Abstract
Stability of α-amylase (α-A), butyrylcholinesterase (BChE), lipase, adenosine deaminase (ADA) and total esterase activity (TEA) in two pools of porcine saliva was studied after 1 and 4 days at 4°C, and after 30, 90 and 360 days at −20° and −80°C. At 4°C, BChE, lipase and TEA were stable less than 1 day, α-A less than 4 days and ADA for up to 4 days. At −20°C, BChE and TEA were stable less than 30 days, α-A and lipase less than 90 days and ADA up to 360 days. At −80°C, TEA was stable less than 30 days, α-A and lipase less than 360 days, and BChE and ADA for up to 360 days.
Keywords: enzymes, pig, saliva, stability, storage condition
Saliva is a biological fluid with increasing applications in Veterinary Medicine and Animal Production because it can be easily collected with minimum stress to the animals. Therefore, many studies have been performed in order to know whether saliva could be used as an alternative to blood, especially in species where blood is difficult to obtain such as pigs [4, 11].
Saliva has an abundant enzymatic activity, since it contains enzymes that can be originated from salivary glands, crevicular gingival fluid, polymorphonuclear leukocytes, epithelial cells, dietary constituents and oral microorganisms [1]. Some of these enzymes have been proven to have potential to be considered as biomarkers of stress or inflammation. For example, salivary alpha-amylase (α-A, E.C. number 3.2.1.1) is related to sympathetic activation both in humans [15] and pigs [6, 13]. In addition, increases in salivary total esterase activity (TEA) have been associated with acute stress and physical exercise in humans [16] and pigs [18]. In pigs, butyrylcholinesterase (BChE, E.C 3.1.1.8) and lipase (E.C. 3.1.1.3) are among the main components of TEA and both increase after stress conditions [17, 18]. On the other hand, TEA, lipase or adenosine deaminase (ADA, E.C. 3.5.4.4) can increase in inflammatory conditions in pigs such as lameness [18, 19].
In spite of the potential use of those enzymatic biomarkers for assessing stress and health status of pigs, their stability in saliva can represent an important limitation for their routine use in practice. Saliva is a non sterile fluid that contains bacteria and proteolytic enzymes that could produce enzyme degradation, affecting the integrity and correct quantification of potential salivary biomarkers. In humans, it has been described that cooling of samples on ice just after collection could protect salivary components from degradation in the first hour until arriving at laboratory to be processed, but this protection is not indefinite [20]. The knowledge of the stability of the analytes in saliva can be really a key point in cases when samples have to be shipped to an external laboratory, they are planned to be stored during long time or even for comparison of results from different experiments. The main aim of this research was to study the stability of the enzymes α-A, BChE, lipase, ADA and TEA in porcine saliva under different storage conditions (refrigerated at 4°C and frozen at −20° or −80°C) in order to evaluate the possible influence of storage conditions on enzyme activities and to determine the optimal way in which each enzyme should be stored.
Saliva was collected from 17 crossbred growing pigs [(Sus scrofa domesticus) (Large White × Large White)]. All animals were vaccinated against Mycoplasma hyopneumoniae (Stellamune Mycoplasma, inactivated Mycoplasma hyopneumoniae NL 1042, Pfizer Animal Health, Madrid, Spain) and Porcine circovirus type 2 (Porcilis® PCV, MSD Animal Health, Boxmeer, The Netherlands) during the phase of lactation. All animals were males in the last phase of fattening with 4 months of age and were housed in the Experimental Farm of the University of Murcia (Murcia, Spain). Pigs were given ad libitum access to a nutritionally balanced diet and water. The animals were housed in pens with a minimum space of 0.65 m2 per animal (Council of Europe. ETS 123. Strasbourg, France: The Council; 1986. [Last accessed January 5, 2014]. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. http://conventions.coe.int/treaty/en/treaties/html/123.htm) and an average temperature of 23 ± 2°C.
From the total of 17 pigs, 10 animals were considered as healthy since they had no evidence of pathology after visual and clinical examinations. The remaining 7 pigs had lameness that was detected because they were not able to set the foot on the ground or to remain standing. The 7 lame animals appeared as a consequence of injuries or fights that sometimes occur in porcine farms. According to local law (RD 1135/2002) those animals were separately housed from the rest in an isolation pen until total recovery; otherwise they were sent to the slaughterhouse.
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Bioethical Commission of Murcia University, Spain) and with the applicable international guidelines for the care and use of animals (European Council Directives regarding the protection of animals used for experimental purposes).
Saliva was collected using Salivette tubes (Sarstedt, Aktiengesellschaft & Co. D-51588 Nümbrecht, Germany) containing a sponge instead of cotton swab (because sponge was less absorbent and released more saliva following centrifugation). The sampled pigs were allowed to chew the sponge, which was clipped to a flexible thin metal rod, until thoroughly moist. Then, the sponges were placed into the Salivette tubes. Salivettes were maintained in ice until arrival at laboratory for processing. Then, the tubes were centrifuged at 3,500 g and 4°C for 10 min to obtain saliva. In order to avoid any possible influence of circadian rhythm in the selected parameters to be measured, all animals were sampled in the same day between 8.00 and 9.00 am. Elapse time before arriving at laboratory for processing was always less than 2 hr.
Two pools of saliva with different enzymatic activities were performed: 500 µl of saliva from each healthy pig were mixed to obtain pool A with low enzymatic activities (5 ml total volume); and 500 µl of saliva from each lame pig were used to perform pool B with high enzymatic activities (3.5 ml total volume) since previous works reported higher TEA, lipase [18] and ADA activities [19] in lame pigs than in healthy ones. Pools A and B were analyzed freshly for the different enzymatic activities. Then, pools were separated into aliquots. The aliquots were kept at three different storage temperatures: 4°, −20° and −80°C. For short-term storage, samples at 4°C were analyzed 1 day and 4 days after sample collection. For long-term storage, samples stored at −20° and −80°C were analyzed at 30, 90 and 360 days after collection. For each time, five aliquots from pool A and five from pool B were analyzed.
α-A was determined by a commercial kit (a-Amylase, OSR6182, Beckman Coulter Inc., Fullerton, CA, U.S.A.) previously validated in pigs [7]; intra-assay coefficient of variation (CV) for this method is 2.1%. BChE was analyzed as previously described [17]; intra-assay CV for this assay is 4.6%. Lipase was determined by a commercially available spectrophotometric method (Lipase, OSR6130, Beckman Coulter Inc.) according to manufacturer’s guidelines; Intra-assay CV for lipase assay is 2.49%. ADA was measured as previously described [19] using a spectrophotometric automated assay which is commercially available (Adenosine Deaminase assay kit, Diazyme Laboratories, Poway, CA, U.S.A.); Intra-assay CV for this assay is 8.3%. TEA was analyzed as previously reported [18]; the intra-assay CV for this assay is 2.6%. All measurements were performed at 37°C in an automated analyzer (Olympus AU400, Olympus Diagnostica GmbH, Ennis, Ireland).
For statistical analysis, enzymatic activities were calculated as percentage of the baseline activities (fresh sample=100% activity). After then, data were evaluated for normality of distribution, using Kolmogorov-Smirnov tests, giving a non-normal distribution. Data were naturally log transformed to assume normal distribution. Two-way ANOVA of repeated measures followed by Dunnett’s multiple comparison test was used to assess whether the percentage of change observed in enzymatic activities were statistically significant between pools and after storage at different temperatures with respect to initial values. Changes that exceeded two intra-assay CV of the assay and had significant differences over time in relation to baseline activity were considered to indicate a lack of acceptable stability for given storage conditions [8]. A value of P<0.05 was used to indicate significance in all analyses. Data analyses were performed using spreadsheet (Excel 2000, Microsoft Corporation, Redmond, WA, U.S.A.) and Graph Pad Software Inc. (GraphPad Prism, version 5 for Windows, Graph Pad Software Inc., San Diego, CA, U.S.A.).
The mean enzymatic activities obtained for the day of collection, respectively for the five aliquots of the pool A vs the five aliquots of the pool B were 353.1 ± 0.8 vs 686.4 ± 4.1 IU/l for α-A activity, 316.5 ± 2.3 vs 865.6 ± 7.2 nmol/ml/min for BChE, 127.8 ± 2.2 vs 151.0 ± 0.9 IU/l for lipase, 713.4 ± 7.8 vs 1,925.0 ± 26.9 IU/l for ADA and 109.1 ± 0.9 vs 216.4 ± 3.6 IU/l for TEA.
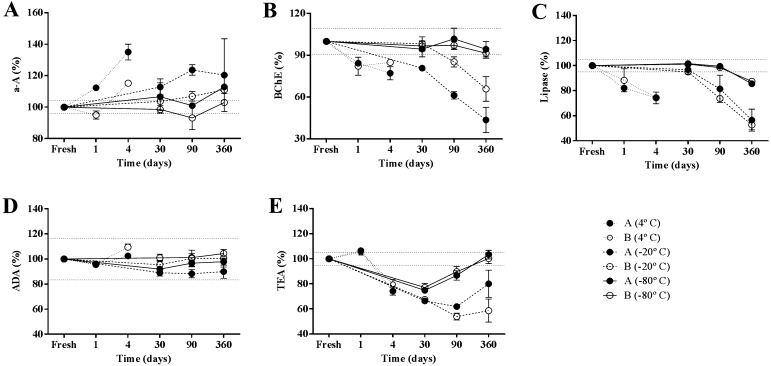
The results obtained for the different enzymatic activities appear in Fig. 1. α-A activity are shown in Fig. 1A. At 4°C, significant increases (P<0.0001 and P<0.01, respectively for pools A and B) at 4 days, exceeding 2 intra-assay CV, were observed. A significant difference was observed between both pools and pool B (with high activity) seemed to be more stable than pool A (P<0.0001). The reproducibility of these results was confirmed by an additional analysis using other four saliva samples (data not shown). The reason for α-A activity increase is unknown and should be further studied, although a bacterial α-A production could be postulated since some bacteria are able to synthesize α-A [2] and bacteria is an usual component of saliva. In addition, possible changes after storage in different α-A isoforms that would have different levels of enzymatic activity could be another possible explanation that should be further studied. It seems that this analyte could be more stable in human saliva, since α-A activity has been found stable for 5 days at room temperature [12] or for 10 days at room temperature or 4°C [14]. Some factors that could influence the higher stability of α-A activity at 4°C in humans compared to pigs could be the different microbial composition, or the fact that salivary specimens in pigs can frequently be contaminated by food or other substances during the collection procedure, which could influence stability. When samples were stored at −20°C, a significant increase was observed at 90 days (P<0.01) and onwards, that was greater than 2CV in both pools, without significant differences between them. At −80°C, a significant increase higher than 2CV was observed at 360 days of storage for pool A (P<0.01) whereas no significant changes were observed for pool B after 360 days, being stability significantly different between both pools (P<0.01). Therefore, it seemed that at −80°C samples with high α-A could be stored up to 360 days, whereas they should be analyzed prior 360 days for those samples with low α-A activity. It could be postulated that the similar mechanisms indicated for explaining the changes of α-A at 4°C could be also involved in the changes observed in frozen samples.
Fig. 1.
Stability of A) α-amylase (α-A), B) butyrylcholinesterase (BChE), C) lipase, D) adenosine deaminase (ADA), and E) total esterase activity (TEA), in two pools of porcine saliva with low (A) and high (B) enzymatic activities stored at different temperatures. Results are expressed in percentages of the baseline activity (fresh sample=100% activity). Each point represents the mean value obtained from five replicates of each pool at any different storage condition and error bars represent the standard deviation of the five replicates. Doted lines indicate 100% ± 2*coefficient of variation of the test.
The results observed for BChE are shown in Fig. 1B. A significant decrease exceeding 2 intra-assay CV was observed after 1 day at 4°C and onwards (P<0.001) in both pools A and B. At −20°C, this enzyme significantly decreased at 30 days in pool A (P<0.01) and at 90 days in pool B (P<0.05) being the changes greater than 2CV. At −80°C, BChE significantly decreased at 360 days only in pool B (P<0.01), although this change was within the range of 2 intra-assay CV. Therefore, at −80°C samples could be stored for up to 1 year for BChE determination. Significant differences were observed between pools A and B (P<0.001) at −20°C but not at −80°C. No studies have been found regarding stability of BChE in saliva of any other species.
Lipase results are shown in Fig. 1C. At 4°C, lipase significantly decreased after the first day of storage in pool A (P<0.01) and pool B (P<0.05), being this change out of the range of 2 intra-assay CV. In frozen samples, significant decrease exceeding 2CV was observed at 90 days of storage at −20°C (P<0.001 and P<0.0001, respectively for pool A and B) and at 360 days of storage at −80°C (P<0.0001 for both pools), without difference in stability between pools A and B. Therefore, at −80°C samples could be stored for 90 days for lipase determinations. No studies have been found regarding lipase stability in saliva of any other species.
Figure 1D shows the results obtained for ADA activity. At 4°C, ADA significantly decreased in both pools (P<0.05) after 1 day, followed by an increase in pool B (P<0.001); although since these changes did not exceed 2 intra-assay CV, the enzyme could be considered as stable at this temperature for up to 4 days. At −20°C significant differences were observed between pools (P<0.01), with pool A showing a significant decrease at 30 days (P<0.01), whereas no significant changes were observed in pool B. These changes did not exceed 2CV. At −80°C, a significant decrease was observed in pool A at 30 days of storage (P<0.001) although these changes did not exceed the interval of 2CV of the assay, whereas no changes were observed in pool B. Therefore, at −80°C samples could be stored for up to 1 year for ADA measurement. A significant difference was observed between pools (P<0.01). In a previous study, ADA activity in porcine saliva has been considered as stable for up to 7 months at −80°C [10].
The results obtained for TEA are shown in Fig. 1E. TEA significantly increased after 1 day of storage at 4°C in pool A (P<0.05) and then decreased in both pools at day 4 (P<0.001). These changes exceeded 2 intra-assay CV. No significant differences were observed between both pools. The reproducibility of these results was again confirmed by the analysis of the four saliva samples measured to corroborate α-A results (data not shown). Significant decreases (P<0.0001) higher than 2CV were observed after 30 days in all frozen samples, although TEA increased and values similar to initial ones were detected in pools stored at −80°C, at 360 days. These results were confirmed by analyzing a group of banked porcine saliva samples stored for different periods at −80°C (three samples stored for 30 days, five for 90 days, two for 240 days and two for 360 days) and the values were compared with those obtained the day of collection (data not shown). Based on these results, TEA seemed to be a labile enzymatic activity that should be analyzed before 30 days of storage at −20 or −80°C. It is difficult to know the reason for the changes in TEA due to its heterogeneity in composition. Since BChE and lipase are contributors to TEA activity and they were not affected at 30 days of storage, it could be postulated that carbonic anhydrase VI or other enzymes that could be contributors to TEA in pigs [18] could be the responsible for these changes, although further studies should be made to corroborate this fact and elucidate the cause of the changes. Significant differences were observed between stability of pools A and B at −20°C (P<0.0001), but stability was similar for both pools at −80°C.
In general, at 4°C the enzymes showed low stability, with the exception of ADA that could remain stable for up to 4 days. The lack of stability of enzymes in refrigerated saliva samples is in line with other analytes in porcine saliva such as chromogranin-A that should be analyzed prior to 2 days when samples are maintained at 4°C [5]. When frozen, enzyme activities were in general more stable at −80° than at −20°C. This fact has been proven for different analytes in porcine saliva such as C-reactive protein and haptoglobin [9]. In addition, in a recent study performed in human salivary samples, several enzymes such as salivary acid phosphatase, tartrate-resistant acid phosphatase, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase showed no significant changes after 28 days at −80°C, whereas significant reductions in all enzyme activities were observed after 14 days at −20°C [3]. However, other analytes such as chromogranin A concentration are equally stable for 1 year at −20 or −80°C in porcine saliva samples [5]. It is important to point out that stability at −80°C was statistically similar for pools A and B, with the exception of α-A and ADA, although in the case of ADA activity the observed changes did not exceed 2CV and therefore they could be attributed to the normal variability of the test. In addition, when all enzymatic activities were corrected by the protein content in the pools, the results were similar to those obtained without correction (data not shown).
From these results it could be concluded that pig saliva samples should be analyzed for enzymatic activities as soon as possible, or frozen at −80°C for long-term storage. Further studies should be done in order to try to improve the short-term storage of the saliva samples, by use of protease inhibitors or other stabilizers that could allow to measure enzymes after 24 hr of storage at 4°C.
Acknowledgments
This study has been granted by the Seneca Foundation of Murcia Region (19894/GERM/15). D. ESCRIBANO was granted by the postdoctoral program ‘Juan de la Cierva’ of the ‘Ministerio de Economía y Competitividad’, Spain. M.D. CONTRERAS-AGUILAR was granted by the predoctoral contract ‘FPU’ of University of Murcia (R-605/2016), Spain.
REFERENCES
- 1.Chauncey H. H.1961. Salivary enzymes. J. Am. Dent. Assoc. 63: 360–368. doi: 10.14219/jada.archive.1961.0215 [DOI] [PubMed] [Google Scholar]
- 2.Divakaran D., Chandran A., Pratap Chandran R.2011. Comparative study on production of a-Amylase from Bacillus licheniformis strains. Braz. J. Microbiol. 42: 1397–1404. doi: 10.1590/S1517-83822011000400022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dos Santos D. R., Souza R. O., Dias L. B., Ribas T. B., de Oliveira L. C. F., Sumida D. H., Dornelles R. C. M., Nakamune A. C. M. S., Chaves-Neto A. H.2018. The effects of storage time and temperature on the stability of salivary phosphatases, transaminases and dehydrogenase. Arch. Oral Biol. 85: 160–165. doi: 10.1016/j.archoralbio.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 4.Escribano D., Fuentes-Rubio M., Cerón J. J.2012. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Invest. 24: 918–923. doi: 10.1177/1040638712455171 [DOI] [PubMed] [Google Scholar]
- 5.Escribano D., Gutiérrez A. M., Fuentes-Rubio M., Cerón J. J.2014. Saliva chromogranin A in growing pigs: a study of circadian patterns during daytime and stability under different storage conditions. Vet. J. 199: 355–359. doi: 10.1016/j.tvjl.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 6.Escribano D., Gutiérrez A. M., Tecles F., Cerón J. J.2015. Changes in saliva biomarkers of stress and immunity in domestic pigs exposed to a psychosocial stressor. Res. Vet. Sci. 102: 38–44. doi: 10.1016/j.rvsc.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 7.Fuentes M., Tecles F., Gutiérrez A., Otal J., Martínez-Subiela S., Cerón J. J.2011. Validation of an automated method for salivary alpha-amylase measurements in pigs (Sus scrofa domesticus) and its application as a stress biomarker. J. Vet. Diagn. Invest. 23: 282–287. doi: 10.1177/104063871102300213 [DOI] [PubMed] [Google Scholar]
- 8.Gröschl M., Wagner R., Rauh M., Dörr H. G.2001. Stability of salivary steroids: the influences of storage, food and dental care. Steroids 66: 737–741. doi: 10.1016/S0039-128X(01)00111-8 [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez A. M., Martínez-Subiela S., Cerón J. J.2011. Evaluation of changes in haptoglobin and C-reactive protein concentrations caused by freezing of saliva and meat juice samples collected from healthy and diseased pigs. Am. J. Vet. Res. 72: 11–17. doi: 10.2460/ajvr.72.1.11 [DOI] [PubMed] [Google Scholar]
- 10.Gutiérrez A. M., De La Cruz-Sánchez E., Montes A., Sotillo J., Gutiérrez-Panizo C., Fuentes P., Tornel P. L., Cabezas-Herrera J.2017. Easy and non-invasive disease detection in pigs by adenosine deaminase activity determinations in saliva. PLoS One 12: e0179299. doi: 10.1371/journal.pone.0179299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzik A. C., Matthews J. O., Kerr B. J., Bidner T. D., Southern L. L.2006. Dietary tryptophan effects on plasma and salivary cortisol and meat quality in pigs. J. Anim. Sci. 84: 2251–2259. doi: 10.2527/jas.2005-292 [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell K., Kammerer M., O’Reilly R., Taylor A., Glover V.2009. Salivary alpha-amylase stability, diurnal profile and lack of response to the cold hand test in young women. Stress 12: 549–554. doi: 10.3109/10253890902822664 [DOI] [PubMed] [Google Scholar]
- 13.Ott S., Soler L., Moons C. P., Kashiha M. A., Bahr C., Vandermeulen J., Janssens S., Gutiérrez A. M., Escribano D., Cerón J. J., Berckmans D., Tuyttens F. A., Niewold T. A.2014. Different stressors elicit different responses in the salivary biomarkers cortisol, haptoglobin, and chromogranin A in pigs. Res. Vet. Sci. 97: 124–128. doi: 10.1016/j.rvsc.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Park J. R., Kim M. H., Woo J., Lee S. J., Song K. E.2008. [Measurement of amylase in saliva collected by salivette]. Korean J. Lab. Med. 28: 438–443 (in Korean). doi: 10.3343/kjlm.2008.28.6.438 [DOI] [PubMed] [Google Scholar]
- 15.Rohleder N., Nater U. M.2009. Determinants of salivary α-amylase in humans and methodological considerations. Psychoneuroendocrinology 34: 469–485. doi: 10.1016/j.psyneuen.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Tecles F., Tvarijonaviciute A., De Torre C., Carrillo J. M., Rubio M., García M., Cugat R., Cerón J. J.2016. Total esterase activity in human saliva: Validation of an automated assay, characterization and behaviour after physical stress. Scand. J. Clin. Lab. Invest. 76: 324–330. doi: 10.3109/00365513.2016.1163417 [DOI] [PubMed] [Google Scholar]
- 17.Tecles F., Escribano D., Martínez-Miró S., Hernández F., Contreras M. D., Cerón J. J.2016. Cholinesterase in porcine saliva: Analytical characterization and behavior after experimental stress. Res. Vet. Sci. 106: 23–28. doi: 10.1016/j.rvsc.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 18.Tecles F., Contreras-Aguilar M. D., Martínez-Miró S., Tvarijonaviciute A., Martínez-Subiela S., Escribano D., Cerón J. J.2017. Total esterase measurement in saliva of pigs: Validation of an automated assay, characterization and changes in stress and disease conditions. Res. Vet. Sci. 114: 170–176. doi: 10.1016/j.rvsc.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 19.Tecles F., Rubio C. P., Contreras-Aguilar M. D., López-Arjona M., Martínez-Miró S., Martínez-Subiela S., Cerón J. J.2018. Adenosine deaminase activity in pig saliva: analytical validation of two spectrophotometric assays. J. Vet. Diagn. Invest. 30: 175–179. doi: 10.1177/1040638717742947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomadaki K., Helmerhorst E. J., Tian N., Sun X., Siqueira W. L., Walt D. R., Oppenheim F. G.2011. Whole-saliva proteolysis and its impact on salivary diagnostics. J. Dent. Res. 90: 1325–1330. doi: 10.1177/0022034511420721 [DOI] [PMC free article] [PubMed] [Google Scholar]