Abstract
A 10-week-old miniature dachshund presented with acute onset of weakness. Electrocardiography showed sustained ventricular tachycardia, and thoracic and abdominal radiography revealed pleural and peritoneal effusion. Echocardiography revealed severely hypokinetic left and right ventricles. Thoracocentesis and abdominocentesis and subsequent transfer to an oxygen chamber yielded no clinical improvement, and the dog died about 1 hr after admission. Gross examination of a longitudinal section through the entire heart revealed poorly demarcated focal or patchy areas of grayish-white tissue infiltrating extensively into the myocardium. Histologically, these lesions were consistent with infiltrative proliferation of neoplastic lymphoid cells. Immunohistochemical staining confirmed the diagnosis of primary cardiac lymphoma (PCL) of T-cell origin. There have been no previous reports of such young dogs with PCL.
Keywords: juvenile dog, primary cardiac lymphoma, T-cell lymphoma
Canine lymphoma usually occurs in multicentric, alimentary, mediastinal, and cutaneous forms, but it rarely involves only the heart and/or pericardium [11]. This type of lymphoma, which primarily affects the heart, is known as primary cardiac lymphoma (PCL) [4]. PCL is uncommon in dogs, comprising approximately 2.5% (34 of 1,383) of all cases of cardiac tumor as revealed by a Veterinary Medical Database search for the period from 1982 to 1995 [10]. This report describes an extremely rare case of PCL in a 10-week-old dog.
A 73-day-old, 1.8-kg male miniature dachshund presented with a 2-day history of weakness and shortness of breath. On physical examination, the dog was found to be slightly smaller in size in comparison with his littermates. The animal was severely depressed and dyspneic, exhibiting open-mouthed breathing, and its abdomen was moderately distended with fluid. The rectal temperature was 38.8°C, and the femoral pulses were too weak to be palpable. Jugular venous distension was detected, and the mucous membranes were pale pink. The heart sounds were muffled on thoracic auscultation. A 6-lead electrocardiogram revealed sustained monomorphic tachycardia (360 beats per min) with QRS complexes of right bundle branch block configuration, assumed to be ventricular tachycardia (VT) (Fig. 1). Thoracic radiography revealed a moderate amount of pleural effusion that obscured the cardiac shadow. Abdominal radiography showed loss of visceral detail, indicating abdominal fluid accumulation. Two-dimensional echocardiography revealed slightly or moderately dilated and severely hypokinetic left and right ventricles (left ventricular fractional shortening <20%) with a small amount of pericardial effusion. Color flow Doppler interrogation of the mitral and tricuspid valves identified mild regurgitation. Thoracocentesis and abdominocentesis (20 ml of light red serous fluid and 50 ml of straw-colored serous fluid were withdrawn, respectively; cytology of the fluids was not performed), and subsequent transfer to an oxygen chamber produced no clinical improvement. While being monitored in the oxygen chamber, the dog continued to exhibit sustained VT for 30 min, which then abruptly degenerated to ventricular fibrillation (VF), and died about 1 hr after admission. The owner consented to have a postmortem examination performed.
Fig. 1.
Six lead electrocardiographic tracings from the 10-week-old dog, showing monomorphic ventricular tachycardia, rate 360 beats per minute, almost regular (bipolar standard limb leads; 50 mm/sec).
At necropsy, there was serosanguineous fluid in the abdominal (210 ml) and thoracic (80 ml) cavities. Macroscopically evident lesions were confined to the liver, lungs, and heart. The liver was markedly congested and enlarged. Both lungs were diffusely congested and edematous with cranioventral segmental and subsegmental areas of collapse. In the heart, poorly demarcated focal or patchy areas of grayish-white tissue covered large areas of the epicardial surface of the left and right ventricles. In a longitudinal section through the entire heart, the grayish-white tissue infiltrated extensively into the ventricular myocardium, showing the appearance of partially or fully confluent lesions (Fig. 2). Progression of such lesions could be seen in the left and right atrial walls to various extents. There was no evidence of lymph node enlargement in the abdominal and thoracic cavities.
Fig. 2.
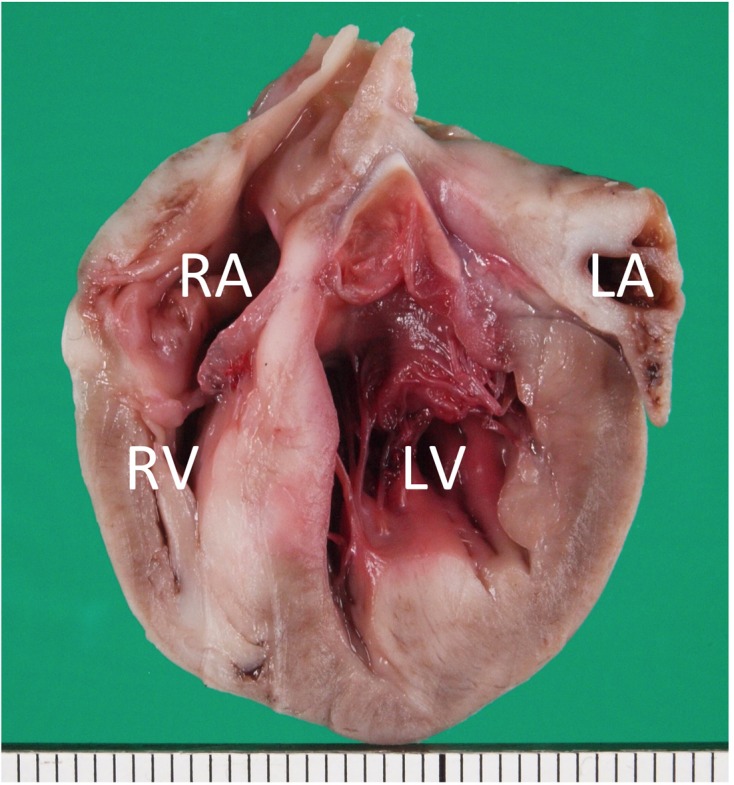
Formalin-fixed heart transected along the long axis, showing extensive infiltration of grayish-white neoplastic tissue into the myocardium of the entire heart. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Scale: 1 mm.
Tissue samples from the heart, lungs, tracheobronchial lymph nodes, thyroid glands, parathyroid glands, esophagus, stomach, small and large intestines, pancreas, liver, mesenteric lymph nodes, spleen, adrenal glands, kidneys, urinary bladder, brain, spinal cord, and skin were fixed in 10% buffered formalin for routine processing; embedded in paraffin wax; sectioned at 5 µm; and stained with hematoxylin and eosin for light microscopy. In addition, the avidin-biotin-peroxidase method (Vectastain; Vector Laboratories, Burlingame, CA, U.S.A.) was employed on paraffin-wax sections of the heart for immunohistochemical identification of the T-cell marker, CD3 (Dako, Glostrup, Denmark), and the B-cell markers, CD 79α and CD20 (Dako).
Histological examination of the heart revealed marked infiltrative proliferation of neoplastic lymphoid cells in the myocardium (Fig. 3A). Sheets of neoplastic round cells separated individual muscle fibers, which were attenuated due to the compressive effects of the neoplastic cells. Many myocytes were necrotic with eosinophilic degeneration of the cytoplasm, loss of cross striations, and pyknotic nuclei. The neoplastic cells had scant to moderate amounts of amphophilic cytoplasm with distinct cell borders. Their round or ovoid nuclei contained 1 to 2 prominent nucleoli and vesicular to coarsely clumped chromatin. Marked anisocytosis and anisokaryosis was evident, but the mitotic rate was relatively low (1 to 2 mitotic figures per high-power field) (Fig. 3B). Immunohistochemical labeling revealed large numbers of CD3-positive neoplastic lymphoid cells (Fig. 4A), whereas fewer number of cells were positive for CD79α (Fig. 4B). All cells stained negative for CD20 (Fig. 4C).
Fig. 3.
(A) Microscopic section taken from the ventricular septum, showing marked infiltrative proliferation of neoplastic lymphoid cells in the myocardium. Sheets of neoplastic round cells separate individual muscle fibers. HE. Bar: 50 µm. (B) The outlined square area in A is shown at higher magnification. HE. Bar: 20 µm.
Fig. 4.
Immunohistochemical labeling of the neoplastic lymphoid cells. Hematoxylin counterstain. Bar: 50 µm. (A) A large number of neoplastic cells stain positively for CD3. (B) Fewer neoplastic cells stain positively for CD79α. (C) All the neoplastic cells are negative for CD20.
Other significant histological findings were evident in the liver and lungs. The liver had centrilobular congestion with swelling and vacuolation of the periportal hepatocytes and dilatation of the lymphatics. The lungs showed mild to moderate congestive edema. The remaining organs were normal. No metastatic lesion or involvement was found in any of the other tissues examined. The results of histopathology and immunohistochemical staining confirmed the diagnosis of PCL to be of T-cell origin. According to the World Health Organization system of classification of canine lymphomas [9], the present case was categorized as peripheral T cell lymphoma not otherwise specified (PTCL-NOS).
PCL is an extremely rare malignancy in dogs; to our knowledge, only four canine cases of PCL, in which a diagnosis was established by necropsy, have been confirmed to date [5,6,7,8]. These cases of PCL involved two 9-year-old and two 11-year-old dogs. In addition, 11 canine cases of presumptive PCL were reported in a retrospective case study, and the dogs ranged in age from 2 to 16 years (median, 8 years) [2]. In a search for cardiac neoplasia in the Veterinary Medical Database at Purdue University, although detailed clinical information was unavailable for 34 individual dogs with cardiac lymphoma, the disease occurred most commonly in dogs less than 7 years of age [10]. The dog in the present study, suffering from PCL, died at the age of 10 weeks; there have been no previous reports of lymphoma primarily affecting the heart in such a young dog.
PCL is also a rare malignancy in humans; fewer than 200 cases were reported during the period between 1949 and 2009 [4]. However, the mortality rate is high when involvement of the myocardial tissue is advanced [3, 4]. PCL is extremely aggressive and can damage whole endocardium, myocardium, epicardium, and/or pericardium. Symptoms of PCL vary depending on the heart site involved; the most common clinical manifestations are pericardial effusion and heart failure. Additionally, many types of arrhythmia can be induced, including atrial fibrillation, atrial flutter, atrioventricular (AV) block, and VT [3, 4]. According to Petrich et al. [4], who reviewed clinical data for 197 human cases of PCL, among cases where the heart rhythm was described, 56% (83 of 149) of patients presented with arrhythmia. The two most common abnormalities were atrial arrhythmia and AV block, at 23 and 22%, respectively. Fatal arrhythmias were detected in 11% of the cases (9 of 83).
In dogs, there are many cases in the veterinary literature reporting cardiac lymphoma as a cause of pericardial effusion [2] and a few cases in which cardiac lymphoma resulted in the development of serious arrhythmia [5,6,7]. The present case was characterized by not only severely hypokinetic left and right ventricles with pericardial fluid accumulation, but also sustained VT, which degenerated to terminal VF in a short period. Microscopy revealed marked infiltrative proliferation of neoplastic lymphoid cells throughout the entire myocardium. Consistent with these observations, premature ventricular complexes, which were followed by complete AV block and then VF, have been reported in a case of infiltrative myocardial lymphoma similar to the present one [6]. Complete AV block has been also reported in two canine cases of PCL with a similar pattern of infiltrative proliferation, the conduction disturbance being attributed to total disappearance of the AV nodal tissue as a result of neoplastic infiltration [5, 7].
The case presented here exhibited PCL of T-cell origin and showed an acute course leading to death. Majority of human PCLs are of B-cell origin, although other cell types, including T-cell lymphoma, have been reported [1, 3]. Chemotherapy is widely used for treatment of human cases of PCL [3, 4]. Clinical remission or cure has been reported for cardiac lymphoma of B-cell origin; however, those of T-cell origin are comparatively insensitive to chemotherapy [1, 3]. On the other hand, a review of all canine cases of confirmed or presumptive PCL revealed six cases of PCL of T-cell origin and only one case of PCL of B-cell origin [5,6,7,8]. Thus, it is likely that there is a considerable difference in the lymphoma cell immunophenotype between human and canine cases of PCL. Because of few reported cases in dogs, there is little information available about the effectiveness of chemotherapy against PCL [2].
In conclusion, there have been no reports involving extremely young dogs with lymphoma primarily affecting the heart. The case presented here suggests that juvenile dogs can also develop PCL.
REFERENCES
- 1.Li B., Li R., Wu B., Chen X., Ni Y., Li W.2012. Primary cardiac T cell lymphoma. J. Card. Surg. 27: 457–460. doi: 10.1111/j.1540-8191.2012.01462.x [DOI] [PubMed] [Google Scholar]
- 2.MacGregor J. M., Faria M. L. E., Moore A. S., Tobias A. H., Brown D. J., de Morais H. S. A.2005. Cardiac lymphoma and pericardial effusion in dogs: 12 cases (1994–2004). J. Am. Vet. Med. Assoc. 227: 1449–1453. doi: 10.2460/javma.2005.227.1449 [DOI] [PubMed] [Google Scholar]
- 3.Miguel C. E., Bestetti R. B.2011. Primary cardiac lymphoma. Int. J. Cardiol. 149: 358–363. doi: 10.1016/j.ijcard.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 4.Petrich A., Cho S. I., Billett H.2011. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer 117: 581–589. doi: 10.1002/cncr.25444 [DOI] [PubMed] [Google Scholar]
- 5.Sasaki T., Kimura Y., Imai T., Machida N.2018. Complete atrioventricular block due to primary cardiac lymphoma in a dog. Jpn. J. Vet. Res. (in press). [Google Scholar]
- 6.Sims C. S., Tobias A. H., Hayden D. W., Fine D. M., Borjesson D. L., Aird B.2003. Pericardial effusion due to primary cardiac lymphosarcoma in a dog. J. Vet. Intern. Med. 17: 923–927. doi: 10.1111/j.1939-1676.2003.tb02534.x [DOI] [PubMed] [Google Scholar]
- 7.Stern J. A., Tobias J. R., Keene B. W.2012. Complete atrioventricular block secondary to cardiac lymphoma in a dog. J. Vet. Cardiol. 14: 537–539. doi: 10.1016/j.jvc.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 8.Tong L. J., Bennett S. L., Thompson D. J., Adsett S. L., Shiel R. E.2015. Right-sided congestive heart failure in a dog because of a primary intracavitary myocardial lymphoma. Aust. Vet. J. 93: 67–71. doi: 10.1111/avj.12289 [DOI] [PubMed] [Google Scholar]
- 9.Valli V. E., San Myint M., Barthel A., Bienzle D., Caswell J., Colbatzky F., Durham A., Ehrhart E. J., Johnson Y., Jones C., Kiupel M., Labelle P., Lester S., Miller M., Moore P., Moroff S., Roccabianca P., Ramos-Vara J., Ross A., Scase T., Tvedten H., Vernau W.2011. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 48: 198–211. doi: 10.1177/0300985810379428 [DOI] [PubMed] [Google Scholar]
- 10.Ware W. A., Hopper D. L.1999. Cardiac tumors in dogs: 1982–1995. J. Vet. Intern. Med. 13: 95–103. [DOI] [PubMed] [Google Scholar]
- 11.Zandvliet M.2016. Canine lymphoma: a review. Vet. Q. 36: 76–104. doi: 10.1080/01652176.2016.1152633 [DOI] [PubMed] [Google Scholar]





