Abstract
Toxoplasma gondii is a highly prevalent protozoon that can infect all warm-blooded animals, including humans. It is frequently used as an Apicomplexan parasite model in research. In this review, the invasion mechanism of T. gondii is described as a representative Apicomplexan parasite. The invasion machinery of T. gondii consists of the moving junction and the glideosome, which is a specific motor system for Apicomplexan parasites. I provide details about the moving junction, parasite-secreted proteins and host adhesion receptors, the glideosome, and calcium signaling, which generates the power for the gliding mobility of T. gondii. A detailed understanding of parasite invasion can be useful for the development of new effective drugs to inhibit this event and disrupt the Apicomplexan life cycle.
Keywords: glideosome, host adhesion receptor, invasion, moving junction, Toxoplasma gondii
TOXOPLASMA GONDII
Toxoplasmosis is a common parasitic disease caused by Toxoplasma gondii [21]. T. gondii is an obligate intracellular parasite of the phylum Apicomplexa, which includes the human and animal parasites Plasmodium, Eimeria, Neospora, Theileria, Babesia, and Cryptosporidium. Approximately one-third of humans worldwide are chronically infected with T. gondii [20, 44]. In healthy individuals, T. gondii infection is usually asymptomatic; however, in pregnant women or people with suppressed immunity, it can be fatal [23]. After infecting its intermediate host, T. gondii differentiates into tachyzoites that rapidly infect host tissues. In principle, most active T. gondii infections are cleared by the immune system or appropriate drugs; however, some tachyzoites differentiates into bradyzoites, which form cysts in the infected tissues. The relatively quiescent tissue cysts may serve as a source for subsequent exacerbations, particularly in immunosuppressed individuals. Unlike most other Apicomplexa, Toxoplasma can invade and replicate in almost all of the nucleated cells of warm-blooded animals. The cell invasion machinery among Apicomplexa parasites is highly conserved.
T. gondii isolates from Europe and North America mainly belong to major three lines, referred to as types I, II, and III. These three genetic types differ in bioactivity. Type I is the most virulent, whereas types II and III have moderate virulence to mouse model. The life cycle of Toxoplasma is illustrated in Fig. 1. In both the intermediate and definitive host feline species, T. gondii replicates quickly and causes acute infection as tachyzoites. Once the parasite is exposed to a stressful environment, which may be the host immune system or particular host cell types, the parasite replicates slowly, evades the immune system in the cyst wall and causes persistent infection as bradyzoites. Host cell invasion by T. gondii involves the sequential secretion of two distinct secretory organelles, termed micronemes and rhoptries (Fig. 2), which are characteristic of the Apicomplexa phylum [9].
Fig. 1.
Schematic image of the life cycle of T. gondii (partially modified from a previous review [24]). In both the intermediate and definitive host feline species, T. gondii replicates quickly and causes acute infection as tachyzoites. Once the parasite is exposed to a stressful environment, the parasite replicates slowly, evades the immune system in the cyst wall, and causes persistent infection as bradyzoites. The bradyzoites in the hosts can be eaten and infect the predator. In the definitive host feline species, gametocytogenesis occurs as well as tachyzoite replication. The macrogamete and microgamete are fertilized and become an oocyst. The oocysts are excreted in the stool of feline species and can be ingested by host thereby leading to infection.
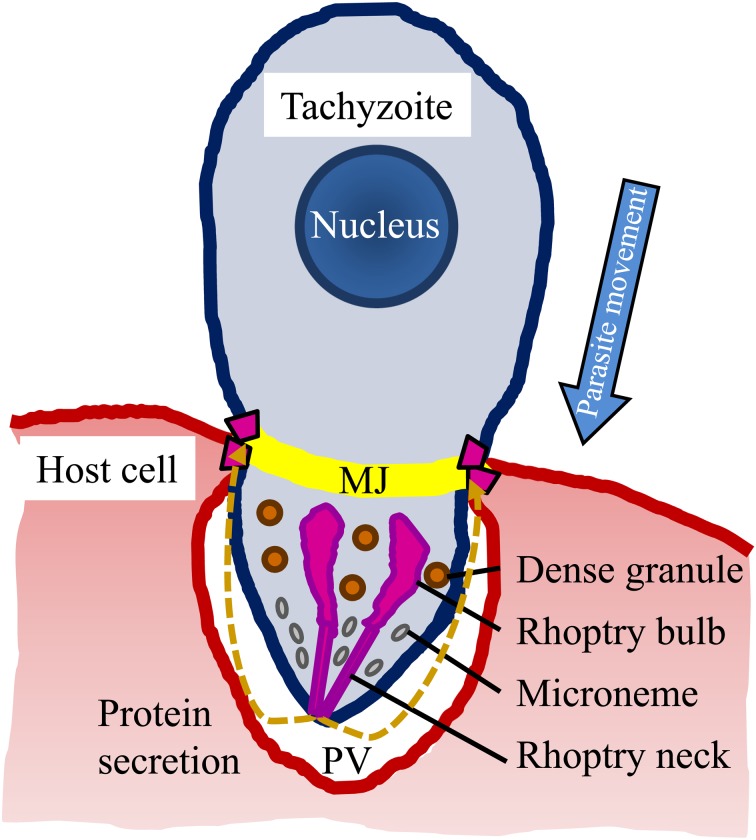
Fig. 2.
The Toxoplasma invasion system. The parasite pushes itself through the moving junction (MJ) and invades via the parasitophorous vacuole (PV), which is formed as an invagination of the host plasma membrane. Parasite proteins are secreted from its organelles (e.g., the microneme, rhoptry, and dense granules). The large arrow indicates the direction of parasite movement.
T. gondii is frequently used as an Apicomplexan parasite model because it can be cultured in almost all types of mammalian cells and has proven to be a valuable experimental research tool. In this review, I described the invasion mechanism of T. gondii as a representative Apicomplexan parasite.
THE MOVING JUNCTION
A key structure for host cell invasion is a tight junction structure known as the moving junction (Fig. 2), which is formed by intimate contact between the apical tip of the tachyzoite and the host cell membrane. As the invasion advances, the tachyzoite propels itself by means of an internal actomyosin motor into the host cell, thereby leading to the formation of a parasitophorous vacuole surrounded by the parasitophorous vacuole membrane inside the host cell [41]. In T. gondii, the moving junction consists of TgRON2, TgRON4, TgRON5, and TgRON8, secreted from rhoptry necks, and apical membrane antigen 1 (TgAMA1), secreted from micronemes (Fig. 3) [1, 4, 27, 39, 45]. TgRON2 is inserted as an integral transmembrane protein into the host plasma membrane, whereas TgRON4, TgRON5, and TgRON8 are exposed to the cytosolic face of the host cell membrane during invasion [4]. The components of this complex are conserved among Apicomplexan species [3, 26, 37], except for TgRON8, which seems to be coccidian-specific [38]. From the study of TgRON8-knockout parasites, it has been proposed that TgRON8 forms a firm intracellular grip that commits the parasite to invasion by anchoring it to the host cytoskeleton [39]. A different study with a TgRON5 conditional knockout strain demonstrated that TgRON5 is required for TgRON2 stability and the proper targeting of TgRON4 [2]. TgRON4 and TgRON5 were not detected at the moving junction during invasion of a TgRON2 conditional knockout parasite [28]. In addition to the importance of TgRON2 and TgRON5, TgRON4 is thought to be crucial for parasite growth as attempts to knock it out have been unsuccessful in T. gondii tachyzoites [1].
Fig. 3.
Details of the MJ in Fig. 2. The glideosome-associated proteins (GAP40, GAP45, and GAP50) form a complex with GAPM in association with alveolin. MLC1 interacts with MyoA with GAP45. This motor complex is anchored to the inner membrane complex (IMC) by GAP40 and GAP50. The small arrow indicates the direction of the MyoA power stroke. The MJ complex is formed by the AMA1-RON complex (RON2, RON4, RON5, and RON8) complex and MIC protein-host cell surface receptors, connected by aldolase. The MJ complex links the host cytoskeletons.
After invasion is completed, the moving junction can still be detected at the posterior pole of the parasite for a few hours [29]. In intracellular parasites, TgRON4, TgRON5, and TgRON8 are exposed on the cytosolic face of the host cell plasma membrane [1, 4, 7, 29]. TgRON4, but not TgAMA1, is associated with the moving junction during ionophore-induced egress [1]. Moreover, TgRON4 shows a typical ring-like signal in conditional TgAMA1 null mutant tachyzoites, indicating that TgAMA1 is not essential for the formation of a functional moving junction [16].
THE INTERACTIONS BETWEEN PARASITE-SECRETED PROTEINS AND HOST ADHESION RECEPTORS
T. gondii uses several adhesion receptors to build a scaffold of host cellular molecules and cellular matrix during the invasion steps (Table 1). TgRON4 is exported to the cytosolic side of the host cell where it can associate with β-tubulin, the cortical host cytoskeleton [43]. TgRON4 interacts with glypican 1, one of the components of the host cell surface, during Toxoplasma invasion [42]. In one study, the ability of T. gondii to infect Chinese hamster ovary (CHO) cells deficient in sialic acids was reduced by 26.9% compared to wild-type cells, indicating that sialic acid is critical for the attachment and invasion of T. gondii [35]. T. gondii microneme protein 1 (TgMIC1) forms a macromolecular complex with TgMIC4 and TgMIC6. Single deletion of the TgMIC1 gene significantly decreases T. gondii invasion of host cells, suggesting an essential role for TgMIC1 in host cell attachment and invasion [10]. Structural analysis of TgMIC1 revealed a novel cell-binding motif called MARR (microneme adhesive repeat region), which provides a specialized structure for glycan discrimination [5]. Structural analysis of TgMIC4 revealed its binding specificity to a variety of galactose-containing carbohydrate ligands [32].
Table 1. The interactions between secreted proteins and host cell receptors during the invasion of T. gondii.
Carbohydrate microarray analyses have shown that TgMIC13, TgMIC1, and its homologue Neospora caninum MIC1, share a preference for α2-3- over α2-6-linked sialyl-N-acetyllactosamine sequences [12]. P104, a PAN/apple domain-containing protein expressed at the apical end of the extracellular parasite, functions as a ligand in the attachment of T. gondii to chondroitin sulfate or other receptors on the host cell, facilitating invasion by the parasite [18]. Immunoprecipitation analyses confirmed the interaction of heat shock cognate protein 70 (HSC70) with T. gondii MAR domain-containing protein 4a (TgMCP4a) [17].
THE GLIDEOSOME
In the T. gondii tachyzoite, the involvement of the myosin motor in the glideosome machinery powers motility and invasion (Fig. 3). The conserved, short single-headed myosin heavy chain A (MyoA) was identified in T. gondii as the motor that was able to generate the rearward traction force critical for motility and entry into host cells [34]. The glideosome is composed of TgMyoA [19], a myosin light chain (TgMLC1), and three gliding-associated proteins, TgGAP45, TgGAP50 [14], and TgGAP40 [11]. TgGAP45 spans the space between the inner membrane complex and the parasite plasma membrane. This motor complex is anchored to the inner membrane complex by TgGAP40, TgGAP50, and GAPs with multiple-membrane spans (TgGAPMs) via an association with alveolin [8]. The connection between the glideosome and adhesion is mediated by aldolase through its actin-binding activity [22]. The micronemal proteins, TgMIC2 and TgAMA1, have been shown to bind to aldolase [6].
CALCIUM SIGNALING
A spike in calcium concentration can mediate signaling by activating specific kinases and coordinating microneme secretion, and thereby has an effect on gliding motility, invasion, and egress [30]. A conditional knockout study revealed the precise function of T. gondii calcium-dependent protein kinase 1 (TgCDPK1) in the calcium-dependent micronemal secretion steps, which are related to egress and invasion [31]. TgCDPK3 is important for egress [13, 33], and the initiation of motility, because it phosphorylates TgMyoA. T. gondii calmodulin-like domain protein kinase isoform 3 (TgCDPKif3: TgCDPK1_2 in ToxoDB (http://toxodb.org/) annotation) is expressed in tachyzoites and localizes to the apical end under extracellular conditions. An in vitro kinase assay demonstrated that TgCDPKif3 can phosphorylate Aldolase 1 [40], a component of the glideosome. More than 20 CDPK-related kinases are encoded in the T. gondii genome [36], and many of them may also contribute to invasion. For example, T. gondii cGMP-dependent protein kinase (TgPKG) is involved in invasion. TgGAP45, which is a member of the glideosome complex, is needed for active host cell penetration and is also phosphorylated in invasive parasites [15]. An in vitro kinase assay demonstrated that T. gondii calmodulin-dependent protein kinase-related kinase (TgCaMKrk) can phosphorylate TgGAP45 [25].
CONCLUSIONS
The T. gondii invasion machinery consists of the moving junction, the glideosome, and parasite-secreted proteins and their corresponding receptors, which unite the moving junction with the glideosome. Signal transduction, mainly calcium signaling, generates the power for the gliding mobility. As the basis for movement is the MyoA/actin motor for T. gondii, one of Apicomplexa, this machinery for movement is specific to some Apicomplexa and interesting. The motility systems including motor complex are also critical for other species to survive. Apicomplexa has a specific motor system. The analysis on Apicomplexan motility system may contribute more knowledge for the motility systems of other species. All of the components of the moving junction and the glideosome have not yet been elucidated. The discovery of more of these components and further analyses of the working system and signal transduction events involving these components are needed. The information obtained will assist in the development of new effective drugs that inhibit invasion and disrupt the Apicomplexan life cycle. Such knowledge would also contribute anti-protozoan strategies including that for malaria.
Acknowledgments
This study was supported by a grant-in-aid for Scientific Research (B) (17H03913) from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan. Some figure illustrations were contributed by Mr. Tatsuya Iwanaga.
REFERENCES
- 1.Alexander D. L., Mital J., Ward G. E., Bradley P., Boothroyd J. C.2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1: e17. doi: 10.1371/journal.ppat.0010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J. R., Chen A. L., Kim E. W., Bradley P. J.2014. RON5 is critical for organization and function of the Toxoplasma moving junction complex. PLoS Pathog. 10: e1004025. doi: 10.1371/journal.ppat.1004025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besteiro S., Dubremetz J. F., Lebrun M.2011. The moving junction of apicomplexan parasites: a key structure for invasion. Cell. Microbiol. 13: 797–805. doi: 10.1111/j.1462-5822.2011.01597.x [DOI] [PubMed] [Google Scholar]
- 4.Besteiro S., Michelin A., Poncet J., Dubremetz J. F., Lebrun M.2009. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog. 5: e1000309. doi: 10.1371/journal.ppat.1000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenschein T. M., Friedrich N., Childs R. A., Saouros S., Carpenter E. P., Campanero-Rhodes M. A., Simpson P., Chai W., Koutroukides T., Blackman M. J., Feizi T., Soldati-Favre D., Matthews S.2007. Atomic resolution insight into host cell recognition by Toxoplasma gondii. EMBO J. 26: 2808–2820. doi: 10.1038/sj.emboj.7601704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher L. E., Bosch J.2015. The apicomplexan glideosome and adhesins - Structures and function. J. Struct. Biol. 190: 93–114. doi: 10.1016/j.jsb.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley P. J., Ward C., Cheng S. J., Alexander D. L., Coller S., Coombs G. H., Dunn J. D., Ferguson D. J., Sanderson S. J., Wastling J. M., Boothroyd J. C.2005. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J. Biol. Chem. 280: 34245–34258. doi: 10.1074/jbc.M504158200 [DOI] [PubMed] [Google Scholar]
- 8.Bullen H. E., Tonkin C. J., O’Donnell R. A., Tham W. H., Papenfuss A. T., Gould S., Cowman A. F., Crabb B. S., Gilson P. R.2009. A novel family of Apicomplexan glideosome-associated proteins with an inner membrane-anchoring role. J. Biol. Chem. 284: 25353–25363. doi: 10.1074/jbc.M109.036772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carruthers V. B., Sibley L. D.1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73: 114–123. [PubMed] [Google Scholar]
- 10.Cérède O., Dubremetz J. F., Soête M., Deslée D., Vial H., Bout D., Lebrun M.2005. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J. Exp. Med. 201: 453–463. doi: 10.1084/jem.20041672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frénal K., Polonais V., Marq J. B., Stratmann R., Limenitakis J., Soldati-Favre D.2010. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe 8: 343–357. doi: 10.1016/j.chom.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Friedrich N., Santos J. M., Liu Y., Palma A. S., Leon E., Saouros S., Kiso M., Blackman M. J., Matthews S., Feizi T., Soldati-Favre D.2010. Members of a novel protein family containing microneme adhesive repeat domains act as sialic acid-binding lectins during host cell invasion by apicomplexan parasites. J. Biol. Chem. 285: 2064–2076. doi: 10.1074/jbc.M109.060988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrison E., Treeck M., Ehret E., Butz H., Garbuz T., Oswald B. P., Settles M., Boothroyd J., Arrizabalaga G.2012. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 8: e1003049. doi: 10.1371/journal.ppat.1003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaskins E., Gilk S., DeVore N., Mann T., Ward G., Beckers C.2004. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 165: 383–393. doi: 10.1083/jcb.200311137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilk S. D., Gaskins E., Ward G. E., Beckers C. J.2009. GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot. Cell 8: 190–196. doi: 10.1128/EC.00201-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannini D., Späth S., Lacroix C., Perazzi A., Bargieri D., Lagal V., Lebugle C., Combe A., Thiberge S., Baldacci P., Tardieux I., Ménard R.2011. Independent roles of apical membrane antigen 1 and rhoptry neck proteins during host cell invasion by apicomplexa. Cell Host Microbe 10: 591–602. doi: 10.1016/j.chom.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 17.Gong H., Kobayashi K., Sugi T., Takemae H., Ishiwa A., Recuenco F. C., Murakoshi F., Xuan X., Horimoto T., Akashi H., Kato K.2014. Characterization and binding analysis of a microneme adhesive repeat domain-containing protein from Toxoplasma gondii. Parasitol. Int. 63: 381–388. doi: 10.1016/j.parint.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 18.Gong H., Kobayashi K., Sugi T., Takemae H., Kurokawa H., Horimoto T., Akashi H., Kato K.2012. A novel PAN/apple domain-containing protein from Toxoplasma gondii: characterization and receptor identification. PLoS One 7: e30169. doi: 10.1371/journal.pone.0030169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herm-Götz A., Weiss S., Stratmann R., Fujita-Becker S., Ruff C., Meyhöfer E., Soldati T., Manstein D. J., Geeves M. A., Soldati D.2002. Toxoplasma gondii myosin A and its light chain: a fast, single-headed, plus-end-directed motor. EMBO J. 21: 2149–2158. doi: 10.1093/emboj/21.9.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill D., Dubey J. P.2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8: 634–640. doi: 10.1046/j.1469-0691.2002.00485.x [DOI] [PubMed] [Google Scholar]
- 21.Hill D. E., Chirukandoth S., Dubey J. P.2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 6: 41–61. doi: 10.1079/AHR2005100 [DOI] [PubMed] [Google Scholar]
- 22.Jewett T. J., Sibley L. D.2003. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol. Cell 11: 885–894. doi: 10.1016/S1097-2765(03)00113-8 [DOI] [PubMed] [Google Scholar]
- 23.Kamau E. T., Srinivasan A. R., Brown M. J., Fair M. G., Caraher E. J., Boyle J. P.2012. A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 56: 5581–5590. doi: 10.1128/AAC.00868-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato K., Sugi T., Iwanaga T.2012. Roles of Apicomplexan protein kinases at each life cycle stage. Parasitol. Int. 61: 224–234. doi: 10.1016/j.parint.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Kato K., Sugi T., Takemae H., Takano R., Gong H., Ishiwa A., Horimoto T., Akashi H.2016. Characterization of a Toxoplasma gondii calcium calmodulin-dependent protein kinase homolog. Parasit. Vectors 9: 405. doi: 10.1186/s13071-016-1676-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp L. E., Yamamoto M., Soldati-Favre D.2013. Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol. Rev. 37: 607–631. doi: 10.1111/1574-6976.12013 [DOI] [PubMed] [Google Scholar]
- 27.Lamarque M., Besteiro S., Papoin J., Roques M., Vulliez-Le Normand B., Morlon-Guyot J., Dubremetz J. F., Fauquenoy S., Tomavo S., Faber B. W., Kocken C. H., Thomas A. W., Boulanger M. J., Bentley G. A., Lebrun M.2011. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 7: e1001276. doi: 10.1371/journal.ppat.1001276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamarque M. H., Roques M., Kong-Hap M., Tonkin M. L., Rugarabamu G., Marq J. B., Penarete-Vargas D. M., Boulanger M. J., Soldati-Favre D., Lebrun M.2014. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat. Commun. 5: 4098. doi: 10.1038/ncomms5098 [DOI] [PubMed] [Google Scholar]
- 29.Lebrun M., Michelin A., El Hajj H., Poncet J., Bradley P. J., Vial H., Dubremetz J. F.2005. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell. Microbiol. 7: 1823–1833. doi: 10.1111/j.1462-5822.2005.00646.x [DOI] [PubMed] [Google Scholar]
- 30.Lourido S., Moreno S. N.2015. The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp. Cell Calcium 57: 186–193. doi: 10.1016/j.ceca.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lourido S., Shuman J., Zhang C., Shokat K. M., Hui R., Sibley L. D.2010. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465: 359–362. doi: 10.1038/nature09022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchant J., Cowper B., Liu Y., Lai L., Pinzan C., Marq J. B., Friedrich N., Sawmynaden K., Liew L., Chai W., Childs R. A., Saouros S., Simpson P., Roque Barreira M. C., Feizi T., Soldati-Favre D., Matthews S.2012. Galactose recognition by the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 287: 16720–16733. doi: 10.1074/jbc.M111.325928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy J. M., Whitehead L., van Dooren G. G., Tonkin C. J.2012. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 8: e1003066. doi: 10.1371/journal.ppat.1003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meissner M., Schlüter D., Soldati D.2002. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298: 837–840. doi: 10.1126/science.1074553 [DOI] [PubMed] [Google Scholar]
- 35.Monteiro V. G., Soares C. P., de Souza W.1998. Host cell surface sialic acid residues are involved on the process of penetration of Toxoplasma gondii into mammalian cells. FEMS Microbiol. Lett. 164: 323–327. doi: 10.1111/j.1574-6968.1998.tb13105.x [DOI] [PubMed] [Google Scholar]
- 36.Nagamune K., Sibley L. D.2006. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol. Biol. Evol. 23: 1613–1627. doi: 10.1093/molbev/msl026 [DOI] [PubMed] [Google Scholar]
- 37.Proellocks N. I., Coppel R. L., Waller K. L.2010. Dissecting the apicomplexan rhoptry neck proteins. Trends Parasitol. 26: 297–304. doi: 10.1016/j.pt.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 38.Straub K. W., Cheng S. J., Sohn C. S., Bradley P. J.2009. Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell. Microbiol. 11: 590–603. doi: 10.1111/j.1462-5822.2008.01276.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub K. W., Peng E. D., Hajagos B. E., Tyler J. S., Bradley P. J.2011. The moving junction protein RON8 facilitates firm attachment and host cell invasion in Toxoplasma gondii. PLoS Pathog. 7: e1002007. doi: 10.1371/journal.ppat.1002007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugi T., Kato K., Kobayashi K., Pandey K., Takemae H., Kurokawa H., Tohya Y., Akashi H.2009. Molecular analyses of Toxoplasma gondii calmodulin-like domain protein kinase isoform 3. Parasitol. Int. 58: 416–423. doi: 10.1016/j.parint.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 41.Suss-Toby E., Zimmerberg J., Ward G. E.1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. U.S.A. 93: 8413–8418. doi: 10.1073/pnas.93.16.8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takemae H., Kobayashi K., Sugi T., Han Y., Gong H., Ishiwa A., Recuenco F. C., Murakoshi F., Takano R., Murata Y., Nagamune K., Horimoto T., Akashi H., Kato K.2018. Toxoplasma gondii RON4 binds to heparan sulfate on the host cell surface. Parasitol. Int. 67: 123–130. doi: 10.1016/j.parint.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 43.Takemae H., Sugi T., Kobayashi K., Gong H., Ishiwa A., Recuenco F. C., Murakoshi F., Iwanaga T., Inomata A., Horimoto T., Akashi H., Kato K.2013. Characterization of the interaction between Toxoplasma gondii rhoptry neck protein 4 and host cellular β-tubulin. Sci. Rep. 3: 3199. doi: 10.1038/srep03199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenter A. M., Heckeroth A. R., Weiss L. M.2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30: 1217–1258. doi: 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyler J. S., Boothroyd J. C.2011. The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathog. 7: e1001282. doi: 10.1371/journal.ppat.1001282 [DOI] [PMC free article] [PubMed] [Google Scholar]