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B, Nat. Biotechnol 2005, 23, 1002. [DOI] [PubMed] [Google Scholar]
- [71].Wittrup A, Lieberman J, Nat. Rev. Genet 2015, 16, 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72] a).Bramsen JB, Laursen MB, Nielsen AF, Hansen TB, Bus C, Langkjaer N, Babu BR, Hojland T, Abramov M, Van Aerschot A, Odadzic D, Smicius R, Haas J, Andree C, Barman J, Wenska M, Srivastava P, Zhou C, Honcharenko D, Hess S, Muller E, Bobkov GV, Mikhailov SN, Fava E, Meyer TF, Chattopadhyaya J, Zerial M, Engels JW, Herdewijn P, Wengel J, Kjems J, Nucleic Acids Res 2009, 37, 2867; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chiu YL, Rana TM, RNA 2003, 9, 1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73] a).Prakash TP, Allerson CR, Dande P, Vickers TA, Sioufi N, Jarres R, Baker BF, Swayze EE, Griffey RH, Bhat B, J. Med. Chem 2005, 48, 4247; [DOI] [PubMed] [Google Scholar]; b) Li B, Luo X, Dong Y, Bio-conjugate Chem 2016, 27, 849; [DOI] [PubMed] [Google Scholar]; c) Kauffman KJ, Mir FF, Jhunjhunwala S, Kaczmarek JC, Hurtado JE, Yang JH, Webber MJ, Kowalski PS, Heartlein MW, DeRosa F, Anderson DG, Biomaterials 2016, 109, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Deleavey GF, Damha MJ, Chem. Biol 2012, 19, 937. [DOI] [PubMed] [Google Scholar]
- [75] a).Calabretta A, Kupfer PA, Leumann CJ, Nucleic Acids Res 2015, 43, 4713; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C, Cell 2015, 161, 1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Strenkowska M, Grzela R, Majewski M, Wnek K, Kowalska J, Lukaszewicz M, Zuberek J, Darzynkiewicz E, Kuhn AN, Sahin U, Jemielity J, Nucleic Acids Res. 2016, 44, 9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77] a).Merki E, Graham MJ, Mullick AE, Miller ER, Crooke RM, Pitas RE, Witztum JL, Tsimikas S, Circulation 2008, 118, 743; [DOI] [PubMed] [Google Scholar]; b) Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST, Lancet 2010, 375, 998. [DOI] [PubMed] [Google Scholar]
- [78].Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, Alfano L, Gomez AM, Lewis S, Kota J, Malik V, Shontz K, Walker CM, Flanigan KM, Corridore M, Kean JR, Allen HD, Shilling C, Melia KR, Sazani P, Saoud JB, Kaye EM, Eteplirsen Study G, Ann. Neurol 2013, 74, 637. [DOI] [PubMed] [Google Scholar]
- [79].Dolgin E, Nat. Biotechnol 2017, 35, 99. [DOI] [PubMed] [Google Scholar]
- [80].Geary RS, Henry SP, Grillone LR, Clin. Pharmacokinet 2002, 41, 255. [DOI] [PubMed] [Google Scholar]
- [81].Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, Yokota T, Mol. Ther 2008, 16, 734. [DOI] [PubMed] [Google Scholar]
- [82].Yu RZ, Gunawan R, Post N, Zanardi T, Hall S, Burkey J, Kim TW, Graham MJ, Prakash TP, Seth PP, Swayze EE, Geary RS, Henry SP, Wang Y, Nucleic Acid Ther 2016, 26, 372. [DOI] [PubMed] [Google Scholar]
- [83].Lorenz C, Hadwiger P, John M, Vornlocher HP, Unverzagt C, Bioorg. Med. Chem. Lett 2004, 14, 4975. [DOI] [PubMed] [Google Scholar]
- [84].Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA, Bioconjugate Chem. 2007, 18, 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xia CF, Boado RJ, Pardridge WM, Mol. Pharmaceutics 2009, 6, 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel’in AV, Milstein S, Taneja N, O’Shea J, Shaikh S, Zhang L, van der Sluis RJ, Jung ME, Akinc A, Hutabarat R, Kuchimanchi S, Fitzgerald K, Zimmermann T, van Berkel TJ, Maier MA, Rajeev KG, Manoharan M, J. Am. Chem. Soc 2014, 136, 16958. [DOI] [PubMed] [Google Scholar]
- [87].Li W, Szoka FC Jr., Pharm. Res 2007, 24, 438. [DOI] [PubMed] [Google Scholar]
- [88].Kotterman MA, Schaffer DV, Nat. Rev. Genet 2014, 15, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89] a). Gao X, Huang L, Biochem. Biophys. Res. Commun 1991, 179, 280; [DOI] [PubMed] [Google Scholar]; b) Katayose S, Kataoka K, Bioconjugate Chem. 1997, 8, 702. [DOI] [PubMed] [Google Scholar]
- [90].Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG, J. Intern. Med 2010, 267, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91] a).Kariko K, Muramatsu H, Keller JM, Weissman D, Mol. Ther 2012, 20, 948; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S, Huppmann M, Mays LE, Illenyi M, Schams A, Griese M, Bittmann I, Handgretinger R, Hartl D, Rosenecker J, Rudolph C, Nat. Biotechnol 2011, 29, 154. [DOI] [PubMed] [Google Scholar]
- [92].Rejman J, Tavernier G, Bavarsad N, Demeester J, De Smedt SC, J. Controlled Release 2010, 147, 385. [DOI] [PubMed] [Google Scholar]
- [93] a).Kauffman KJ, Dorkin JR, Yang JH, Heartlein MW, DeRosa F, Mir FF, Fenton OS, Anderson DG, Nano Lett. 2015, 15, 7300; [DOI] [PubMed] [Google Scholar]; b) Fenton OS, Kauffman KJ, McClellan RL, Appel EA, Dorkin JR, Tibbitt MW, Heartlein MW, DeRosa F, Langer R, Anderson DG, Adv. Mater 2016, 28, 2939; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, Fenton OS, Zhang Y, Olejnik KT, Yesilyurt V, Chen D, Barros S, Klebanov B, Novobrantseva T, Langer R, Anderson DG, Nat. Commun 2014, 5, 4277; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D, Nano Lett. 2017, 17, 1326; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Luo X, Li B, Zhang X, Zhao W, Bratasz A, Deng B, McComb DW, Dong Y, Nanoscale 2017, 9, 1575; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Zhang X, Li B, Luo X, Zhao W, Jiang J, Zhang C, Gao M, Chen X, Dong Y, ACS Appl. Mater Interfaces 2017, 9, 25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lu JJ, Langer R, Chen J, Mol. Pharmaceutics 2009, 6, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mui BL, Tam YK, Jayaraman M, Ansell SM, Du X, Tam YY, Lin PJ, Chen S, Narayanannair JK, Rajeev KG, Manoharan M, Akinc A, Maier MA, Cullis P, Madden TD, Hope MJ, Mol. Ther. Nucleic Acids 2013, 2, e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, Hoekstra D, Mol. Ther 2005, 11, 801. [DOI] [PubMed] [Google Scholar]
- [97] a).Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, Zhu H, Siegwart DJ, Angew. Chem 2017, 56, 1059; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ, Nat. Biotechnol 2010, 28, 172. [DOI] [PubMed] [Google Scholar]
- [98].Fenton OS, Kauffman KJ, Kaczmarek JC, McClellan RL, Jhunjhunwala S, Tibbitt MW, Zeng MD, Appel EA, Dorkin JR, Mir FF, Yang JH, Oberli MA, Heartlein MW, DeRosa F, Langer R, Anderson DG, Adv. Mater 2017, 29, 1606944. [DOI] [PubMed] [Google Scholar]
- [99].Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fouger olles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG, Nat. Biotechnol 2008, 26, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG, Proc. Natl. Acad. Sci. USA 2010, 107, 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101] a).Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, Merkel TJ, Luthi AJ, Patel PC, Cutler JI, Daniel WL, Scott AW, Rotz MW, Meade TJ, Giljohann DA, Mirkin CA, Stegh AH, Sci. Transl. Med 2013, 5, 209ra152; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Randeria PS, Seeger MA, Wang XQ, Wilson H, Shipp D, Mirkin CA, Paller AS, Proc. Natl. Acad. Sci. USA 2015, 112, 5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Iordanov H, Mirkin CA, Paller AS, Proc. Natl. Acad. Sci. USA 2012, 109, 11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103] a).Dahlman JE, Kauffman KJ, Langer R, Anderson DG, Adv. Genet 2014, 88, 37; [DOI] [PubMed] [Google Scholar]; b) Yan Y, Xiong H, Zhang X, Cheng Q, Siegwart DJ, Biomacromolecules 2017, 18, 4307. [DOI] [PubMed] [Google Scholar]
- [104] a).Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, Kjems J, Mol. Ther 2006, 14, 476; [DOI] [PubMed] [Google Scholar]; b) Guo J, Cheng WP, Gu J, Ding C, Qu X, Yang Z, O’Driscoll C, Eur. J. Pharm. Sci 2012, 45, 521. [DOI] [PubMed] [Google Scholar]
- [105] a).Behr JP, Chimia 1997, 51, 34; [Google Scholar]; b) Rehman Z, Zuhorn IS, Hoekstra D, J. Controlled Release 2013, 166, 46. [DOI] [PubMed] [Google Scholar]
- [106].Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP, Proc. Natl. Acad. Sci. US A 1995, 92, 7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107] a).Dahlman JE, Barnes C, Khan O, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, Bader A, Bogorad RL, Yin H, Racie T, Dong Y, Jiang S, Seedorf D, Dave A, Sandu KS, Webber MJ, Novobrantseva T, Ruda VM, Lytton-Jean AKR, Levins CG, Kalish B, Mudge DK, Perez M, Abezgauz L, Dutta P, Smith L, Charisse K, Kieran MW, Fitzgerald K, Nahrendorf M, Danino D, Tuder RM, von Andrian UH, Akinc A, Schroeder A, Panigrahy D, Kotelianski V, Langer R, Anderson DG, Nat. Nanotechnol 2014, 9, 648; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Khan OF, Zaia EW, Yin H, Bogorad RL, Pelet JM, Webber MJ, Zhuang I, Dahlman JE, Langer R, Anderson DG, Angew. Chem 2014, 53, 14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108] a).Anderson DG, Lynn DM, Langer R, Angew. Chem 2003, 42, 3153; [DOI] [PubMed] [Google Scholar]; b) Kaczmarek JC, Patel AK, Kauffman KJ, Fenton OS, Webber MJ, Heartlein MW, DeRosa F, Anderson DG, Angew. Chem 2016, 55, 13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang LL, Sloand JN, Gaffey AC, Venkataraman CM, Wang Z, Trubelja A, Hammer DA, Atluri P, Burdick JA, Bio-macromolecules 2017, 18, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Conde JO, Atliano N, Song M, Artzi HS, N., Nat. Mater 2016, 15, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111] a).Forbes DC, Peppas NA, J. Controlled Release 2012, 162, 438; [DOI] [PubMed] [Google Scholar]; b) Forbes DC, Peppas NA, Macromol. Biosci 2014, 14, 1096. [DOI] [PubMed] [Google Scholar]
- [112].Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, Park A, Yang J, Suresh S, Bizhanova A, Gupta A, Bolukbasi MF, Walsh S, Bogorad RL, Gao G, Weng Z, Dong Y, Koteliansky V, Wolfe SA, Langer R, Xue W, Anderson DG, Nat. Biotechnol 2016, 34, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yin H, Song CQ, Suresh S, Wu Q, Walsh S, Rhym LH, Mintzer E, Bolukbasi MF, Zhu LJ, Kauffman K, Mou H, Oberholzer A, Ding J, Kwan SY, Bogorad RL, Zatsepin T, Koteliansky V, Wolfe SA, Xue W, Langer R, Anderson DG, Nat. Biotechnol 2017, 35, 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Iwamoto T, Biol. Pharm. Bull 2013, 36, 715. [DOI] [PubMed] [Google Scholar]
- [115].Corrie PG, Medicine 2008, 36, 24. [Google Scholar]
- [116].Airley R, Cancer Chemotherapy, Wiley-Blackwell; 2009. [Google Scholar]
- [117] a).Langer R, Pharmacol. Ther 1983, 21, 35; [DOI] [PubMed] [Google Scholar]; b) Ren PW, Ju XJ, Xie R, Chu LY, J. Colloid Interface Sci 2010, 343, 392; [DOI] [PubMed] [Google Scholar]; c) Ma M, Chiu A, Sahay G, Doloff JC, Dholakia N, Thakrar R, Cohen J, Vegas A, Chen D, Bratlie KM, Dang T, York RL, Hollister-Lock J, Weir GC, Anderson DG, Adv. Healthcare Mater 2013, 2, 667; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Brigger ID, Dubernet C, Couvreur P, Adv. Drug Delivery Rev 2012, 64, 24. [DOI] [PubMed] [Google Scholar]
- [118].Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacik I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Nat. Mater 2015, 14, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Niinomi M, Metall. Mater. Trans. A 2002, 33, 477. [Google Scholar]
- [120].Conrad HJ, Seong WJ, Pesun IJ, J. Prosthet. Dent 2007, 98, 389. [DOI] [PubMed] [Google Scholar]
- [121].Kelly JR, Benetti P, Aust. Dent. J 2011, 56, 84. [DOI] [PubMed] [Google Scholar]
- [122] a).Stuart MA, Huck WT, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S, Nat. Mater 2010, 9, 101; [DOI] [PubMed] [Google Scholar]; b) Yan X, Wang F, Zheng B, Huang F, Chem. Soc. Rev 2012, 41, 6042; [DOI] [PubMed] [Google Scholar]; c) de Las Heras Alarcon C, Pennadam S, Alexander C, Chem. Soc. Rev 2005, 34, 276. [DOI] [PubMed] [Google Scholar]
- [123].Young RJL, Lovell PA, Introduction to Polymers, CRC Press, Boca Raton, FL, USA: 2011. [Google Scholar]
- [124].Kessel AB-T, Ben-Tal N, Introduction to Proteins: Structure, Function, and Motion, CRC Press, Boca Raton, FL, USA: 2010. [Google Scholar]
- [125].Peacock AJ, Handbook of Polyethylene: Structures, Properties, and Applications, Marcel Dekker Inc., USA: 2000. [Google Scholar]
- [126] a).Matyjaszewski K, Macromolecules 2012, 45, 4015; [Google Scholar]; b) Siegwart DJ, Oh JK, Matyjaszewski K, Prog. Polym. Sci 2012, 37, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, Perrier S, Chem. Rev 2009, 109, 5402. [DOI] [PubMed] [Google Scholar]
- [128].Nicolas JG, Guillaneuf Y, Lefay C, Bertin D, Gigmes D, Charleux B, Prog. Polym. Sci 2013, 38, 63. [Google Scholar]
- [129].Bielawski CWG, Grubbs RH, Prog. Polym. Sci 2007, 32, 1. [Google Scholar]
- [130].Gauthier MA, Gibson MI, Klok HA, Angew. Chem 2009, 48, 48. [DOI] [PubMed] [Google Scholar]
- [131] a).Mura S, Nicolas J, Couvreur P, Nat. Mater 2013, 12, 991; [DOI] [PubMed] [Google Scholar]; b) Zaffaroni A, Med. Res. Rev 1981, 1, 373; [DOI] [PubMed] [Google Scholar]; c) Folkman J, Long DM, J. Surg. Res 1964, 4, 139. [DOI] [PubMed] [Google Scholar]
- [132].Priya James HJ, John R, Alex A, Anoop KR, Acta Pharm. Sin. B 2014, 4, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wu G, Fang YZ, Yang S, Lupton JR, Turner ND, J. Nutr 2004, 134, 489. [DOI] [PubMed] [Google Scholar]
- [134].Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA, Nat. Mater 2004, 3, 183. [DOI] [PubMed] [Google Scholar]
- [135].Meng F, Hennink WE, Zhong Z, Biomaterials 2009, 30, 2180. [DOI] [PubMed] [Google Scholar]
- [136] a).Zhao M, Biswas A, Hu B, Joo KI, Wang P, Gu Z, Tang Y, Biomaterials 2011, 32, 5223; [DOI] [PubMed] [Google Scholar]; b) Miyata K, Kakizawa Y, Nishiyama N, Harada A, Yamasaki Y, Koyama H, Kataoka K, J. Am. Chem. Soc 2004, 126, 2355. [DOI] [PubMed] [Google Scholar]
- [137].Shim MS, Xia Y, Angew. Chem 2013, 52, 6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Broaders KE, Grandhe S, Frechet JM, J. Am. Chem. Soc 2011, 133, 756. [DOI] [PubMed] [Google Scholar]
- [139].Cao WW, Wang L, Xu H, Nano Today 2015, 10, 717. [Google Scholar]
- [140].Cheng G, He Y, Xie L, Nie Y, He B, Zhang Z, Gu Z, Int. J. Nanomed 2012, 7, 3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z, Biomaterials 2016, 85, 152. [DOI] [PubMed] [Google Scholar]
- [142].Schmaljohann D, Adv. Drug Delivery Rev 2006, 58, 1655. [DOI] [PubMed] [Google Scholar]
- [143].Lee H, Son SH, Sharma R, Won YY, J. Phys. Chem. B 2011, 115, 844. [DOI] [PubMed] [Google Scholar]
- [144] a).Sun W, Jiang T, Lu Y, Reiff M, Mo R, Gu Z, J. Am. Chem. Soc 2014, 136, 14722; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hoy MRR, Roche EJ, US Patent 5489436, 1996. [Google Scholar]
- [145].Chen LQ, Pagel MD, Adv. Radiol 2015, 2015, 206405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yang SC, Chen D, Li N, Mei X, Qi X, Li H, Xu Q, Lu J, J. Mater. Chem 2012, 22, 25354. [Google Scholar]
- [147].Fu QX, Xu J, Ladewig K, Henderson TMA, Qiao GG, Polym. Chem 2015, 6, 35. [Google Scholar]
- [148].Wang S, Wang H, Liu Z, Wang L, Wang X, Su L, Chang J, Nanoscale 2014, 6, 7635. [DOI] [PubMed] [Google Scholar]
- [149] a).Ulery BD, Nair LS, Laurencin CT, J. Polym. Sci., Part B: Polym. Phys 2011, 49, 832; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kamath KRP, Park K, Adv. Drug Delivery Rev 1993, 11, 59. [Google Scholar]
- [150] a).Gajanayake T, Olariu R, Leclere FM, Dhayani A, Yang Z, Bongoni AK, Banz Y, Constantinescu MA, Karp JM, Vemula PK, Rieben R, Vogelin E, Sci. Transl. Med 2014, 6, 249ra110; [DOI] [PubMed] [Google Scholar]; b) Kim HJ, Zhang K, Moore L, Ho D, ACS Nano 2014, 8, 2998; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jiang TM, Mo R, Bellotti A, Zhou J, Gu Z, Adv. Funct. Mater 2014, 24, 2295; [Google Scholar]; d) Biswas A, Joo KI, Liu J, Zhao M, Fan G, Wang P, Gu Z, Tang Y, ACS Nano 2011, 5, 1385; [DOI] [PubMed] [Google Scholar]; e) Gu Z, Yan M, Hu B, Joo KI, Biswas A, Huang Y, Lu Y, Wang P, Tang Y, Nano Lett. 2009, 9, 4533; [DOI] [PubMed] [Google Scholar]; f) Linderoth L, Peters GH, Madsen R, Andresen TL, Angew. Chem 2009, 48, 1823; [DOI] [PubMed] [Google Scholar]; g) Hu J, Zhang G, Liu S, Chem. Soc. Rev 2012, 41, 5933; [DOI] [PubMed] [Google Scholar]; h) Ulijn RV, J. Mater. Chem 2006, 23, 2217; [Google Scholar]; i) de la Rica R, Aili D, Stevens MM, Adv. Drug Delivery Rev 2012, 64, 967. [DOI] [PubMed] [Google Scholar]
- [151] a).Supper S, Anton N, Boisclair J, Seidel N, Riemenschnitter M, Curdy C, Vandamme T, Eur. J. Pharm. Biopharm 2014, 88, 361; [DOI] [PubMed] [Google Scholar]; b) Fujishige SK, Kubota K, Ando I, J. Phys. Chem 1989, 93, 3311; [Google Scholar]; c) Wiltsey C, Christiani T, Williams J, Scaramazza J, Van Sciver C, Toomer K, Sheehan J, Branda A, Nitzl A, England E, Kadlowec J, Iftode C, Vernengo J, Acta Biomater. 2015, 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152] a).Gandhi AP, Paul, Sen SO, Sen KK, Asian J. Pharm. Sci 2015, 10, 99; [Google Scholar]; b) Roy D, Brooks WL, Sumerlin BS, Chem. Soc. Rev 2013, 42, 7214. [DOI] [PubMed] [Google Scholar]
- [153] a).Chen CY, Kim TH, Wu WC, Huang CM, Wei H, Mount CW, Tian Y, Jang SH, Pun SH, Jen AK, Biomaterials 2013, 34, 4501; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kakkar AT, Traverso G, Farokhzad OC, Weissleder R, Langer R, Nat. Rev. Chem 2017, 8, 0063; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chilkoti A, Dreher MR, Meyer DE, Raucher D, Adv. Drug Delivery Rev 2002, 54, 613; [DOI] [PubMed] [Google Scholar]; d) Wang CF, Flynn NT, Langer R, Adv. Mater 2004, 13, 1074; [Google Scholar]; e) Meyer DE, Chilkoti A, Nat. Biotechnol 1999, 17, 1112. [DOI] [PubMed] [Google Scholar]
- [154] a).Arruebo MF-P, Fernandez-Pacheco R, Ibarra MR, Santamaria J, Nano Today 2007, 2, 22; [Google Scholar]; b) Hoare T, Santamaria J, Goya GF, Irusta S, Lin D, Lau S, Padera R, Langer R, Kohane DS, Nano Lett. 2009, 9, 3651; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kost J, Wolfrum J, Langer R, J. Biomed. Mater. Res 1987, 21, 1367; [DOI] [PubMed] [Google Scholar]; d) Ye F, Barrefelt A, Asem H, Abedi-Valugerdi M, El-Serafi I, Saghafian M, Abu-Salah K, Alrokayan S, Muhammed M, Hassan M, Biomaterials 2014, 35, 3885; [DOI] [PubMed] [Google Scholar]; e) Li X, Li H, Liu G, Deng Z, Wu S, Li P, Xu Z, Xu H, Chu PK, Biomaterials 2012, 33, 3013; [DOI] [PubMed] [Google Scholar]; f) Cheng R, Meng F, Deng C, Klok HA, Zhong Z, Bio-materials 2013, 34, 3647; [DOI] [PubMed] [Google Scholar]; g) Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T, Aoki I, Nishiyama N, Kataoka K, Nat. Nanotechnol 2016, 11, 724. [DOI] [PubMed] [Google Scholar]
- [155] a).Kondaveeti S, Cornejo DR, Petri DF, Colloids Surf. B 2016, 138, 94; [DOI] [PubMed] [Google Scholar]; b) Baez CAA, Aracely C, Cruz IEL, Padilla MCR, Gonzalez JMA, J. Nanotechnol 2014, 2014, 313415; [Google Scholar]; c) Sharma S, Chockalingam S, Sanpui P, Chattopadhyay A, Ghosh SS, Adv. Healthcare Mater 2014, 3, 106; [DOI] [PubMed] [Google Scholar]; d) Mir M, Ishtiaq S, Rabia S, Khatoon M, Zeb A, Khan GM, Ur Rehman A, Ud Din F,Nanoscale Res. Lett 2017, 12, 500; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Edelman ER, Kost J, Bobeck H, Langer R, J. Biomed. Mater. Res 1985, 19, 67. [DOI] [PubMed] [Google Scholar]
- [156].Mahdavinia GR, Etemadi H, Soleymani F, Carbohydr. Polym 2015, 128, 112. [DOI] [PubMed] [Google Scholar]
- [157].Anselmo AC, Mitragotri S, Bioeng. Transl. Med 2016, 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158] a).Mitragotri S, Nat. Rev. Drug Discovery 2005, 4, 255; [DOI] [PubMed] [Google Scholar]; b) Zakrewsky M, Mitragotri S, J. Controlled Release 2016, 242, 80; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ferrara K, Pollard R, Borden M, Annu. Rev. Biomed. Eng 2007, 9, 415; [DOI] [PubMed] [Google Scholar]; d) Chen WD, Du J, Sci. Rep 2013, 3, 2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159] a).Moncion A, Lin M, O’Neill EG, Franceschi RT, Kripfgans OD, Putnam AJ, Fabiilli ML, Biomaterials 2017, 140, 26; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Prausnitz MR, Mitragotri S, Langer R, Nat. Rev. Drug Discovery 2004, 3, 115. [DOI] [PubMed] [Google Scholar]
- [160] a).Geers BD, Dewitte H, De Smedt SC, Lentacker I, J. Controlled Release 2012, 164, 248; [DOI] [PubMed] [Google Scholar]; b) Liu HL, Fan CH, Ting CY, Yeh CK, Theranostics 2014, 4, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161] a).Iqbal D, Samiullah MH, Materials 2013, 6, 116; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim MS, Diamond SL, Bioorg. Med. Chem. Lett 2006, 16, 4007; [DOI] [PubMed] [Google Scholar]; c) Lin CC, Anseth KS, Pharm. Res 2009, 26, 631; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Science 2009, 324, 59; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Jochum FD, Theato P, Chem. Soc. Rev 2013, 42, 7468; [DOI] [PubMed] [Google Scholar]; f) Timko BP, Arruebo M, Shankarappa SA, McAlvin JB, Okonkwo OS, Mizrahi B, Stefanescu CF, Gomez L, Zhu J, Zhu A, Santamaria J, Langer R, Kohane DS, Proc. Natl. Acad. Sci. USA 2014, 111, 1349; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Lee TT, Garcia JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, Garcia AJ, Nat. Mater 2015, 14, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Marturano VV, Cerruti P, Giamberini M, Tylkowski B, Ambrogi V, Polymers 2017, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ercole FD, Davis TP, Evans RA, Polym. Chem 2010, 9, 1. [Google Scholar]
- [164].Pearson SV, Vitucci D, Khine YY, Dag A, Lu H, Save M, Billon L, Stenzel MH, Eur. Polym. J 2015, 69, 616. [Google Scholar]
- [165] a).Qazi TH, Rai R, Boccaccini AR, Biomaterials 2014, 35, 9068; [DOI] [PubMed] [Google Scholar]; b) Svirskis D, Travas-Sejdic J, Rodgers A, Garg S, Controlled Release J 2010, 146, 6. [DOI] [PubMed] [Google Scholar]
- [166].Simon DT, Kurup S, Larsson KC, Hori R, Tybrandt K, Goiny M, Jager EW, Berggren M, Canlon B, Richter-Dahlfors A, Nat. Mater 2009, 8, 742. [DOI] [PubMed] [Google Scholar]
- [167] a).Wang FL, Lai YH, Han MY, Macromolecules 2004, 37, 3222; [Google Scholar]; b) Izumi AN, Nomura R, Masuda T, Macrmolecules 2001, 34, 4342. [Google Scholar]
- [168] a).Ge J, Neofytou E, Cahill TJ 3rd, Beygui RE, Zare RN, ACS Nano 2012, 6, 227; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) George PM, LaVan DA, Burdick JA, Chen C, Liang E, Langer R, Adv. Mater 2006, 18, 577; [Google Scholar]; c) Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A, J. Pharm. Pharmacol 2007, 59, 311. [DOI] [PubMed] [Google Scholar]
- [169] a).Abidian MR, Kim DH, Martin DC, Adv. Mater 2006, 18, 405; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wadhwa R, Lagenaur CF, Cui XT, J. Controlled Release 2006, 110, 531; [DOI] [PubMed] [Google Scholar]; c) Balogh D, Tel-Vered R, Freeman R, Willner I, J. Am. Chem. Soc 2011, 133, 6533. [DOI] [PubMed] [Google Scholar]
- [170] a).Lei P, Padmashali RM, Andreadis ST, Biomaterials 2009, 30, 3790; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Khare AR, Peppas NA, J. Biomater. Sci., Polym. Ed 1993, 4, 275; [DOI] [PubMed] [Google Scholar]; c) Torres-Lugo MP, Peppas NA, Macromolecules 1999, 32, 6646; [Google Scholar]; d) Podual KP, Peppas NA, Polym. Int 2005, 54, 581; [Google Scholar]; e) Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM, Chem. Rev 1999, 99, 3181; [DOI] [PubMed] [Google Scholar]; f) Khare AR, Peppas NA, Biomaterials 1995, 16, 559; [DOI] [PubMed] [Google Scholar]; g) Podual KD, Doyle FJ, Peppas NA, Polymer 2000, 11, 3975; [Google Scholar]; h) Gu Z, Dang TT, Ma M, Tang BC, Cheng H, Jiang S, Dong Y, Zhang Y, Anderson DG, ACS Nano 2013, 7, 6758; [DOI] [PubMed] [Google Scholar]; i) Podual K, Doyle FJ 3rd, Peppas NA, J. Controlled Release 2000, 67, 9; [DOI] [PubMed] [Google Scholar]; j) Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z, Proc. Natl. Acad. Sci. US Am 2015, 112, 8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Campbell KT, Hadley DJ, Kukis DL, Silva EA, PloS One 2017, 12, e0181484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Janeway CA, 1989, 54, 1. [Google Scholar]
- [173].Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, Biomaterials Science: An Introduction to Materials in Medicine, Academic Press, USA: 2012. [Google Scholar]
- [174].Anderson JM, Rodriguez A, Chang DT, Semin. Immunol 2008, 20, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Andorko JI, Jewell CM, Bioeng. Transl. Med 2017, 2, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Williams DF, Biomaterials 2008, 29, 2941. [DOI] [PubMed] [Google Scholar]
- [177].Hotaling NA, Tang L, Irvine DJ, Babensee JE, Annu. Rev. Biomed. Eng 2015, 17, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Janeway CA, Travers P, Walport M, Shlomchik MJ, Immunobiology: The Immune System in Health and Disease, Garland Science, USA: 2001. [Google Scholar]
- [179].Medzhitov R, Nat. Rev. Immunol 2001, 1, 135. [DOI] [PubMed] [Google Scholar]
- [180] a).Eisenbarth SCC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA, Nature 2008, 453, 1122; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E, Nat. Immunol 2008, 9, 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Martinon F, Mayor A, Tschopp J, Annu. Rev. Immunol 2009, 27, 229. [DOI] [PubMed] [Google Scholar]
- [182].Hirano M, Das S, Guo P, Cooper MD, Adv. Immunol 2011, 109, 125. [DOI] [PubMed] [Google Scholar]
- [183].Murphy K, Travers P, Walport M, Janeway’s Immunobiology 7th Edition, Garland Science, USA: 2007. [Google Scholar]
- [184].Iwasaki A, Medzhitov R, Nat. Immunol 2015, 16, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Serhan CN, Chiang N, Dalli J, Levy BD, Cold Spring Harbor Perspect. Biol 2014, 7, a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN, Nature 2009, 461, 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA, Curr. Opin. Pharmacol 2009, 9, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Moore LB, Kyriakides TR, Adv. Exp. Med. Biol 2015, 865, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Klopfleisch R, Jung F, J. Biomed. Mater. Res., Part A 2017, 105, 927. [DOI] [PubMed] [Google Scholar]
- [190].Anderson JM, McNally AK, Sem. Immunol 2011, 33, 221. [DOI] [PubMed] [Google Scholar]
- [191].Goodman SB, Biomaterials 2007, 28, 5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Mitchell RN, Biomater. Sci 2013, II.2.3512. [Google Scholar]
- [193] a).Littman DR, Rudensky AY, Cell 2010, 140, 845; [DOI] [PubMed] [Google Scholar]; b) Josefowicz SZ, Lu LF, Rudensky AY, Annu. Rev. Immunol 2012, 30, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Rabinovich GA, Gabrilovich D, Sotomayor EM, Annu. Rev. Immunol 2007, 25, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Bryers JD, Giachelli CM, Ratner BD, Biotechnol. Bioeng 2012, 109, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Babensee JE, Paranjpe A, J. Biomed. Mater. Res., Part A 2005, 74, 503. [DOI] [PubMed] [Google Scholar]
- [197].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD, Jiang S, Nat. Biotechnol 2013, 31, 553. [DOI] [PubMed] [Google Scholar]
- [198].Dziki JL, Wang DS, Pineda C, Sicari BM, Rausch T, Badylak SF, J. Biomed. Mater. Res., Part A 2017, 105, 138. [DOI] [PubMed] [Google Scholar]
- [199].Wen Y, Waltman A, Han H, Collier JH, ACS Nano 2016, 10, 9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Gallorini S, Berti F, Parente P, Baronio R, Aprea S, D’Oro U, Pizza M, Telford JL, Wack A, J. Immunol 2007, 179, 8208. [DOI] [PubMed] [Google Scholar]
- [201] a).Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG, Nat. Biotechnol 2016, 34, 345; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vegas AJ, Veiseh O, Gurtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG, Nat. Med 2016, 22, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, Shirwan H, Garcia AJ, Sci. Adv 2017, 3, e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Barclay AN, Brown MH, Nat. Rev. Immunol 2006, 6, 457. [DOI] [PubMed] [Google Scholar]
- [204].Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE, Science 2013, 339, 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Murphy KN, Immunobiology, Garland Science, USA: 2012. [Google Scholar]
- [206].Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK, Proc. Natl. Acad. Sci. USA 2015, 112, E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Tostanoski LH, Chiu YC, Gammon JM, Simon T, Andorko JI, Bromberg JS, Jewell CM, Cell Rep. 2016, 16, 2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208] a).Serhan CN, FASEB J. 2017, 31, 1273; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Serhan CN, Chiang N, Dalli J, Semin. Immunol 2015, 27, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, Desai TA, Conte MS, J. Vasc. Surg 2017, 65, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Sok MCP, Tria MC, Olingy CE, San Emeterio CL, Botchwey EA, Acta Biomater 2017, 53, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokzhad O, Tabas I, Sci. Transl. Med 2015, 7, 275ra220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Aresta-Dasilva S, Griffin M, Jhunjhunwala S, Webber M, Siebert S, Tang K, Chen M, Langan E, Dholokia N, Thakrar R, Qi M, Oberholzer J, Greiner DL, Langer R, Anderson DG, Nat. Mater 2017, 16, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Chen EY, Chu S, Gov L, Kim YK, Lodoen MB, Tenner AJ, Liu WF, J. Mater. Chem. B 2017, 5, 1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ruella M, Kenderian SS, BioDrugs 2017, 31, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215] a).Lim WA, June CH, Cell 2017, 168, 724; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gill S, June CH, Immunol. Rev 2015, 263, 68; [DOI] [PubMed] [Google Scholar]; c) Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH, Sci. Transl. Med 2012, 4, 132ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].McHugh KJ, Nguyen TD, Linehan AR, Yang D, Behrens AM, Rose S, Tochka ZL, Tzeng SY, Norman JJ, Anselmo AC, Xu X, Tomasic S, Taylor MA, Lu J, Guarecuco R, Langer R, Jaklenec A, Science 2017, 357, 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT, Nat. Biotechnol 2015, 33, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD, Stephan MT, J. Clin. Invest 2017, 127, 2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Petrilli V, Tschopp J, O’Neill LA, Lavelle EC, Proc. Natl. Acad. Sci. USA 2009, 106, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Schanen BC, Karakoti AS, Seal S, Drake DR 3rd, Warren WL, Self WT, ACS Nano 2009, 3, 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Irvine DJ, Swartz MA, Szeto GL, Nat. Mater 2013, 12, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Gu L, Mooney DJ, Nat. Rev. Cancer 2016, 16, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ, Nat. Biotechnol 2015, 33, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224] a).Ali OAE, Emerich D, Dranoff G, Mooney DJ, Sci. Transl. Med 2009, 1, 8ra19; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ, Nat. Mater 2009, 8, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Dolgin E, Nature 2013, 504, S16. [DOI] [PubMed] [Google Scholar]
- [226].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Nature 2014, 507, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Kosmides AK, Meyer RA, Hickey JW, Aje K, Cheung KN, Green JJ, Schneck JP, Biomaterials 2017, 118, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Sunshine JC, Perica K, Schneck JP, Green JJ, Biomaterials 2014, 35, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S, J. Controlled Release 2015, 220, 141. [DOI] [PubMed] [Google Scholar]
- [230].Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, Ijiro K, Sawa H, ACS Nano 2013, 7, 3926. [DOI] [PubMed] [Google Scholar]
- [231].Kakizawa Y, Lee JS, Bell B, Fahmy TM, Acta Biomater. 2017, 57, 136. [DOI] [PubMed] [Google Scholar]
- [232].Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Husemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Tureci O, Sahin U, Nature 2016, 534, 396. [DOI] [PubMed] [Google Scholar]
- [233].Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, Proc. Natl. Acad. Sci. USA 2016, 113, E6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234] a).Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ, Nat. Med 2010, 16, 1035; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ, Biomaterials 2012, 33, 5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Jones RB, Mueller S, Kumari S, Vrbanac V, Genel S, Tager AM, Allen TM, Walker BD, Irvine DJ, Biomaterials 2017, 117, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ, ACS Nano 2017, 11, 3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237] a).Yu J, Xu X, Yao F, Luo Z, Jin L, Xie B, Shi S, Ma H, Li X, Chen H, Int. J. Pharm 2014, 470, 151; [DOI] [PubMed] [Google Scholar]; b) Fleming AB, Saltzman WM, Clin. Pharmacokinet 2002, 41, 403; [DOI] [PubMed] [Google Scholar]; c) Painbeni T, Venier-Julienne MC, Benoit JP, Eur. J. Pharm. Biopharm 1998, 45, 31; [DOI] [PubMed] [Google Scholar]; d) Chahal JSK, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S, Ploegh HL, Anderson DG, Proc. Natl. Acad. Sci. USA 2016, 113, E4133; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Xu J, Strandman S, Zhu JX, Barralet J, Cerruti M, Biomaterials 2015, 37, 395; [DOI] [PubMed] [Google Scholar]; f) Sung JC, Padilla DJ, Garcia-Contreras L, Verberkmoes JL, Durbin D, Peloquin CA, Elbert KJ, Hickey AJ, Edwards DA, Pharm. Res 2009, 26, 1847; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Bellinger A, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee YL, Mazdiyasni H, Kogan L, Barman R, Cleveland C, Booth L, Bensel T, Minahan D, Hurowitz HM, Tai T, Daily J, Nikolic B, Wood L, Eckhoff PA, Langer R, Traverso G, Sci. Transl. Med 2016, 8, 365ra157; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Mohideen M, Quijano E, Song E, Deng Y, Panse G, Zhang W, Clark MR, Saltzman WM, Biomaterials 2017, 144, 144; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238] a).Morales JO, Fathe KR, Brunaugh A, Ferrati S, Li S, Montenegro-Nicolini M, Mousavikhamene Z, McConville JT, Prausnitz MR, Smyth HDC, AAPS J 2017, 19, 652; [DOI] [PubMed] [Google Scholar]; b) Anselmo AC, Mitragotri S, Bioeng. Transl. Med 2016, 1, 10; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Malaterre V, Ogorka J, Loggia N, Gurny R, Eur. J. Pharm. Biopharm 2009, 73, 311; [DOI] [PubMed] [Google Scholar]; d) Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Pharm. Res 2016, 33, 2373. [DOI] [PubMed] [Google Scholar]