Abstract
Brain disease is one of the greatest threats to public health. Brain theranostics is recently taking shape, indicating the treatments of stroke, inflammatory brain disorders, psychiatric diseases, neurodevelopmental disease, and neurodegenerative disease. However, several factors, such as lack of endophenotype classification, blood-brain barrier (BBB), target determination, ignorance of biodistribution after administration, and complex intercellular communication between brain cells, make brain theranostics application difficult, especially when it comes to clinical application. So, a more thorough understanding of each aspect is needed. In this review, we focus on recent studies regarding the role of exosomes in intercellular communication of brain cells, therapeutic effect of graphene quantum dots, transcriptomics/epitranscriptomics approach for target selection, and in vitro/in vivo considerations.
Keywords: Theranostics, Radiotheranostics, Brain, Exosome, Graphene, Transcriptomics
Brain disease is one of the greatest threats to public health. Global disease burden of the neuropsychiatric diseases are well reported and depression is the most serious problem, as its years lived with disability (YLD) is the largest for advanced, recently developed, and developing countries [1–3]. Neurodevelopmental diseases such as autism spectrum disorder (ASD) or attention deficit hyperkinetic disorders (ADHD) follows depression as a second entity with great burden, since they arise during childhood, not only to the affected children but also to their families suffer for the lifetime [4]. Neurodegenerative diseases occur in the late stages of peoples’ lives and thus the third important problem in terms of YLD [1].
Brain theranostics, which might have been misunderstood as brain cancer treatment, is recently taking shape, indicating the treatments of stroke [5], trauma [6], and other inflammatory brain disorders [7, 8]. Brain theranostics should also have held great promises in the field of psychiatric diseases [9], neurodevelopmental disease, and neurodegenerative disease with motor or memory impairments [10–13]. The emerging field of brain theranostics involves concepts associated with monitoring biomarker levels in tissue and engineering probes for improved diagnosis and treatment efficacy. However, there are several factors that make brain theranostics application difficult, especially when it comes to clinical application.
First, many brain diseases are not well classified by their endophenotypes, which are recently established especially for schizophrenia [14, 15]. Though germline abnormalities are suspected to cause the diseases [16] and many somatic mutation–based causes are known to present psychopathology [16, 17], the disease onset is late and the environmental factors’ contribution to pathogenesis has not been elucidated yet even for Alzheimer’s disease (AD) [18]. As yet, the classification and thus the prognostication of the brain diseases are based on symptom complexes and the diagnosis is much like a syndrome.
Second, neuropsychiatric, neurodevelopmental, and neurodegenerative diseases have relatively intact blood-brain barrier (BBB) [19–22]. BBB is a unique endothelial barrier that acts as a security system, separating the circulating blood from the brain and extracellular fluid. BBB prohibits more than 90% of small-molecule drugs and nearly all of large-molecule neurotherapeutics to enter the inside of the brain, which is a great concern for the novel drug candidates [23, 24]. Although investigators have been focusing on the surrogate parameters of BBB penetration as is well-known octanol extraction used in radiopharmaceuticals evaluation to represent the permeability of novel drug candidates, it is still challenging to accurately predict and design characteristics of CNS-targeting drugs in their BBB penetration [25]. There have been three ways of approach to overcome this ambiguity of the BBB: (1) understanding the BBB in much deeper detail at the molecular level, (2) simulation of the in vitro BBB using microfluidic chips and testing new biopharmaceuticals on these chips, and, lastly, (3) ignoring the BBB problem while hoping that BBB shall be in any way disturbed in vivo in brain diseases especially in their advanced stages. The last one prevailed when the investigators try to develop new drugs using small molecules, peptides, or monoclonal antibodies [24, 26]. Using monoclonal antibodies to treat AD was an obvious example in that the %ID/g of monoclonal antibodies were reported as 0.1%, but these drugs went on to clinical trials and failed [24, 27, 28]. The same drugs are also tested for treating mild cognitive impairment (MCI) to modify the disease course, preventing MCI conversion to AD. However, brain uptake of solanezumab or bapineuzumab will be less favorable and how much effect can be expected, with the almost-intact BBB of MCI resisting the drug penetration, is questionable.
In vitro BBB models conventionally used a relatively simple transwell system consisting of a monolayer of endothelial cells. However, recent use of 3D models, on the microfluidic chip, using a combination of pericytes and endothelial cells enabled realistic BBB models in vitro [29–31]. In silico methods are recently applicable in prediction of BBB permeability [32]. Starting with simple regression models based on a calculation of lipophilicity and polar surface area, investigators developed partial least squares–based methods to grid-based approaches to predict the BBB permeation of novel drugs. Recent advances in the artificial neural networks make these approaches gain more importance [33]. In vivo methodology to measure total brain concentration and brain/plasma ratio of novel drug candidates were used in to evaluate novel brain therapeutics. In vivo experiments provide the most reliable information for assessing brain penetration of drug candidates; however, due to the huge number of molecules produced by combinatorial chemistry, they cannot be applied as an effective screening in the early stage of drug development [34]. Thus, the combined use of in silico method, in vitro microfluidic chips, and classical in vivo models are necessary to unravel the future promises of novel drug candidates.
Third, determining the targets is also a huge obstacle in the development of the novel brain therapeutics. Small molecules or peptides and monoclonal antibodies cannot be tested easily for their efficacy in vitro since their pharmacological effects take place in such a brisk way or in an incipient way that no one can understand the biological significance of these changes in vivo. Target cells are so heterogeneous in vivo that choosing the right cells and the correct target molecules are highly warranted [35]. Previously for AD, amyloid plaques of extracellular spaces were considered to be the optimal target for vaccines or monoclonal antibodies [26, 36], and we have many diagnostic imaging agents, which had the similar shapes of methylene blue which are intercalated with amyloid plaques [37–39]. Tau fibrillary tangle was another target; however, tau targeting therapeutics are not yet introduced as there is another barrier for therapeutic drugs, i.e., cellular membranes, as tau tangles are mostly intracellular bodies [40–45].
Selection of the target in the brain had been almost always against the membrane macromolecules such as receptors, transporters, and epitopes residing in the neuronal surface [46]. Brain imaging with C-11- and F-18-labeled positron emission tomography (PET) ligands targeted dopamine receptors or dopamine transporters of dopaminergic neurons and peripheral benzodiazepine receptors of microglial cells [47–51]. These classic imaging methods using PET or sometimes single-photon emission computed tomography (SPECT), I-123 fluoropropyl CIT for dopamine transporters [51, 52], are now at the stage of expansion to explore more targets by the transcriptomic approaches. Single-cell transcriptomics with various RNA sequencing (RNA-seq) methods are used to underpin this approach [53–57].
What we need to do is to develop the methods to explore the target by just narrowing down methods of incriminating the cells and the intracellular molecular events. Molecular imaging, more specifically molecular biology–based imaging will render appropriate mouse models to us so that we could use them to confirm the biological consequences of novel drug candidates [58]. In this case, novel drug candidates can be small molecules which are examined through the chemical library, peptides, either natural or non-natural, sequence based but manufactured by engineering methods, or monoclonal antibodies, murine to human, and also the nanoparticles on their own or with surface modification.
Fourth, the real problem resides in the ignorance that we do not have enough information about the whereabouts or biodistribution of novel drug candidates when they are injected systemically [59]. With these novel drug candidates, after a thorough test for the feasibility for use in in vivo and especially in humans, we need to check the pharmacokinetics and thus biodistribution. Here, radiotheranostics come in [60]. By labeling radionuclides to the novel therapeutic candidates, we are going to be able to follow the whereabouts of these novel drug candidates. In case of radiolabeled nanoparticles, physical or chemical core labeling was reported, but capsular labeling or surface modifications were considered more practical [61–66]. Surface modifications could be done either by chemical or physical methods; however, the physical method using a micelle-encapsulation method tended to prevail as it removed cumbersome sequential steps of separation and reaction [61, 67]. In case of monoclonal antibodies, obviously the chelator linker–based methods are the solution [68, 69]. However, when labeling chemical compound drugs, chelator-based labeling itself affects the change in in vivo biodistribution and binding affinity. Thus, we need to confirm that the chelator-bound peptides of antibodies perform the same as native peptides/antibodies. If we use gamma- or positron-emitting radionuclides, we can figure out the tissue uptakes of radionuclides and the biodistribution of the drugs while estimating the concentration of drug candidates in specific regions of the brain [70, 71]. Biological effects can be visualized and imaged using in vivo molecular imaging methods repeatedly and at the last moment can be measured ex vivo using sacrificed animals [72].
What we need is the definitive methods to confirm the novel drug candidates are working at which molecular level, within the cells either in the cytoplasm or even in the nucleus, at the specific region of the brain for a period of the time to take effect upon the behavior of the animals. For this purpose, model to model differences should be taken into account as was reported for AD model mice, where amyloid plaques were deposited in different areas of the brains in APP23, Tg2576, and PS1/APP mice [73]. The behaviors of the model mice should later be translated to those of humans such as thought, emotion, and (motor) behaviors. Obviously, we do not have suitable animal models for the neuropsychiatric or brain diseases, which makes the translation of the in vivo findings of animals to humans in clinical situations very difficult. What we have are the models for translation of the pharmacokinetics and pharmacodynamics of novel drug candidates from mice to humans. These models include physiologically based pharmacokinetic model and target-mediated drug disposition model as well as scale-based ones [74–77].
Finally, a more comprehensive understanding of intercellular communication between brain cells, including glial cells, neurons, and endothelial cells of BBB, complicates the development of novel brain therapeutics [78, 79]. The biological effects of monoclonal antibodies should be interpreted again considering the action of the exosomes which are secreted and taken up by neurons and their progenitors and glial cells. Peptide propagation model [80] and the role of exosomes especially from microglia [81] need to be the included in the schematic analysis of the efficacy of the novel drug candidates [82]. Exosomes have been considered to be the carrier to deliver the intra-exosomal materials such as nucleic acids or peptides and the perturbation of these processes might control the propagation of tau hopefully to mitigate the neurodegenerative disease process [83]. Kamerkar et al. reported that, compared to liposomes, exosomes exhibited superior ability as a drug delivery conveyor and showed better tumor growth suppression for pancreatic cancer therapy [84]. This study highlighted that the presence of CD47 expression on exosomes allows evasion from non-specific uptake by immune cells, resulting in increased circulation time in the bloodstream. Consequently these properties may be able to increase the efficacy of brain delivery. Microglia-derived extracellular vesicles, which is the generic term of exosomes or microvesicles irrespective of the sizes, were speculated to exert supportive or aggravating roles to the degenerating neurons in AD model mice [81]. Exosomes are obviously performing their physiologic roles in healthy states but once pathologic processes begin, they will be involved in the progress of the neurodegenerative processes and then the therapeutics to inhibit the transfer of intra-exosomal materials will ameliorate the disease pathology [85]. Or if exosomes play the role of inhibiting the propagation in neurodegenerative processes, enhancing the activity of microglia-derived exosomal propagation will be necessary as therapeutics [82]. These hopeful predictions seem mutually contradictory now and should be sorted out very soon.
In case of exosomes, the research opportunities are in in vitro condition, which means the production of microfluidic chips to test and predict the in vivo behavior of the exosomes, which might be developed themselves as novel therapeutics, carrying intra-exosomal biomolecules to the targets. In brain theranostics, the targets are brain cells of neurons, microglia, activated astrocytes, and oligodendrocytes. The differentiation of these targets is important for the exosomes as novel therapeutics as well as crossing BBB as novel drug candidates.
By taking examples, we will explain further the above schemes and their sub-schemes of doing brain theranostics and radiotheranostics for the items warranting more detailed explanations.
Delivery to the Brain of the Novel Drug Candidates
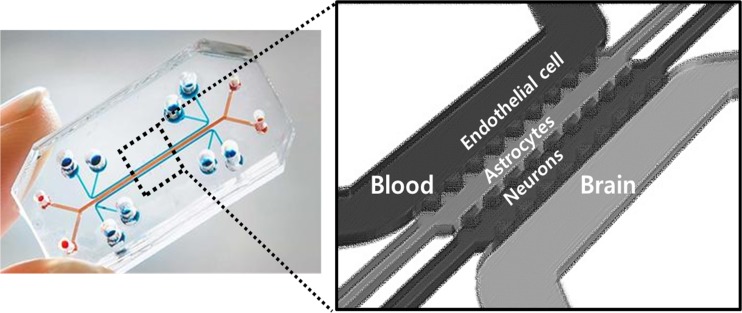
The problem of delivery to the brain lies in the combination of the insufficient knowledge about the structural and functional characteristics of BBB and the unscrupulous approach of applying novel drug candidates by just jumping to the conclusion that the novel candidates work at the behavioral level of mouse models [79, 86]. In addition to the paracellular pathway of water-soluble agents, transcellular pathways of lipid-soluble agents or transport proteins–mediated materials (such as glucose, amino acids, or nucleosides), transcellular receptor–mediated (insulin, transferrin) and adsorptive transcytosis pathways using lipid raft–mediated endocytosis or micropinocytosis (albumin, plasma proteins) are known [21, 22, 87]. Novel drug candidates should be tested for how much amounts are delivered to the brain after systemic injection in in vivo models, and then the mechanism should be understood in in vitro equipment of microfluidic devices using inhibitors of receptor-mediated pinocytosis (chlorpromazine), lipid raft–mediated endocytosis (flilipin III or nystatin) or macropinocytosis (cytochalasin D) [21]. Endogenous nanocarrier of exosomes or exogenous nanoparticles of inorganic or organic composition have been proposed, and thus, this mechanistic investigation came to be warranted [66]. The Transwell model was not sufficient for the understanding of BBB permeation and two- or three-channel microfluidic chip would serve to delineate the mechanism [88–92]. These chips are under active development and will soon be available (Fig. 1).
Fig. 1.
Concept of a 3D BBB model on the microfluidic chip
Exosomes as Brain Theranostics Agents and Radiotheranostics
As exosomes are excreted by all the cells and are also taken up by all the cells in the body, we need to know the specificity of these intercellular interactions which are not yet clearly understood [93]. Thus, the investigation of using exosomes are divided into two approaches; one is to isolate exosomes from cell lines or primary cell cultures and then inject directly or systemically to the recipient model animals and explore the effects [94–96], and the other is to deplete the source cells or to inhibit the synthesis of exosomes and to explore their consequences [82]. Investigators still do not know whether the administration of xeno- or allo- or autologous exosomes are different in their interaction with the recipient cells, though major histocompatibility complex (MHC) molecules are at the exosomal surface membranes, and confine their study in in vitro models with the cells of the same origin, or use the nude or severe combined immunodeficiency (SCID) mice as recipients to circumvent this ignorance [97–100]. Specificity of the uptake of exosomes and their consequent biological effects on the recipient cells is to be elucidated soon. The biodistribution or pharmacokinetics of administered exosomes is monitored in tissues of the sacrificed animals using fluorescent dyes even at later times after administration [96, 101]. The problem is that despite stable labeling at the surface of the exosomal membranes lipophilic fluorescent dyes are released from the injected exosomes and recirculate in the body to confound the distribution of the intact exosomes [102]. In our previous study, the brain was suggested to reveal that the ex vivo fluorescent imaging studies are not appropriate because Tc-99m HMPAO-labeled exosomes do not demonstrate localization of labeled exosomes in the brain but DiI-labeled exosomes showed fluorescent activities very well (Fig. 2) [103].
Fig. 2.
Imaging of dual tracer-labeled exosome-mimetic nanovesicles showed that the accumulation pattern of radiotracer was different from fluorescence imaging. Fluorescent image showed a considerable signal in the brain, while radionuclide-labeled exosome indicated almost no accumulation in the brain. (Adapted with permission from Ref [103])
Tc-99m HMPAO act as lipophilic agents which cross the exosome membranes easily (almost transparently) and then within the exosomes they convert to hydrophilic forms which stay there as a fixed agent to the interior of these exosomes [104]. While we follow the radioactivity using SPECT, we can trace the whereabouts of intact exosomes. Radioactivity stayed with exosomes in vitro in plasma until 4 h after mixing. However, the in vivo radioactivity does reveal the location of Tc-99m HMPAO instead of exosomes, and we should be cautious that radioactivity automatically visualized the exosomal distribution. It is reassuring that Tc-99m is almost never released from HMPAO in vivo. As was expected, the Tc-99m radioactivity appeared in the intestines and this should be interpreted either that the labeled exosomes were disintegrated in the liver and intra-exosomal Tc-99m HMPAO was released and excreted via hepatobiliary pathways or that the labeled exosomes containing Tc-99 m HMPAO was excreted as an entity via hepatobiliary pathways. Though there is no definite evidence, we think that the first hypothesis has a higher possibility, as there has been no report that the endocytosed exosomes are passing through, in their intact form, to the other side of the cells. When the investigators were trying to use exosomes as delivery vehicles for brain theranostics, they hypothesize implicitly that this unlikely mechanism of transcytosis of exosomes are going to work and exosomes may carry their cargos to the target cells especially across BBB. Whether this is the case or not should be elucidated.
Another approach of inhibiting exosome production by depleting the source cells is promising, as this adopts the discipline of classic molecular biology. The best scenario was the selection of the most promising model to reveal the mechanism of intercellular interactions, which can be applied later to explain also the cell-to-cell interactions via exosomes. For example, the tau propagation model was used to unravel the role of microglia and their excreted exosomes [20, 82]. Among the dominance of the reports in the literature based on the hypotheses considering amyloid oligomer and hyperphosphorylated tau as pathologic causes in a cell-autonomous manner, or at most only between neurons, the rise of the importance of the cell-to-cell interactions between brain cells or the involvement of non-neuronal cells as possible players in AD was fresh and new. Thus, the report by Asai and colleagues that the depletion of microglia and the inhibition of exosome synthesis could halt tau propagation attracted much attention [82]. For AD, the dichotomous role of microglia-derived exosomes is hypothesized and the modulation of the microglia using classical small-molecule drugs are being investigated [81, 105].
Graphene Quantum Dots as Brain Theranostics and Radiotheranostics
Nanoparticles had been proposed to be used as carriers carrying novel or established drugs as cargos [106]. Investigations had focused on the binding of the drug candidates physically or chemically to the cores or surfaces of nanoparticles. These nanoparticles have the capability of being taken up to the site of interest (mainly the tumors) and of releasing the drugs at the extracellular milieu or of being taken up again by the tumor cells to release the drugs intracellularly [107, 108]. If the cargo drugs are nucleic acids such as siRNAs or aptamers, efficient delivery system to the target is necessary to minimize the off-target effects [109]. For example, siRNAs are known to be cytostatic, and thus, though they once had been considered as the optimal and promising therapeutics exploiting their sequence-specific targeting capability, this characteristic rather hindered their further use as definitive therapeutics owing to the documented off-target effects with larger administered doses [110, 111]. Among many nanoparticles, a few can be used for in vivo, for example, iron oxide nanoparticles [112, 113], upconversion nanoparticles [114, 115], and organic ones such as liposomes [116] and their endogenous counterparts, exosomes. Recently, the biomedical application of graphene, reduced graphene oxide (rGO), or graphene oxide (GO) has grown rapidly, due to their stability, large surface area, and biocompatibility [117, 118]. GO, if injected systemically to the animals, will be surrounded by corona proteins and meet innate immune cells [119]. Neutrophils will engulf or trap GO using neutrophil extracellular trap and metabolize GO into smaller ones using their myeloperoxidase [120]. GO can bind easily to nucleic acids, based on π-π interaction, and once their size are reduced to several nanometers, they do emit fluorescence by themselves and are called graphene quantum dots [121].
Graphene oxide quantum dots (GQD) were suggested to be a good carrier, considering their low toxicity and high hydrophobicity [118, 122, 123]. Interestingly, GQD was working as novel drug candidates themselves in several reports, recently [12, 13, 124]. GQD was found to intercalate in the fibrillary proteins and disintegrate the fibrils into monomers [12]. With the clear evidence and reproducibility in the in vitro works, Kim et al. showed that intraperitoneal injection of GQD prohibited α-synuclein (α-syn) fibril propagation in the peptide propagation model of Parkinson’s disease [12]. The in vivo permeability of the BBB was studied by using biotin-labeled GQD, and showed that GQDs distributed in the entire CNS region. However, it is notable that a relatively low concentration (0.7–1 nmol/ml) of GQD in the brain was enough to prohibit peptide fibril propagation, while the equal amount of PFFs and GQD were needed to show the effect of the fibril disintegration in the in vitro study. It could be due to the lack of α-syn clearance mechanisms in the in vitro system, while in the brain, α-syn monomers can be cleared by multiple pathways including the activation of central innate immunity. The same disparity was also discussed in the study regarding 4-(2-hydroxyethyl)-1-piperazinepropanesulphonic acid (EPPS), small molecule, which has been proven to disaggregate amyloid-β [125].
Recent accumulating evidence suggests that central innate immunity, specifically disease-associated microglia (DAM), might have a protective role in the field of neurodegenerative disease [126, 127]. Altered systemic immune signals, due to systemic injection of macromolecules or nanoparticles, can affect microglia in steady state, consequently changing the disease course [128]. So, the research opportunities reside in the elucidation of the interaction of GOs with immune cells, mainly innate immune cells of the body and the metabolism of GOs (Fig. 3). Innate immune cells take care of the GOs [119, 120], and at the same time, they will be modulated by GOs [129]. The size of GOs, as well as their propensity for carboxyl and hydroxyl residues, was reported to be their major factor of interaction with innate immune cells such as macrophages or neutrophils [130, 131]. However, the impurity of lipopolysaccharides, endotoxins in GOs is another important factor to determine their interaction with the immune system [132, 133]. Graphenes, in contrast to GOs, will make a hydrophobic mass and will be surrounded by corona proteins and will be engulfed by mononuclear phagocytic system (MPS) of the body [118]. As is well known, MPS resides not only in the liver, spleen, and bone marrow but also in the brain as microglia and in the peritoneum too.
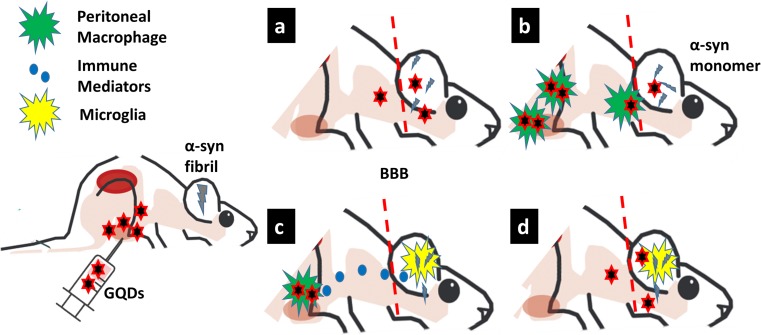
Fig. 3.
Illustration of conceptual hypothesis showing interaction of graphene quantum dots (GQD) with immune cells, mainly innate immune cells of brain and periphery, and how it may enhance the clearance of fibril. After intraperitoneal injection of GQD, by direct systemic circulation (a) or after engulfment of intraperitoneal macrophage (b), it may penetrate through blood-brain barrier and disintegrate the fibrils. However, there may be indirect activation of microglia, by intraperitoneal macrophage–induced immune mediators (c) or GQD itself (d), accelerating the fibril clearance
The intraperitoneal environment of small animals is very much influenced by the environment of the animals, whether they are in the aseptic milieu, or in a normal environment with usual microbiome. Also, intraperitoneal environments are with resident macrophages working as first-line defense against the invaders, and thus, the intraperitoneal injection of GQD in sufficient amount may change the characteristics of peritoneal macrophage and activate the innate immunity [12, 134]. This finding which heralded the novel utility of the unique drug, GQD, based on an entirely different composition, i.e., heterogeneous, from the classic drugs, which had been homogenous in composition, is expected to be reproduced in the follow-up studies. The mechanism of action of using GQDs as drugs themselves is soon to be scrutinized while paying more attention to their possible interaction with the immune system and the physiological responses of the body to the administered GQDs by thorough examination of their pharmacokinetics.
Radiolabeled GQDs are going to be a surrogate to enlighten the exact distribution after their intraperitoneal injection or even intravenous injection [135]. As is known, the corona proteins are going to wrap the intravenous GQD and will activate the immune system [136], and while the peripheral immune activation or tolerance is going to influence the brain’s immune system. Radiolabeled GQDs should represent the behavior of the unlabeled GQDs after their administration. Thus, the formulated problem to be solved in this issue is unconventional, since there are possibilities of the radiolabeled GQDs behaving more variously than the radiolabeled small molecules, which is quite familiar to the nuclear medicine investigators [137–139]. Cu-64, Ga-68, or I-125 were reported to be used as the label for GO or GQD [139–141]. GO and GQD should be tested using microfluidic chips which were made for testing permeability of BBB and double labeling of fluorescent dye and radionuclide are recommended. The effect of corona wrapping and the effect of neutrophils or myeloperoxidases can also be investigated and shall be compared with in vivo experiment results.
Determination of Disease Target for Brain Theranostics Using Transcriptomics and Epitranscriptomics
As the amyloid hypothesis or tau hypothesis for AD is in question, the development of novel drug candidates is in trouble [142, 143]. Investigators are seeking for the breakthrough, and as this endeavor cannot depend on the preset hypothesis, the investigation should lead to an exploratory research.
Fortunately, we have seen the growing interest and great progress in the field of omics in its technical and analytical arms. Technically, finally we have tools for single-cell transcriptomics for floating cells (C1 or 10X) with drop-seq technology [144–146]. Out of bulk transcriptomics, one can trace the location and their messenger RNAs (mRNAs) or long non-coding RNAs (lncRNAs) expressions, or using floating cell microfluidic technology, ignoring the location information, one can find the specific transcriptome per individual cells [147, 148]. Analytical methods using principal component analysis (PCA), multidimensional scaling (MDS), independent component analysis (ICA), t-distributed stochastic neighbor embedding (t-SNE) or topological data analysis (TDA) were introduced to summarize the transcriptomics information useful for cell clustering [149–151]. This is important that previously, the surface membrane proteins were mainly used for classifying the cells of interest, of which immune cells were classified using FACS (fluorescence-activated cell sorter) with already known clusters of differentiation (CD) markers. Now, we have full armamentarium for characterizing cells in the brain to classic cell types of neurons, astrocytes, microglia, and oligodendrocytes using transcriptomes and further to neurons of many subtypes, activated astrocytes (A1 cells), diverse subtypes of microglial cells including DAM, or monocyte-derived macrophages [126, 152, 153]. Temporal changes of radial cells, neural stem cells, and neural progenitor cells can also be characterized using transcriptome data [154]. RNA sequencing methods, a.k.a. RNA-seq, had shown exponential growth and diversification and almost replaced microarray analysis [155]. This is also the additional developments of omics technology having innovated genomic and epigenomic analysis.
Understanding of biological effects at the single-cell level, according to differential transcriptome expression, is needed to explain the human brain functional diversity [152]. This has been a conundrum for scientists as the same type of cells in other organs perform the same function, but in the brain, even the same neurotransmitter-emitting cells, such as glutamatergic or GABAergic neurons, and immune cells, such as microglia or astrocyte, perform different functions according to the location [152, 156, 157]. How these are done are not exactly understood but just assumed to be functioning as is. The mechanism is beyond our understanding until recently. This mechanism is now to be interpreted cautiously with the idea proposed by the investigators studying lncRNAs and epitranscriptomics based on single-cell RNA-seq studies [158–160]. Once brain targets, either on the cell surface membrane or intracellularly, are on hand, the method to reach these targets needs to be investigated.
Recent reports are saying that the expression of many lncRNAs is the characteristic of the brain, especially mammalian or human [161, 162]. According to the statistical analysis conducted as part of the GENCODE Project, while half of all the transcriptomes are mRNAs, thus number around 20,000 but the proteomes are 200,000, long non-coding RNA genes numbered 15,779 (version 28 released in 2017) [163, 164]. The difference between coding RNAs (mRNAs) and non-coding RNAs resided mostly in the brain as the amount of lncRNAs ranged around one thousandth, species non-conserved [163–165]. In addition, 6-N-methyl adenosine (m6A), which is the most abundant internal modifications in mRNAs and lncRNAs, has recently been recast in the scientific field [166]. Especially, m6A is known to have writer, reader, and eraser proteins and propensity of 3′-UTR (untranslated region) of the transcribed RNAs [167].
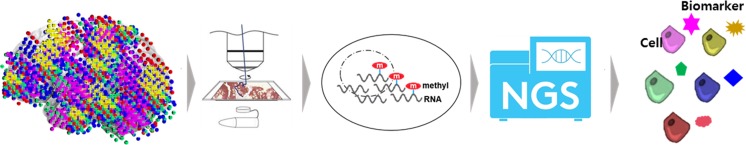
Targets of brain theranostics are going to be yielded by thorough genomic and epigenomic analysis of the big data derived from single-cell RNA-seq as was reported in the kidney recently [57] and are going to lead to the development of drug candidates for brain theranostics (Fig. 4). Once its sensitivity of detection limit improves and is adopted to spatial transcriptomics, it will open the huge possibility of determining the targets, either membrane or intracellular locations thereof [168]. This advance will call for the parallel development and advance of producing radiopharmaceutical tracers which are ambidextrous for both therapy and diagnostic imaging. Brain theranostics and radiotheranostics will be sure to depend on this progress and, at first, will be tried in the small animal models.
Fig. 4.
Determination of theranostics brain targets using transcriptomics and epitranscriptomics
Data and Analysis Aspects of Brain Theranostics and Radiotheranostics
Here, we would add two points for the success of brain theranostics and radiotheranostics. One is the availability of public database available to the appropriate qualified investigators. Once one hypothesis-testing or another hypothesis-generating investigation is completed and published in prestigious journals, the data for this investigation will become available to the public. If we set up a hypothesis and are willing to invest our time and earnestness for good scientific purpose, we can depend on these public data as the start-up trial. GEO (Genomic Expression Omnibus) or Genotype-Tissue Expression (GTEx) or Database of Genotypes and Phenotypes (dbGaP) are some of these databases. However, there are yet few transcriptome databases for brain studies. The Allen Institute has the largest database of brain transcriptomics [169, 170].
Another point is that we now have new colleagues, who are very efficient and tireless, the artificial intelligence (AI) [171–173]. Among AI, machine learning had progressed and evolved to yield deep learning. The deep-learning algorithms are mostly available in public website (https://github.com), as an example is ADAGE (Analysis using Denoising AutoEncoders for Gene Expression) in https://github.com/greenelab/adage. Once investigators develop their own data and analysis tools, they can download other investigators’ data or other algorithms. The difference of AI deep-learning algorithm from human intelligence is that humans are very good at learning fast with scanty data on very early period of learning and at missing data imputation. Clinicians or humans are good generative entity, and on trial to mimic this human quality, deep learning recently introduced the variational autoencoder (VAE) networks [174] and generative adversarial network (GAN) [175] over conventional convolutional neural networks (CNN). Humans are also good at relational reasoning and deep learning is exploring the implementation of relational CNN or neural scene networks [176, 177].
Comments about Animal Models for Brain Theranostics/ Radiotheranostics
In neurodegenerative mouse models such as 5XFAD, PS1/APP, or APP23, the precipitation of amyloid plaques are diverse, and in another model of MAPT, the tau tangles develop variably between individuals and along the timeline [178, 179]. In spontaneously hypertensive rat (SHR), the development of attention deficit behavior was also varying between individual rats [180, 181]. We needed to introduce individual imaging in these mice and rats as well as behavior tests. This inter-strain or inter-individual difference should be understood in various aspects of the drug action such as %ID/g in brain tissue, pharmacokinetics/pharmacodynamics based on classic metabolomics, or circulation time in the blood and excretion which is called ADME (absorption, distribution, metabolism and excretion). However, brain radiotheranostics will sort out, from the beginning, having a role as a discriminating factor or parameter of the novel brain theranostics drug candidates, which might be aptamers, peptides, petidomimetics, nanoparticles, or the combination of any of these. The final goal is to intervene or influence the brain pathologic processes, and this can happen after novel drugs’ crossing BBB or just influencing the peripheral bodily system such as innate immune cells.
Once in vivo physiology and immunology are understood well and thus we came to be versatile using this knowledge at our fingertips, we still need the testbed of our novel theranostics / radiotheranostics candidates for fast success. Though we are going to target the microglia or activated astrocyte A1 cells, the final object to be tamed and modulated is the neurons. The neuronal changes of genome, epigenome, and also transcriptome are to be modeled in transgenic mouse models.
Neurons are now known to produce mRNA and lncRNA and transport these materials to their dendrites, and there, they do modify the RNAs [182, 183]. This epitranscriptomics, the study of modified RNAs, are recently paid attention to by many investigators. The modified RNAs might be the final holy grail of the behavior production in animals or thought, emotion, and behavior generation in humans. This had been noted long before but the scientific community still does not have a breakthrough as molecular imaging for modified RNAs is yet beyond our reach so far.
Conclusion
Brain theranostics and radiotheranostics will open a new gateway to the nuclear medicine for the brain. Nuclear medicine for the brain will move on from its prior growth of implementing functional or abnormal protein (amyloid and tau) imaging to the therapy-incorporated disciplines. Acetazolamide brain perfusion SPECT for cerebrovascular diseases and PET and ictal SPECT for finding epileptogenic zones for epilepsy surgery had been the theranostics (therapy-guiding) nuclear medicine for brain. However, we have many more brain diseases waiting for contribution of nuclear medicine and this is now classified as neuropsychiatric diseases or neurodevelopmental diseases or the brain diseases without any abnormality in brain imaging including CT, MRI, PET, SPECT, and any other imaging modalities. Among these, nuclear medicine modalities can be the most likely discipline which can contribute to the introduction of the novel therapy to these diseases. In this manuscript, we explained why and how for this possibility by taking examples of exosome and graphene, and nevertheless, this description is neither the last nor the least. Futuristic CRIPR/Cas-based genomic editing might be introduced to treat the intractable brain diseases in any phase of the lifetime of the affected patients and then PET or SPECT might pinpoint the targets and this was not included in this perspective. The time will come to include this currently apparently imaginary discipline in brain theranostics and radiotheranostics soon.
Acknowledgements
None.
Conflict of Interest
Dong Soo Lee and Minseok Suh declare that there is no conflict of interest.
Ethical Approval
All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
As a review article, obtaining informed consent was waived.
References
- 1.Baingana F, Al'Absi M, Becker AE, Pringle B. Global research challenges and opportunities for mental health and substance-use disorders. Nature. 2015;527:S172–S177. doi: 10.1038/nature16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Depression and other common mental disorders: global health estimates. 2017.
- 4.Davidson LL, Grigorenko EL, Boivin MJ, Rapa E, Stein A. A focus on adolescence to reduce neurological, mental health and substance-use disability. Nature. 2015;527:S161–S166. doi: 10.1038/nature16030. [DOI] [PubMed] [Google Scholar]
- 5.Kim CK, Kim T, Choi IY, Soh M, Kim D, Kim YJ, et al. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl. 2012;51:11039–11043. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- 6.Bailey ZS, Nilson E, Bates JA, Oyalowo A, Hockey KS, Sajja V, et al. Cerium oxide nanoparticles improve outcome after in vitro and in vivo mild traumatic brain injury. J Neurotrauma. 2016. [DOI] [PMC free article] [PubMed]
- 7.Kang DW, Kim CK, Jeong HG, Soh M, Kim T, Choi IY, et al. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017;10:2743–2760. [Google Scholar]
- 8.Ghalamfarsa G, Hojjat-Farsangi M, Mohammadnia-Afrouzi M, Anvari E, Farhadi S, Yousefi M, et al. Application of nanomedicine for crossing the blood-brain barrier: theranostic opportunities in multiple sclerosis. J Immunotoxicol. 2016;13:603–619. doi: 10.3109/1547691X.2016.1159264. [DOI] [PubMed] [Google Scholar]
- 9.Milanesi E, Maj C, Bocchio-Chiavetto L, Maffioletti E. Nanomedicine in psychiatry: new therapeutic opportunities from research on small RNAs. Drug Dev Res. 2016;77:453–457. doi: 10.1002/ddr.21344. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HJ, Kim D, Seo K, Kim YG, Han SI, Kang T, et al. Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson’s disease. Angew Chem Int Ed Engl. 2018. [DOI] [PubMed]
- 11.Hegazy MA, Maklad HM, Samy DM, Abdelmonsif DA, El Sabaa BM, Elnozahy FY. Cerium oxide nanoparticles could ameliorate behavioral and neurochemical impairments in 6-hydroxydopamine induced Parkinson’s disease in rats. Neurochem Int. 2017;108:361–371. doi: 10.1016/j.neuint.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Yoo JM, Hwang H, Lee J, Lee SH, Yun SP, et al. Graphene quantum dots prevent alpha-synucleinopathy in Parkinson’s disease. Nat Nanotechnol. 2018. [DOI] [PMC free article] [PubMed]
- 13.Xiao S, Zhou D, Luan P, Gu B, Feng L, Fan S, et al. Graphene quantum dots conjugated neuroprotective peptide improve learning and memory capability. Biomaterials. 2016;106:98–110. doi: 10.1016/j.biomaterials.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Allen AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD. Endophenotypes in schizophrenia: a selective review. Schizophr Res. 2009;109:24–37. doi: 10.1016/j.schres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiLalla LF, McCrary M, Diaz E. A review of endophenotypes in schizophrenia and autism: the next phase for understanding genetic etiologies. Am J Med Genet C Semin Med Genet. 2017;175:354–361. doi: 10.1002/ajmg.c.31566. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PF. Schizophrenia and the dynamic genome. Genome Med. 2017;9:22. doi: 10.1186/s13073-017-0416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insel TR. Brain somatic mutations: the dark matter of psychiatric genetics? Mol Psychiatry. 2014;19:156–158. doi: 10.1038/mp.2013.168. [DOI] [PubMed] [Google Scholar]
- 18.Chung J, Wang XL, Maruyama T, Ma YY, Zhang XL, Mez J, et al. Genome-wide association study of Alzheimer’s disease endophenotypes at prediagnosis stages. Alzheimers Dement. 2018;14:623–633. doi: 10.1016/j.jalz.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, et al. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. 2017;12:5. doi: 10.1186/s13024-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 23.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehlin D, Fang XT, Cato L, Antoni G, Lannfelt L, Syvanen S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat Commun. 2016;7:10759. doi: 10.1038/ncomms10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geldenhuys WJ, Mohammad AS, Adkins CE, Lockman PR. Molecular determinants of blood-brain barrier permeation. Ther Deliv. 2015;6:961–971. doi: 10.4155/tde.15.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemere CA. Immunotherapy for Alzheimer’s disease: hoops and hurdles. Mol Neurodegener. 2013;8:36. doi: 10.1186/1750-1326-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dyck CH. Anti-amyloid-beta monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Biol Psychiatry. 2018;83:311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang S, Lee SR, Ko J, Son K, Tahk D, Ahn J, et al. A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci Rep. 2017;7. [DOI] [PMC free article] [PubMed]
- 30.Wang JD, Khafagy E, Khanafer K, Takayama S, Elsayed MEH. Organization of endothelial cells, pericytes, and astrocytes into a 3D microfluidic in vitro model of the blood-brain barrier. Mol Pharm. 2016;13:895–906. doi: 10.1021/acs.molpharmaceut.5b00805. [DOI] [PubMed] [Google Scholar]
- 31.Kim Jeong Ah, Kim Hong Nam, Im Sun-Kyoung, Chung Seok, Kang Ji Yoon, Choi Nakwon. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics. 2015;9(2):024115. doi: 10.1063/1.4917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ecker GF, Noe CR. In silico prediction models for blood-brain barrier permeation. Curr Med Chem. 2004;11:1617–1628. doi: 10.2174/0929867043365071. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JB. Machine learning methods in chemoinformatics. Wiley Interdiscip Rev Comput Mol Sci. 2014;4:468–481. doi: 10.1002/wcms.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bicker J, Alves G, Fortuna A, Falcao A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm. 2014;87:409–432. doi: 10.1016/j.ejpb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Coman H, Nemes B. New therapeutic targets in Alzheimer’s disease. Int J Gerontol. 2017;11:2–6. [Google Scholar]
- 36.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 37.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of Florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004;3:519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- 39.Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 2015;11:964–974. doi: 10.1016/j.jalz.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Hanger DP, Lau DH, Phillips EC, Bondulich MK, Guo T, Woodward BW, et al. Intracellular and extracellular roles for tau in neurodegenerative disease. J Alzheimers Dis. 2014;40(Suppl 1):S37–S45. doi: 10.3233/JAD-132054. [DOI] [PubMed] [Google Scholar]
- 41.Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brendel M, Yousefi BH, Blume T, Herz M, Focke C, Deussing M, et al. Comparison of (18)F-T807 and (18)F-THK5117 PET in a mouse model of tau pathology. Front Aging Neurosci. 2018;10:174. doi: 10.3389/fnagi.2018.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada R, Okamura N, Furumoto S, Furukawa K, Ishiki A, Tomita N, et al. 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J Nucl Med. 2016;57:208–214. doi: 10.2967/jnumed.115.164848. [DOI] [PubMed] [Google Scholar]
- 44.Okamura N, Furumoto S, Harada R, Tago T, Yoshikawa T, Fodero-Tavoletti M, et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med. 2013;54:1420–1427. doi: 10.2967/jnumed.112.117341. [DOI] [PubMed] [Google Scholar]
- 45.Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–322. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J, Ifuku M, Noda M, Guilarte TR. Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia. 2011;59:219–230. doi: 10.1002/glia.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prante O, Maschauer S, Banerjee A. Radioligands for the dopamine receptor subtypes. J Labelled Comp Radiopharm. 2013;56:130–148. doi: 10.1002/jlcr.3000. [DOI] [PubMed] [Google Scholar]
- 50.Liu GJ, Middleton RJ, Hatty CR, Kam WW, Chan R, Pham T, et al. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014;24:631–653. doi: 10.1111/bpa.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaasinen V, Vahlberg T. Striatal dopamine in Parkinson disease: a meta-analysis of imaging studies. Ann Neurol. 2017;82:873–882. doi: 10.1002/ana.25103. [DOI] [PubMed] [Google Scholar]
- 52.Tatsch K, Poepperl G. Nigrostriatal dopamine terminal imaging with dopamine transporter SPECT: an update. J Nucl Med. 2013;54:1331–1338. doi: 10.2967/jnumed.112.105379. [DOI] [PubMed] [Google Scholar]
- 53.Poulin JF, Tasic B, Hjerling-Leffler J, Trimarchi JM, Awatramani R. Disentangling neural cell diversity using single-cell transcriptomics. Nat Neurosci. 2016;19:1131–1141. doi: 10.1038/nn.4366. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science. 2018;360:176–182. doi: 10.1126/science.aam8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayer C, Hafemeister C, Bandler RC, Machold R, Batista Brito R, Jaglin X, et al. Developmental diversification of cortical inhibitory interneurons. Nature. 2018;555:457–462. doi: 10.1038/nature25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science. 2017;358:1318–1323. doi: 10.1126/science.aap8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360:758–763. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 59.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2:554–571. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee DS. Radionanomedicine. Springer Internatioenal Publishing 2018.
- 61.Lee YS, Kim YI, Lee DS. Future perspectives of radionanomedicine using the novel micelle-encapsulation method for surface modification. Nucl Med Mol Imaging. 2015;49:170–173. doi: 10.1007/s13139-015-0358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee DS, Im HJ, Lee YS. Radionanomedicine: widened perspectives of molecular theragnosis. Nanomedicine. 2015;11:795–810. doi: 10.1016/j.nano.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Sun G, Xu J, Hagooly A, Rossin R, Li Z, Moore DA, et al. Strategies for optimized radiolabeling of nanoparticles for in vivo PET imaging. Adv Mater. 2007;19:3157–3162. [Google Scholar]
- 64.Yang LK, Sundaresan G, Sun MH, Jose P, Hoffman D, McDonagh PR, et al. Intrinsically radiolabeled multifunctional cerium oxide nanoparticles for in vivo studies. J Mater Chem B. 2013;1:1421–1431. doi: 10.1039/c2tb00404f. [DOI] [PubMed] [Google Scholar]
- 65.Lee DS, Suh M, Lee YS. Radiolabeling method: core/surface labeling, chemical and physical labeling. Radionanomedicine. Springer International Publishing; 2018.
- 66.Chen Daiqin, Hong Hao. Radionanomedicine. Cham: Springer International Publishing; 2018. Surface Modification of Radionanomedicine; pp. 185–207. [Google Scholar]
- 67.Lee YK, Jeong JM, Hoigebazar L, Yang BY, Lee YS, Lee BC, et al. Nanoparticles modified by encapsulation of ligands with a long alkyl chain to affect multispecific and multimodal imaging. J Nucl Med. 2012;53:1462–1470. doi: 10.2967/jnumed.111.092759. [DOI] [PubMed] [Google Scholar]
- 68.van Dongen GA, Visser GW, Lub-de Hooge MN, de Vries EG, Perk LR. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist. 2007;12:1379–1389. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- 69.Rijpkema M, Boerman OC, Oyen WJG. Tumor targeting using radiolabeled antibodies for image-guided drug delivery. Curr Drug Targets. 2015;16:625–633. doi: 10.2174/1389450115666141029234200. [DOI] [PubMed] [Google Scholar]
- 70.Perkins AC, Frier M. Radionuclide imaging in drug development. Curr Pharm Des. 2004;10:2907–2921. doi: 10.2174/1381612043383476. [DOI] [PubMed] [Google Scholar]
- 71.Varnas K, Varrone A, Farde L. Modeling of PET data in CNS drug discovery and development. J Pharmacokinet Pharmacodyn. 2013;40:267–279. doi: 10.1007/s10928-013-9320-6. [DOI] [PubMed] [Google Scholar]
- 72.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higuchi M, Maeda J, Ji B, Maruyama M, Okauchi T, Tokunaga M, et al. In-vivo visualization of key molecular processes involved in Alzheimer’s disease pathogenesis: insights from neuroimaging research in humans and rodent models. Biochim Biophys Acta. 1802;2010:373–388. doi: 10.1016/j.bbadis.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Shi SJ. Biologics: an update and challenge of their pharmacokinetics. Curr Drug Metab. 2014;15:271–290. doi: 10.2174/138920021503140412212905. [DOI] [PubMed] [Google Scholar]
- 75.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Iyer S, Fielder PJ, Davis JD, Deng R. Projecting human pharmacokinetics of monoclonal antibodies from nonclinical data: comparative evaluation of prediction approaches in early drug development. Biopharm Drug Dispos. 2016;37:51–65. doi: 10.1002/bdd.1952. [DOI] [PubMed] [Google Scholar]
- 77.Mager DE, Woo S, Jusko WJ. Scaling pharmacodynamics from in vitro and preclinical animal studies to humans. Drug Metab Pharmacokinet. 2009;24:16–24. doi: 10.2133/dmpk.24.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaham S. Glia-neuron interactions in nervous system function and development. Curr Top Dev Biol. 2005;69:39–66. doi: 10.1016/S0070-2153(05)69003-5. [DOI] [PubMed] [Google Scholar]
- 79.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 80.Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trotta T, Panaro MA, Cianciulli A, Mori G, Di Benedetto A, Porro C. Microglia-derived extracellular vesicles in Alzheimer's disease: a double-edged sword. Biochem Pharmacol. 2018;148:184–192. doi: 10.1016/j.bcp.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 82.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao TT, Zhang WW, Jiao B, Pan CZ, Liu XX, Shen L. The role of exosomes in the pathogenesis of Alzheimer’ disease. Transl Neurodegener. 2017;6.
- 84.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarko DK, McKinney CE. Exosomes: origins and therapeutic potential for neurodegenerative disease. Front Neurosci. 2017;11:82. doi: 10.3389/fnins.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neuwelt E, Abbott N, Abrey L, Banks WA, Blakley B, Davis T, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 87.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 88.Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 89.Shin Y, Yang K, Han S, Park HJ, Seok Heo Y, Cho SW, et al. Reconstituting vascular microenvironment of neural stem cell niche in three-dimensional extracellular matrix. Adv Healthc Mater. 2014;3:1457–1464. doi: 10.1002/adhm.201300569. [DOI] [PubMed] [Google Scholar]
- 90.Han S, Yang K, Shin Y, Lee JS, Kamm RD, Chung S, et al. Three-dimensional extracellular matrix-mediated neural stem cell differentiation in a microfluidic device. Lab Chip. 2012;12:2305–2308. doi: 10.1039/c2lc21285d. [DOI] [PubMed] [Google Scholar]
- 91.Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc. 2012;7:1247–1259. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oh HJ, Shin Y, Chung S, Hwang DW, Lee DS. Convective exosome-tracing microfluidics for analysis of cell-non-autonomous neurogenesis. Biomaterials. 2017;112:82–94. doi: 10.1016/j.biomaterials.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 93.Lee C, Hwang DW, Gho YS. Endogenous radionanomedicine: extracellular vesicles. Radionanomedicine. Springer International Publishing 2018.
- 94.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tkach M, Kowal J, Thery C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond Ser B Biol Sci. 2018;372. [DOI] [PMC free article] [PubMed]
- 98.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 99.Zeng F, Morelli AE. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin Immunopathol. 2018. [DOI] [PMC free article] [PubMed]
- 100.Synowsky SA, Shirran SL, Cooke FGM, Antoniou AN, Botting CH, Powis SJ. The major histocompatibility complex class I immunopeptidome of extracellular vesicles. J Biol Chem. 2017;292:17084–17092. doi: 10.1074/jbc.M117.805895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morishita M, Takahashi Y, Nishikawa M, Takakura Y. Pharmacokinetics of exosomes-an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J Pharm Sci. 2017;106:2265–2269. doi: 10.1016/j.xphs.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 102.Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6. [DOI] [PMC free article] [PubMed]
- 103.Choi H, Lee DS. Illuminating the physiology of extracellular vesicles. Stem Cell Res Ther. 2016;7:55. doi: 10.1186/s13287-016-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci Rep. 2015;5:15636. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 106.Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 107.Nichols JW, Bae YH. Odyssey of a cancer nanoparticle: from injection site to site of action. Nano Today. 2012;7:606–618. doi: 10.1016/j.nantod.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 109.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 110.Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 111.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Yang BY, Moon SH, Seelam SR, Jeon MJ, Lee YS, Lee DS, et al. Development of a multimodal imaging probe by encapsulating iron oxide nanoparticles with functionalized amphiphiles for lymph node imaging. Nanomedicine (Lond). 2015;10:1899–1910. doi: 10.2217/nnm.15.41. [DOI] [PubMed] [Google Scholar]
- 114.Chen F, Bu W, Cai W, Shi J. Functionalized upconversion nanoparticles: versatile nanoplatforms for translational research. Curr Mol Med. 2013;13:1613–1632. doi: 10.2174/1566524013666131111122133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seo HJ, Nam SH, Im HJ, Park JY, Lee JY, Yoo B, et al. Rapid hepatobiliary excretion of micelle-encapsulated/radiolabeled upconverting nanoparticles as an integrated form. Sci Rep. 2015;5. [DOI] [PMC free article] [PubMed]
- 116.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 117.Liu JQ, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9:9243–9257. doi: 10.1016/j.actbio.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 118.Yoo JM, Hwang DW, Hong BH. Graphene-based nanomaterials. Radionanomedicine: Springer International Publishing; 2018. [Google Scholar]
- 119.Mukherjee SP, Bottini M, Fadeel B. Graphene and the immune system: a romance of many dimensions. Front Immunol. 2017;8:673. doi: 10.3389/fimmu.2017.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mukherjee SP, Gliga AR, Lazzaretto B, Brandner B, Fielden M, Vogt C, et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale. 2018;10:1180–1188. doi: 10.1039/c7nr03552g. [DOI] [PubMed] [Google Scholar]
- 121.Sun HJ, Wu L, Wei WL, Qu XG. Recent advances in graphene quantum dots for sensing. Mater Today. 2013;16:433–442. [Google Scholar]
- 122.Iannazzo D, Pistone A, Salamo M, Galvagno S, Romeo R, Giofre SV, et al. Graphene quantum dots for cancer targeted drug delivery. Int J Pharm. 2017;518:185–192. doi: 10.1016/j.ijpharm.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 123.Zheng XT, Ananthanarayanan A, Luo KQ, Chen P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small. 2015;11:1620–1636. doi: 10.1002/smll.201402648. [DOI] [PubMed] [Google Scholar]
- 124.Liu YB, Xu LP, Dai WH, Dong HF, Wen YQ, Zhang XJ. Graphene quantum dots for the inhibition of beta amyloid aggregation. Nanoscale. 2015;7:19060–19065. doi: 10.1039/c5nr06282a. [DOI] [PubMed] [Google Scholar]
- 125.Kim HY, Kim HV, Jo S, Lee CJ, Choi SY, Kim DJ, et al. EPPS rescues hippocampus-dependent cognitive deficits in APP/PS1 mice by disaggregation of amyloid-beta oligomers and plaques. Nat Commun. 2015;6:8997. doi: 10.1038/ncomms9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–90.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 127.Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173:1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 128.Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556:332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen GY, Yang HJ, Lu CH, Chao YC, Hwang SM, Chen CL, et al. Simultaneous induction of autophagy and toll-like receptor signaling pathways by graphene oxide. Biomaterials. 2012;33:6559–6569. doi: 10.1016/j.biomaterials.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 130.Ma J, Liu R, Wang X, Liu Q, Chen Y, Valle RP, et al. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano. 2015;9:10498–10515. doi: 10.1021/acsnano.5b04751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou HJ, Zhao K, Li W, Yang N, Liu Y, Chen CY, et al. The interactions between pristine graphene and macrophages and the production of cytokines/chemokines via TLR- and NF-kappa B-related signaling pathways. Biomaterials. 2012;33:6933–6942. doi: 10.1016/j.biomaterials.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 132.Li Y, Boraschi D. Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomedicine (Lond). 2016;11:269–287. doi: 10.2217/nnm.15.196. [DOI] [PubMed] [Google Scholar]
- 133.Mukherjee Sourav P., Lozano Neus, Kucki Melanie, Del Rio-Castillo Antonio E., Newman Leon, Vázquez Ester, Kostarelos Kostas, Wick Peter, Fadeel Bengt. Detection of Endotoxin Contamination of Graphene Based Materials Using the TNF-α Expression Test and Guidelines for Endotoxin-Free Graphene Oxide Production. PLOS ONE. 2016;11(11):e0166816. doi: 10.1371/journal.pone.0166816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diez-Orejas R, Feito MJ, Cicuendez M, Rojo JM, Portoles MT. Differential effects of graphene oxide nanosheets on Candida albicans phagocytosis by murine peritoneal macrophages. J Colloid Interface Sci. 2018;512:665–673. doi: 10.1016/j.jcis.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 135.Hwang DW. Radio-graphene in theranostic perspectives. Nucl Med Mol Imaging. 2017;51:17–21. doi: 10.1007/s13139-016-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee DS, Shin YK. Innate immunity to nanomaterials. Radionanomedicine. Springer International Publishing 2018.
- 137.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1.
- 138.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 139.Shi S, Xu C, Yang K, Goel S, Valdovinos HF, Luo H, et al. Chelator-free radiolabeling of nanographene: breaking the stereotype of chelation. Angew Chem Int Ed Engl. 2017;56:2889–2892. doi: 10.1002/anie.201610649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hong H, Zhang Y, Engle JW, Nayak TR, Theuer CP, Nickles RJ, et al. In vivo targeting and positron emission tomography imaging of tumor vasculature with (66)Ga-labeled nano-graphene. Biomaterials. 2012;33:4147–4156. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang K, Feng L, Hong H, Cai W, Liu Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat Protoc. 2013;8:2392–2403. doi: 10.1038/nprot.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du XG, Wang XY, Geng MY. Alzheimer’s disease hypothesis and related therapies. Transl Neurodegener. 2018;7. [DOI] [PMC free article] [PubMed]
- 143.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 144.Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161:1187–1201. doi: 10.1016/j.cell.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Petukhov V, Guo J, Baryawno N, Severe N, Scadden DT, Samsonova MG, et al. DropEst: pipeline for accurate estimation of molecular counts in droplet-based single-cell RNA-seq experiments. Genome Biol. 2018;19:78. doi: 10.1186/s13059-018-1449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356. [DOI] [PMC free article] [PubMed]
- 148.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S. et al., Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018. [DOI] [PMC free article] [PubMed]
- 149.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rizvi AH, Camara PG, Kandror EK, Roberts TJ, Schieren I, Maniatis T, et al. Single-cell topological RNA-seq analysis reveals insights into cellular differentiation and development. Nat Biotechnol. 2017;35:551–560. doi: 10.1038/nbt.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 154.Dulken BW, Leeman DS, Boutet SC, Hebestreit K, Brunet A. Single-cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Rep. 2017;18:777–790. doi: 10.1016/j.celrep.2016.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 156.Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep. 2018;22:832–847. doi: 10.1016/j.celrep.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 157.Ben Haim L, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017;18:31–41. doi: 10.1038/nrn.2016.159. [DOI] [PubMed] [Google Scholar]
- 158.Kim DH, Marinov GK, Pepke S, Singer ZS, He P, Williams B, et al. Single-cell transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. Cell Stem Cell. 2015;16:88–101. doi: 10.1016/j.stem.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mattick JS. The central role of RNA in human development and cognition. FEBS Lett. 2011;585:1600–1616. doi: 10.1016/j.febslet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 163.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.GENCODE, v28. 2017. https://www.gencodegenes.org/stats/current.html.
- 165.Qureshi IA, Mattick JS, Mehler MF. Long non-coding RNAs in nervous system function and disease. Brain Res. 2010;1338:20–35. doi: 10.1016/j.brainres.2010.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu N, Pan T. RNA epigenetics. Transl Res. 2015;165:28–35. doi: 10.1016/j.trsl.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu N, Pan T. N6-methyladenosine-encoded epitranscriptomics. Nat Struct Mol Biol. 2016;23:98–102. doi: 10.1038/nsmb.3162. [DOI] [PubMed] [Google Scholar]
- 168.Lein E, Borm LE, Linnarsson S. The promise of spatial transcriptomics for neuroscience in the era of molecular cell typing. Science. 2017;358:64–69. doi: 10.1126/science.aan6827. [DOI] [PubMed] [Google Scholar]
- 169.ABI. Allen brain institute cell types webpage. 2017. http://celltypes.brain-map.org. [DOI] [PMC free article] [PubMed]
- 170.Grillner S, Ip N, Koch C, Koroshetz W, Okano H, Polachek M, et al. Worldwide initiatives to advance brain research. Nat Neurosci. 2016;19:1118–1122. doi: 10.1038/nn.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88. doi: 10.1016/j.media.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 172.Min S, Lee B, Yoon S. Deep learning in bioinformatics. Brief Bioinform. 2017;18:851–869. doi: 10.1093/bib/bbw068. [DOI] [PubMed] [Google Scholar]
- 173.Wang H, Raj B. On the origin of deep learning. arXiv preprint arXiv:170207800. 2017.
- 174.Kingma DP, Welling M. Auto-encoding variational bayes. arXiv preprint arXiv:13126114. 2013.
- 175.Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S et al., editors. Generative adversarial nets. Adv Neural Inf Process Syst. 2014.
- 176.Santoro A, Raposo D, Barrett DG, Malinowski M, Pascanu R, Battaglia P et al., editors. A simple neural network module for relational reasoning. Adv Neural Inf Process Syst. 2017.
- 177.Eslami SMA, Jimenez Rezende D, Besse F, Viola F, Morcos AS, Garnelo M, et al. Neural scene representation and rendering. Science. 2018;360:1204–1210. doi: 10.1126/science.aar6170. [DOI] [PubMed] [Google Scholar]
- 178.Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer’s disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 180.van den Bergh FS, Bloemarts E, Chan JS, Groenink L, Olivier B, Oosting RS. Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol Biochem Behav. 2006;83:380–390. doi: 10.1016/j.pbb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 181.Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Gallegos-Cari A, Castillo C. Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Rev Neurosci. 2011;22:365–371. doi: 10.1515/RNS.2011.024. [DOI] [PubMed] [Google Scholar]
- 182.Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron. 2015;88:861–877. doi: 10.1016/j.neuron.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 183.Sambandan S, Akbalik G, Kochen L, Rinne J, Kahlstatt J, Glock C, et al. Activity-dependent spatially localized miRNA maturation in neuronal dendrites. Science. 2017;355:634–637. doi: 10.1126/science.aaf8995. [DOI] [PubMed] [Google Scholar]