Abstract
Insulin degludec (IDeg) is a new insulin formulation that facilitates long-term control of glucose level in humans. In this study, we investigated the effects of IDeg on glycemic control in dogs. Its time-action profiles were monitored in healthy dogs using an artificial pancreas apparatus under euglycemic conditions. At 9.0–13.5 hr post-IDeg injection, an indistinct peak of glucose level was detected. Moreover, the action of IDeg was persistent for >20 hr. Both IDeg and neutral protamine Hagedorn insulin (NPH) lowered blood glucose concentrations in diabetic dogs, but IDeg caused postprandial hyperglycemia and a somewhat lower preprandial glucose level than that caused by NPH. IDeg might be ineffective in concurrently preventing postprandial hyperglycemia and preprandial hypoglycemia in a single-agent administration.
Keywords: glucose infusion rate, glucose-lowering effect, long-acting insulin
Insulin degludec (IDeg) is a new basal form of insulin that generates soluble multihexamers after subcutaneous injection, which results in an ultra-long duration of action to reduce glucose in human patients with diabetes [12, 16, 20]. IDeg has the same amino acid sequence as that of human insulin, with the exception of threonine at position 30 in the B chain, which has been removed (Des-B30), and a 16-carbon fatty diacid (hexadecanoic diacid) attached to lysine at position 29 via a glutamic acid linker [17, 18]. This formulation allows IDeg to form multiple hexamers that bind to albumin in the blood, which makes them dissociate slowly after subcutaneous injection and increases their duration of action [10]. In human studies, the half-life of IDeg is longer than that of insulin glargine after subcutaneous administration (25 vs. 12 hr, respectively), and IDeg has a duration of action of > 42 hr [5, 8, 9]. For this reason, there is a reduced risk of severe hypoglycemic episodes in humans undergoing IDeg therapy [3, 19]. However, the effect of IDeg has not been investigated in a veterinary scenario.
Insulin injection is commonly used as an effective treatment for long-term glycemic control in diabetic dogs [4]. Previously, our laboratory showed a clear difference among three other insulin preparations in healthy dogs by examining their time-action profiles. Insulin detemir (IDet) had the longest profile, followed by insulin glargine (IGla) and neutral protamine Hagedorn insulin (NPH) [11, 13]. However, the time-action profiles of IDeg have not been investigated in dogs.
Therefore, in this study, we investigated the pharmacodynamic properties of IDeg in dogs. First, to reveal the basal activity of IDeg in healthy dogs, we determined the time-action profile of subcutaneously injected IDeg. Second, we investigated whether IDeg could reduce glucose level in insulin-dependent diabetic dogs by comparing its effects against that of the most commonly used insulin preparation, NPH.
Five healthy dogs [three castrated males and two spayed females; body weight (BW): 6.6–13.5 kg; age: 3–8 years old] and four dogs with diabetes mellitus treated with insulin (one castrated, experimentally induced diabetic male beagle, two castrated male miniature dachshunds with juvenile-onset diabetes, and one spayed female miniature schnauzer with juvenile-onset diabetes; BW: 3.1–12.4 kg; age: 6–12 years old) served as the healthy and diabetic groups, respectively. Diabetes was induced in one dog by an intravenous administration of 25 mg/kg of Streptozotocin (10 nmol/l in Na-citrate buffer, pH 4.0) as previously described [2]. All diabetic dogs were confirmed with diabetes mellitus for >5 years and were defined with clinical signs (polyuria and polydipsia) and documentation of persistent fasting hyperglycemia (>250 mg/dl) and glucosuria. Before use in our study, the diabetic dogs were treated with subcutaneous injections of NPH (Eli Lilly Japan K.K., Kobe, Japan) at 0.26–0.41 units/kg twice daily (at 08:00 and 20:00 hr) to maintain fair-to-good diabetic control (serum glycated albumin of 18.4–26.6%) [14, 15]. Previously, our laboratory determined a normal reference range of 11.4–11.9% for serum Glycated Albumin (%) (95% C.I.) in normal healthy dogs [14, 15].
All dogs were fed a commercial diet (Select Protein, Royal Canin Japon, Tokyo, Japan) twice daily (at 08:00 and 20:00 hr) to maintain an ideal body condition score (a score of 5–6/9) and BW (kg). Caloric intake was set at 0.5 × (1.2–2.0) × RER (BW0.75 × 70), with RER referring to resting energy requirement in each feeding period. The Nippon Veterinary and Life Science University Animal Research Committee approved this study (Acceptance Number: 26S-61 and 27S-57).
To confirm the time-action profile of IDeg in healthy dogs, we used five dogs without diabetes. Prior to use, the healthy dogs were subjected to over 12 hr fasting. We then inserted catheters into the left cephalic vein to collect blood samples, and into the right cephalic vein to inject glucose with physiological saline. These catheters were connected to an artificial pancreas apparatus (Nikkiso STG-55, Nikkiso Co., Ltd., Tokyo, Japan) to monitor and adjust blood glucose concentration. After the artificial pancreas apparatus was connected, each dog was allowed to settle down for 30 min, during which blood glucose concentration stabilized to within the normal range (68–90 mg/dl). Each dog then received a single subcutaneous injection (0.5 units/kg) of IDeg under euglycemic glucose clamp conditions. Glucose infusion rates (GIR) (mg/kg/min) were recorded for up to 20 hr, relative to the rate of glucose administered (mg) per BW (kg) per min. This method followed that described by Sako et al. [13], which is considered to be a gold standard for measuring insulin time-action profiles in humans and dogs [6, 7, 11].
All four diabetic dogs were fasted for 12 hr prior to the experiments. Diabetic dogs were fed diet and injected with NPH or IDeg. Insulin was provided as a single injection twice daily at a 12-hr interval (at 08:00 and 20:00 hr) after feeding. To determine the proper insulin dose of NPH or IDeg for each diabetic dog, we conducted a preliminary examination before beginning this portion of our study. The initial NPH dose was determined on the basis of the latest daily insulin dose (0.26–0.41 units/kg twice a day), which was replicated for the initial IDeg dose. The ideal dose of insulin maintained the blood glucose concentration between 100 and 250 mg/dl throughout day and night [4]. However, IDeg is a very long-acting form of insulin with no pronounced peak effect; thus, it may be not effective in preventing both postprandial hyperglycemia and preprandial hypoglycemia. Therefore, we adjusted the insulin dose for each dog to meet the following conditions: (1) no clinical hypoglycemia, and (2) lowest blood glucose concentration of <150 mg/dl [4].
After two weeks of insulin dose adjustment in the preliminary examination, the main test was performed in two phases, with each phase lasting up to seven consecutive days. In the first phase (days 1–7), diabetic dogs were treated with various doses of NPH (0.26, 0.29, 0.40, or 0.41 units/kg) as a single injection twice daily at a 12-hr interval (08:00 and 20:00 hr). In the second phase (days 8–14), the same dogs were treated with various doses of IDeg (0.10, 0.21, 0.31, or 0.33 units/kg) as a single injection twice daily at the same time of day.
Preprandial blood samples were obtained from each dog via the jugular vein at days seven and fourteen (for the main test) and at 1-hr intervals between 0 and 12 hr after feeding. Blood samples for glucose measurement were collected into polypropylene tubes and allowed to clot at 20 ± 5°C for 15 min. After clotting, blood samples were centrifuged immediately at 1,700 × g for 10 min at 4°C to obtain serum samples, which were promptly stored at −80°C until further use. Serum glucose concentrations were measured using a Wako2 Glucose test commercial kit (Wako Pure Chemical Industries, Tokyo, Japan), according to the manufacturer’s protocol.
Data are presented as mean ± standard deviation (SD). Total area under the curve (AUC) between 0 and 20 hr represents glucose infusion value (mg/kg), which was calculated by the trapezoidal rule of GIR (mg/kg/min) × min [1]. To determine the percentage of time each dog was hyperglycemic or hypoglycemic throughout the day, we calculated the percentage of glucose values below 60 mg/dl or above 400 mg/dl from 13 blood glucose measurements. Statistical significance was determined using paired t-tests, one-way repeated measures ANOVA, or two-way repeated measures ANOVA, followed by the Bonferroni post-hoc test, (GraphPad Prism 5 analysis software, GraphPad Software, Inc., San Diego, CA, U.S.A.). Differences were considered to be significant at values of P<0.05.
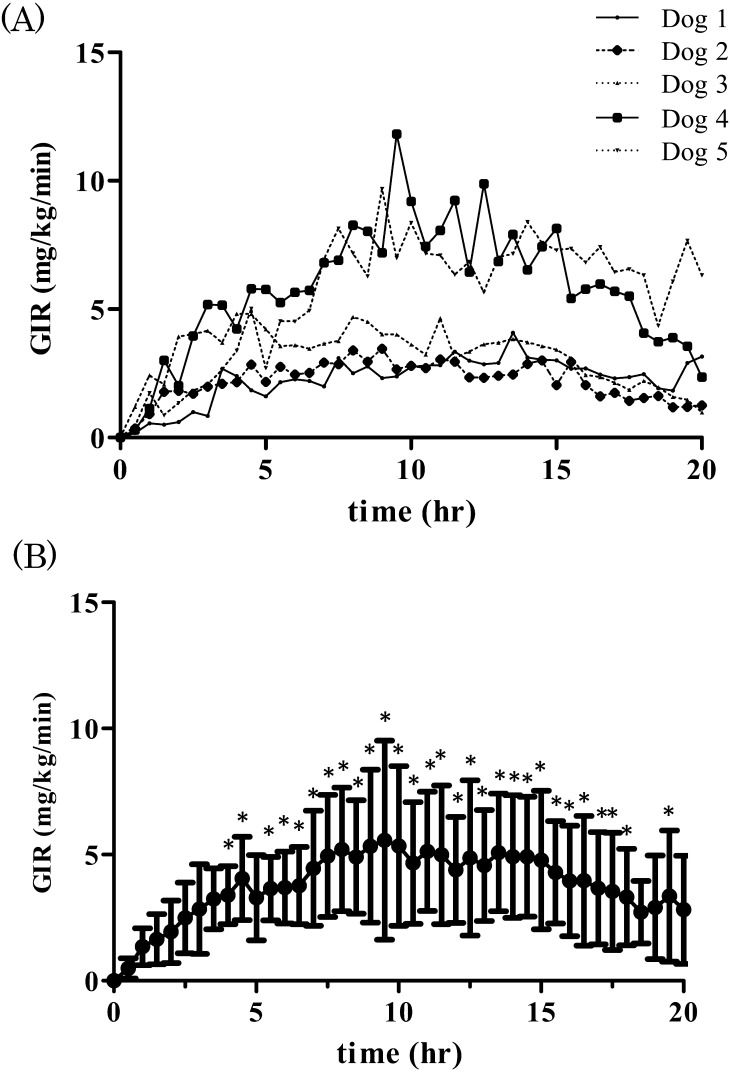
The time-action profiles of IDeg in individual dogs (Fig. 1A) and the mean ± SD of GIR after IDeg injection (Fig. 1B) are presented to show the variability of the action of IDeg. GIR increased significantly 4, 4.5, 5.5–18.0, and 19.5 hr after subcutaneous injection of IDeg (one-way repeated measures ANOVA and Bonferroni post-hoc test, P<0.05) (Fig. 1B). Moreover, insulin was persistent for >20 hr.
Fig. 1.
Time-action profiles of insulin degludec over a 20-hr period in each individual (A) and the mean ± SD (n=5) in healthy dogs (B). Results are presented as glucose infusion rate (GIR) over time after treatment with 0.5 units/kg insulin degludec. Higher values of GIR indicate stronger insulin action. Asterisk indicates significant difference (P<0.05, one-way repeated measures ANOVA and Bonferroni post-hoc test) compared to the value at 0 hr.
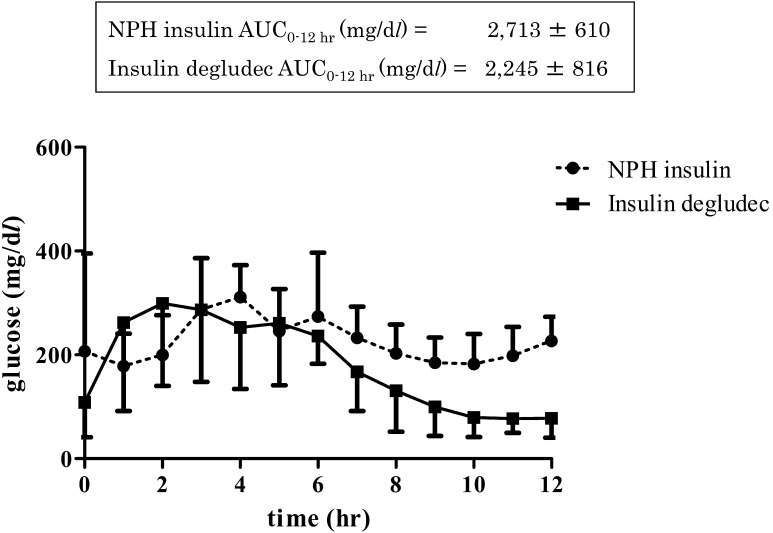
Diabetic dogs were maintained with injection of NPH at 0.34 ± 0.08 units/kg/day (min-max: 0.26–0.41) or IDeg at 0.24 ± 0.11 units/kg/day (0.10–0.33). Temporal analysis in the four diabetic dogs indicated no significant difference in glucose profiles between the IDeg and NPH, as analyzed by two-way repeated measures ANOVA (Fig. 2). In addition, no significant difference was observed between NPH and IDeg mean glucose AUC at 0–12 hr after administration, as analyzed by paired t-test (Fig. 2). Furthermore, there was no significant difference in mean glucose concentration, time spent in hyperglycemia (blood glucose >400 mg/dl), time spent in hypoglycemia (blood glucose <60 mg/dl), and maximum and minimum glucose concentrations between NPH and IDeg in the four diabetic dogs, as analyzed by paired t-test. IDeg maintained a mean glucose concentration of 180 ± 65 mg/dl, whereas NPH maintained 225 ± 53 mg/dl in all diabetic dogs. The maximum and minimum glucose concentrations were 356 ± 98 and 132 ± 70 mg/dl, respectively, after NPH treatment, and 362 ± 98 and 59 ± 20 mg/dl, respectively, after IDeg treatment. The percentage of time spent in glucose levels greater than 400 mg/dl for NPH and IDeg were 3.8 ± 7.7 and 11.5 ± 18.3%, respectively. Moreover, dogs treated with NPH and IDeg spent 0 and 11.5 ± 23.1% of time with glucose concentrations less than 60 mg/dl, respectively.
Fig. 2.
Comparison of glucose concentrations at 1-hr intervals (mean ± SD) after injection of neutral protamine Hagedorn insulin (NPH) or insulin degludec (IDeg) to four insulin-dependent diabetic dogs. Inset indicates total area under the curve (0–12 hr) of NPH and IDeg.
An indistinct peak was detected at 9.0–13.5 hr post-subcutaneous injection of IDeg to healthy dogs. At that point, the general effect of insulin was maintained during the observation period. Furthermore, we showed that the time-action profile of IDeg in healthy dogs lasted for more than 20 hr, confirming similar trends reported using IGla and IDet [11, 13]. Therefore, IDeg appeared to have a duration of action of >20 hr without any pronounced peak effects.
In our previous study, there is a clear difference in time-action profiles between NPH, IGla, and IDet in healthy dogs, even though all insulin preparations required time to show peak effects [11, 13]. In those studies, subcutaneous injection of those insulins caused distinct peaks of mean maximum GIR: 12 (at 5 hr post-NPH injection), 11 (at 7 hr post-IGla injection), and 20 mg/kg/min (at 7 hr post-IDet injection). In contrast, in the present study, the mean maximum GIR peaked at 5.5 mg/kg/min at 9.5 hr post-IDeg injection. Although it is difficult to compare these results because different healthy dogs were used in both studies, the mean maximum GIR following treatment with IDeg was less than half of that of the other drugs. Thus, it was indicated that IDeg had a long-lasting effects in modulating glucose levels, which may lead to its use as a novel treatment for diabetes in dogs.
Because mean glucose levels were similar in the NPH and IDeg groups (225 and 180 mg/dl, respectively), we determined that both insulin preparations can reduce glucose level in diabetic dogs. Furthermore, maximum glucose level was similar between both insulin preparations. However, minimum glucose level with IDeg (59 ± 20 mg/dl) was lower than that with NPH (132 ± 70 mg/dl, mean ± SD), though not significantly different. Furthermore, dogs treated with NPH did not show hypoglycemia (blood glucose <60 mg/dl) during the monitoring period. Meanwhile, mean temporal glucose concentration following IDeg administration showed minimum values preprandially (77 mg/dl at 11 hr) (Fig. 2). Furthermore, during the observation period (12 hr), hypoglycemia (<60 mg/dl) occurred 11.5% of the time after IDeg injection, although this was not significantly different than that caused by NPH. In healthy dogs, subcutaneously injected IDeg might be absorbed slowly and it showed a continuous glucose-lowering effect [5]. This continuous insulin action did not correspond to physiological postprandial insulin secretion in dogs. Therefore, IDeg did not concurrently prevent postprandial hyperglycemia and preprandial hypoglycemia in a single-agent administration in diabetic dogs.
Limitations of our study include the small number of dogs used and lack of repetition of experiments in both the healthy and diabetic groups. As such, because of the large biological variability among animals and the small sample size used in our study, the clinical efficiency of IDeg treatment might remain uncertain. Moreover, variation under external circumstances (multiple courses of blood sampling per day) may have led to stress-induced glycemic changes.
In conclusion, our results clearly showed that IDeg exhibited a long-lasting effect in healthy and diabetic dogs, similar to its effects in human patients. However, IDeg might be not be effective to concurrently prevent postprandial hyperglycemia and preprandial hypoglycemia in a single-agent administration in diabetic dogs.
REFERENCES
- 1.Altman D. G.1999. Some common problems in medical research. pp. 396–439. In: Practical Satistics for Medical Research, Chapman and Hall/CRC, Boca Raton. [Google Scholar]
- 2.Arai T., Nakamura M., Magori E., Fukuda H., Mizutani H., Kawakami E., Sako T.2002. Changes in activities of enzymes related to energy metabolism in peripheral leukocytes of diabetic dogs with glycemic control by intensive insulin treatment. Res. Vet. Sci. 73: 183–186. doi: 10.1016/S0034-5288(02)00104-2 [DOI] [PubMed] [Google Scholar]
- 3.Birkeland K. I., Home P. D., Wendisch U., Ratner R. E., Johansen T., Endahl L. A., Lyby K., Jendle J. H., Roberts A. P., DeVries J. H., Meneghini L. F.2011. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care 34: 661–665. doi: 10.2337/dc10-1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman E. C., Nelson R. W., Reusch C., Scoot-Moncrieff J. C.2015. Canine diabetes mellitus. pp. 213–257. In: Canine and Feline Endocrinology, 4th ed. [Google Scholar]
- 5.Haahr H., Heise T.2014. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin. Pharmacokinet. 53: 787–800. doi: 10.1007/s40262-014-0165-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinemann L., Linkeschova R., Rave K., Hompesch B., Sedlak M., Heise T.2000. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 23: 644–649. doi: 10.2337/diacare.23.5.644 [DOI] [PubMed] [Google Scholar]
- 7.Heise T., Nosek L., Rønn B. B., Endahl L., Heinemann L., Kapitza C., Draeger E.2004. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 53: 1614–1620. doi: 10.2337/diabetes.53.6.1614 [DOI] [PubMed] [Google Scholar]
- 8.Jonassen I., Havelund S., Hoeg-Jensen T., Steensgaard D. B., Wahlund P. O., Ribel U.2012. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm. Res. 29: 2104–2114. doi: 10.1007/s11095-012-0739-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtzhals P., Heise T., Strauss H., Bottcher S., Granhall C., Haahr H., Jonassen I.2011. Multi-hexamer formation is the underlying basis for the ultra-long glucose-lowering effect of insulin degludec. Diabetologia 54: S426. [Google Scholar]
- 10.Madhu S. V., Velmurugan M.2013. Future of newer basal insulin. Indian J. Endocrinol. Metab. 17: 249–253. doi: 10.4103/2230-8210.109690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori A., Sako T., Lee P., Motoike T., Iwase K., Kanaya Y., Fukuta H., Mizutani H., Arai T.2008. Comparison of time-action profiles of insulin glargine and NPH insulin in normal and diabetic dogs. Vet. Res. Commun. 32: 563–573. doi: 10.1007/s11259-008-9059-5 [DOI] [PubMed] [Google Scholar]
- 12.Russell-Jones D., Gall M. A., Niemeyer M., Diamant M., Del Prato S.2015. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: A meta-analysis of seven clinical trials. Nutr. Metab. Cardiovasc. Dis. 25: 898–905. doi: 10.1016/j.numecd.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 13.Sako T., Mori A., Lee P., Oda H., Saeki K., Miki Y., Kurishima M., Mimura K., Nozawa S., Mizutani H., Makino Y., Ishioka K., Arai T.2011. Time-action profiles of insulin detemir in normal and diabetic dogs. Res. Vet. Sci. 90: 396–403. doi: 10.1016/j.rvsc.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Sako T., Mori A., Lee P., Sato T., Mizutani H., Takahashi T., Kiyosawa Y., Tazaki H., Arai T.2009. Serum glycated albumin: Potential use as an index of glycemic control in diabetic dogs. Vet. Res. Commun. 33: 473–479. doi: 10.1007/s11259-008-9193-0 [DOI] [PubMed] [Google Scholar]
- 15.Sako T., Mori A., Lee P., Takahashi T., Izawa T., Karasawa S., Furuuchi M., Azakami D., Mizukoshi M., Mizutani H., Kiyosawa Y., Arai T.2008. Diagnostic significance of serum glycated albumin in diabetic dogs. J. Vet. Diagn. Invest. 20: 634–638. doi: 10.1177/104063870802000517 [DOI] [PubMed] [Google Scholar]
- 16.Shimoda S., Sato M., Sekigami T., Motoshima H., Yoshimura R., Fukuda K., Matsuo Y., Noda H., Okubo M., Ichimori S., Fujisawa K., Fukunaga M., Araki E., Kumamoto Insulin Degludec Observational (KIDUNA) Study Group. 2016. A 1-year, prospective, observational study of Japanese outpatients with type 1 and type 2 diabetes switching from insulin glargine or detemir to insulin degludec in basal-bolus insulin therapy (Kumamoto Insulin Degludec Observational study). J. Diabetes Investig. 7: 703–710. doi: 10.1111/jdi.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steensgaard D. B., Schluckebier G., Strauss H. M., Norrman M., Thomsen J. K., Friderichsen A. V., Havelund S., Jonassen I.2013. Ligand-controlled assembly of hexamers, dihexamers, and linear multihexamer structures by the engineered acylated insulin degludec. Biochemistry 52: 295–309. doi: 10.1021/bi3008609 [DOI] [PubMed] [Google Scholar]
- 18.Tambascia M. A., Eliaschewitz F. G.2015. Degludec: the new ultra-long insulin analogue. Diabetol. Metab. Syndr. 7: 57. doi: 10.1186/s13098-015-0037-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thuillier P., Alavi Z., Kerlan V.2015. Long-term safety and efficacy of insulin degludec in the management of type 2 diabetes. Diabetes Metab. Syndr. Obes. 8: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urakami T., Mine Y., Aoki M., Okuno M., Suzuki J.2017. A randomized crossover study of the efficacy and safety of switching from insulin glargine to insulin degludec in children with type 1 diabetes. Endocr. J. 64: 133–140. doi: 10.1507/endocrj.EJ16-0294 [DOI] [PubMed] [Google Scholar]