Abstract
A new cell line (GS-1) was developed from the spleen tissue of the orange-spotted grouper, Epinephelus coioides applied for viral infection studies of fish ranavirus and megalocytivirus. The cells proficiently multiplied in Leibovitz’s L-15 medium supplemented with 10% fetal bovine serum at temperatures between 20°C and 32°C. Morphologically, the cell line comprised fibroblast-like cells, and this was confirmed by immunostaining with vimentin, fibronectin, and desmin antibodies. The optimal temperature for grouper iridovirus (GIV) and infectious spleen and kidney necrosis virus (ISKNV) proliferation in GS-1 cells was 25°C, and the highest titer of GIV was 108.4 TCID50/ml, and the highest titer of ISKNV was 105.2 TCID50/ml. Electron micrographs showed that the mean diameter of GIV virions was 180−220 nm, which was larger than ISKNV virions (160−200 nm). Negatively stained GIV particles possessed an envelope structure that was assembled by the three-layered structure with an inner electron-dense core surrounded by a lighter coat (mean diameter, 27 ± 3 nm). The highest GIV-induced mortality of groupers occurred at 25°C, whereas the highest ISKNV-induced mortality occurred at 30°C. In summary, GS-1 cell line is a valuable tool for isolating and investigating fish ranavirus and megalocytivirus in the same host system.
Keywords: fish cell line, grouper, iridoviruses, pathogenesis
Groupers are economically important fish species of the aquaculture industry in south-east Asian regions. The Food and Agriculture Organization estimates that the market demand for orange-spotted groupers may reach 100,000 tons in 2020 [9]. Taiwan grouper production has progressed and reached a high production of approximately 25,749 tons during the last decade [40]. Although grouper culture keeps growing in Asia, its development is constrained by the limited availability of fingerlings, mainly because commercial fish farming has been severely hit by epidemics associated with viruses. Nervous necrosis virus (NNV) [3] and iridoviruses cause high mortality in larval and juveniles stages, and several iridoviruses have been reported in Taiwan [2, 5, 26]. The grouper iridoviruses causing severe mass mortality in Taiwan are primarily included in two different genera, namely, ranavirus and megalocytivirus [14]. Ranaviruses, including Singapore grouper iridovirus (SGIV) [6] and grouper iridovirus (GIV) [26], are known to infect marine fish. Megalocytiviruses, including the red sea bream iridovirus (RSIV) [38], infectious spleen and kidney necrosis virus (ISKNV) [11, 15, 39], orange-spotted grouper iridovirus (OSGIV) [17], grouper sleepy disease iridovirus (GSDIV) [25], turbot reddish body iridovirus (TRBIV) [36] and Korean flounder iridovirus (FLIV) [7], infect marine and freshwater fish. One iridovirus isolate, named as Taiwan grouper iridovirus (TGIV), has been classified to the genus of megalocytivirus and exhibited 99.3% identity to the major capsid protein gene of ISKNV [21]. The TGIV can replicate and cause a cytopathic effect (CPE) in a red-spotted grouper embryo cell line (KRE-3), but its infectivity rapidly lost during serial passages [5]. Two cell lines derived from the kidney tissue of different grouper species are suitable for cultivation of the GIV [13, 19]. The epithelial-like cells derived from the mandarin fish fry (MFF-1) could produce high titers of ISKNV, and the virus particles were observed with a diameter of approximately 150–170 nm in the cytoplasm of the infected cells [8]. Previous studies indicated that piscine ranavirus is much easier to cultivate in many fish cell lines; however, that the cultivation of megalocytivirus is not easy [8, 16]. The same situation has been observed in Taiwanese strains over the past two decades. To the present, it has not been reported that any cell lines are sensitive to both GIV and ISKNV. In this study, we established a novel cell line (GS-1) which was susceptible to GIV and ISKNV, and was applied for the in vitro and in vivo studies of fish ranavirus and megalicytivirus.
MATERIALS AND METHODS
Establishment of the primary GS-1 cell line
Healthy orange-spotted grouper, Epinephelus coioides, weighing 15 g each were collected from a fish farm and were used for primary cell culture in this study. The method used to develop the GS-1 cell line was similar to that described for the GK [19] cell line. Once a confluent monolayer formed in the primary culture, the cells were trypsinized with 0.25% trypsin solution and subcultured with a ratio of 1:2 in culture medium (Leibovitz’s L-15 with 20% fetal bovine serum FBS, 200 IU/ml penicillin and 200 µg/ml streptomycin ; Life Technologies Co., Carlsbad, CA, U.S.A.) at 25°C. After 10 passages, the concentration of FBS was decreased from 20 to 10% for the following 20 passages and the concentration of penicillin was reduced to 100 IU/ml and streptomycin was reduced to 100 µg/ml. The GS-1 cell line has been subcultured for more than 50 passages, and was cultured in 175 cm2 tissue culture flasks at 1:3 split ratio every 3 or 4 days at 25°C.
Characterization of cell growth
Cell growth of the GS-1 cells at the 30th passage were assessed at four culture temperatures (20, 24, 28 and 32°C) for 7 days, and using four FBS concentrations (2, 5, 10 and 15%) in L-15 medium at 28°C for 7 days. The cells were seeded into 25 cm2 cell culture flasks at an initial density of 1 × 105 cells per flask and incubated at different temperatures. Triplicate flasks were trypsinized and counted by 12 independent haemocytometer counts at 0, 1, 3, 5 and 7 days after subculturing. Analogous procedures were performed for determining the effects of various concentrations of FBS. The mean number of cells and standard deviations were calculated.
Immunofluorescence microscopy assay
Immunophenotyping of the GS-1 cell line was carried out at the 30th passage following the method described previously [10] with some modifications. Briefly, the cells were grown on coverslips in six-well plates for 24 hr and then fixed with 4% paraformaldehyde at 4°C for 1 hr and permeabilized with 0.2% Triton-X 100 at room temperature for 15 min. After blocking with 2% bovine serum albumin (BSA) in PBS at room temperature for 2 hr, the cells were then incubated with four different primary antibodies of mouse-anti-cytokeratin, mouse-anti-vimentin, mouse-anti-fibronectin and rabbit-anti-desmin (Sigma-Aldrich Co., St. Louis, MO, U.S.A.) at a dilution of 1:200 at room temperature for 2 hr to characterize the cell line. Subsequently, the cells were rinsed three times with PBS and were incubated with the appropriate secondary antibodies of goat-anti-mouse FITC-conjugated or goat-anti-rabbit FITC-conjugated (Sigma-Aldrich) at a dilution of 1:320 in PBS containing 1% BSA at room temperature for 1 hr. In control coverslips, only PBS with 1% BSA was used in place of the primary antibodies. Diaminophenylindole at a final concentration of 1 µg/ml was used to stain DNA in nuclei. All samples were observed with a Carl Zeiss fluorescence microscope.
Virus isolation
The virus strains GIV/90/grouper and ISKNV/105-2955/grouper were isolated from sick juveniles of Epinephelus coioides in the field, and were used in this study. For virus isolation, five individual spleen tissues were thawed and homogenized with 10-fold volume of sterile PBS (pH7.4). The homogenate was centrifuged at 1,500 rpm for 15 min at 4°C and filtered through 0.22 µm membrane filter. Briefly, 0.5 ml of supernatant was inoculated into 2 × 105 GS-1 cells in 6-wells plates and for 1 hr adsorption at room temperature. These inoculated cells were respectively incubated at 20, 25 and 30°C for 7 days and examined daily for the presence of CPE in triplicate. Cultures with appearance of CPE were frozen at −70°C for further PCR analysis. The blind passages were carried out once for another 7 days for the inoculated cells in which no CPE was observed. Regardless of whether CPE was observed, the inoculated cells were subjected to the PCR after the blind passage.
Viral confirmation by conventional PCR
The methods were described in previous studies [14] and also used to confirm the infection of inoculated cells and experimentally challenged fishes. PCR products were sequenced using an ABI PRISM 377 DNA sequencer with a BigDye Terminator Kit (Applied Biosystems, Foster City, CA, U.S.A.). Sequences of the viral genes were examined for identity with the published sequences and submitted to GenBank database.
Viral replication efficiency in vitro
Virus in the L-15 medium with 2%FBS was inoculated into 24-well plates which were pre-seeded with 2 × 104 GS-1 cells, and the multiplicity of infection (MOI) was 0.1. The culture plates were respectively incubated at 20, 25 and 30°C for 7 days and examined daily for the appearance of CPE. The culture supernatant of virus-infected cells collected at a 2-day interval to measure the titers of virus in triplicate. Viral titers were determined by using 50% tissue culture infective dose (TCID50) method in a 96-well culture plate [35].
Electron microscopy
After appearance of advanced CPE, the virus-infected cells were harvested, pelleted by centrifugation at 3,000 rpm for 10 min, and fixed with 2.5% glutaraldehyde in cacodylate buffer (0.1 M, pH 7.2) for 2 hr at 4°C. The fixed cells were washed with fresh cacodylate buffer and rinsed in PBS (0.1 M, pH7.2) for 10 min three times, then post-fixed in 1% osmium tetroxide for 1 hr. After being rinsed four times with PBS for 15 min, the fixed cells were dehydrated in graded ethyl alcohol (50, 75, 90, 95, and 100%) and embedded in epoxy resin. Ultrathin sections were cut with an ultra-microtome (Reicher-Jung, Vienna, Australia) and stained with saturated aqueous uranyl acetate and lead citrate. Briefly, a 100 µl drop of suspension and double-distilled water mixed and using an airfuge A-100/30 rotor (Beckman, Palo Alto, CA, U.S.A.) for centrifugation was carried out at 90,000 rpm for 5 min. Next, the supernatant was discarded and the pellet was resuspended in 25 µl of double-distilled water. A drop of purified virus solution was applied onto a carbon coated formvar-filmed copper grid. The grid was dried by blotting its edge with a filter paper, and the grid was negatively stained with 2% phosphotungstic acid (pH 6.8) for 1 min. Preparation of cell culture supernatant for negative staining was performed as described previously [33]. Specimens were examined using an electron microscope (JEM-1000CX II, JEOL Ltd., Tokyo, Japan) and photomicrographs were taken.
Experimental infection in vivo
A total of 120 healthy orange-spotted groupers with an average body weight of 5.2 ± 1.2 g were used for the pathogenicity test. Prior to the experiment, the groupers were sampled randomly and tested by PCR to confirm the absence of iridovirus infections. The fish were divided into six groups. For each groups, the fish were reared in a 60-liter glass tank with an air-pumped circulating seawater system with water temperature at either 25 ± 0.5°C or 30 ± 0.5°C. Each fish of the first and second groups were intraperitoneally (IP) injected the GIV and ISKNV isolates with 0.1 ml of 104 TCID50, and then reared at 25°C. Fish of the third and fourth groups were challenged with each of the same virus and kept at 30°C. Fish of the fifth and sixth groups were injected with the same volume of culture medium without virus and kept at 25 and 30°C, respectively, as the negative control groups. The fish were observed daily for mortality for 14 days. Virus in the dead fish was examined by PCR. Statistical analysis was carried out using Fisher’s exact test on mortality.
RESULTS
Primary culture and subculture of GS-1 cells
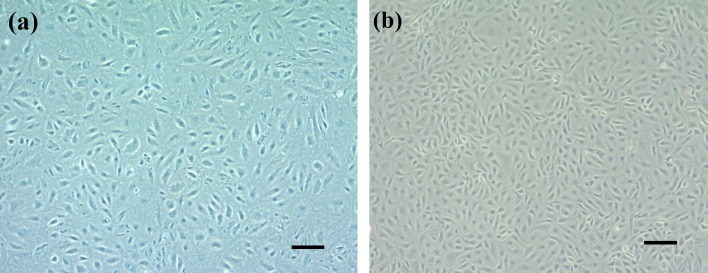
Spleen-derived primary cells of orange-spotted grouper attached to the culture flask on the second day after seeding and a monolayer was formed after 20 days. Cells were then passaged 10 times in the L-15 medium plus 20% FBS at a ratio of 1:2 every 5−7 days, as determined by the cell growth rate. After the 10th passage, these cells rapidly propagated and thereafter were adapted to 10% FBS culture medium and passaged at 1:3 every 5 days. Morphologically, the initial subcultures comprised fibroblastic and epithelial-like cells (Fig. 1a). After the 30th passage, the cell line mainly comprised fibroblast-like cells (Fig. 1b).
Fig. 1.
Morphology of GS-1 cells. (a) Cultures at the 10th passage comprised of fibroblastic and epithelial-like cells. (b) Cultures at the 30th passage mainly comprised of fibroblast-like cells (bar=100 µm).
Growth kinetics at different temperature and concentration of FBS
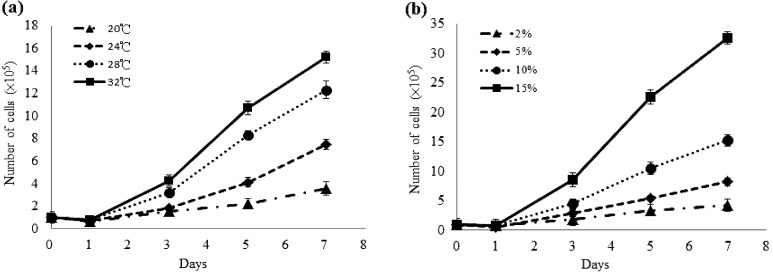
The GS-1 cells were able to grow at temperatures between 20 and 32°C, with the fastest replication occurring at 32°C (Fig. 2a). The growth rate of GS-1 cells increased with FBS concentration from 2 to 15% at 28°C. Cells exhibited population stability at 2% FBS, relatively good growth at 5 and 10% FBS, and maximum growth at 15% FBS (Fig. 2b).
Fig. 2.
Growth kinetics of the grouper spleen-derived GS-1 cell line at the 30th passage. (a) Effect of temperature on cell growth in the L-15 medium containing 10% fetal bovine serum. (b) Effect of fetal bovine serum concentration on cell growth in the L-15 medium at 28°C.
Cell typing of the GS-1 cells
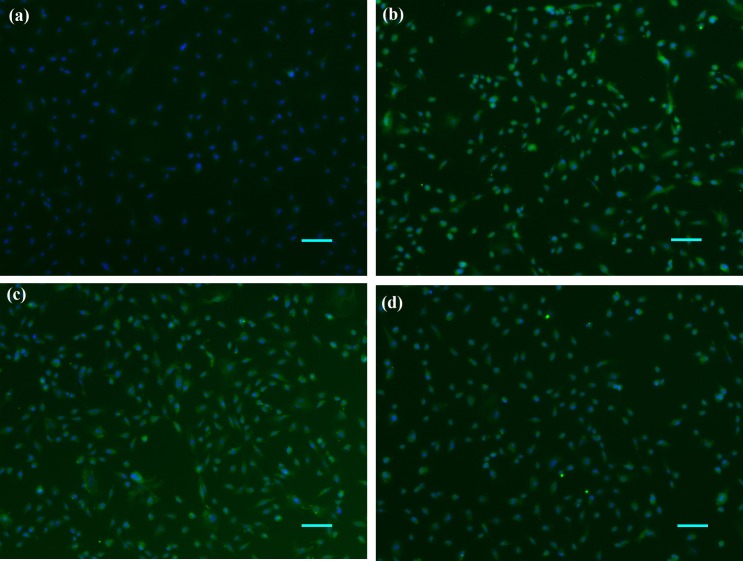
Antibodies against cytokeratin, vimentin, fibronectin, and desmin were used for immunophenotyping. No reactivity against the epithelial cell marker cytokeratin was observed (Fig. 3a), whereas strong fluorescence signals were observed with vimentin (Fig. 3b), fibronectin (Fig. 3c) and desmin antibodies (Fig. 3d) at the 30th passage.
Fig. 3.
Immunocytochemical staining of GS-1 cells using antibodies against (a) cytokeratin, (b) vimentin, (c) fibronectin, and (d) desmin (green). The cell nuclei were counterstained with diaminophenylindole (DAPI; blue) (bar=100 µm).
Viral confirmation by conventional PCR
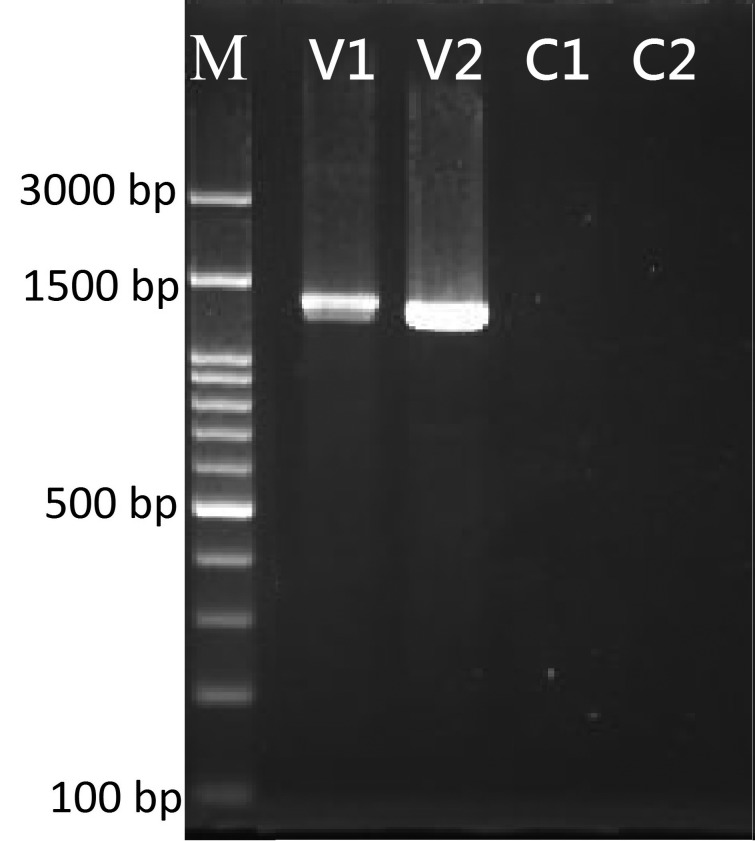
Full-length DNA sequences of the major capsid protein gene were amplified from viral isolates, yielding a 1,392 bp product from GIV and a 1,362 bp product from ISKNV (Fig. 4). Sequence analysis of GIV/90/grouper and ISKNV/105-2955/grouper showed 100 and 99% identities to match the iridovirus reference strains of GIV (Accession number AF371960) and ISKNV (Accession number AF371960), respectively. These GIV/90/grouper and ISKNV/105-2955/grouper sequences were deposited in GenBank under accession numbers KU510328 and KY074549, respectively.
Fig. 4.
The amplified sequences of the grouper iridovirus (GIV) and infectious spleen and kidney necrosis virus (ISKNV) using specific primer to confirm the virus isolates responsible for the infections in GS-1 cells. M: 100 bp DNA ladder; V1: GIV/90/grouper isolate (1,392 bp); V2: ISKNV/105-2955/grouper isolate (1,362 bp); and C1and C2: negative control for the individual reactions.
Cytopathic effect induced by GIV and ISKNV in GS-1 cells
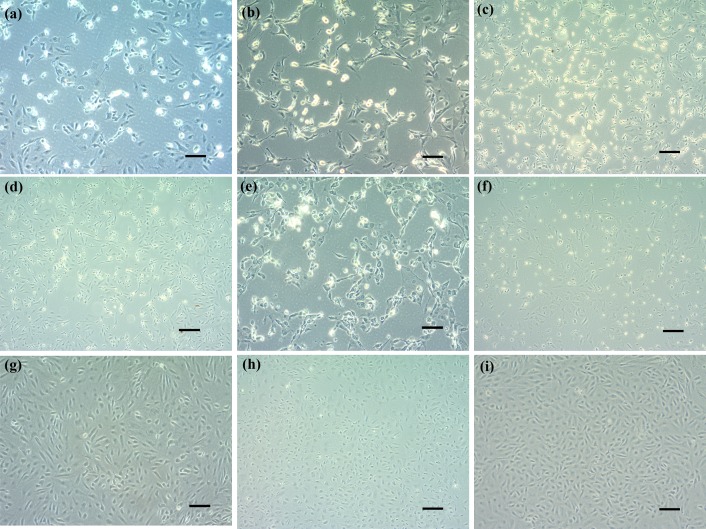
The GIV-induced CPE was observed in GS-1 cells from 20 to 30°C (Fig. 5a–c) and the first CPE has appeared at 25°C since 24 hr post-inoculation (hpi). Initially, CPE developed as foci areas of rounded, granular, and refractive cells. CPE spread through the cell sheet over 72 hr to form a network of degenerated cells, and the cells completely disintegrated by 4 days post-inoculation (dpi).
Fig. 5.
Cytopathic effects induced by GIV and ISKNV in GS-1 cells. (a) GIV-infected GS-1 cells grown at 20°C for 3 days post-inoculation (dpi). (b) GIV-infected GS-1 cells grown at 25°C for 3 dpi. (c) GIV-infected GS-1 cells grown at 30°C for 5 dpi. (d) ISKNV-infected GS-1 cells grown at 20°C for 2 dpi. (e) ISKNV-infected GS-1 cells grown at 25°C for 3 dpi. (f) ISKNV-infected GS-1 cells grown at 30°C for 3 dpi. (g) Mock-infected cell at 20°C. (h) Mock-infected cell at 25°C. (i) Mock-infected cell at 30°C (bar=100 µm).
Following ISKNV infection at 25°C, GS-1 cell morphology has changed since 12 hpi. Cell shrinkage and rounding appeared first, and then the number of rounded cells markedly increased by 24 hpi. The ISKNV-induced CPE was characterized by cell rounding, cell fusion and cytolysis, which were observed at 20 and 25°C, but not at 30°C (Fig. 5d–f). Finally, numerous rounded cells fused and broke up on 3 dpi. Uninfected GS-1 cells showed no morphological changes during this period of observation at a different temperature (Fig. 5g–i).
The same CPE was reproduced, and the positive PCR signal was detected, after the serial 5 blind passages in GIV- and ISKNV-inoculated groups at 20, 25 and 30°C. However, this CPE was not observed from the ISKNV-inoculated GS-1 cells at 30°C after the third passage in the GS-1 cells.
The replication kinetics of GIV and ISKNV in GS-1 cells
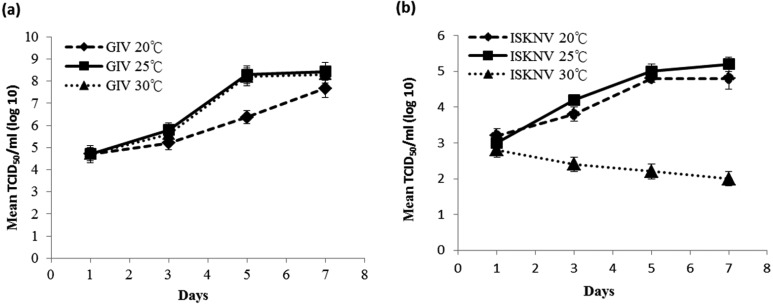
The optimum temperature for the proliferation of GIV in the GS-1 cells was between 20 to 30°C, which induced the viral titers of 107.7 TCID50/ml and the maximum titer of 108.4 TCID50/ml on 7 dpi at 25°C (Fig. 6a). Viral titers of ISKNV inoculated in the GS-1 cells on 7 dpi were 104.8 TCID50/ml at 20°C and 105.2 TCID50/ml at 25°C (Fig. 6b). When cells were cultured at 30°C, ISKNV multiplication was only observed during the first 2 days, with lower virus yields compared with 2 dpi after longer incubation time.
Fig. 6.
Replication kinetics of GIV and ISKNV in GS-1 cells cultured at different temperatures. The virus titer is indicated as the mean ± standard deviation (n=3).
Electron microscopy of GIV and ISKNV infected GS-1 cells
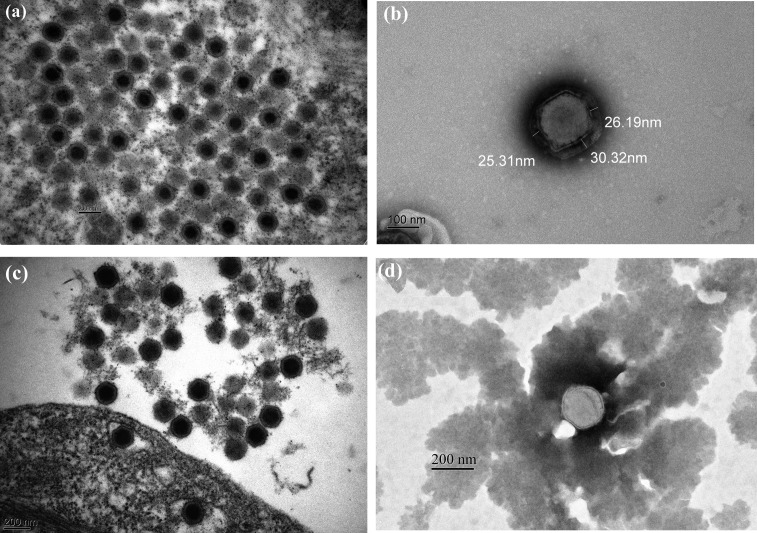
Examination of ultrathin sections of the GIV-infected GS-1 cell at 1 dpi revealed large amounts of virus particles were scattered throughout the cytoplasm as individual viruses or in paracrystalline arrays at assembly sites. The mean diameter of the GIV mature icosahedral particles were approximately 180–220 nm and containing full electron-dense cores (n=10) (Fig. 7a). In the supernatant, enveloped GIV showed a typical hexagonal shape and three-layered structure with an inner electron-dense core surrounded by a lighter coat (mean diameter, 27 ± 3 nm) (Fig. 7b). The diameter of the virus particle and mature nucleocapsids were approximately 210–220 and 140–150 nm, respectively (Fig. 7b). Numerous ISKNV virus particles with the icosahedral morphology of 160–200 nm in mean diameter were observed in the infected GS-1 cells at 1 dpi and the virions size were smaller than GIV (n=10) (Fig. 7c). The negative-stained ISKNV particle exhibited a two-layered membrane structure with 190–200 nm diameter sizes. The diameter of ISKNV mature nucleocapsids was approximately 170–180 nm, and was larger than that of GIV particles (Fig. 7d).
Fig. 7.
Electron micrographs of ultrathin sections and negatively stained virus particles from infected GS-1 cells. (a) Ultrathin section of a GIV-infected GS-1 cell showed the mean diameter of the mature icosahedral particles were approximately 180−220 nm and containing full electron-dense cores (n=10) (bar=200 nm). (b) A negatively stained GIV particle with 210−220 nm diameter exhibiting a three-layered structure with an inner electron-dense core surrounded by a lighter coat (mean diameter, 27 ± 3 nm) (bar=100 nm). (c) Numerous ISKNV virus particles with the icosahedral morphology of 160−200 nm in mean diameter were observed in the infected GS-1 cells and the virions size were smaller than GIV (n=10) (bar=200 nm). (d) A negative-stained ISKNV particle exhibited a two-layered membrane structure and the diameter of mature nucleocapsids was determined approximately 170−180 nm that was larger than GIV (bar=200 nm).
Accumulated mortality of groupers after challenge with GIV or ISKNV
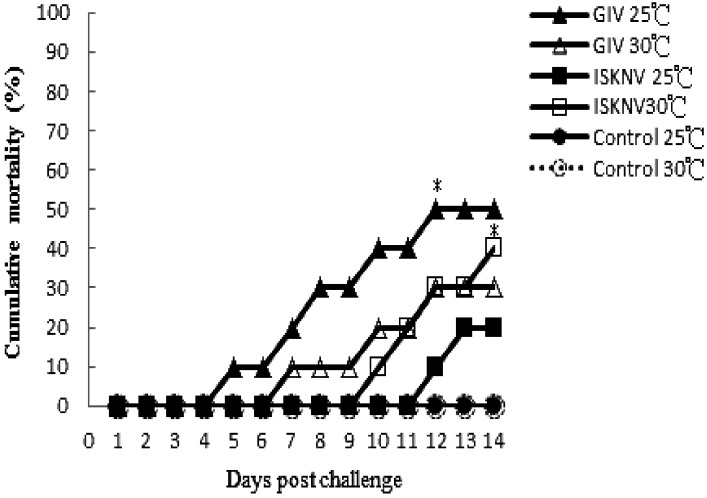
The cumulative mortality of GIV-infected fish incubated at 25°C reached 50% by 12 dpi, which was higher than at 30°C. Between 7 and 14 days after infection, the mortality rate increased from 20 to 50% at 25°C and from 10 to 30% at 30°C; the rates were significantly higher than those in control groups (P<0.05 by Fisher’s exact test).
In ISKNV-infected fish, cumulative mortality at 14 dpi was 40% at 30°C and 20% at 25°C (Fig. 8). Viruses were detected by PCR in pooled organs (spleen and kidney) from both dead and surviving fish following GIV and ISKNV infection. No viruses were detected in the target organs of either control group.
Fig. 8.
Cumulative mortality of E. coioides injected with GIV and ISKNV isolates and maintained at different water temperatures. Each group contained 20 fish. Cumulative mortality of GIV-infected fish reached 50% by 12 dpi at 25°C (closed triangle), which is higher than that at 30°C (open triangle). The asterisk indicates that mortality was significantly higher in GIV- and ISKNV-infected groups than in control groups (P<0.05). In ISKNV-challenged groups, the cumulative mortality was higher at 30°C (open square) than at 25°C (closed square) by 14 dpi. No mortality was observed among the two control groups (circle).
DISCUSSION
Cell lines are valuable tools for studying cellular responses and viral pathogenicity in fish species. The spleen is the target organ for fish iridoviruses [4, 16]. A published cell line designed as GS, derived from the spleen of Epinephelus coioides, showed high sensitivity to SGIV and NNV in vitro [34]. Different from GS cell line, GS-1 cell line was very sensitive to the infection and proliferation of GIV and ISKNV, belonging to ranavirus and megalocytivirus of Iridovirdae, respectively. Further, the GIV and ISKNV isolated from the GS-1 cell were applied to assess the investigation of the iridovirus–host interaction in vivo.
Morphologically, the initial GS-1 subcultures comprised fibroblastic and epithelial-like cells, but fibroblast-like cells accounted for the majority of the cell line after the 30th passage. Immunocytochemistry showed strong immunopositivity for vimentin, fibronectin, and desmin, consistent with our previous observation that GS-1 cells mainly comprise fibroblast-like cells. Similar phenotypic shifts have been demonstrated in grouper heart (GH) and grouper fin (GF) cells from the tropical grouper E. awoara [18]. The presence of immunocytochemistry in GS-1 cells confirmed the fibroblast-cell origin.
The GS-1 cell line grew well at a wide range of temperatures, with fastest growth at 32°C. No significant growth was observed at 20°C, similar to most established tropical fish cell lines [19, 31]. This adaptability is advantageous for studies of infection by viruses with a range of optimal growth temperatures. FBS is essential for the in vitro expansion of primary cultures. Growth rates of GS-1 cells increased with FBS concentration, as also observed in previous reports regarding the establishment of cell lines from marine fish [18, 30]. Our results showed that as little as 2% FBS can support the maintenance of GS-1 cells and be applied to routine isolation.
The susceptibility results indicated that the GIV appeared more susceptible to GS-1 than ISKNV in vitro. GS-1 cells efficiently produced CPE between 20°C and 30°C with yields of 107.7 TCID50/ml and the maximum titer was 108.4 TCID50/ml at 25°C. It has not been reported that different temperatures affect GIV proliferation capability in vitro. This result indicates that GIV is stable in GS-1 cells over a wide temperature range from 20 to 30°C in vitro. On the other hand, GS-1 cells infected with ISKNV yielded a lower titer of 105.2 TCID50/ml and only observed CPE at 20 and 25°C, but the appearance of CPE was not stable at 30°C. Our findings were consistent with previous studies in rock bream, (Oplegnathus fasciatus) embryo (RoBE-4) cell line, where RSIV isolation in RoBE-4 cells cultured at 20 or 25°C reached 106.0 TCID50/ml, with substantially lower titers at 30°C [28]. RSIV yielded from grunt fin (GF) cells gradually decreased by multiple subculturing at 15 or 30°C, and the optimum temperature to produce RSIV was 25°C [27, 29]. Our results shown in this study indicated that ISKNV proliferation at 25°C was better than that at 30°C in vitro. Similarly, it has been reported that the faster progression of apoptosis induced by high temperature in the TGIV-infected cells restricted the propagation of the virus [22]. Therefore, we speculated that the lower titer of ISKNV-infected GS-1 cells cultured at 30°C might be due to the same reason.
Electron microscopy revealed an envelope structure encapsulating GIV particles in infected GS-1 cells. Previous ultrastructural studies on ranavirus also revealed enveloped virus particles in the early stages of infection by frog virus 3 and SGIV infection [1, 32, 33]. In this study, the GIV possessed an envelope structure that was assembled by the three-layered structure with an inner electron-dense core surrounded by a lighter coat, and the results are in accordance with the published report that the mature SGIV viral particles released through budding and become enveloped virions [23]. In our ultrastructural studies, the mean diameter of GIV viral particles was 180–220 nm, substantially larger than ISKNV viral particles (160–200 nm). The negative-stained virions of ISKNV exhibited a two-layered membrane structure, similar to an RSIV-type megalocytivirus from spotted knifejaw termed SKIV [37]. These observations further confirm that the GS-1 cell line is suitable for the proliferation of both GIV and ISKNV. In this study, the outer membrane layers of GIV was first found to be different from that of ISKNV.
The cumulative mortality of GIV-infected groupers incubated at 25°C was higher than that at 30°C, which indicates that the GIV replication in vivo was consistent with in vitro. In addition, the GIV-infected groupers demonstrate more virulence than ISKNV-infected groupers at 25°C. These findings are consistent with the previous comparative study reporting that GIV has a much higher virulence than TGIV [20]. It has also been reported that ISKNV-induced mortality of mandarinfish only occurred at water temperature above 20°C [12]. The comparative pathogenicity of Ranavirus (GIV-R) and Megalocytivirus-type GIV (GIV-M) revealed that the GIV-M induced higher mortalities (97.5%) than GIV-R (85%) at 28°C [24]. Many factors may affect the interaction of virus and host in vivo, including water temperatures, virus-induced apoptosis, fish species, virus strains. The best temperature for viral replication in vitro and the highest mortality of virus-infected host fish in vivo might be different. We suggested that high water temperatures (30°C) might suppress defense system of the groupers, and result in high mortality. More research is needed to clarify the pathogenesis mechanism of ISKNV in groupers.
In vitro, GS-1 cell line exhibits high susceptibility and proliferation ability for GIV than ISKNV. In vivo, the highest mortality induced by GIV in groupers occurred at 25°C, whereas that by ISKNV was at 30°C. These data suggest that the GS-1 cell line will become a powerful tool for diagnosis and pathogenesis of the two iridoviruses in the same host system.
Acknowledgments
The authors thank Dr. Fan Lee for his comments on this manuscript. This study was granted by projects 104AS-10.3.1-HI-H8 and 105AS-10.3.1-HI-H6 of the Council of Agriculture, Executive Yuan, Taiwan.
REFERENCES
- 1.Bandín I., Dopazo C. P.2011. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet. Res. (Faisalabad) 42: 67. doi: 10.1186/1297-9716-42-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao C. B., Pang V. F.1997. An outbreak of an iridovirus-like infection in cultured grouper (Epinephelus spp.) in Taiwan. J. Chinese Soc. Vet. Sci. 23: 411–422. [Google Scholar]
- 3.Chi S. C., Shieh J. R., Lin S. J.2003. Genetic and antigenic analysis of betanodaviruses isolated from aquatic organisms in Taiwan. Dis. Aquat. Organ. 55: 221–228. doi: 10.3354/dao055221 [DOI] [PubMed] [Google Scholar]
- 4.Choi S. K., Kwon S. R., Nam Y. K., Kim S. K., Kim K. H.2006. Organ distribution of red sea bream iridovirus (RSIV) DNA in asymptomatic yearling and fingerling rock bream (Oplegnathus fasciatus) and effects of water temperature on transition of RSIV into acute phase. Aquaculture 256: 23–26. doi: 10.1016/j.aquaculture.2006.01.026 [DOI] [Google Scholar]
- 5.Chou H. Y., Hsu C. C., Peng T. Y.1998. Isolation and characterization of a pathogenic iridovirus from cultured grouper (Epinephelus sp.) in Taiwan. Fish Pathol. 33: 201–206. doi: 10.3147/jsfp.33.201 [DOI] [Google Scholar]
- 6.Chua F. H. C., Ng M. L., Ng K. L., Loo J. J., Wee J. Y.1994. Investigation of outbreaks of a novel disease, ‘Sleepy Grouper Disease’, affecting the brown-spotted grouper, Epinephelus tauvina Forskal. J. Fish Dis. 17: 417–427. doi: 10.1111/j.1365-2761.1994.tb00237.x [DOI] [Google Scholar]
- 7.Do J. W., Cha S. J., Kim J. S., An E. J., Lee N. S., Choi H. J., Lee C. H., Park M. S., Kim J. W., Kim Y. C., Park J. W.2005. Phylogenetic analysis of the major capsid protein gene of iridovirus isolates from cultured flounders Paralichthys olivaceus in Korea. Dis. Aquat. Organ. 64: 193–200. doi: 10.3354/dao064193 [DOI] [PubMed] [Google Scholar]
- 8.Dong C., Weng S., Shi X., Xu X., Shi N., He J.2008. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 135: 273–281. doi: 10.1016/j.virusres.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 9.FAO. 2018. Fisheries statistics and information. Topics fact sheets, FAO Fisheries and Aquaculture Department. http://www.fao.org/fishery/ [accessed on Decemer 22, 2015].
- 10.Gong J., Huang Y., Huang X., Ouyang Z., Guo M., Qin Q.2011. Establishment and characterization of a new cell line derived from kidney of grouper, Epinephelus akaara (Temminck & Schlegel), susceptible to Singapore grouper iridovirus (SGIV). J. Fish Dis. 34: 677–686. doi: 10.1111/j.1365-2761.2011.01281.x [DOI] [PubMed] [Google Scholar]
- 11.He J. G., Wang S. P., Zeng K., Huang Z. J., Chan S. M.2000. Systemic disease caused by an iridovirus-like agent in cultured mandarinfish, Siniperca chuatsi (Basilewsky), in China. J. Fish Dis. 23: 219–222. doi: 10.1046/j.1365-2761.2000.00213.x [DOI] [Google Scholar]
- 12.He J. G., Zeng K., Weng S. P., Chan S. M.2002. Experimental transmission, pathogenicity and physical–chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 204: 11–24. doi: 10.1016/S0044-8486(01)00639-1 [DOI] [Google Scholar]
- 13.Huang S. M., Tu C., Kuo S. T., Kuo H. C., Chou C. C., Chang S. K.2016. Establishment and characterization of a novel kidney-cell line from orange-spotted grouper, Epinephelus coioides, and its susceptibility to grouper iridovirus. J. Mar. Sci. Res. Dev. 6: 2–7. [Google Scholar]
- 14.Huang S. M., Tu C., Tseng C. H., Huang C. C., Chou C. C., Kuo H. C., Chang S. K.2011. Genetic analysis of fish iridoviruses isolated in Taiwan during 2001−2009. Arch. Virol. 156: 1505–1515. doi: 10.1007/s00705-011-1017-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z., Tang J., Li M., Fu Y., Dong C., Zhong J. F., He J.2012. Immunological evaluation of Vibrio alginolyticus, Vibrio harveyi, Vibrio vulnificus and infectious spleen and kidney necrosis virus (ISKNV) combined-vaccine efficacy in Epinephelus coioides. Vet. Immunol. Immunopathol. 150: 61–68. doi: 10.1016/j.vetimm.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 16.Ito T., Yoshiura Y., Kamaishi T., Yoshida K., Nakajima K.2013. Prevalence of red sea bream iridovirus among organs of Japanese amberjack (Seriola quinqueradiata) exposed to cultured red sea bream iridovirus. J. Gen. Virol. 94: 2094–2101. doi: 10.1099/vir.0.052902-0 [DOI] [PubMed] [Google Scholar]
- 17.Lü L., Zhou S. Y., Chen C., Weng S. P., Chan S. M., He J. G.2005. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper, Epinephelus coioides. Virology 339: 81–100. doi: 10.1016/j.virol.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 18.Lai Y. S., John J. A. C., Lin C. H., Guo I. C., Chen S. C., Fang K., Lin C. H., Chang C. Y.2003. Establishment of cell lines from a tropical grouper, Epinephelus awoara (Temminck & Schlegel), and their susceptibility to grouper irido- and nodaviruses. J. Fish Dis. 26: 31–42. doi: 10.1046/j.1365-2761.2003.00434.x [DOI] [PubMed] [Google Scholar]
- 19.Lai Y. S., Murali S., Ju H. Y., Wu M. F., Guo I. C.2000. Two iridovirus-susceptible cell lines established from kidney and liver of grouper, Epinephelus awoara (Temminck & Schlegel), and partial characterization of grouper iridovirus. J. Fish Dis. 23: 379–388. doi: 10.1046/j.1365-2761.2000.00247.x [DOI] [Google Scholar]
- 20.Leu J. H., Wu M. H., Chou H. Y.2013. A comparative study between ranavirus and megalocytivirus infections in orange-spotted grouper (Epienphelus coioides). J. Mar. Sci. Technol. 21: 58–64. [Google Scholar]
- 21.Liu H. I., Chiou P. P., Gong H. Y., Chou H. Y.2015. Cloning of the Major Capsid Protein (MCP) of Grouper Iridovirus of Taiwan (TGIV) and Preliminary Evaluation of a Recombinant MCP Vaccine against TGIV. Int. J. Mol. Sci. 16: 28647–28656. doi: 10.3390/ijms161226118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H. I., Lin Y. C., Chiou P. P., Chou H. Y.2015. Temperature-dependent pathogenicity of grouper Iridovirus of Taiwan (TGIV). J. Mar. Sci. Technol. 24: 637–644. [Google Scholar]
- 23.Liu Y., Tran B. N., Wang F., Ounjai P., Wu J., Hew C. L.2016. Visualization of assembly intermediates and budding vacuoles of singapore grouper iridovirus in grouper embryonic cells. Sci. Rep. 6: 18696. doi: 10.1038/srep18696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H., Peng C., Su Y., Feng J., Guo Z.2016. Isolation of a Ranavirus-type grouper iridovirus in mainland China and comparison of its pathogenicity with that of a Megalocytivirus-type grouper iridovirus. Aquaculture 463: 145–151. doi: 10.1016/j.aquaculture.2016.05.032 [DOI] [Google Scholar]
- 25.Mahardika K., Zafran, Yamamoto A., Miyazaki T.2004. Susceptibility of juvenile humpback grouper Cromileptes altivelis to grouper sleepy disease iridovirus (GSDIV). Dis. Aquat. Organ. 59: 1–9. doi: 10.3354/dao059001 [DOI] [PubMed] [Google Scholar]
- 26.Murali S., Wu M. F., Guo I. C., Chen S. C., Yang H. W., Chang C. Y.2002. Molecular characterization and pathogenicity of a grouper iridovirus (GIV) isolated from yellow grouper, Epinephelus awoara (Temminck & Schlegel). J. Fish Dis. 25: 91–100. doi: 10.1046/j.1365-2761.2002.00343.x [DOI] [Google Scholar]
- 27.Nakajima K., Sorimachi M.1994. Biological and Physico-chemical Properties of the Iridovirus Isolated from Cultured Red Sea Bream, Pagrus major. Fish Pathol. 29: 29–33. doi: 10.3147/jsfp.29.29 [DOI] [Google Scholar]
- 28.Oh S. Y., Nishizawa T.2016. Establishment of rock bream Oplegnathus fasciatus embryo (RoBE-4) cells with cytolytic infection of red seabream iridovirus (RSIV). J. Virol. Methods 238: 1–5. doi: 10.1016/j.jviromet.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 29.Oh S. Y., Nishizawa T.2016. Multiple Passages of Grunt Fin Cells Persistently Infected with Red Seabream Iridovirus (RSIV) at 15ºC or 30ºC to Yield Uninfected Cells. J. Aquat. Anim. Health 28: 214–221. doi: 10.1080/08997659.2016.1208120 [DOI] [PubMed] [Google Scholar]
- 30.Ou-Yang Z. L., Huang X. H., Huang E. Y., Huang Y. H., Gong J., Sun J. J., Qin Q. W.2010. Establishment and characterization of a new marine fish cell line derived from red-spotted grouper Epinephelus akaara. J. Fish Biol. 77: 1083–1095. doi: 10.1111/j.1095-8649.2010.02749.x [DOI] [PubMed] [Google Scholar]
- 31.Parameswaran V., Ishaq Ahmed V. P., Shukla R., Bhonde R. R., Sahul Hameed A. S.2007. Development and characterization of two new cell lines from milkfish (Chanos chanos) and grouper (Epinephelus coioides) for virus isolation. Mar. Biotechnol. (NY) 9: 281–291. doi: 10.1007/s10126-006-6110-9 [DOI] [PubMed] [Google Scholar]
- 32.Qin Q. W., Chang S. F., Ngoh-Lim G. H., Gibson-Kueh S., Shi C., Lam T. J.2003. Characterization of a novel ranavirus isolated from grouper Epinephelus tauvina. Dis. Aquat. Organ. 53: 1–9. doi: 10.3354/dao053001 [DOI] [PubMed] [Google Scholar]
- 33.Qin Q. W., Lam T. J., Sin Y. M., Shen H., Chang S. F., Ngoh G. H., Chen C. L.2001. Electron microscopic observations of a marine fish iridovirus isolated from brown-spotted grouper, Epinephelus tauvina. J. Virol. Methods 98: 17–24. doi: 10.1016/S0166-0934(01)00350-0 [DOI] [PubMed] [Google Scholar]
- 34.Qin Q. W., Wu T. H., Jia T. L., Hegde A., Zhang R. Q.2006. Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J. Virol. Methods 131: 58–64. doi: 10.1016/j.jviromet.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 35.Reed L. J., Muench H.1938. A simple method of estimating fifty per cent endpoits. Am. J. Epidemiol. 27: 493–497. doi: 10.1093/oxfordjournals.aje.a118408 [DOI] [Google Scholar]
- 36.Shi C. Y., Wang Y. G., Yang S. L., Huang J., Wang Q. Y.2004. The first report of an iridovirus-like agent infection in farmed turbot, Scophthalmus maximus, in China. Aquaculture 236: 11–25. doi: 10.1016/j.aquaculture.2003.11.007 [DOI] [Google Scholar]
- 37.Shuang F., Luo Y., Xiong X. P., Weng S., Li Y., He J., Dong C.2013. Virions proteins of an RSIV-type megalocytivirus from spotted knifejaw Oplegnathus punctatus (SKIV-ZJ07). Virology 437: 89–99. doi: 10.1016/j.virol.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 38.Wang C. S., Shih H. H., Ku C. C., Chen S. N.2003. Studies on epizootic iridovirus infection among red sea bream, Pagrus major (Temminck & Schlegel), cultured in Taiwan. J. Fish Dis. 26: 127–133. doi: 10.1046/j.1365-2761.2003.00441.x [DOI] [PubMed] [Google Scholar]
- 39.Wang Y. Q., Lü L., Weng S. P., Huang J. N., Chan S. M., He J. G.2007. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Arch. Virol. 152: 763–773. doi: 10.1007/s00705-006-0870-4 [DOI] [PubMed] [Google Scholar]
- 40.Young B. C., Yeh S. P., Chung H. R., Lee P. P.2015. The current status of grouper culture operations and cost analysis of the industry in Taiwan. Jacobs J. Aquaculture Res. 1: 1–6. [Google Scholar]