Abstract
Intracellular labile iron is an iron species which is not or weakly bound to proteins and depicts an important effect on homeostatic regulation in cells. An excess or deficiency of iron can cause oxidative damage to key cellular biomolecules. The behavior and concentrations of labile iron are difficult to monitor, but the specific redox state of the Fe ions is relevant to the physiological and pathological properties that we would like to study. We have developed a series of turn-on type fluorescent probes that are highly selective to the labile Fe(II) ions, and we have tested their applications to cellular level imaging. These probes are based on N-oxide chemistry with a range of fluorophores that depict optimal performance for specific applications. Herein, I review the recent progress of our research and discuss prospects for future work to understand the relation between intracellular ion and oxidative stress.
Keywords: iron, fluorescence imaging, fluorescent probe, oxidative stress
I. Introduction
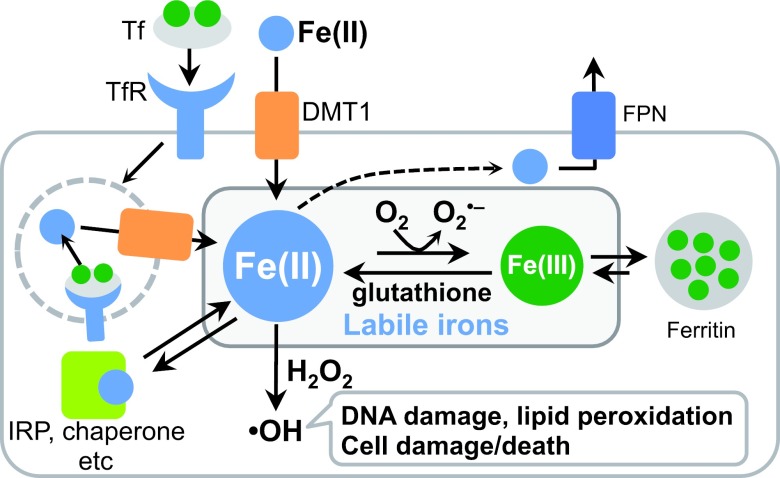
Iron is the most abundant transition metal among the essential trace elements in the human body, and each adult body contains approximately 4 g of iron. Most of the iron in the human body is stored in proteins such as ferritin or acts as cofactors of physiologically essential proteins such as hemoglobin and cytochrome P450 [22, 29, 38, 40]. These facts indicate that the biological systems exploit the unique redox properties of iron while preventing undesirable Fe-induced redox reactions by embedding the Fe ions inside appropriate proteins. Meanwhile, a small proportion of iron in the body that that flows freely in intracellular fluid or that is only weakly protein-bound is referred as labile ion (Fig. 1) [7, 13, 21]. Labile iron contributes to homeostatic regulation of cellular iron through activation and deactivation of iron-responsive proteins (IRPs). Excess labile iron, however, can induce oxidative stress because of the insufficient protection for the redox activity. The subcellular iron-regulation system strictly controls the concentration of labile iron to avoid iron-induced oxidative damage; therefore, dysfunction of the iron homeostasis can easily cause cellular damage and cell death, which may eventually lead to severe diseases, such as cancer and some neurodegenerative diseases [9, 35, 41]. The Fe ions exists in a stable manner in the form of Fe(II) and Fe(III) ions; however, the labile iron species mainly comprise Fe(II) ions rather than Fe(III) ions. Fe(II) ions are more abundant in the intracellular environment because they are more water-soluble than Fe(III) ions and because of the abundance of intracellular reductants such as glutathione. Iron transporters and chaperones that recognize Fe(II) ions as their substrates also increase the abundance of intracellular Fe(II) ions (Fig. 1) [7, 13]. However, Fe(II) ions are highly relevant in case of oxidative stress because of the Fenton reaction in which an Fe(II) ion reacts with hydrogen peroxide to generate highly reactive oxygen species [10, 19]. This reaction causes severe oxidative damages to DNA, proteins, and lipids [6, 11]. In this context, the selective detection of Fe(II) ions in cells is desirable for understanding both the physiological and pathological properties of the cellular labile iron species in living cells. Fluorescent probes are powerful tools for measuring the subcellular levels of Fe ions in living cells. However, only a few fluorescent probes that can detect Fe(II) ions with a turn-on response in a redox-state selective manner [8, 15]. This limitation is caused due to the intrinsic tendency of Fe(II) ions to quench fluorescence and due to relatively weak binding property according the Irving–Williams series. We have established a new molecular methodology based on N-oxide chemistry to achieve turn-on and redox-state selective fluorescent detection of Fe(II) ions in living cells [14, 16, 25, 26]. In this review, I describe a series of novel fluorescent probes developed in our group and their applications to living cells and tissues.
Fig. 1.
Schematic diagram of the cellular iron homeostasis. Tf: transferrin; TfR: transferrin receptor; IRP: iron-responsive element-binding protein; FPN: ferroportin; DMT1: divalent metal ion transporter 1.
II. Classical Probes with Turn-off Readout
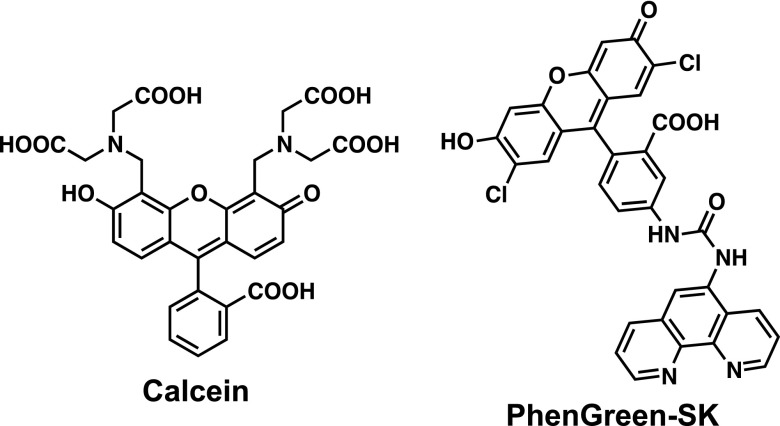
The design of fluorescent probes depicts several requirements such as solubility, cell-membrane permeability, and low toxicity. Selectivity against a target is among the most important properties that should be considered because of the extensive variety of biological molecules in the intracellular medium, including oxidants (reactive oxygen species), reductants, proteins, DNA, and lipids. Even though iron is the most abundant transition metals in the human body, iron is only present in trace amounts, which indicates that a fluorescent probe to achieve the current objective must be highly selective to Fe(II) ions rather than to other more-abundant biological molecules. The first generation of fluorescent probes to detect labile iron is represented by Calcein [5, 33, 34] and PhenGreen-SK [27, 28] (Fig. 2). Although both of these probes show turn-off response while chelating with Fe ions and suffer from poor metal selectivity, they significantly contribute to the evidence, which supports the concept of chelatable iron and labile iron. Other fluorescent probes with turn-off readouts have also been thoroughly reviewed elsewhere [8, 42].
Fig. 2.
Structures of Calcein and PhenGreen-SK
III. RhoNox-1 as the First Turn-on Fluorescent Probe and Its Biological Applications
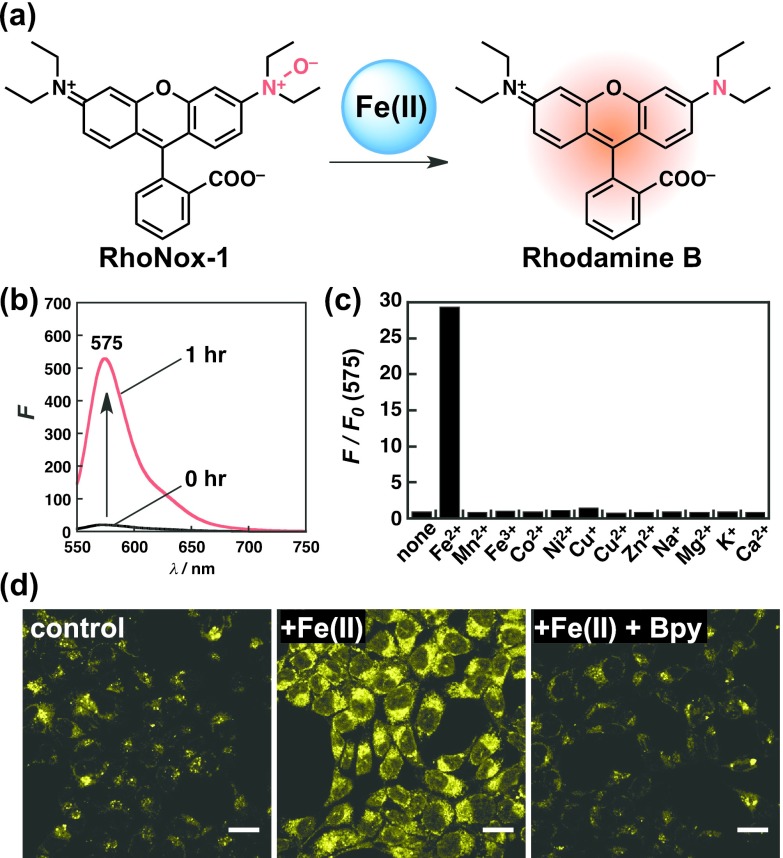
We first overcame the challenges of turn-off readout and poor selectivity with the development of RhoNox-1 [14]. This probe can detect Fe(II) ions with a highly selective turn-on readout by exploiting the chemical reaction of the tertiary amine N-oxide and Fe(II) ions. N-oxide is known as a protective group for tertiary amines, and it can be deprotected by Fe(II)-assisted reductive deoxygenation reactions. Because the Fe(II) ions, which depict a redox state of iron that is less than that of Fe(III) ions, are favored to perform the deoxygenation reaction, we expected this reaction to work as a kind of selective molecular switch that responds to Fe(II) ions. Based on this hypothesis, we designed RhoNox-1 using a strongly fluorescent rhodamine B (quantum yield > 0.8) as the parent fluorophore and an N-oxidized tertiary amine moiety at one of the two amino groups (Fig. 3a). The installation of N-oxide attenuates the fluorescence of RhoNox-1 (quantum yield = 0.01) through multiple mechanisms, including a break of the π-conjugation in the fluorophore, photo-induced electron transfer (PET), and twisted intramolecular charge transfer (TICT). RhoNox-1 depicts only weak fluorescence intensity (and quantum yield), and the addition of Fe(II) ions triggers a maximum of thirty-fold recovery of fluorescence after a period of 1 hr because the deoxygenation reaction regenerates the strongly fluorescent rhodamine B (Fig. 3b). The most useful advantage of RhoNox-1 is its high selectivity to Fe(II) ions. As illustrated in Fig. 3c, the fluorescence response was only induced by Fe(II) ions, whereas other first-row transition metal cations, including Fe(III) ions, did not trigger a response. Furthermore, we applied RhoNox-1 to live cell imaging and successfully monitored the subcellular labile Fe(II) ions with turn-on readout (Fig. 3d) [14].
Fig. 3.
(a) Structure of RhoNox-1 and the mechanism by which it detects the Fe(II) ions. (b) Fluorescence spectra of RhoNox-1 before (black line) and after (red line) the addition of Fe(II) ions. (c) Metal selectivity test of RhoNox-1 over other metal ions. Fluorescence intensities at 575 nm after incubation for 1 hr with each metal ion are plotted. (d) Fluorescence microscopy images of RhoNox-1-stained HepG2 cells that were treated with no Fe(II) (left), Fe(II) (middle), and Fe(II) with 2,2'-bipyridyl (Bpy). All the cells were stained with RhoNox-1. Bars = 25 μm.
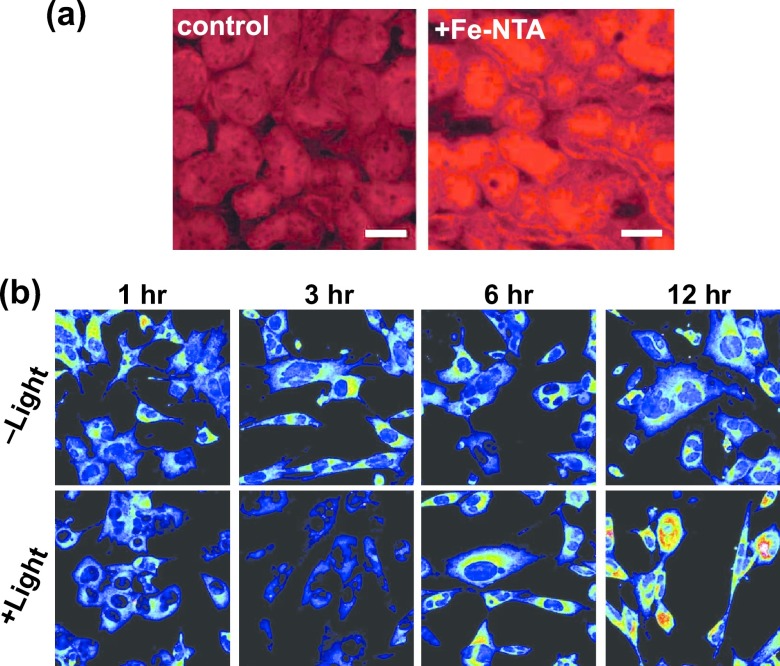
We further applied RhoNox-1 to perform histochemical detection of the labile Fe(II) ions in a tissue sample that was obtained from rats treated with ferric nitrilotriacetate (Fe-NTA) as an iron-induced carcinogenesis model in collaboration with Toyokuni et al. [24]. Intraperitoneal injection of Fe-NTA induces renal carcinogenesis through the oxidative damage to the DNA. This oxidative damage is caused by Fe(II) ions via the Fenton chemistry [35, 36]. The frozen sections of the proximal renal tubules that were prepared from the Fe-NTA injected rats were stained with RhoNox-1. We observed a significantly higher fluorescence signal in the Fe-NTA-treated samples than that was observed in the control samples (Fig. 4a). Noted that the probe worked only in case of freshly prepared frozen sections and not in case of paraffin preparations, which is understandable because the labile Fe(II) ions would be easily leaked out during the paraffin preparation process.
Fig. 4.
(a) Fluorescence microscopy images of RhoNox-1-stainded rat renal proximal tubules that were captured 1 hr after the intraperitoneal injection of Fe(III)-NTA. Bars = 40 μm. (b) Fluorescence microscopy images of RhoNox-1-stained 661W cells after exposure to white fluorescent light every time.
Another collaborative study of RhoNox-1 monitored the fluctuation of the labile Fe(II) ions during light irradiation on retina cells (661W cells) as a pathological model of dry-type aged macular disease (AMD) model [18]. In this test, light irradiation caused the time-dependent fluctuation of the labile Fe(II) ions in the photoreceptor cells. An initial decrease in the subcellular concentration of labile Fe(II) ions (1–3 hr) was followed by a gradual increase (6–12 hr) prior to the cell death (Fig. 4b). Furthermore, we found that the increase in the subcellular concentration of labile Fe(II) ions was correlated to the cell death, which was inhibited by the treatment with 2,2'-bipyridyl, a chelator of Fe(II), at the moment when the increase of labile Fe(II) began. Studies that used RhoNox-1 were expanded to study the subcellular concentrations of the labile Fe(II) ions during plasma-activated medium-induced cell injury [1], ovalbumin-induced peritonitis [20], and some other pathological models [12, 17, 32, 37, 39].
We have also developed a variant of RhoNox-1, HMRhoNox-M, that improves upon the turn-on response using a regulated spirocyclization profile [25]. This probe has been successfully applied to monitoring the labile Fe(II) ions in several contexts, including transferrin-delivered labile Fe(II) [25], non-thermal plasma-induced elevation of Fe(II) in mesothelioma cells [30], and labile Fe(II) in ovarian endometriosis-associated stromal cells [23].
IV. Various Probes with N-oxide Chemistry and Its Application
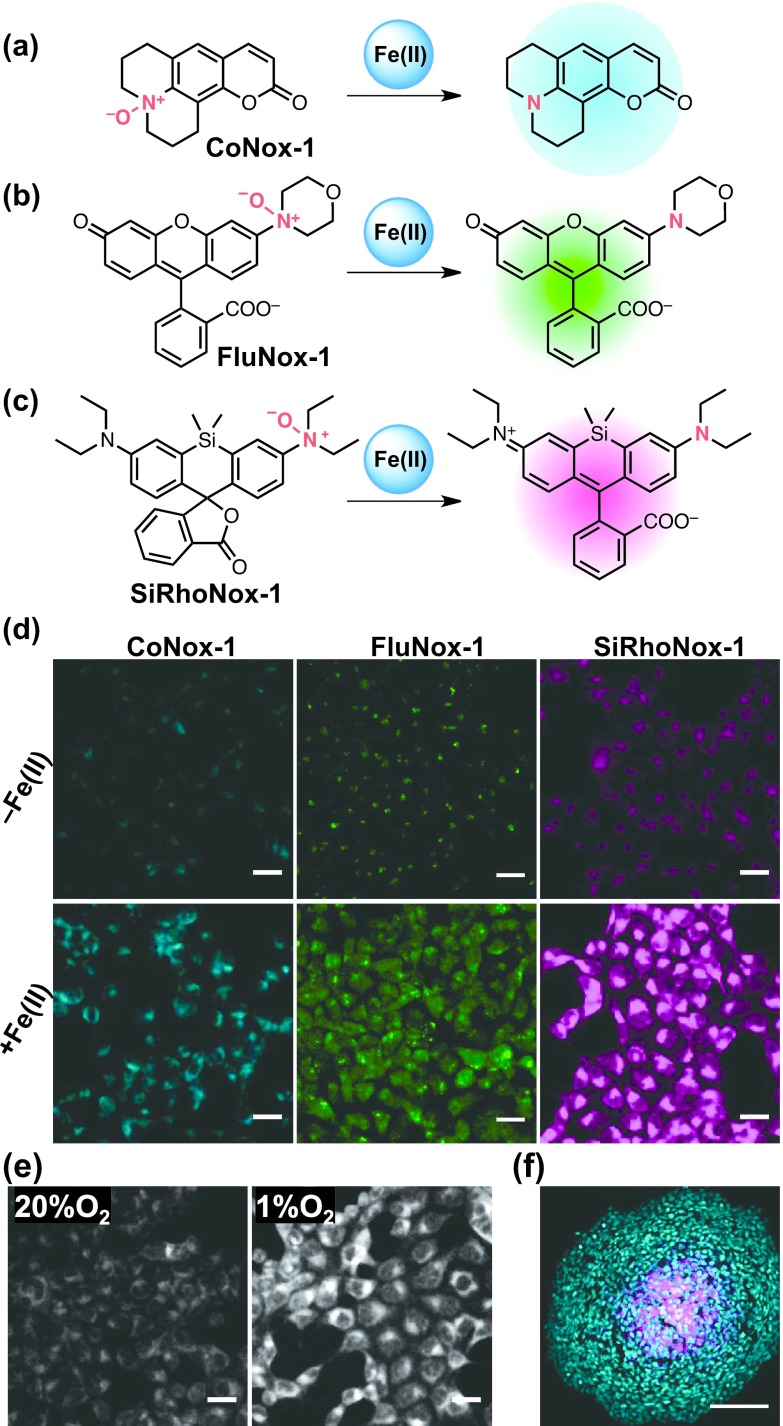
We expected that the N-oxide strategy could be generalized for usage with various fluorophores that have tertiary amine(s) within their π-conjugation system. This expectation led to the establishment of a color palette of N-oxide-based Fe(II) fluorescent probes, CoNox-1, FluNox-1, and SiRhoNox-1, which ranged from blue to deep red (Fig. 5a–c) [16]. All of these probes exhibited highly selective turn-on responses to Fe(II) ion in both the cuvettes and cells (Fig. 5d). This suggests that our N-oxide-chemistry-based strategy is applicable to a variety of fluorophores that contain π-conjugation systems with dialkylarylamine. Among the probes, SiRhoNox-1 showed a significantly better fluorescence response than that exhibited by RhoNox-1. SiRhoNox-1 was applied successfully to visualize the fluctuations of the subcellular redox balance between the Fe(II) and Fe(III) ions as the oxygen concentration varied (Fig. 5e). An elevation in the concentration of the labile Fe(II) ions in the hypoxic region of a 3D-cultured spheroid was detected using SiRhoNox-1 (Fig. 5f). Thus, we have determined that N-oxide is a molecular switch that is generally applicable N-oxide, for redox state-selective detection of the labile Fe(II) ions in cells.
Fig. 5.
(a–c) Structures of CoNox-1, FluNox-1, and SiRhoNox-1 and their detection mechanisms. (d) Fluorescence microscopy images of HepG2 cells that were treated with no Fe(II) (upper) and Fe(II) (lower). The cells were stained with CoNox-1 (left), Ac-FluNox-1 (middle), and SiRhoNox-1 (right), respectively. Bars = 25 μm. (e) Fluorescence microscopy images of SiRhoNox-1-stained HepG2 cells that were incubated under 20% (left) and 1% of oxygen concentration for 2 hr. Bars = 25 μm. (f) Fluorescence microscopy images of a spheroid of HepG2 cells. Blue color and red colors indicate the signals derived from DAPI (nucleus stain) and SiRhoNox-1 (Fe(II)), respectively. Note that the center of the spheroid is stained specifically by the usage of SiRhoNox-1. Bar = 100 μm.
V. Mem-RhoNox as a Cellular Membrane-anchoring Fe(II) Fluorescent Probe
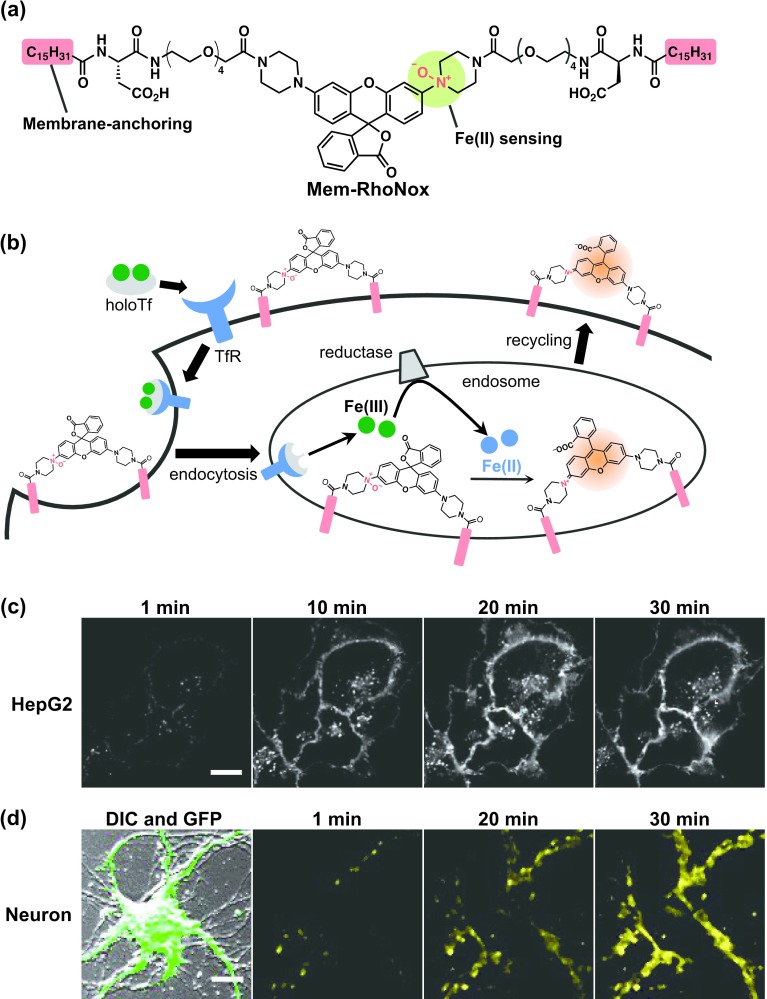
Transferrin-induced endocytosis is a primary pathway for ion uptake [38]. As described previously, because Fe ions can potentially cause undesired oxidative damage, mammalian cells have acquired sophisticated machinery for iron uptake. A transferrin molecule can hold a pair of Fe(III) ions and releases them inside the endosome; however, the redox state of the released iron has not yet been measured in detail. We developed Mem-RhoNox to allow intra-endosomal detection of the labile Fe(II) ions. We used this probe to visualize the generation of Fe(II) ions inside the endosome during transferrin-triggered endocytosis. Mem-RhoNox is a membrane-anchoring fluorescent probe that is sensitive to the Fe(II) ions [26]. The probe has an N-oxide-incorporated rhodamine scaffold that incorporates two palmitoyl groups as the cellular membrane-anchoring domain (Fig. 6a, b). Mem-RhoNox is retained on the cell surface, and retention onto the cell membrane is completely lost without the anchoring arms. We successfully conducted real-time detection of the Fe(II) ions generated on the cell membrane and that were released from transferrin inside the endosome in HepG2 cells (Fig. 6c). Because transferrin-mediated endocytosis is the primary pathway for iron uptake in neuronal cells, we applied Mem-RhoNox to monitor the transferrin-delivered Fe(II) ions in primary cultured neurons. In this test, the probe allowed visualization of the generation of Fe(II) ions during the endocytosis (Fig. 6d).
Fig. 6.
(a) The structure of Mem-RhoNox. (b) Schematic diagram of the mechanism by which Mem-RhoNox detects transferrin (Tf)-delivered Fe(II) ions. (c) Time-lapse fluorescence imaging of the Tf-triggered release of Fe(II) ions in HepG2 cells that were stained with Mem-RhoNox. Bar = 10 μm. (d) Time-lapse fluorescence images of the Tf-triggered release of Fe(II) ions in primary-cultured mouse neurons that were stained with Mem-RhoNox and green fluorescent protein (GFP). The neurons were visualized using an overlapped image of GFP and differential interference contrast (DIC) (left). Bars = 10 μm.
VI. Conclusion
This study reviews our group’s development of fluorescent probes for selective detection and live imaging of the subcellular labile Fe(II) ions by utilizing the N-oxide chemistry. In addition to our N-oxide-based fluorescent probes, the Fe(II)-selective fluorescent probes that were based on endoperoxide chemistry [2, 3, 31] have been reported by other group, who have also provided a discussion regarding their biological applications [4]. Iron is essential to perform homeostatic regulation; a surplus or deficiency of iron can cause several serious diseases. Study of the synergistic reactions of iron with other redox-relevant molecules, such as reactive oxygen, nitrogen, and sulfur species, should elucidate the redox-signal network and the manner in which biological systems maintain homeostasis. Thus, the development of fluorescent probes that can monitor the labile iron species with redox-state specificity will contribute to understanding both the physiological and pathological properties of the labile iron species. We have successfully developed the labile Fe(II)-specific fluorescent probes. Imaging studies have revealed the changes in intracellular labile Fe(II) ions in terms of pathological and physiological properties. In future, we plan to develop imaging probes that can be applied to in vivo/ex vivo studies as well as to organelle-specific monitoring and neuroscience. The type of probe would signify considerable progress in the pathological and physiological studies of iron and oxidative stress.
VII. Conflicts of Interest
There are no conflicts of interest to declare.
VIII. Acknowledgments
This work was financially supported by JSPS KAKENHI (No. 25702050 for T. H.) from the Ministry of Education, Culture, Sports, Science and Technology Japan (MEXT), by the Naito Foundation (for T.H.), and by the Kato Memorial Bioscience Foundation (for T.H.).
IX. References
- 1.Adachi T., Nonomura S., Horiba M., Hirayama T., Kamiya T., Nagasawa H. and Hara H. (2016) Iron stimulates plasma-activated medium-induced A549 cell injury. Sci. Rep. 6; 20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron A. T., Loehr M. O., Bogena J. and Chang C. J. (2016) An endoperoxide reactivity-based FRET probe for ratiometric fluorescence imaging of labile iron pools in living cells. J. Am. Chem. Soc. 138; 14338–14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aron A. T., Heffern M. C., Lonergan Z. R., Vander Wal M. N., Blank B. R., Spangler B., Zhang Y., Park H. M., Stahl A., Renslo A. R., Skaar E. P. and Chang C. J. (2017) In vivo bioluminescence imaging of labile iron accumulation in a murine model of Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. U S A 114; 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aron A. T., Reeves A. G. and Chang C. J. (2018) Activity-based sensing fluorescent probes for iron in biological systems. Curr. Opin. Chem. Biol. 43; 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breuer W., Epsztejn S. and Cabantchik Z. I. (1995) Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 270; 24209–24215. [DOI] [PubMed] [Google Scholar]
- 6.Burkitt M. J. and Mason R. P. (1991) Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: an ESR spin-trapping investigation. Proc. Natl. Acad. Sci. U S A 88; 8440–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabantchik Z. I. (2014) Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 5; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter K. P., Young A. M. and Palmer A. E. (2014) Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 114; 4564–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon S. J. and Stockwell B. R. (2014) The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10; 9–17. [DOI] [PubMed] [Google Scholar]
- 10.Enami S., Sakamoto Y. and Colussi A. J. (2014) Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. U S A 111; 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell B. and Gutteridgeb J. M. C. (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 307; 108–112. [DOI] [PubMed] [Google Scholar]
- 12.Hattori Y., Mukaide T., Jiang L., Kotani T., Tsuda H., Mano Y., Sumigawa S., Hirayama T., Nagasawa H., Kikkawa F. and Toyokuni S. (2015) Catalytic ferrous ion in amniotic fluid as a predictive marker of human maternal-fetal disorders. J. Clin. Biochem. Nutr. 56; 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hider R. C. and Kong X. (2013) Iron speciation in the cytosol: an overview. Dalton Trans. 42; 3220–3229. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama T., Okuda K. and Nagasawa H. (2013) A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 4; 1250–1256. [Google Scholar]
- 15.Hirayama T. and Nagasawa H. (2017) Chemical tools for detecting Fe ions. J. Clin. Biochem. Nutr. 60; 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirayama T., Tsuboi H., Niwa M., Miki A., Kadota S., Ikeshita Y., Okuda K. and Nagasawa H. (2017) A universal fluorogenic switch for Fe(II) ion based on N-oxide chemistry permits the visualization of intracellular redox equilibrium shift towards labile iron in hypoxic tumor cells. Chem. Sci. 8; 4858–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda Y., Horinouchi Y., Hamano H., Hirayama T., Kishi S., Izawa-Ishizawa Y., Imanishi M., Zamami Y., Takechi K., Miyamoto L., Ishizawa K., Aihara K. I., Nagasawa H., Tsuchiya K. and Tamaki T. (2017) Dietary iron restriction alleviates renal tubulointerstitial injury induced by protein overload in mice. Sci. Rep. 7; 10621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamura T., Hirayama T., Tsuruma K., Shimazawa M., Nagasawa H. and Hara H. (2014) Hydroxyl radicals cause fluctuation in intracellular ferrous ion levels upon light exposure during photoreceptor cell death. Exp. Eye Res. 129; 24–30. [DOI] [PubMed] [Google Scholar]
- 19.Imlay J. A., Chin S. M. and Linn S. (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240; 640–642. [DOI] [PubMed] [Google Scholar]
- 20.Ito F., Nishiyama T., Shi L., Mori M., Hirayama T., Nagasawa H., Yasui H. and Toyokuni S. (2016) Contrasting intra- and extracellular distribution of catalytic ferrous iron in ovalbumin-induced peritonitis. Biochem. Biophys. Res. Commun. 476; 600–606. [DOI] [PubMed] [Google Scholar]
- 21.Kakhlon O. and Cabantchik Z. I. (2002) The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic. Biol. Med. 33; 1037–1046. [DOI] [PubMed] [Google Scholar]
- 22.Lippard, S. J. and Berg, J. M. (1994) Principles of Bioinorganic Chemistry. University Science Books, Mill Valley, CA. [Google Scholar]
- 23.Mori M., Ito F., Shi L., Wang Y., Ishida C., Hattori Y., Niwa M., Hirayama T., Nagasawa H., Iwase A., Kikkawa F. and Toyokuni S. (2015) Ovarian endometriosis-associated stromal cells reveal persistently high affinity for iron. Redox Biol. 6; 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukaide T., Hattori Y., Misawa N., Funahashi S., Jiang L., Hirayama T., Nagasawa H. and Toyokuni S. (2014) Histological detection of catalytic ferrous iron with the selective turn-on fluorescent probe RhoNox-1 in a Fenton reaction-based rat renal carcinogenesis model. Free Radic. Res. 48; 990–995. [DOI] [PubMed] [Google Scholar]
- 25.Niwa M., Hirayama T., Okuda K. and Nagasawa H. (2014) A new class of high-contrast Fe(II) selective fluorescent probes based on spirocyclized scaffolds for visualization of intracellular labile iron delivered by transferrin. Org. Biomol. Chem. 12; 6590–6597. [DOI] [PubMed] [Google Scholar]
- 26.Niwa M., Hirayama T., Oomoto I., Wang D. O. and Nagasawa H. (2018) Fe(II) ion release during endocytotic uptake of iron visualized by a membrane-anchoring Fe(II) fluorescent probe. ACS Chem. Biol. 13; 1853–1861. [DOI] [PubMed] [Google Scholar]
- 27.Petrat F., de Groot H. and Rauen U. (2000) Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch. Biochem. Biophys. 376; 74–81. [DOI] [PubMed] [Google Scholar]
- 28.Petrat F., Weisheit D., Lensen M., De Groot H., Sustmann R. and Rauen U. (2002) Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. Biochem. J. 362; 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz I. J., Chen C., Paw B. H. and Hamza I. (2010) Iron and porphyrin trafficking in heme biogenesis. J. Biol. Chem. 285; 26753–26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L., Ito F., Wang Y., Okazaki Y., Tanaka H., Mizuno M., Hori M., Hirayama T., Nagasawa H., Richardson D. R. and Toyokuni S. (2017) Non-thermal plasma induces a stress response in mesothelioma cells resulting in increased endocytosis, lysosome biogenesis and autophagy. Free Radic. Biol. Med. 108; 904–917. [DOI] [PubMed] [Google Scholar]
- 31.Spangler B., Morgan C. W., Fontaine S. D., Vander Wal M. N., Chang C. J., Wells J. A. and Renslo A. R. (2016) A reactivity-based probe of the intracellular labile ferrous iron pool. Nat. Chem. Biol. 12; 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takenaka M., Suzuki N., Mori M., Hirayama T., Nagasawa H. and Morishige K. (2017) Iron regulatory protein 2 in ovarian endometrial cysts. Biochem. Biophys. Res. Commun. 487; 789–794. [DOI] [PubMed] [Google Scholar]
- 33.Tenopoulou M., Kurz T., Doulias P.-T., Galaris D. and Brunk U. T. (2007) Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? Biochem. J. 403; 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas F., Serratrice G., Béguin C., Aman E. S., Pierre J. L., Fontecave M. and Laulhère J. P. (1999) Calcein as a fluorescent probe for ferric iron. Application to iron nutrition in plant cells. J. Biol. Chem. 274; 13375–13383. [DOI] [PubMed] [Google Scholar]
- 35.Torti S. V. and Torti F. M. (2013) Iron and cancer: more ore to be mined. Nat. Rev. Cancer 13; 342–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyokuni S. (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 100; 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsugawa H., Mori H., Matsuzaki J., Masaoka T., Hirayama T., Nagasawa H., Sakakibara Y., Suematsu M. and Suzuki H. (2015) Nordihydroguaiaretic acid disrupts the antioxidant ability of Helicobacter pylori through the repression of SodB activity in vitro. Biomed. Res. Int. 2015; 734548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J. and Pantopoulos K. (2011) Regulation of cellular iron metabolism. Biochem. J. 434; 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Okazaki Y., Shi L., Kohda H., Tanaka M., Taki K., Nishioka T., Hirayama T., Nagasawa H., Yamashita Y. and Toyokuni S. (2016) Role of hemoglobin and transferrin in multi-wall carbon nanotube-induced mesothelial injury and carcinogenesis. Cancer Sci. 107; 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang M., Yang H., Guan H., Kato T., Mukaisho K., Sugihara H., Ogasawara K., Terada T. and Tooyama I. (2017) Characterization of a novel monoclonal antibody against human mitochondrial ferritin and its immunohistochemical application in human and monkey substantia nigra. Acta Histochem. Cytochem. 50; 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zecca L., Youdim M. B. H., Riederer P., Connor J. R. and Crichton R. R. (2004) Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5; 863–873. [DOI] [PubMed] [Google Scholar]
- 42.Zhu H., Fan J., Wang B. and Peng X. (2015) Fluorescent, MRI, and colorimetric chemical sensors for the first-row d-block metal ions. Chem. Soc. Rev. 44; 4337–4366. [DOI] [PubMed] [Google Scholar]