Abstract
We previously demonstrated that transmissible gastroenteritis virus (TGEV) could induce apoptosis through caspase signaling. However, apoptosis was not completely prevented by caspases inhibitors, suggesting that there may be a caspase-independent pathway involved in TGEV-induced cell apoptosis. In this study, we investigated the regulation of apoptosis-inducing factor (AIF) on TGEV-induced apoptotic pathway. Results indicated that AIF translocated from the mitochondria to nucleus during TGEV infection, and the AIF inhibitor, N-phenylmaleimide (NP), significantly attenuated the apoptosis. In addition, the translocation of AIF was inhibited by Veliparib (ABT-888), an inhibitor of poly (ADP-ribose) polymerase (PARP). And the reactive oxygen species (ROS) scavenger, pyrrolidinedithiocarbamic (PDTC), redistributed AIF in the mitochondria and nucleus in TGEV-infected cells. Moreover, the protein levels in nucleus and the mRNA levels of AIF were inhibited in the presence of the p53 inhibitor, pifithrin-α (PFT-α) or in TGEV-infected p53−/−cells. Furthermore, TGEV-induced apoptosis was blocked by combination of three or more inhibitors, such as pan caspase inhibitor Z-VAD-FMK, NP, ABT-888, PDTC, PFT-α, to treat PK-15 cells. Taken together, these results suggest that the p53- and ROS-mediated AIF pathway and caspase-dependent pathway were involved in TGEV-induced apoptosis.
Keywords: AIF, apoptosis, p53, ROS, TGEV
Apoptosis-inducing factor (AIF), normally locates in the inner mitochondrial membrane and exert a cytoprotective role [17, 25, 26]. Recently, in contrast to its cytoprotective role in the mitochondria, AIF was found to translocate from the mitochondria to the cytosol, and subsequently to the nucleus, serving as a powerful proapoptotic trigger [3, 13]. Upon induction of various apoptotic stimuli, AIF partly bound to DNA, stimulated DNase activity and triggered condensation and large-scale fragmentation of chromatin, to induce apoptosis [25, 27, 30]. However, the proapoptotic effects were not prevented by caspase inhibitors, suggesting that AIF mediated a caspase-independent manner to trigger nuclear apoptosis [5, 16, 25]. Numerous lines of research have showed that a caspase-independent manner is involved in various stimuli-induced apoptotic programs [5, 6], including viruses [10, 20].
Transmissible gastroenteritis virus (TGEV) belongs to the alphacoronavirus genus in the Coronaviridae family according to viral taxonomy of International committee of taxonomy of viruses [1, 19]. After infecting piglets, TGEV then replicates in enterocytes, subsequently results in lethal watery diarrhea and dehydration [7]. When infects several cell lines in vitro, TGEV could induce morphological and biochemical changes in these cells [7, 9], and these findings were consistent with in vivo pathologic changes. We previously proved that TGEV infection induced apoptosis by caspase signaling [7]. p53 and reactive oxygen species (ROS) play pivotal roles in TGEV-induced cell apoptosis [8, 12]. However, TGEV-induced apoptosis is not completely inhibited by caspases inhibitors, suggesting that a caspase-independent pathway may be involved in TGEV-induced cell apoptosis. Thus, the study presented here stemmed from our previous findings and investigated the role of AIF in TGEV-induced apoptosis. Our results demonstrated that ROS and p53 levels mediated AIF released to the nucleus from the mitochondria during TGEV infection, thereby leading to apoptosis.
MATERIALS AND METHODS
Cells, virus and antibodies
PK-15 cells were cultured in Dulbecco Minimal Essential Medium (DMEM) with 10% heat-inactivated newborn bovine serum (Gibco BRL, Now York, MD, U.S.A.), at 37°C in a humidified 5% CO2 chamber. The TGEV was used as previously described by Ding et al. [7]. Primary antibodies against AIF (sc-13116), β-actin (sc-69879), Cox4 (sc-376731), p53 (sc-4246), PARP-1 (sc-8007), and histone H3 (sc-517576) were purchased from Santa Cruz Biotechnology (Santa Cruz, Delaware, CA, U.S.A.). The antibody against TGEV N protein was prepared by our laboratory. Fluorescein Isothiocyanate (FITC), Rhodamine and horseradish peroxidase-conjugated secondary antibodies were purchased from The Thermo Scientific Pierce (Pierce, Rockford, IL, U.S.A.). N-phenylmaleimide (NP) and pifithrin-α (PFT-α) were purchased from Sigma (Sigma-Aldrich, Burlington, MA, U.S.A.). Z-VAD-FMK and Veliparib (ABT-888) were purchased from MedChemExpress (MedChemExpress, Monmouth Junction, NJ, U.S.A.). Pyrrolidinedithiocarbamic (PDTC) was purchased from Merck (Merck KGaA, Darmstadt, Germany).
Immunofluorescence assay
Cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.2% Triton X-100 for 10 min. Then the cells were incubated with a primary antibody against AIF (1:100) or TGEV N at 4°C overnight after blocked with 5% serum albumin for 30 min. Samples were washed three times with phosphate-buffered saline (PBS). Subsequently, secondary antibody (Rhodamine-conjugated IgG) was incubated for 1 hr in the dark. Then, 4’, 6-diamidino-2-phenylindole (DAPI) were used to counterstain cell nuclei for 10 min. Images were taken under a fluorescence microscope (AMG EVOS, Hatfield, PA, U.S.A.).
RNA analysis by qRT-PCR
RNA extraction and qRT-PCR were performed as our previously described [7]. Specific primers that were used are as follows: AIF sense, TGTGGAACGTCTTCAACCGA; AIF antisense, AGAGGAAGGCTGCGCAATAA. Actin sense GGACTTCGAGCAGGAGATGG; actin antisense: AGGAAGGAGGGCTGGAAGAG.
Western blot analysis
The mitochondrial and nuclear proteins were isolated as the description of the Mitochondria Fractionation Kit and the CelLytic™ NuCLEAR™ Extraction Kit (Sigma-Aldrich, Burlington, MA, U.S.A.), respectively. Protein concentrations were measured using the BCA Protein Assay Reagent (Pierce, Rockford, IL, U.S.A.). The Western blot assay was conducted as we described previously [7].
Flow cytometry analysis
The detection of apoptosis was performed as described by Ding et al [8]. Summarily, harvested cells were washed with PBS for three times and then resuspended in 500 µl binding buffer, followed by the addition of 5 µl of Annexin V-FITC and 5 µl of PI. After incubation for 30 min at room temperature, a total of 10,000 cells were examined. The positive cells was analyzed by flow cytometry using Beckman CytoFLEX FCM (Beckman Coulter, Brea, CA, U.S.A.).
CRISPR/cas9 KO PK-15 cells
The CRISPR/Cas9 system (Genome Engineering. Broad Institute Cambridge, MA, U.S.A.) was used to mutate p53 gene, according to the description by Xu et al [29]. Briefly, annealed fragments of gRNA (F: AGTCACGAACTGGCTGGATG, R: TCAGTGGCTTGACCGACCTAC) were cloned into lentiCRISPRv2 plasmid (Addgene, Cambridge, MA, U.S.A.), then with the psPAX2 packaging plasmid co-transfected into HEK293T cells to generate the lentivirus. Lentiviral titers were measured by qPCR. Lentiviral-infected PK15 cell lines grown in puromycin DMEM medium were used for selecting p53 knockout cells. Puromycin-resistant PK15 cells were selected and amplified and were subsequently examined by Western blot.
RESULTS
TGEV infection induced AIF translocation to the nucleus
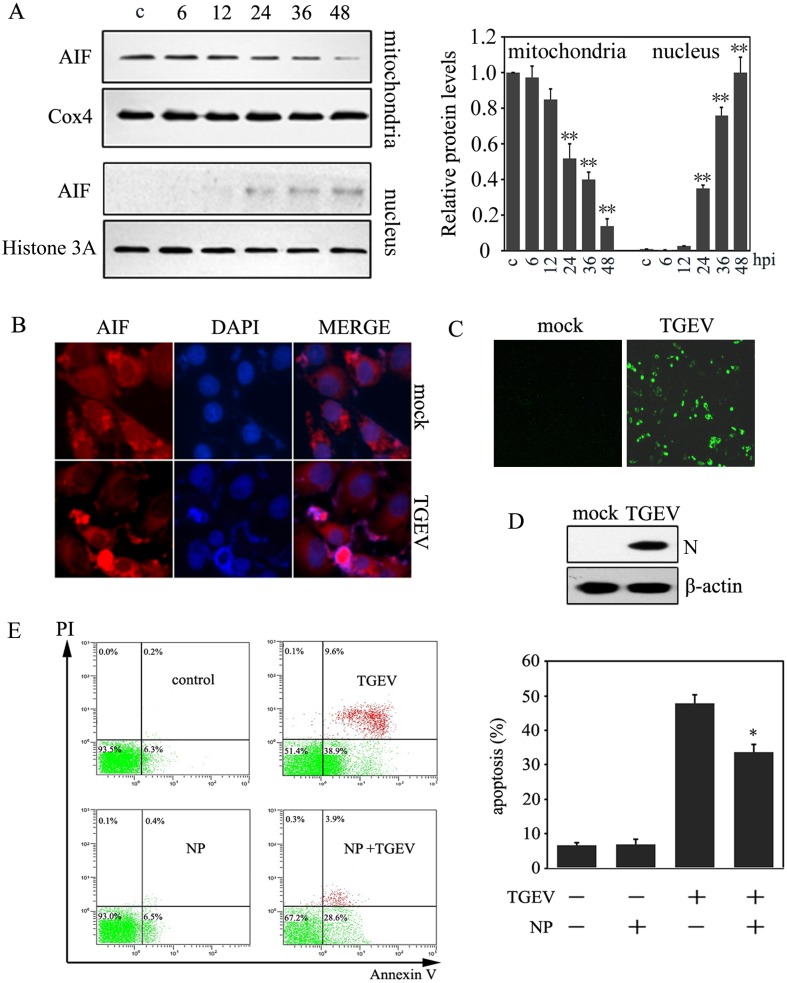
Our previous study demonstrated that caspase inhibitors did not completely prevent apoptosis in TGEV-infected cells [7]. Thus, to investigate whether AIF pathway was activated in TGEV-induced apoptosis in PK-15 cells, the protein levels of AIF in mitochondrial and nuclear fractions were measured by Western blot. Results showed that the mitochondrial fraction levels of AIF decreased significantly after 24 hr of infection with TGEV, while the nuclear fraction of AIF increased after 24 hr post infection, and keep rising with infection time (Fig. 1A).
Fig. 1.
The activation of AIF during TGEV infection. (A) PK-15 cells were collected at different infection times (MOI=10). The mitochondrial and nuclear proteins were extracted, and the cell lysates were analyzed by Western blot. Histone H3 and Cox4 were used as nuclear and mitochondria internal controls, respectively. The protein expression of AIF in the mitochondria and nucleus was calculated by densitometry of the corresponding bands after normalization to Cox4 and histone, respectively (right panel). Results are expressed as means ± SD from three independent experiments. **P<0.01 versus control. (B) Cells were fixed for immunofluorescence when infected with TGEV for 36 hr post infection. AIF were stained red, and cellular nuclei were stained blue. (C) Cells were fixed for immunofluorescence when infected with TGEV for 36 hr post infection. TGEV N protein were stained green. (D) Cells were collected when infected with TGEV for 36 hr post infection, then were subjected to Western blot analysis for TGEV N protein. (E) The effect of the AIF inhibitor, NP, on apoptosis. Cells were incubated with 10 µM NP for 1 hr, and then were infected with TGEV for 36 hr, subsequently cells were subjected to flow cytometric analysis. The percentages shown are the proportion of apoptotic cells (right panel). *P<0.05 versus TGEV infection. All data are means ± SD of results from three independent experiments.
To further confirm AIF translocation to the nucleus during TGEV infection, immunofluorescence was performed with an antibody against AIF (red fluorescence). As predicted, AIF translocated to the nucleus after TGEV infection (Fig. 1B), suggesting that AIF was activated in TGEV-infected PK-15 cells. In addition, to confirm the AIF activation was induced by TGEV infection, we measured the TGEV N protein levels by immunofluorescence and Western blot assays. Results showed that TGEV N protein were observed in TGEV-infected PK15 cells (Fig. 1C and 1D).
To verify whether AIF activation was involved in TGEV-induced apoptosis, the AIF inhibitor, NP, was used to co-treat PK-15 cells with TGEV, and the apoptotic rate was measured. Our results showed that NP partly abrogated TGEV-induced apoptosis (Fig. 1C). These results suggest that AIF pathway might be involved in TGEV-induced apoptosis.
PARP-1 mediated AIF translocation during TGEV-induced apoptosis
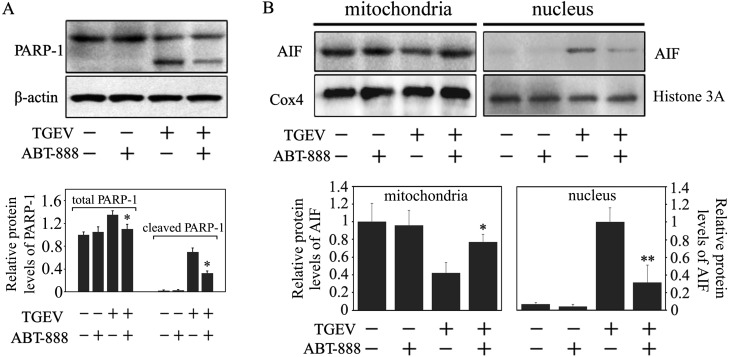
Previous studies demonstrated that Poly (ADP-ribose) polymerase 1 (PARP-1) contributed to the release of AIF from the mitochondria to nucleus, activating the AIF apoptotic pathway [11]. Our previous research showed that TGEV infection induced PARP-1 activation and subsequent cleavage [7]. To determine whether the translocation of AIF was mediated by PARP-1 during TGEV-induced apoptosis, the PARP-1 inhibitor, ABT-888, was used to co-treat PK-15 cells with TGEV. Western blot assay was used to measure the AIF protein levels in the nucleus. As shown in Fig. 2A, ABT-888 markedly inhibited the total protein and cleaved protein levels of PARP-1, compared with TGEV infection alone, however the total protein levels of PARP-1 were no significant difference in ABT-888- and TGEV- co-treated PK-15 cells, compared with ABT-888 treatment alone. Moreover, ABT-888 significantly inhibited the activation of PARP-1 and the AIF translocation was prevented by ABT-888 (Fig. 2B). These results indicated that PARP-1 may be crucial in the AIF translocation during TGEV infection.
Fig. 2.
The effect of ABT-888, a PARP-1 inhibitor, on the translocation of AIF. (A) PK-15 cells were incubated with 20 µM ABT-888 for 1 hr and subsequently infected with TGEV for 36 hr. Cells were collected and then subjected to Western blot analysis for PARP-1. The total protein and cleaved protein levels of PARP-1 were calculated by densitometry of the corresponding bands after normalization to β-actin. (B) Cells were treated as in (A). The mitochondrial and nuclear proteins were extracted from the collected cells, and then subjected to Western blot analysis. AIF protein expression in the mitochondria and nucleus was calculated by densitometry of the corresponding bands after normalization to Cox4 and Histone H3, respectively. (C) All data are means ± SD of triplicates from three independent experiments. *P<0.05, **P<0.01 versus TGEV infection without ABT-888.
ROS regulated PARP-AIF activation in TGEV-infected PK-15 cells
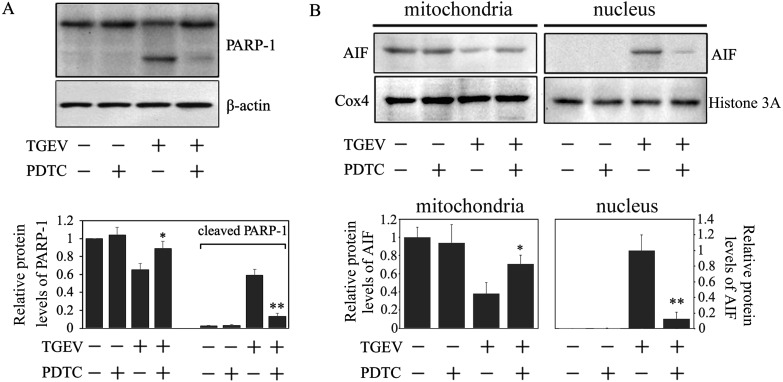
Virus infection could induce ROS accumulation [8, 24], and excessive ROS could change mitochondrial permeability transition pore (mPTP) and activate PARP-1, thereby leading to AIF translocation into the nucleus [17, 28]. Our previous work revealed that ROS is an important early mediator in TGEV-induced apoptosis [8]. To further explore the roles of ROS accumulation in the AIF-mediated apoptotic pathway during TGEV infection, we investigated the effects of PDTC, an ROS scavenger, on the cleavage of PARP-1 and AIF translation. As predicted, PDTC significantly attenuated the cleavage of PARP-1 (Fig. 3A), as evidenced by the redistribution of AIF in the mitochondria and nucleus in PK-15 cells infected by TGEV (Fig. 3B). The result indicated that ROS may play a vital role in TGEV-induced AIF activation.
Fig. 3.
ROS regulated PARP-AIF activation. (A) The effect of PDTC on the activation of PARP-1. Cells were treated with PDTC (100 µM) and infected with TGEV for 36 hr. Cell lysates were subjected to Western blot analysis. The expression of PARP-1 and cleaved PARP-1 was calculated by densitometry of the corresponding bands after normalization to actin (lower panel). (B) The effect of PDTC on the translocation of AIF. PDTC were co-treated PK-15 cells with TGEV for 36 hr. The mitochondrial and nuclear proteins were analyzed by Western blot. AIF protein levels in the mitochondria and nucleus were calculated by densitometry of the corresponding bands after normalization to Cox4 and Histone H3, respectively (lower panel). All data are means ± SD of results from three independent experiments. *P<0.05, **P<0.01 versus TGEV infection without PDTC.
p53 mediated AIF mRNA expression and regulated the AIF translocation
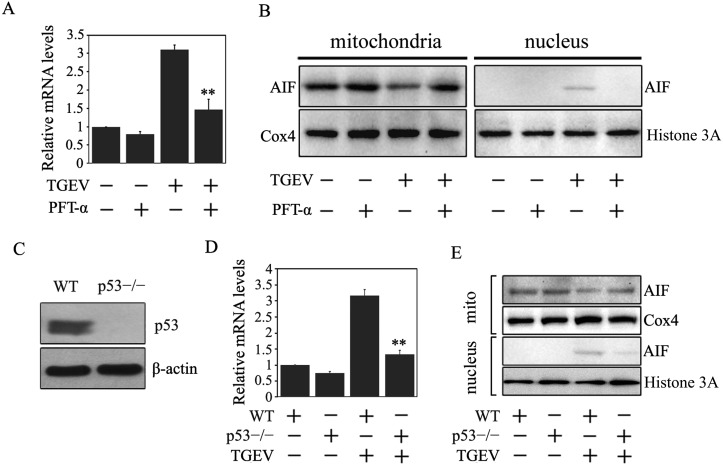
Our previous research showed that TGEV infection can activate ROS/p53- mediated apoptotic pathways [8]. In addition, several lines of research showed that AIF could be regulated by p53 [25, 31]. We therefore sought to whether p53 regulated the AIF expression. To that end, we used the p53 inhibitor, PFT-α, to co-treat the cells with TGEV and analyzed the AIF mRNA levels. Result showed that the mRNA levels of AIF decreased in the presence of PFT-α compared to those without PFT-α (Fig. 4A). To further explore the effect of p53 on AIF translocation, the AIF protein levels in the mitochondria and the nucleus were detected by Western blot. Our results showed that PFT-α treatment significantly reduced AIF protein levels in the nucleus (Fig. 4B), suggesting that p53 may be involved in AIF activation upon TGEV infection.
Fig. 4.
p53 mediated AIF mRNA expression and AIF translocation. (A) The effect of PFT-α on the mRNA expression of AIF. Cells were pretreated with 20 µM of PFT-α for 1 hr, subsequently co-treated with TGEV for 36 hr. The total RNA were extracted, reverse transcribed, and analyzed by qRT-PCR. The gene expression levels were presented after normalizing with β-actin. Values are mean ± SD of three different experiments performed in triplicate. **P<0.01 versus TGEV infection only. (B) The effect of PFT-α on the translocation of AIF. Cells were treated as above. The mitochondrial and nuclear proteins levels were subjected to Western blot analysis. (C) The expression of p53 after knockout. Wildtype PK15 cells (WT) and the puromycin-resistant cells containing gRNA (p53−/−) were subjected to Western blot analysis. (D) The mRNA expression of AIF in TGEV-infected WT PK15 and p53−/−PK-15 cells. Cells were collected when they were infected with TGEV for 36 hr. The total RNA in the cells was extracted, reverse transcribed, and analyzed by qRT-PCR. Data are mean ± SD of three different experiments performed in triplicate. **P<0.01 versus TGEV-infected WT PK-15 cells. (E) The translocation of AIF in TGEV-infected WT PK-15 and p53−/−PK-15 cells. Cells were treated as in (D), then the lysates were analyzed by Western blot.
To rule out the contribution of the PFT-α inhibitor on the virus, we used the CRISPR/Cas9 system to mutate the p53 gene. Result showed that the p53 protein was completely eliminated by the gRNA-generated clone (Fig. 4C). Next, the wild-type PK15 cells (WT) and the PK15 p53 knockout cells (PK15p53−/−) were infected with TGEV, and the activation of AIF was detected by qPCR and Western blot. As shown in Fig. 4D and 4E, AIF mRNA expression and the translocation of AIF were reduced markedly in the absence of p53. The findings were consistent with the changes associated with PFT-α treatment.
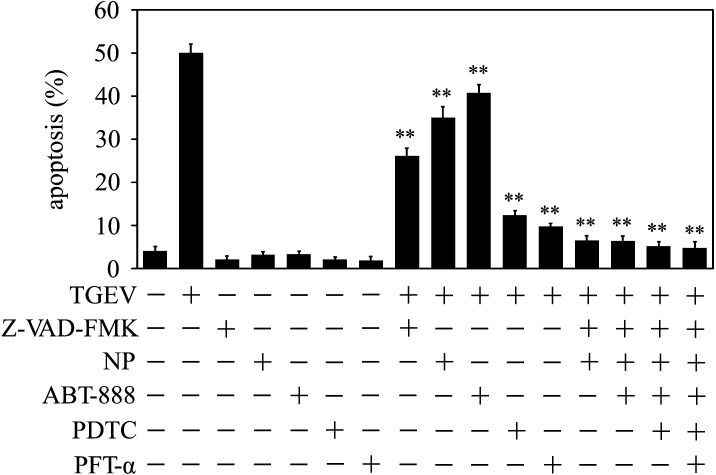
Combined inhibitors of caspase-dependent and caspase-independent pathway blocked TGEV-induced apoptosis
To further confirm that TGEV infection activated both caspase-dependent pathway and AIF pathway, we combined these inhibitors to treat the cells, and then measured the apoptotic rate upon TGEV infection. Result showed that TGEV-induced apoptosis was almost completely inhibited by combination of pan caspase inhibitor Z-VAD-FMK and AIF inhibitor NP treatment, compared to TGEV infection alone. The same blocking were also observed when the cells were treated by combination of three or more inhibitors, such as Z-VAD-FMK, NP, ABT-888, ROS scavenger PDTC, p53 inhibitor PFT-α (Fig. 5). The result is further evidence that caspase-dependent and caspase-independent pathway were included in TGEV-induced apoptosis, and, p53 and ROS played vital roles during apoptosis process.
Fig. 5.
Combined inhibitors of caspase-dependent and caspase-independent pathway blocked TGEV-induced apoptosis. Cells were cultured in six well plates, and incubated with Z-VAD-FMK (20 µM), NP (10 µM), ABT-888 (20 µM), PDTC (100 µM), PFT-α (20 µM) for 1 hr respectively, or co-treated by combination of these inhibitors. Then the cells were infected with TGEV for 36 hr, subsequently cells were subjected to flow cytometric analysis. The percentages shown are the proportion of apoptotic cells. **P<0.01 versus TGEV infection. All data are means ± SD of results from three independent experiments.
DISCUSSION
AIF can participate in apoptosis upon translocation to the nucleus [4, 5, 14]. It binds to DNA in the nucleus, and induces chromatin condensation and DNA fragmentation by recruiting downstream nucleases, resulting in apoptosis [11]. Actually, AIF is considered to play a centric role in regulating caspase-independent pathways in cells [4, 6]. Our present study showed that TGEV infection activated AIF, which was released to the nucleus, thereby resulting in apoptosis. Combination the inhibitors of pan caspase and AIF blocked the apoptosis. In agreement with our previous research [7], we demonstrated that TGEV-induced apoptosis involved a caspase-independent pathway as well as caspase-dependent pathways in infected cells. Similar findings were seen with the rabies virus and the vesicular stomatitis virus [10, 20].
Several researches indicated that PARP-1 can induce poly (ADP-ribosyl) ation translocation to the mitochondria, then triggering AIF translocation to the nucleus, resulting in DNA fragmentation, to activate the AIF apoptotic pathway [11, 28], Consistent with these results, the current study showed that PARP-1 inhibition could reduce the redistribution of AIF in the mitochondria and nucleus, suggesting that PARP-1 may be a prerequisite for TGEV-induced translocation of AIF. However, the total protein levels of PARP-1 were no significant difference in ABT-888 and TGEV co-treated PK-15 cells, compared with ABT-888 treatment alone, but remarkably decreased when compared to that TGEV infection alone. And also the cleaved PARP-1 were not absolutely inhibited in TGEV-infected cells. These results suggest that TGEV might activate PARP-1 to induce AIF translocation, while the cleavage of PARP-1 was partly promoted by caspases.
In multiple signaling pathways, ROS is believed to be a second messenger [23]. Many studies have shown that high levels of ROS can change the mitochondrial permeability transition pores (mPTPs) and activate PARP-1, leading to AIF translocation to the nucleus [18, 21]. We previously demonstrated that TGEV-induced ROS accumulation reduced mitochondrial membrane potential, leading to apoptosis [8]. In the present study, we show that the inhibition of ROS by PDTC attenuated the translocation of AIF, indicating that ROS may be an important factor in a caspase-independent cell apoptosis pathway during TGEV infection.
The accumulation of ROS may initiate cellular safeguard machinery p53 [15]. The signal would be returned to the mitochondria, leading to the increase of mitochondrial outer membrane permeabilization, subsequently the release of pro-apoptotic factors, thus triggering the apoptosis cascade [15]. p53 not only takes effects on caspase-dependent apoptosis [2, 22], but also drives the efficient activation of AIF through regulating AIF gene expression, to mediate a caspase-independent death pathway [25]. In the current study, the p53 inhibitor, PFT-α, significantly reduced the mRNA levels of AIF and decreased the protein levels in the nucleus, and the similar results were also found in TGEV-infected p53−/− cells. These results showed that p53 might mediate the transcription of AIF, and induce AIF translocation to the nucleus.
In the present study, we elaborated that TGEV infection induced AIF translocation to the nucleus, which may be mediated by p53 activation and ROS accumulation. These results provided further insight into the mechanism of TGEV-induced cell death.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 31502036), Hainan Natural Science Foundation (Grant No. 318MS046; 20153086), Hainan Science Research Project of Higher Education (No. Hnky2016-15).
REFERENCES
- 1.Adams M. J., Lefkowitz E. J., King A. M., Carstens E. B.2014. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2014). Arch. Virol. 159: 2831–2841. doi: 10.1007/s00705-014-2114-3 [DOI] [PubMed] [Google Scholar]
- 2.Amaral J. D., Xavier J. M., Steer C. J., Rodrigues C. M.2010. The role of p53 in apoptosis. Discov. Med. 9: 145–152. [PubMed] [Google Scholar]
- 3.Candé C., Cecconi F., Dessen P., Kroemer G.2002. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J. Cell Sci. 115: 4727–4734. doi: 10.1242/jcs.00210 [DOI] [PubMed] [Google Scholar]
- 4.Candé C., Cohen I., Daugas E., Ravagnan L., Larochette N., Zamzami N., Kroemer G.2002. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84: 215–222. doi: 10.1016/S0300-9084(02)01374-3 [DOI] [PubMed] [Google Scholar]
- 5.Candé C., Vahsen N., Garrido C., Kroemer G.2004. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ. 11: 591–595. [DOI] [PubMed] [Google Scholar]
- 6.Cregan S. P., Dawson V. L., Slack R. S.2004. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23: 2785–2796. doi: 10.1038/sj.onc.1207517 [DOI] [PubMed] [Google Scholar]
- 7.Ding L., Xu X., Huang Y., Li Z., Zhang K., Chen G., Yu G., Wang Z., Li W., Tong D.2012. Transmissible gastroenteritis virus infection induces apoptosis through FasL- and mitochondria-mediated pathways. Vet. Microbiol. 158: 12–22. doi: 10.1016/j.vetmic.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L., Zhao X., Huang Y., Du Q., Dong F., Zhang H., Song X., Zhang W., Tong D.2013. Regulation of ROS in transmissible gastroenteritis virus-activated apoptotic signaling. Biochem. Biophys. Res. Commun. 442: 33–37. doi: 10.1016/j.bbrc.2013.10.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eleouet J. F., Chilmonczyk S., Besnardeau L., Laude H.1998. Transmissible gastroenteritis coronavirus induces programmed cell death in infected cells through a caspase-dependent pathway. J. Virol. 72: 4918–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadaleta P., Perfetti X., Mersich S., Coulombié F.2005. Early activation of the mitochondrial apoptotic pathway in Vesicular Stomatitis virus-infected cells. Virus Res. 109: 65–69. doi: 10.1016/j.virusres.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 11.Hong S. J., Dawson T. M., Dawson V. L.2004. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci. 25: 259–264. doi: 10.1016/j.tips.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 12.Huang Y., Ding L., Li Z., Dai M., Zhao X., Li W., Du Q., Xu X., Tong D.2013. Transmissible gastroenteritis virus infection induces cell apoptosis via activation of p53 signalling. J. Gen. Virol. 94: 1807–1817. doi: 10.1099/vir.0.051557-0 [DOI] [PubMed] [Google Scholar]
- 13.Joza N., Susin S. A., Daugas E., Stanford W. L., Cho S. K., Li C. Y., Sasaki T., Elia A. J., Cheng H. Y., Ravagnan L., Ferri K. F., Zamzami N., Wakeham A., Hakem R., Yoshida H., Kong Y. Y., Mak T. W., Zúñiga-Pflücker J. C., Kroemer G., Penninger J. M.2001. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410: 549–554. doi: 10.1038/35069004 [DOI] [PubMed] [Google Scholar]
- 14.Kim J., Soh J.2009. Cadmium-induced apoptosis is mediated by the translocation of AIF to the nucleus in rat testes. Toxicol. Lett. 188: 45–51. doi: 10.1016/j.toxlet.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 15.Liu B., Chen Y., St Clair D. K.2008. ROS and p53: a versatile partnership. Free Radic. Biol. Med. 44: 1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenzo H. K., Susin S. A., Penninger J., Kroemer G.1999. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 6: 516–524. doi: 10.1038/sj.cdd.4400527 [DOI] [PubMed] [Google Scholar]
- 17.Miramar M. D., Costantini P., Ravagnan L., Saraiva L. M., Haouzi D., Brothers G., Penninger J. M., Peleato M. L., Kroemer G., Susin S. A.2001. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 276: 16391–16398. doi: 10.1074/jbc.M010498200 [DOI] [PubMed] [Google Scholar]
- 18.Murahashi H., Azuma H., Zamzami N., Furuya K. J., Ikebuchi K., Yamaguchi M., Yamada Y., Sato N., Fujihara M., Kroemer G., Ikeda H.2003. Possible contribution of apoptosis-inducing factor (AIF) and reactive oxygen species (ROS) to UVB-induced caspase-independent cell death in the T cell line Jurkat. J. Leukoc. Biol. 73: 399–406. doi: 10.1189/jlb.0702335 [DOI] [PubMed] [Google Scholar]
- 19.Pratelli A.2011. The evolutionary processes of canine coronaviruses. Adv. Virol. 2011: 562831. doi: 10.1155/2011/562831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarmento L., Tseggai T., Dhingra V., Fu Z. F.2006. Rabies virus-induced apoptosis involves caspase-dependent and caspase-independent pathways. Virus Res. 121: 144–151. doi: 10.1016/j.virusres.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 21.Schriewer J. M., Peek C. B., Bass J., Schumacker P. T.2013. ROS-mediated PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia-reperfusion. J. Am. Heart Assoc. 2: e000159. doi: 10.1161/JAHA.113.000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuler M., Bossy-Wetzel E., Goldstein J. C., Fitzgerald P., Green D. R.2000. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 275: 7337–7342. doi: 10.1074/jbc.275.10.7337 [DOI] [PubMed] [Google Scholar]
- 23.Simon H. U., Haj-Yehia A., Levi-Schaffer F.2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5: 415–418. doi: 10.1023/A:1009616228304 [DOI] [PubMed] [Google Scholar]
- 24.Souza F. N., Blagitz M. G., Latorre A. O., Ramos Sanchez E. M., Batista C. F., Weigel R. A., Renno F. P., Sucupira M. C., Della Libera A. M.2012. Intracellular reactive oxygen species production by polymorphonuclear leukocytes in bovine leukemia virus-infected dairy cows. J. Vet. Med. Sci. 74: 221–225. doi: 10.1292/jvms.11-0246 [DOI] [PubMed] [Google Scholar]
- 25.Stambolsky P., Weisz L., Shats I., Klein Y., Goldfinger N., Oren M., Rotter V.2006. Regulation of AIF expression by p53. Cell Death Differ. 13: 2140–2149. doi: 10.1038/sj.cdd.4401965 [DOI] [PubMed] [Google Scholar]
- 26.Susin S. A., Lorenzo H. K., Zamzami N., Marzo I., Snow B. E., Brothers G. M., Mangion J., Jacotot E., Costantini P., Loeffler M., Larochette N., Goodlett D. R., Aebersold R., Siderovski D. P., Penninger J. M., Kroemer G.1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446. doi: 10.1038/17135 [DOI] [PubMed] [Google Scholar]
- 27.Wang X., Yang C., Chai J., Shi Y., Xue D.2002. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science 298: 1587–1592. doi: 10.1126/science.1076194 [DOI] [PubMed] [Google Scholar]
- 28.Wu X., Deng G., Li M., Li Y., Ma C., Wang Y., Liu X.2015. Wnt/β-catenin signaling reduces Bacillus Calmette-Guerin-induced macrophage necrosis through a ROS -mediated PARP/AIF-dependent pathway. BMC Immunol. 16: 16. doi: 10.1186/s12865-015-0080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D., Du Q., Han C., Wang Z., Zhang X., Wang T., Zhao X., Huang Y., Tong D.2016. p53 signaling modulation of cell cycle arrest and viral replication in porcine circovirus type 2 infection cells. Vet. Res. (Faisalabad) 47: 120. doi: 10.1186/s13567-016-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye H., Cande C., Stephanou N. C., Jiang S., Gurbuxani S., Larochette N., Daugas E., Garrido C., Kroemer G., Wu H.2002. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat. Struct. Biol. 9: 680–684. doi: 10.1038/nsb836 [DOI] [PubMed] [Google Scholar]
- 31.Yu J. Y., Zheng Z. H., Son Y. O., Shi X., Jang Y. O., Lee J. C.2011. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol. In Vitro 25: 1654–1663. doi: 10.1016/j.tiv.2011.07.002 [DOI] [PubMed] [Google Scholar]