Abstract
Tumor cells evolve multiple sophisticated mechanisms to escape immune surveillance, one of which is to establish tolerogenic microenvironment by recruiting certain immune suppressive cells such as regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs). Tregs are subpopulation of CD4+ T cells, which specialize in suppressing immune responses and preventing autoimmune damage to collateral tissue. Emerging evidence suggests that Treg cell number increases in various types of cancer, which correlates with tumor grade and poor patient prognosis. This review will focus on discussion of the origins and features of tumor-infiltrating Treg cells. Ultimately, these features may provide insight into potential therapeutic intervention by targeting Treg cells to invigorate immune response against tumor.
Keywords: Regulatory T cells, Treg origin, Treg metabolism, tumor microenvironment
Origins of tumor-infiltrating Treg cells
Treg cells, as well as other subsets of T cells, can develop in thymus by the stimulation of self-antigens. These Tregs are termed as thymic Treg cells or natural Treg cells (tTregs or nTregs). The critical role of thymic Treg cells in maintaining immunological tolerance has been demonstrated by the observation that thymectomy of 3-day old neonatal mice induced T-cell-mediated autoimmunity [1]. On the other hand, Tregs cells can be differentiated from naïve T cells upon antigen stimulation under certain cytokine conditions in peripheral tissues. These Treg cells are termed as peripheral Treg cells (pTregs) or induced Treg cells (iTregs) [2,3]. Peripheral Treg cells phenotypically resemble thymic Tregs, and both of them have similar phenotypic characteristics and comparable suppressive function in suppressing immune responses [4]. Maintaining of Treg suppressive function requires the expression of transcription factor Foxp3 [5], whose function itself is regulated by a variety of post-translational modifications [6-8], and Treg lineage stability is also determined by the establishment of Treg cell-specific CpG hypomethylation pattern induced by T cell receptor stimulation [9].
In the context of tumor, there are several possible origins accounting for the increased Treg cell numbers in tumor:
Treg cells are selectively recruited to tumor sites by a variety of chemokines
Hypoxia is a common feature of tumor microenvironment, which drives angiogenesis and promotes tumor progression. Hypoxia induced the expression of chemotactic factors CCL28 in ovarian cancer and liver cancer, which promoted the recruitment of CCR10+ Treg cells and the establishment of immune suppressive environment. Moreover, hypoxia enhanced the constitutive secretion of VEGFA by tumor-infiltrating Treg cells, which promoted a pro-angiogenic tumor milieu as well [10]. More recent studies suggested that FOXP3-expressing pancreatic ductal adenocarcinoma (PDAC) recruited Treg cells by secretion of CCL5, and selective depletion of Treg cells by CCL5 blockade may facilitate the antitumor immune response in PDAC patients [11]. CCL8/CCR5 signaling axis drove Treg recruitment to the lungs of mice bearing metastatic primary tumors and influenced the development of tumor metastases [12]. Treg cells could also be recruited to the tumor in a CCR5-dependent manner and contributed to skin squamous cell carcinoma (SSC) and colorectal cancer (CRC) development [13]. The CCR4/CCL22 axis was highlighted for Treg accumulation in mouse melanoma metastasis model. Interestingly, cutaneous overexpression of Ccl22 by gene gun vaccination could divert Treg cells away from tumor and ameliorate tumor metastasis, therefore inhibiting autoimmune side effects caused by immune checkpoint therapeutics [14].
There are some tumor-associated cells involved in the recruitment of Treg cells through the production of chemokines. Tumor-associated macrophages (TAMs) produced a large amount of CCL20 to enrich CCR6+ regulatory T cells and promoted the development of colorectal cancer [15]. Similarly, macrophages and microglia within the glioma microenvironment produced CCL2 which was critical for recruiting suppressive CCR4+ Treg as well as CCR2+Ly-6C+ monocytic MDSCs [16]. Besides, the clinical and preclinical data showed that Indoleamine 2,3-dioxygenase (IDO) expression in brain tumor increased the recruitment of Treg cells to promote tumor outgrowth. Inversely, IDO deficiency decreased Treg recruitment and enhanced T-cell-mediated tumor rejection [17].
Conventional CD4+ T cells are converted to Treg cells in tumor
It was reported that IDO-expressing acute myeloid leukemia (AML) cells could directly convert CD4+CD25- T cells into CD4+CD25+ Treg cells expressing surface CTLA-4 and FOXP3 mRNA. In fact, CTLA-4 signal is crucial for Foxp3+ Treg development [18]. The treatment of IDO inhibitor 1-methyl tryptophan (1-MT) abrogated this effect and impaired T-cell tolerance in A20-bearing mice [19]. In non-Hodgkin lymphoma (NHL), NHL cells themselves were responsible for the increased numbers of Treg cells, and could induce Treg cells from CD25- PBMCs in vitro [20]. Similarly, in follicular lymphoma (FL), malignant B cells alone, without artificial TCR stimulation, could induce conventional T cells to express Foxp3 with regulatory function via a cell-cell contact fashion. Moreover, T cells isolated from FL or normal peripheral blood were equally susceptible to being converted by tumor B cells, indicating that this effect was independent of the T cell background [21]. Malignant B cells were also reported to induce the conversion of CD4+CD25- T cells to Treg cells through PD-1/B7-H1 pathway in B-Cell Non-Hodgkin Lymphoma [22]. Exposure to immature dendritic cells (DCs) which loaded with apoptotic Cutaneous T-cell lymphoma (CTCL) cells conferred CD4+ CTCL cells acquiring regulatory T cell phenotypes, such as expression of CD25, CTLA-4, Foxp3 and secretion of IL-10 and TGF-β. Inversely, blocking the expression or transport of DC MHC Class 2 inhibited CTCL cell from adopting Treg features [23].
However, whether this effect is specific for blood cancer or general among other types of cancers remains elusive. A recent study found that suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumor-associated Treg cells which were converted from IL-17A+Foxp3neg cells in ovarian or colorectal cancer-bearing mice. These suppressive cells exerted active aerobic glycolysis, which is metabolically characteristic of Th cells [24]. On the other hand, studies with transgenic and knockout animals demonstrated that some genes play cell-intrinsic roles in determining Treg cell-lineage stability. Therefore, deficiency of these genes can convert Treg cells into Teff cells inversely with enhanced anti-tumor immunity. For instance, Helios deficiency within CD4 Treg cells led to instability of intratumoral but not systemic Tregs, and conversion of these Tregs into Teff cells within the transplantable melanoma (B16/F10) or colon adenocarcinoma (MC38) [25]. Similarly, Treg specific deletion of Nrp1 gene drove Treg cell fragility with increasing production of pro-inflammatory cytokine IFN-γ in tumor without impacting Treg cell function elsewhere in the body, indicating Nrp1 signaling could be specifically targeted to modulate intratumoral Treg cell activity and enhance anti-tumor immune response [26]. Moesin, a member of the ezrin-radixin-moesin (ERM) family of proteins, plays a critical role in augmenting optimal TGF-β signaling and facilitates efficient development of iTreg cell. Moesin-deficient mice were protected against recurrence of B16 melanoma tumor after adoptive T cell transfer due to the impaired conversion to FOXP3+ iTregs and compromised suppressive capacity [27]. Although these studies suggest the potential perturbation of Treg cell stability and conversion of Treg cells in animal tumor models through targeting a certain specific signaling pathway, how to manipulate Treg stability and design related therapeutic intervention for human cancer therapy remain to be further explored.
Tumor microenvironment promotes Treg cell proliferation and stability
Tumor microenvironment contains a range of factors that facilitate Treg cell proliferation. The increased Treg cell numbers in tumor are attributed to the cell activation and expansion, which is supported by the analysis of the TCR repertoires from tumor-infiltrating effector and regulatory T cells. TC-1 tumor-infiltrating Teff and Treg cells displayed biased TCR repertoire respectively and characteristic of antigen-driven clonal expansions [28]. Similarly, Treg cells were significantly enriched in the chemical carcinogen 3-methylcholanthrene (MCA)-induced tumors (fibrosarcomas). TCR repertoires of antigen-experienced tumor-infiltrating Tconvs and Tregs were largely distinct and non-overlapping, implying that tumor microenvironment promotes Treg cell proliferation or survival rather than conversion from Tconvs cells in tumors [29]. In addition, activated Treg cells (defined as CD4+CD45RA-Foxp3high) could be preferentially expanded in colon cancer (CC) and non-small cell lung cancer (NSCLC). However, these Treg cells were phenotypically plastic with the expression of RORγt transcription factor and production of pro-inflammatory cytokine IL-17 [30,31].
In CT26 and MC-38 tumor-bearing mice, there was a skewed Treg/Tconv ratio in the tumor compared with spleen or blood. The expression of Ki-67, a marker of cell proliferation, was elevated in intra-tumoral Treg cells relative to Tconv cells, which was associated with the increased Treg abundance in tumor [32]. Consistently, in comparison with conventional T cells, Treg cells adopted a metabolic advantage based on the combination of glycolysis and fatty acid synthesis and oxidation for their energy demands, therefore, preferentially proliferated in the hostile tumor microenvironment [33].
The origins of Tregs in tumor may vary according to different types of tumors. Therefore, to reconcile these hypothesis about the origins of tumor-infiltrating Treg, it necessities to discuss the inherent phenotypes and metabolic features of these Treg cells.
Features of tumor-infiltrating Treg cells
CpG hypomethylation of the Foxp3 Treg-specific demethylated region (TSDR) is a hallmark of stable nTregs, which distinguishes nTreg cells from other cell types including TGFβ-inducing iTreg cells and activated FOXP3-expressing effector T cells [34]. The tumor- infiltrating Tregs from a range of mouse tumors and human tumors (NSCLC and ovarian) exhibited a uniform pattern of Foxp3 TSDR hypomethylation. Moreover, TGF-β neutralization by TGF-β-Trap did not impact intratumoral Treg frequency and accumulation, indicating that nTreg-like cells rather than iTregs predominantly populate tumors in mice and humans [32]. By using methylation-specific quantitative polymerase chain reaction (MS-qPCR) assay, a similar study has demonstrated that a majority of these suppressive Treg cells are functional nTreg cells in the human colorectal cancer [35]. These observations are in line with the hypothesis of recruitment rather than conversion about the origins of Treg cells in tumor.
As mentioned above, given Treg cells are highly activated and proliferative in animal cancer models or cancer patients, tumor-infiltrating Treg cells require metabolic reprogramming to support their function and expansion. Tumor microenvironment is metabolically abnormal due to the poor replenishment of nutrients such as glucose, glutamine and tryptophan while being enriched with lactic acid and kynurenines [36]. Alessia Angelin et al. reported that Treg cells adopted a selective metabolic advantage in low-glucose, high-lactate environments which is characteristic of the tissues with ischemic injury or the microenvironment surrounding solid tumors. Foxp3 expression induced in iTreg cells or retrovirally transduced in general T cells increased oxygen consumption rates (OCR) and oxidative phosphorylation (OXPHOS) for their energy production, while inhibited Myc expression and glycolysis in these cells. However, Treg division and suppressive function were unaffected by the exposure to L-lactate which was enriched in the tumor microenvironment. Of note, Treg metabolic advantage did not depend upon the ability to use L-lactate as an alternative fuel source when glucose was sparse, but rather, the resistance to the depletion of intracellular NAD pool resulting from the oxidation of L-lactate to pyruvate by lactate dehydrogenase (LDH), which significantly impairing Teff function and proliferation on the contrary [37].
The mitochondrial metabolism plays a critical role in T cell activation. Mitochondrial reactive oxygen species (mROS) specifically derived from complex III were required for CD4+ T cell activation in vitro and antigen-specific CD4+ and CD8+ T cell expansion in vivo (Sena, et al. 2013). Oxidative stress is an additional metabolic feature in the tumor microenvironment, which can shape the biological behaviors of tumor-infiltrating Treg cells. A recent study found that ovarian-cancer-infiltrating Treg cells showed high mitochondrial activity and produced higher amounts of intracellular ROS compared with that in conventional T cells. Treg cells were found relatively more sensitive to oxidative stress in the tumor microenvironment than conventional T cells. Oxidative stress induced Treg cell apoptosis. Interestingly, the apoptotic Treg cells were more efficient than non-apoptotic Treg cells at suppressing T cell activation and cytokines release. Mechanically, these apoptotic Treg cells could release and convert a large amount of ATP into immunosuppressive adenosine via CD39 and CD73 [38]. On the other hand, another study suggested that upon T cell activation, ROS mediated SENP3 accumulation and triggered the deSUMOylation of BACH2, which contributed to Treg cell effector programs and stability. Thus, genetic deficiency of SENP3 in Treg cells or pharmacologic inhibition of ROS with treatment of antioxidant N-acetylcysteine (NAC) in tumor-bearing mice could enhance anti-tumor immune response [39]. Therefore, it might be interesting to know whether ROS-mediated SENP3 signaling exactly accounts for the Treg cell apoptosis in the context of tumor as described in the earlier study.
However, Ilenia Pacella et al. recently proposed that, unlike tumor-infiltrating Tconv cells, Treg cells could preferentially preserve the glycolytic activity in the tumor microenvironment. Tumor-infiltrating Treg cells in an implanted colon carcinoma mouse model accumulated intracellular lipid content through upregulating the rate of lipid biosynthesis, thus, they have the advantage of concomitant engagement of glucose and lipid metabolic routes, which fueled their preferential expansion in tumor. Additionally, the authors also found that tumor-infiltrating Treg cells highly expressed the receptor OX40 which sustained Treg fitness and promoted the expansion of stable and suppressive Tregs in the tumor [33].
Conclusion
It is now well-documented that Treg cells contribute to the establishment of immune suppressive tumor microenvironment and promote the growth of various types of tumors. The origins of Treg cells in tumor are diverse according to different cancer types (Figure 1). Tumor-infiltrating Treg cells are highly activated and proliferative. Treg cells have metabolic advantages to adopt to the tumor microenvironment. Understanding the origins and phenotypic features of tumor-infiltrating Treg cells may, therefore, help us to design therapeutic strategies targeting Treg cells to augment anti-tumor immune responses in combination of other immunotherapies. To this end, there are two interesting questions remained to be answered: (1) How do Treg cells sustain their lineage stability under extremely stressful conditions? In fact, Treg cells may become unstable and plastic under certain conditions such as hypoxia. Hypoxia induced hypoxia-inducible factor 1 (HIF-1), a key metabolic sensor, which enhanced Th17 differentiation but inhibited Treg differentiation during T cell lineage commitment [40]. (2) What are the potential interplays between Treg cells with other type cells such as malignant tumor cells, stroma cells and immune cells? Tumor-infiltrating immune cells are heterogeneous among tumor types, and vary from patient to patient. Tumor-infiltrating Treg cells can promote immune tolerance by suppressing tumor-associated DC immunogenicity in pancreatic ductal adenocarcinoma (PDA) [41]. Answers to these questions would help us better understand the biology of tumor-infiltrating Treg cells and design rational clinic interventions for tumor therapy.
Figure 1.
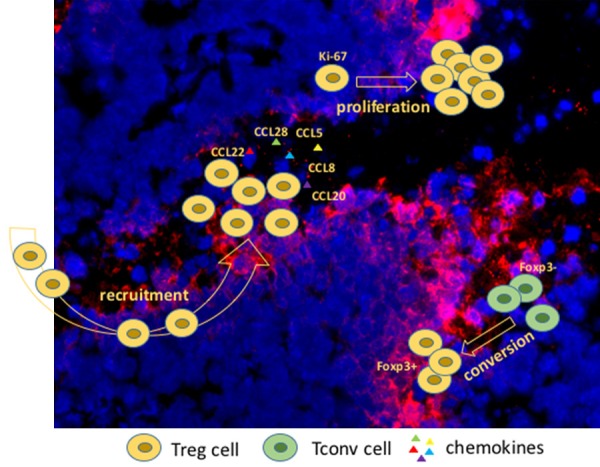
Model of origins of tumor-infiltrating regulatory T cells. Tumor tissue (blue) was stained with DAPI, hypoxic area (red) was labelled with Pimonidazole.
Acknowledgements
The author apologizes to those whose publications could not be cited here because of space limitation. The author thanks Dr. Yan Xiao at Alliance Pharma in USA and Dr. Song Guo Zheng at the Pennsylvania State University for their critical reading of the manuscript. This work is supported by Start Funding of Peking University Health Science Center (BMU2018YJ011).
Disclosure of conflict of interest
None.
References
- 1.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+ CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 4.Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol. 2013;6:116–123. [PMC free article] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 6.Deng G, Xiao Y, Zhou Z, Nagai Y, Zhang H, Li B, Greene MI. Molecular and biological role of the FOXP3 N-terminal domain in immune regulation by T regulatory/suppressor cells. Exp Mol Pathol. 2012;93:334–338. doi: 10.1016/j.yexmp.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng G, Nagai Y, Xiao Y, Li Z, Dai S, Ohtani T, Banham A, Li B, Wu SL, Hancock W, Samanta A, Zhang H, Greene MI. Pim-2 kinase influences regulatory T cell function and stability by mediating Foxp3 protein N-terminal phosphorylation. J Biol Chem. 2015;290:20211–20220. doi: 10.1074/jbc.M115.638221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Y, Nagai Y, Deng G, Ohtani T, Zhu Z, Zhou Z, Zhang H, Ji MQ, Lough JW, Samanta A, Hancock WW, Greene MI. Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Rep. 2014;7:1471–1480. doi: 10.1016/j.celrep.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Lang M, Zhao T, Feng X, Zheng C, Huang C, Hao J, Dong J, Luo L, Li X, Lan C, Yu W, Yu M, Yang S, Ren H. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3(+)Treg cells in pancreatic ductal adenocarcinoma. Oncogene. 2017;36:3048–3058. doi: 10.1038/onc.2016.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halvorsen EC, Hamilton MJ, Young A, Wadsworth BJ, LePard NE, Lee HN, Firmino N, Collier JL, Bennewith KL. Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. Oncoimmunology. 2016;5:e1150398. doi: 10.1080/2162402X.2016.1150398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward ST, Li KK, Hepburn E, Weston CJ, Curbishley SM, Reynolds GM, Hejmadi RK, Bicknell R, Eksteen B, Ismail T, Rot A, Adams DH. The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer. 2015;112:319–328. doi: 10.1038/bjc.2014.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klarquist J, Tobin K, Farhangi Oskuei P, Henning SW, Fernandez MF, Dellacecca ER, Navarro FC, Eby JM, Chatterjee S, Mehrotra S, Clark JI, Le Poole IC. Ccl22 diverts T regulatory cells and controls the growth of melanoma. Cancer Res. 2016;76:6230–6240. doi: 10.1158/0008-5472.CAN-16-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J, Wang H. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P, Han Y, Wu M, Zhang L, Horbinski CM, Ahmed AU, Lesniak MS. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76:5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 19.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 20.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 21.Ai WZ, Hou JZ, Zeiser R, Czerwinski D, Negrin RS, Levy R. Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. Int J Cancer. 2009;124:239–244. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, Wu J, Bi L, Xiong S, Gao S, Yin L, Jiang L, Chen C, Yu K, Zhang S. Malignant B cells induce the conversion of CD4+CD25- T cells to regulatory T cells in B-cell non-Hodgkin lymphoma. PLoS One. 2011;6:e28649. doi: 10.1371/journal.pone.0028649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, Edelson RL. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 24.Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, Bartlett DL, Obermajer N. Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) Treg cells are a source of tumour-associated Treg cells. Nat Commun. 2017;8:14649. doi: 10.1038/ncomms14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ. Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci U S A. 2016;113:6248–6253. doi: 10.1073/pnas.1604765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-gamma drives treg fragility to promote anti-tumor Immunity. Cell. 2017;169:1130–1141. e1111. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansa-Addo EA, Zhang Y, Yang Y, Hussey GS, Howley BV, Salem M, Riesenberg B, Sun S, Rockey DC, Karvar S, Howe PH, Liu B, Li Z. Membrane-organizing protein moesin controls Treg differentiation and antitumor immunity via TGF-beta signaling. J Clin Invest. 2017;127:1321–1337. doi: 10.1172/JCI89281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res. 2012;72:3557–3569. doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- 29.Hindley JP, Ferreira C, Jones E, Lauder SN, Ladell K, Wynn KK, Betts GJ, Singh Y, Price DA, Godkin AJ, Dyson J, Gallimore A. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011;71:736–746. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips JD, Knab LM, Blatner NR, Haghi L, DeCamp MM, Meyerson SL, Heiferman MJ, Heiferman JR, Gounari F, Bentrem DJ, Khazaie K. Preferential expansion of pro-inflammatory Tregs in human non-small cell lung cancer. Cancer Immunol Immunother. 2015;64:1185–1191. doi: 10.1007/s00262-015-1725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waight JD, Takai S, Marelli B, Qin G, Hance KW, Zhang D, Tighe R, Lan Y, Lo KM, Sabzevari H, Hofmeister R, Wilson NS. Cutting edge: epigenetic regulation of Foxp3 defines a stable population of CD4+ regulatory T cells in tumors from mice and humans. J Immunol. 2015;194:878–882. doi: 10.4049/jimmunol.1402725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacella I, Procaccini C, Focaccetti C, Miacci S, Timperi E, Faicchia D, Severa M, Rizzo F, Coccia EM, Bonacina F, Mitro N, Norata GD, Rossetti G, Ranzani V, Pagani M, Giorda E, Wei Y, Matarese G, Barnaba V, Piconese S. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc Natl Acad Sci U S A. 2018;115:E6546–E6555. doi: 10.1073/pnas.1720113115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Turbachova I, Hamann A, Olek S, Huehn J. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo C, Li Z, Xu Y, Wang Y, Li Q, Peng J, Zheng H, Wu P, Li B, Cai S. Higher FOXP3-TSDR demethylation rates in adjacent normal tissues in patients with colon cancer were associated with worse survival. Mol Cancer. 2014;13:153. doi: 10.1186/1476-4598-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singer K, Gottfried E, Kreutz M, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ 3rd, Kopinski PK, Wang L, Akimova T, Liu Y, Bhatti TR, Han R, Laskin BL, Baur JA, Blair IA, Wallace DC, Hancock WW, Beier UH. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25:1282–1293. e1287. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis C, Liu JR, Kryczek I, Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, Lao Y, Teng XL, Li S, Zhou Y, Wang F, Guo X, Deng S, Chang Y, Wu X, Liu Z, Chen L, Lu LM, Cheng J, Li B, Su B, Jiang J, Li HB, Huang C, Yi J, Zou Q. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat Commun. 2018;9:3157. doi: 10.1038/s41467-018-05676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 2017;20:558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]


