Abstract
Purpose
The aim of the study was to compare response evaluation criteria in solid tumours 1.1 (RECIST 1.1), positron emission tomography response criteria in solid tumours (PERCIST), European organisation for research and treatment of cancer (EORTC), and MD Anderson (MDA) criteria for response assessment by Gallium 68-prostate-specific membrane antigen positron emission tomography-computed tomography (Ga68-PSMA PET-CT) in metastatic adenocarcinoma prostate cancer (mPCa) patients with biochemical progression.
Methods
Eighty-eight mPCa patients with pre and post treatment Ga68-PSMA PET-CT were included. A ≥ 25% increase and ≥ 2 ng/ml above the nadir if prostate specific antigen (PSA) drop or ≥ 2 ng/ml above the baseline if PSA does not drop was considered as biochemical progression. RECIST 1.1 and MDA criteria for morphology and PERCIST and EORTC criteria for molecular response were investigated. Percentages of progressive disease (PD), partial response (PR), and stable disease (SD) were calculated. Chi-square test was used for statistical significance.
Results
Proportion of PD, SD, and PR by RECIST 1.1 and MDA criteria were 44 (50.57%), 39 (44.83%), 4 (4.6%), and 33 (39.76%), 48 (57.83%), 2 (2.41%) respectively. Proportion of PD, SD, and PR by PERCIST and EORTC criteria were 71 (80.68%), 11 (12.50%), 6 (6.82%), and 74 (84.09%), 8 (9.09%), 6 (6.82%) respectively. Chi-square test showed statistically significant (P < 0.05) higher proportion of progression detected by both molecular criteria as compare to both morphological criteria.
Conclusion
We concluded that for Ga68-PSMA PET-CT response evaluation, molecular criteria performed better than morphological criteria in mPCa patient with PSA progression.
Electronic supplementary material
The online version of this article (10.1007/s13139-018-0548-3) contains supplementary material, which is available to authorized users.
Keywords: RECIST 1.1, PERCIST, EORTC, MDA, 68Ga-PSMA PET-CT, Response assessment
Introduction
Assessment of response to treatment is critical in oncological practice. Many response evaluation criteria have been proposed in the literature and it is crucial to adopt one of these in the given setting based on a number of factors [1]. Response evaluation criteria in solid tumours 1.1 (RECIST 1.1) is well established and it performs well in assessment of tumour shrinkage as a criteria for response [2]. Other evaluation criteria assessing the activity of tumour have also been used [3]. Positron emission tomography response criteria in solid tumours (PERCIST) is one of the molecular criteria proposed by Wahl et al. for fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT) based response evaluation [4]. Gallium 68-prostate-specific membrane antigen (Ga68-PSMA) PET-CT is a new molecular imaging technique for assessment of prostate cancer (PCa) and it is being frequently used in staging and detecting recurrence with encouraging results [5–8]. However, its role in assessment of treatment response has not been well investigated so far. In this article, we have tried to evaluate the ideal criterion among RECIST 1.1, PERCIST, European organisation for research and treatment of cancer (EORTC), and MD Anderson (MDA) for Ga68-PSMA PET-CT response assessment in metastatic PCa patients with biochemical progression.
Methods
We evaluated 190 patients retrospectively undergoing systemic treatment, who underwent Ga68-PSMA PET-CT during July 2014–Dec 2017 referred for evaluation of response. Patients’ data were retrieved from computerised patient record system (CPRS) and picture archiving and communication system (PACS). In final analysis, 88 patients of metastatic adenocarcinoma prostate with biochemical progression, pre and post treatment Ga68-PSMA PET-CT were included. A ≥ 25% increase and ≥ 2 ng/ml above the nadir if prostate specific antigen (PSA) drop, or ≥ 2 ng/ml above the baseline if PSA does not drop, was considered as biochemical progressive disease (PD) [9]. For time interval between two Ga68-PSMA PET-CT scans, we decided arbitrary to include those with ≤ 12 months to avoid a long interval time between two studies. Patients with no change in treatment in-between two PET-CT studies were included in the analysis.
Imaging Protocol
Standard protocol for in-house synthesis of Ga68-PSMA and PET-CT acquisition was used [10, 11]. PSMA-11 was acquired from advanced biochemical compounds (ABx) and labelling was done in IQS-fluidic labelling module (iTG) using 1.11 GBq iTG self-shielded Ga68 generator. Two MBq/kg body weight of labelled PSMA-11 was injected intravenously and after 1 h of injection; a full body scan (vertex to mid-thigh) was acquired with a dedicated full ring hybrid PET-CT system (Biograph TruePoint40 with LSO crystal from Siemens Healthcare at Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India) with 4 min per bed position in three-dimensional mode. A non-contrast enhanced CT scan (100 mAs and 120 kVp) was used for attenuation correction and anatomical interpretation. Each scan was reconstructed using iterative reconstruction (two iterations and 12 subsets).
Image Interpretation
All Ga68-PSMA PET-CT studies were reinterpreted independently by two nuclear medicine physicians and one radiologist. Increased PSMA uptake in comparison to background, not in areas of normal bio-distribution, was taken as positive for disease. No size criterion was used for PET interpretation. Single voxel maximum standard uptake value normalised to body weight (SUVmax) was recorded. For CT, soft tissue lesion other than the lymph node, with more than 1 centimeter (cm) size in longest axis, was considered measurable while for lymph node, it was ≥ 1 cm in the shortest axis. Bony lesions per patient were categorised as per following criteria: number, type, and PSMA avidity. Number of bony lesions was divided in to three groups, namely, single site, 2–10 sites, and > 10 sites. Type of bone metastasis were classified as sclerotic, lytic, mixed, and marrow while PSMA avidity was grouped as all lesions, most lesions (≥ 10), and a few lesions (< 10).
Response Criteria
RECIST 1.1 was used for morphological response evaluation [2]. Target lesion was defined as ≥ 1-cm well-defined lesion for soft tissue in longest axis and ≥ 1.5 cm in shortest axis for lymph node. The largest sum of diameter (SoD) of five target lesions with maximum two lesions per organ was evaluated. Sclerotic or lytic/sclerotic (mixed type) bone metastases were considered non-measurable (NM) lesions. Greater than or equal to thirty percent decrease in SoD was considered as partial response (PR) while ≥ 20% increase was considered as PD. Change in-between PR and PD (< − 30% and < + 20%) was considered as stable disease (SD). In case of bone only disease (NM disease), SD was considered for equivocal or no change. New bone lesion in view of PSA progression was considered as PD.
PERCIST 1.0 was used with modification for PSMA PET response evaluation [4]. Highest SUVmax was recorded for both PET studies irrespective of number of lesions. It might be two different lesions in a comparison. A drop of ≥ 30% in highest SUVmax was considered as PR while a ≥ 30% increase was considered as PD. New PSMA avid lesion was also considered as PD. Change in-between PR and PD (< − 30% and < + 30%) was considered as SD.
EORTC criterion was also used for PET response evaluation [12]. Highest SUVmax was recorded for both PET studies irrespective of number of lesions. A drop of ≥ 25% in highest SUVmax was considered as PR while a ≥ 25% increase was considered as PD. New PSMA avid lesion was also considered as PD. Change in-between PR and PD (< − 25% and < + 25%) was considered as SD.
Bone is the most common site of distant metastases in PCa and almost all patients with PCa develop bone metastasis during the course of their disease [13, 14]. There is however no clear method of response assessment for bony disease in RECIST 1.1 except for a lytic lesion with soft tissue component. Therefore, to overcome this limitation, we decided to use a criterion for bone metastases developed by The University of Texas MD Anderson Cancer Center (MDA criterion) [15]. In this criterion, PR is defined as development of a sclerotic rim or partial sclerotic fill-in of lytic lesions. Greater than or equal to fifty percent decrease in measurable lesions or ≥ 50% subjective decrease in the size of ill-defined lesions on CT was also considered as PR. Greater than or equal to twenty-five percent increase in measurable lesions or ≥ 25% subjective increase in the size of ill-defined lesion or new bone lesion was considered as PD. A new bone lesion in view of PSA progression was also considered as PD. Change in-between PR and PD (< − 50% and < + 25%) or equivocal change was considered as SD.
Statistical Analysis
Mean ± standard deviation, median, range (minimum to maximum), and inter-quartile range (IQR) were presented for quantitative data and absolute frequencies with percentages for categorical data. Pre and post treatment changes in PSA, RECIST SoD, and SUVmax for individual patient was tabulated. Percentages of PD, PR, and SD based on the different criteria (RECIST, PERCIST, EORTC, and MDA) were calculated. Chi-square test was used for statistical significance between morphological and molecular criteria for proportion of PD. P value < 0.05 was considered statistically significant. SPSS version 21 (IBM New York) was used for the entire statistical analysis.
Results
Mean ± standard deviation, median, range (Min-Max), and IQR for age and Gleason score for 88 patients were 66.18 ± 8.17, 66.5, 51–89, 60–71.50 years and 8.12 ± 1.06, 8, 6–10, 7–9 respectively. Baseline characteristic of Ga68-PSMA PET-CT were presented in Table 1. Bone metastasis was seen in 82/88 (93.18%) of our patients. Distant lymph node metastasis was seen in 5/88 patients. While, there was no lymph node metastasis in 36 (40.91%) patients in our study. Visceral metastasis was present in 15 patients in our study group. Other than the liver and lung, visceral metastases were also seen in pleura, muscle, omentum, and adrenal. For types of treatment categorisation, first-line hormone drugs were grouped together while for other lines of treatment, individual frequency was calculated. We also found that by RECIST 1.1 criterion, 35 patients had NM disease only and 4 patients had measurable disease but not enough to qualify as target lesion.
Table 1.
Showing baseline characteristic of Ga68-PSMA PET-CT scans (n = 88)
The results of baseline and post treatment Ga68-PSMA PET-CT studies, PSA values, and time interval are tabulated in supplementary Table 1. Mean ± standard deviation, median, range (Min-Max), and IQR of time interval between two Ga68-PSMA PET-CT scans were 5.06 ± 2.64, 4, 2–12, and 3–7 months. Pre (baseline or nadir) and post treatment mean ± standard deviation, median, range (min-max), and IQR of PSA (ng/ml) were 42.17 ± 115.32, 10.45, 0.1–989, and 4.05–30.75 and 160.12 ± 439.67, 36.15, 2.5–3910, and 14.40–183.50 respectively. Pre and post treatment mean ± standard deviation, median, range (min-max), and IQR of RECIST SoD (cm) were 4.35 ± 2.32, 3.9, 1.2–12, 2.60–5.40 and 5.32 ± 3.54, 4, 1.2–16.4, 3.15–6.35 respectively. Pre and post treatment mean ± standard deviation, median, range (min-max), and IQR of highest SUVmax were 31.75 ± 26.33, 23, 1.2–132.4, 10.95–45.85 and 34.64 ± 28.77, 24.8, 2.1–140.6, 14.15–45.15 respectively. In RECIST 1.1 criterion-based PD, we noticed 6/44 patients were termed as PD due to appearance of new bony lesion in view of rising PSA while it was 10/33 in MDA criterion. Therefore, help of biochemical parameter was required to interpret our morphological criteria results. Contrary to this, it was not the case with molecular criteria, where we were able to interpret better, either by increase in SUVmax above to cut-off level or by new PSMA avid lesion. PERCIST and EORTC criteria helped us to interpret 35 patients as PD due to new PSMA avid lesion.
We also calculated the proportion of PD, SD, and PR by the different criteria as shown in Table 2. We noticed that the proportion of PD was higher by both PERCIST and EORTC criteria as compared to RECIST 1.1 and MDA criteria when compared to PSA value. It was also seen that both RECIST 1.1 and MDA criteria underestimated the disease status when compared to the PSA values. The statistical significance between these results was calculated by Chi-square test comparing the proportion of PD by various criteria (Table 3). We found that there was statistically significant (P < 0.05) higher proportion of progression detected by both molecular criteria (PERCIST and EORTC) as compared to both morphological criteria (RECIST 1.1 and MDA). No statistically significant difference was seen in proportion of PD comparing RECIST 1.1 Vs MDA. In PERCIST Vs EORTC, higher percentage of PD reported by EORTC criteria which was due to lower cut-off for defining PD for EORTC (25% Vs 30%). However, this was not statistically significant. Two to seven percent of patients showed PR by all criteria in spite of PSA progression.
Table 2.
Showing proportion of PD, SD, and PR by various criteria
| RECIST 1.1 | MDA | PERCIST | EORTC | |
|---|---|---|---|---|
| PD | 44 (50.57%) | 33 (39.76%) | 71 (80.68%) | 74 (84.09%) |
| SD | 39 (44.83%) | 48 (57.83%) | 11 (12.50%) | 8 (9.09%) |
| PR | 4 (4.6%) | 2 (2.41%) | 6 (6.82%) | 6 (6.82%) |
Table 3.
Showing results of Chi-square test for comparing various response criteria for proportion of PD
| Response criteria | P value |
|---|---|
| RECIST Vs PERCIST | < .0001 |
| RECIST Vs EORTC | < .0001 |
| RECIST Vs MDA | 0.207 |
| PERCIST Vs EORTC | 0.692 |
| MDA Vs PERCIST | < .0001 |
| MDA Vs EORTC | < .0001 |
Discussion
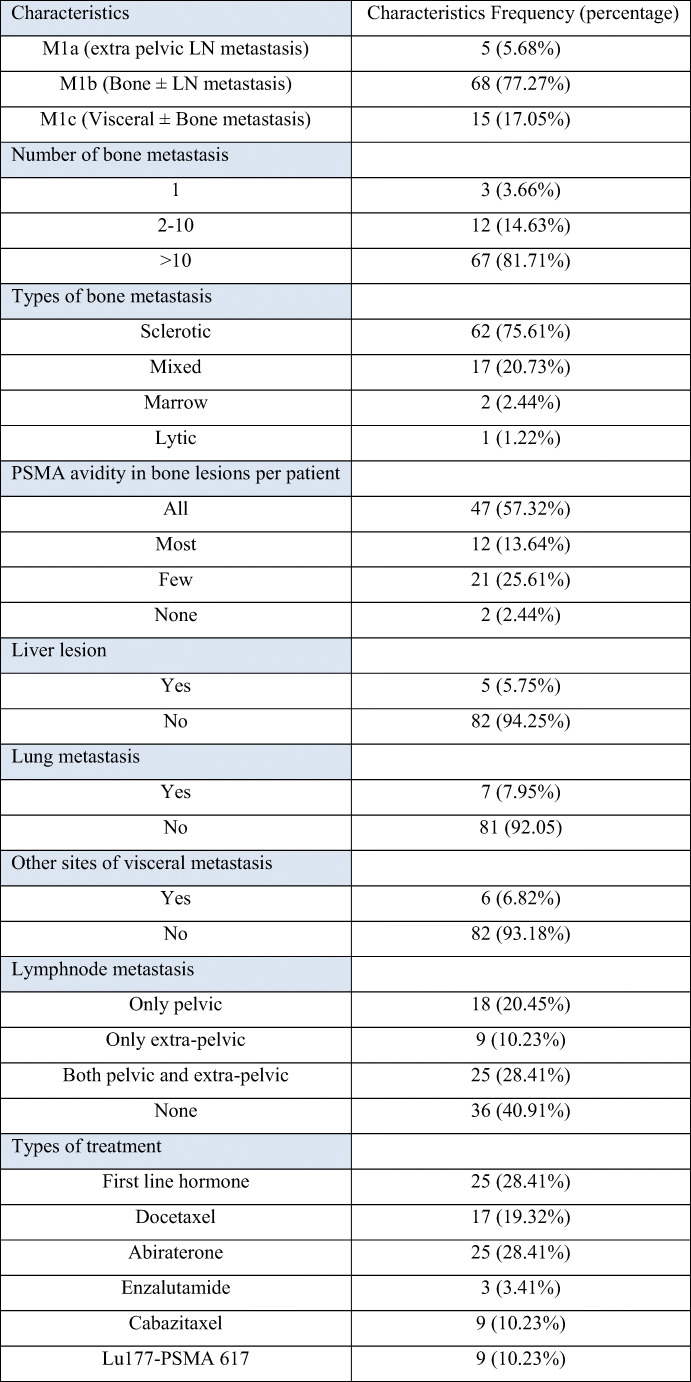
PCa is the second most common cancer in men and third most frequent cause of cancer-related death worldwide [16]. Generally, patients with early stage has good prognosis while most late-stage patients develop resistance to standard treatments and progresses at various time interval [17]. Identifying these patients early is critical in providing optimum management. Serum PSA is a tumour marker for PCa and is frequently used for response assessment during treatment. Rising PSA is an indicator of disease progression with the cut-off value as described by prostate cancer working group 3 (PCWG-3) [8]. PSA however fails to show the distribution of disease and changes occurring at each site [18]. It is important to know the volume of disease and the sites of progression for deciding optimal management. Hence, in this situation, imaging-based response assessment is helpful. Commonly used evaluating criterion is based on morphological changes evaluated by RECIST 1.1 as per the guidelines [16]. There are many instances of discordant findings between RECIST 1.1 criterion and actual disease status (Fig. 1). This is due to the fact that molecular changes appear much earlier than morphological changes.
Fig. 1.
Ga68-PSMA PET-CT maximum intensity projection (a, b) and axial images (c–f). Fifty-seven-year-old prostate cancer patient on Firmagon from last 4 months showed PSA increase from baseline 5.4 to 18.5 ng/ml. Baseline images (a, c, e) showed a few mildly PSMA avid sub-centimetre right pelvic and aortocaval lymph nodes. Post treatment images (b, d, f) showed increase PSMA avidity in up to centimetre-sized lymph nodes and a new PSMA avid sub-centimetre para-esophageal lymph node. Findings suggested stable disease by RECIST 1.1 while progressive disease by PERCIST and EORTC criteria
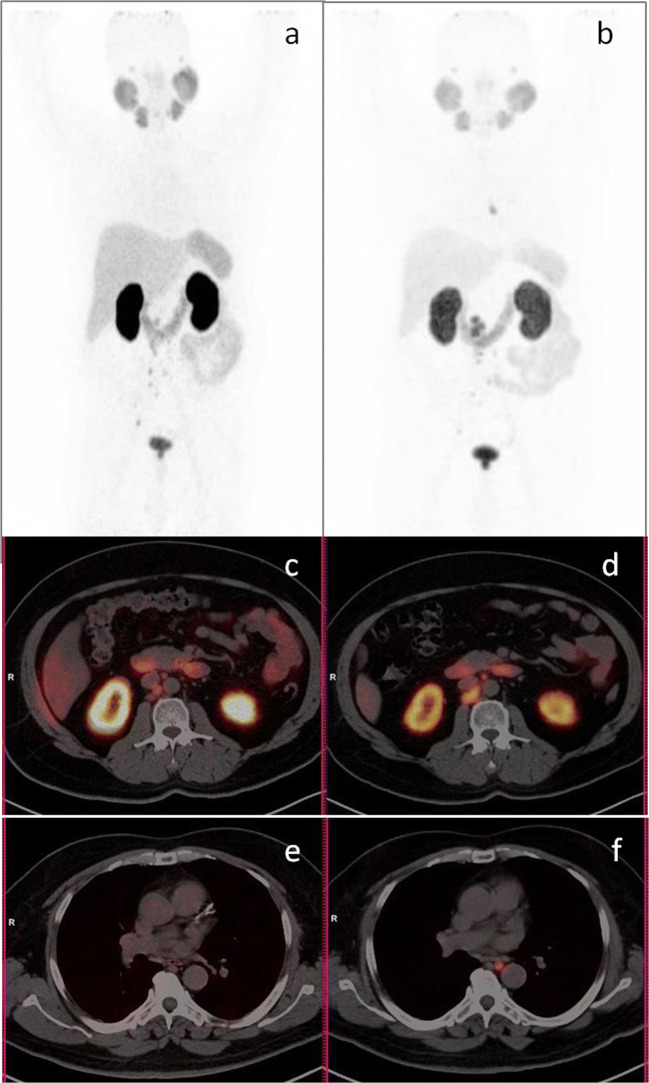
PCa has a unique tropism for bone metastasis which contributes to major disease burden [17]. In most instances, bone metastases are sclerotic or mixed (sclerotic/lytic) in nature, while pure lytic lesions are rare [18]. Most end-stage patients are expected to have multiple bone metastases. In our group, 82/88 patients had bone metastasis, while 67/82 patients had multiple (> 10) bone lesions. In RECIST criterion, no clear method for response assessment is given for sclerotic or mixed lesions except for lytic lesion with measurable soft tissue component. It is indeed important as sclerotic and mixed lesions patients are a majority and constitute 96.34% (79/82) in our group. Lytic lesion without soft tissue component was seen in 1/82 patient. Rest two patients had PSMA avid marrow lesions where morphological criteria underestimate the disease burden. At the same time, it is difficult to analyse the presence of active disease by changes in morphology (Fig. 2). In our patients group, 47/82 patients had all PSMA avid bone lesions and two patients had PSMA non-avid bone lesions as well. In remaining 33/82 patients, not all bone lesions were PSMA avid.
Fig. 2.
Ga68-PSMA PET-CT maximum intensity projection (a, b) and sagittal (c, d) images. Fifty-six-year-old patient, post 3 cycles of cabazitaxel showed PSA increase from 30.5 to 205.0 ng/ml. Baseline images (a, c) showed multiple sclerotic lesions with PSMA uptake in a few lesions. Post treatment images (b, d) showed multiple sclerotic lesions with PSMA uptake in most. Findings suggested stable disease by RECIST and MDA criteria while progressive disease by PERCIST and EORTC criteria
Another challenge for assessment by morphological-based criteria was a new sclerotic bone lesion. Such lesion might be due to healing process or disease progression. Hence, for both RECIST 1.1 and MDA criteria, it is important to know the clinical and biochemical status and the status of other disease sites to characterise a new bone lesion. As already mentioned before, 6/44 and 10/33 PD patients by RECIST and MDA criteria respectively had new sclerotic bone lesions and in view of rising PSA, these were considered as progressive lesions. On the contrary, by molecular criteria, it was easier to interpret these new lesions. PSMA avid new bone lesion was considered as PD while non-avid new lesion was considered as healing response. A possibility of tumour cells actually getting de-differentiated and losing PSMA expression is also a remote possibility [19].
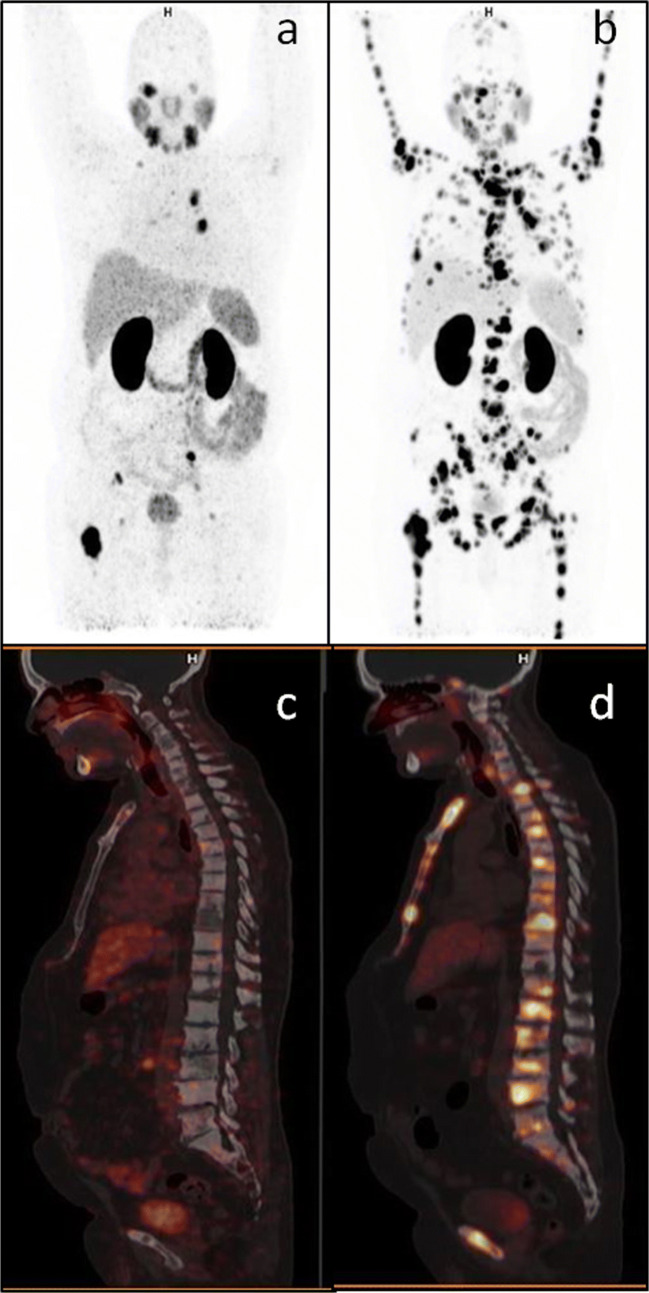
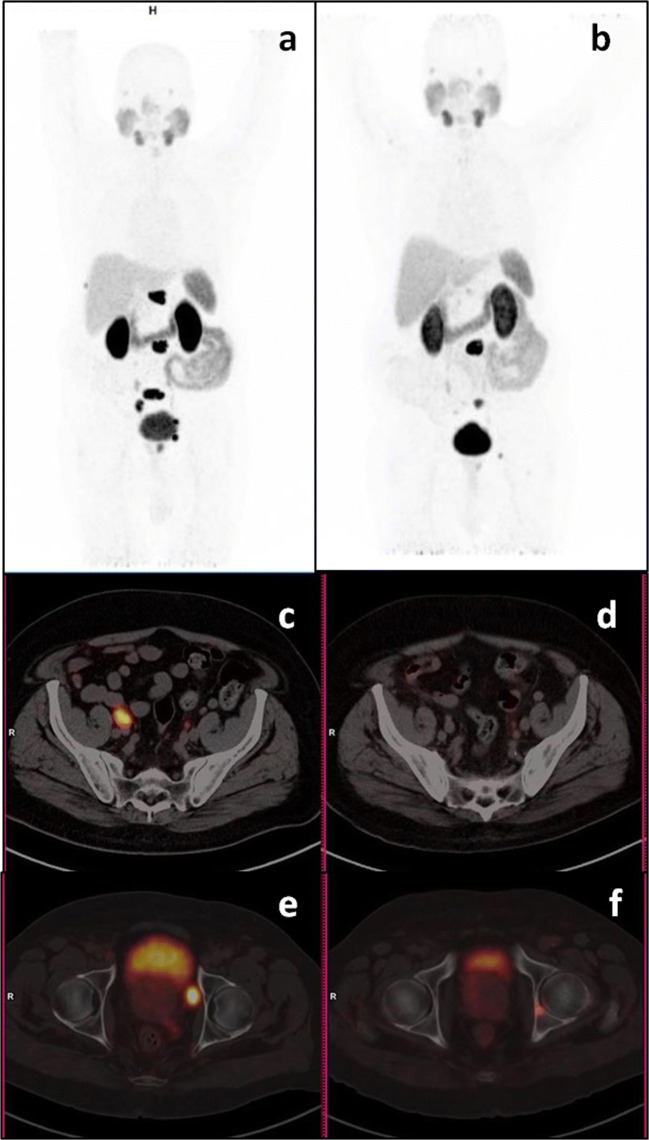
In our study, we have arbitrarily included those patients with ≤ 12-month time interval between two Ga68-PSMA PET-CT scans. This was mainly to avoid long interval time and change in treatment in between, which might lead to false interpretation. This false interpretation would be more common with long interval time between two scans (Fig. 3). Hence, either an interim scan or short duration between two scans and more important no change in treatment becomes important to avoid misinterpretation.
Fig. 3.
Ga68-PSMA PET-CT maximum intensity projection (a, b) and axial images (c–f). Eighty-one-year-old prostate cancer patient on Firmagon from last 11 months showed PSA increase from nadir 0.8 to 3.4 ng/ml. Baseline images (a, c, e) showed PSMA avid prostate lesion, pelvic lymph nodes, and a few sclerotic bone lesions. Post treatment images (b, d, f) showed response in prostate, lymph nodes, and earlier seen bone lesions; however, a new PSMA avid lesion is seen in left acetabulum. Findings suggested pseudo-response by RECIST 1.1 due to long interval time (11 months) while progressive disease by PERCIST and EORTC (due to new bone lesion) criteria
In our study, we have seen a few partial responses by all criteria despite taking PSA PD patients only. By RECIST 1.1 criteria, 4/88 patients were reported as PR with interval time between scans being 2, 7, 11, and 12 months (Supplementary table). Two of these patients with interval time of 11 and 12 months actually showed PD by both molecular criteria, hence these were misinterpreted by RECIST 1.1 due to long interval time. We have also reported 6/88 patients as PR by both molecular criteria. Interval time between two scans of these 6 patients was 2, 2, 3, 7, 7, and 12 months. Similar to morphological criteria, it was expected as misinterpretation for 12-month interval time scans. However, for 2- to 3-month interval time scans, it was difficult to explain as misinterpretation. Rather, it might be due to de-differentiation and loss of PSMA expression on tumour cell but with maintained PSA expression. Further research in indicated to find out the right interval time to minimise these misinterpretation. We propose that there should be less than 6-month interval time between two scans for response assessment.
There is not much literature available on response assessment by Ga68-PSMA PET-CT in metastatic PCa. Seitz et al. described preliminary results on response assessment using Ga68-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy [20]. They reported a better concordance between biochemical response (BR) and radiological response (RR) by PET than by CT alone. However, the difference in the results between PET and CT was not statistically significant (P = 0.7). This was attributed to the small sample size. Two other small studies have described the role of serial Ga68-PSMA PET-CT in response assessment to radiotherapy in recurrent nodal disease [21, 22]. These studies show reductions in SUV in most treated lesions as indicative of response. A few studies have also reported role of F18-Choline PET-CT in PCa response assessment to abiraterone [23], enzalutamide [24], and docetaxel [25]. They have also reported better concordance between BR and PET responses than BR and CT responses.
There were a few limitations in our study. Firstly, there were different treatment regimes given to the patients in our group which may cause difference in the pattern of response. In PCa, bone is the predominant site of metastasis and hence morphological criteria tend to underestimate changes in the bone lesions due to known limitations which were also seen in our groups. In molecular response criteria, we have considered only one highest SUVmax per study for statistical analysis. There were instances that many lesions have shown decrease in SUVmax while one lesion showed increase in SUVmax and become more than the first study highest value. Hence, it was qualified as PD for response analysis. In other scenario, many lesions showed decrease in SUVmax while some or one lesion showed increase in SUVmax but still remain less than the first study highest value. Hence, it was qualified as PR or SD in response analysis. Therefore, we realised that evaluating one highest value might not represent the real picture of molecular response in all sites. Thus, in future, we will have to take multiple lesions into analysis; however, we need to find out how many lesions and whether these target lesions will be based on first PET study to see decrease in SUVmax or will be based on second PET study to considered smallest increase in SUVmax.
Conclusion
Our results have shown that for using Ga68-PSMA PET-CT for response evaluation, molecular criteria are better than morphological criteria in metastatic PCa patient in the PSA progression subgroup. However, further clarifications will be required on number of lesions for molecular criteria and the best interval time between two scans to avoid false interpretations. Therefore, prospective studies are warranted to recommend molecular criteria for Ga68-PSMA PET-CT-based response evaluation for metastatic PCa patients in clinical practice.
Electronic Supplementary Material
(DOCX 33 kb)
Compliance with Ethical Standards
Conflict of Interest
Manoj Gupta, Partha Sarathi Choudhury, Harish Chandra Goel, S Avinash Rao, and Sudhir Rawal declare that they have no conflict of interest.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional review board waived the need to obtain informed consent for this retrospective study.
References
- 1.Curran SD, Muellner AU, Schwartz LH. Imaging response assessment in oncology. Cancer Imaging. 2006;6:S126–S130. doi: 10.1102/1470-7330.2006.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Carnaghi C, Sclafani F, Basilico V, Doherty M. Response assessment in oncology: limitations of anatomic response criteria in the era of tailored treatments. Q J Nucl Med Mol Imaging. 2011;55:589–602. [PubMed] [Google Scholar]
- 4.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–1250. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Wester HJ, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 6.Perera M, Papa N, Christidis D, Hofman MS, Bolton D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Gupta M, Choudhury PS, Hazarika D, Rawal S. A comparative study of 68Gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: an initial experience. World J Nucl Med. 2017;16:186–191. doi: 10.4103/1450-1147.207272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hruby George, Eade Thomas, Emmett Louise, Ho Bao, Hsiao Ed, Schembri Geoff, Guo Linxin, Kwong Carolyn, Hunter Julia, Byrne Keelan, Kneebone Andrew. 68 Ga-PSMA-PET/CT staging prior to definitive radiation treatment for prostate cancer. Asia-Pacific Journal of Clinical Oncology. 2018;14(4):343–346. doi: 10.1111/ajco.12872. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amor-Coarasa A, Schoendorf M, Meckel M, Vallabhajosula S, Babich JW. Comprehensive quality control of the ITG 68Ge/68Ga generator and synthesis of 68Ga-DOTATOC and 68Ga-PSMA-HBED-CC for clinical imaging. J Nucl Med. 2016;57:1402–1405. doi: 10.2967/jnumed.115.171249. [DOI] [PubMed] [Google Scholar]
- 11.Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. doi: 10.1007/s00259-017-3670-z. [DOI] [PubMed] [Google Scholar]
- 12.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 13.Rigaud J, Tiguert R, Le Normand L, Karam G, Glemain P, Buzelin JM, et al. Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol. 2002;168:1423–1426. doi: 10.1016/S0022-5347(05)64465-5. [DOI] [PubMed] [Google Scholar]
- 14.Soloway MS, Hardeman SW, Hickey D, aymond J, Todd B, Soloway S, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::AID-CNCR2820610133>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92. doi: 10.7150/jca.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomark Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 17.Sridhar SS, Freedland SJ, Gleave ME, Higano C, Mulders P, Parker C, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65:289–299. doi: 10.1016/j.eururo.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Emmenegger U, Ko Y-J. PSA-based treatment response criteria in castration-resistant prostate cancer: promises and limitations. Can Urol Assoc J. 2009;3:375–376. doi: 10.5489/cuaj.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronsert P, Reichel K, Ruf J. Loss of PSMA expression in non-neuroendocrine dedifferentiated acinar prostate cancer. Clin Nucl Med. 2018;43:526–528. doi: 10.1097/RLU.0000000000002100. [DOI] [PubMed] [Google Scholar]
- 20.Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, et al. Preliminary results on response assessment using (68)Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2017;45:602–612. doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]
- 21.Zschaeck S, Wust P, Beck M, Wlodarczyk W, Kaul D, Rogasch J, et al. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat Oncol. 2017;12:140. doi: 10.1186/s13014-017-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumann R, Koncz M, Luetzen U, Krause F, Dunst J. Oligometastases in prostate cancer: metabolic response in follow-up PSMA-PET-CTs after hypofractionated IGRT. Strahlenther Onkol. 2018;194:318–324. doi: 10.1007/s00066-017-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Giorgi U, Caroli P, Burgio SL, Menna C, Conteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5:12448–12458. doi: 10.18632/oncotarget.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, et al. (18)F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–1283. doi: 10.1007/s00259-015-3042-5. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzenböck SM, Eiber M, Kundt G, Retz M, Sakretz M, Kurth J, et al. Prospective evaluation of [11C]choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2105–2113. doi: 10.1007/s00259-016-3439-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)