Abstract
Dementia is known as loss of cellular communications in the brain at a region caused by multi-factorial diseases and pathogenic infections. Approximately eighty percent reported cases of Alzheimer’s disease are followed by vascular dementia. The common symptoms of dementia include memory loss, concentration problems, thinking, and language solving situations. Dementia is a multifactorial disease but based on latest research; various reports have been published describing the linkage and role of viruses, prions and miRNAs in neurodegeneration and neurodegenerative disorders resulting into dementia and due to this we selected to review and provide latest information related to dementia. MiRNAs are small non-coding RNAs carrying genetic regulatory information contributing to neurological disorders among human and animals. A prion is an infectious agent made of protein material. Recently, it has been reported that prions play a significant role in signaling processes, resulting in amyloidogenesis and neurological disorders. Viruses attack human immune system and central nervous system and affect classical pathways of neurodegenerative diseases. Comprehensive understandings of the expression profiles and activities of these miRNAs, Prions, Viruses will illuminate their roles as potential therapeutic targets in neurodegeneration and may lead to the discovery of breakthrough treatment strategies for neurodegenerative disorders and dementia. The provided information will further be significant not only in neuro-scientific research, but also in designing and development of management strategies for dementia.
Keywords: Viruses, Prions, miRNAs, Neurodegeneration, Dementia
Introduction
Dementia is not a specific disease, but it is a general and collective term used for loss of memory caused by physical changes in the brain and other mental abilities which interferes the daily life of old age people. Dementia is a symptom of several disorders in the brain. Dementias can be caused by brain cell death, and neurodegenerative disease-progressive brain cell death that happens over time is associated with most dementias. Additionally, dementia is also caused by Pathogenic infections like Prions, virus infection like HIV, and reversible factors. Currently, it has been a serious challenge and approximately 47.5 million people suffer with dementia globally. There is no specific therapy available to cure dementia. Dementia has been divided into four stages such as mild cognitive impairment, mild dementia, moderate dementia, and severe dementia. In dementia, various symptoms appear such as loss and decline in memory with thinking ability as well as loss of skills to perform basic daily activities. Globally, 60–80% of reported Alzheimer’s disease (AD) cases are followed by stroke-induced vascular dementia and known as other most common dementia Alzheimer’s disease is the most common type of dementia but there are many other types of dementia such as; dementia with lewy bodies, Cruetzfeldt–Jakob disease (CJD), Down’s syndrome, frontotemporal dementia (FTD), Parkinson’s disease (PD), Hungtinton’s disease (HD), posterior cortical atrophy, vascular dementia, Amyotrophic lateral sclerosis (ALS), Korsakoff syndrome, Traumatic brain injury, and mixed dementia. Many risk factors are involved leading to dementia such as age, family history, Down’s syndrome, and mild cognitive impairment. In some cases, dementia or dementia like symptoms can be reversed by many factors such as infections and immune disorders, metabolic problems and endocrine abnormalities, nutritional deficiencies, reactions to medications, subdural hematomas, poisoning, brain tumors, anoxia, and normal-pressure hydrocephalus. Dementia leads to many complications like; inadequate nutrition, pneumonia, inability to perform self-care tasks, personal safety challenges and finally death. In addition to these pathological hallmarks and diagnostic lesions, the AD brain is typified by impaired synaptic function, neuro-inflammation, and neuronal loss, which ultimately contribute to the full expression of dementia. Additionally, FTD is also known as a serious neurodegenerative disease of human personality and language and severe atrophy of the frontal and temporal brain lobes. Many other conditions like thyroid problems and vitamin deficiencies are also known to cause dementia symptoms but it can be cured with the passage of time [49]. Pathogenic infections like viruses are known to cause neurodegenerative disorders [55]. Currently, many viruses are also well known to play a significant role in neurodegeneration and neurological disorders disease progression and finally lead to dementia. Viruses are known to have a direct or indirect role in the development of neurological disorders. A viral infection spreads systemically and enters into the immune system as well as another organ. They can severely affect neurons and CNS resulting in development of neurological disorders. There are many viruses reported to be associated with neurological disorders and they are known as Borna Disease Virus (BDV), Cytomegalovirus (CMV), Enterovirus, H5N1, Hepatitis virus, HIV, Herpes Simplex Virus (HSV), Influenza Virus, Picornavirus and West Nile Virus (WNV) [55].
Additionally, prions are also known to cause disease like Creutzfeldt–Jakob disease and leads to dementia. A prion is known as an infectious proteinaceous agent which transfers biological information in both healthy and diseased cells and causes disease like a virus infection. In 1982, the term prion was given by Nobel prize winner Dr. Stanley Prusiner [88]. The prion contains a single protein known as PrP. This protein folded into two different conformations, one is known as normal protein PrP = PrPc and the other is known as diseased protein (PrP = PrPsc − scrapie) and cause deadly neurodegenerative disease in both human and animals [9]. Prion disease is a collective condition affecting the CNS and develops neurodegenerative diseases (NDD) in both humans and animals. Recently, prion has been defined as “proteinaceous nucleating particles” [39, 65, 108, 109]. Generally, a neurodegenerative disease is age dependent and contains filamentous inclusions as major component known as amyloid-β (Aβ), tau and a-synuclein [39, 108]. Based on many published reports and evidence; it is well known that prion and prion-like disease arise from changes in the normal protein conformation into infectious and self-propagating stages and contributes significantly to the emergence of neurodegenerative disease in both human and animals [89, 90]. The role and association of prion in humans and animals NDD are reported in many published papers. The human diseases associated with prion are known as Creutzfeldt–Jakob disease (CJD), Gerstmann–Straussler–Scheinker syndrome (GSS), Kuru, Fatal Familial Insomnia (FFI), FTD, AD, ALS and HD and animal disease are known as Scrapie, Bovine Spongiform Encephalopathy (BSE) and Chronic wasting disease (CWD) [6, 39, 40].
Apart from viruses and prions, microRNAs are also known to be associated with neurological disorders [22, 71]. miRNAs are ~ 18–25 nucleotides (nt) long and have essential information for gene regulation and expression in both animals and plants [48]. The existence of miRNA was reported 14 years back, during the study of larval development in the nematode Caenorhabditis Elegans. This small non-coding (nc) RNA was discovered by Ambrosa and Tuschl. The involvement of miRNAs in neurological disorders has recently been reported [22, 71]. Recently, some miRNAs have been identified and isolated from CSF and blood serum and provided valuable information about AD diagnosis, and it was further observed that there is no general consensus for their expression pattern, either by up-regulation or down-regulation and provided major challenges in the profiling of miRNAs in the CNS disorders [5, 19, 73]. Approximately 2550 novel miRNAs have been isolated and characterized from humans, but only highly selective miRNA population appears to be differentially expressed in CNS disorders [5, 73]. The miRNAs are well known to play an important role in gene expression, controlling of aging and disease development but they are not highly selective for their nucleotides sequences arrangement and their abundance and specificity in the tissue and they vary in different population [73]. Molecular biology-based techniques like direct RNA sequencing, RT-PCR, Northern Blot, miRNAs-based microarray helped in the identification of several miRNAs in CNS diseases and currently, around 45 miRNAs altered in the AD have been detected [20, 21, 47, 115]. It has been only about 8 years since the first reports of altered miRNA abundance; speciation and complexity in the human CNS in aging brain and in AD have emerged. Some dominantly inherited cases of AD are caused by mutations in the gene encoding the amyloid precursor protein (APP), the cleavage of which gives rise to Ab. In these cases, dysfunction of APP precedes dysfunction of tau. In contrast, mutations in MAPT, the tau gene, give rise to dominantly inherited frontotemporal dementia and Parkinsonism, with abundant tau inclusions in the absence of Ab plaques. Recent findings have suggested instead that non-cell-autonomous processes play an important part in AD and PD. Inclusions are thought to form in a small number of cells and-given enough time and, perhaps, a genetic predisposition-spread in a deterministic manner to distant brain regions. The prion concept appears to apply to all human neurodegenerative diseases with abnormal protein assemblies, including AD and PD. This has brought unity to the field and changed the way we think about these diseases. It has been known for some time that a seed can template aggregation of the homologous protein [39]. In this review we have provided the latest information about the role of viruses, prions and miRNAs in neurodegeneration and neurodegenerative disorders leading to dementia. Dementia is a widespread disease. The role and linkage of viruses, prions and microRNAs in neurodegenerative disorders leading to dementia are reported well in many published papers from various parts of the world. Currently, the detailed understanding of disease mechanisms, an early disease diagnosis, development of biomarkers and an effective disease treatment is an urgently need.
Types of dementia
Currently, there are many types of dementia has been reported.
A: Alzheimer’s disease (AD): AD is the most common form of dementia. It is hallmarked by the loss of neurons in the cerebral cortex and sub-cortical regions and the formation of neurofibrillary tangles and plaques in brain.
B: Dementia with Lewy bodies: This is a neurodegenerative condition linked to abnormal structures in the brain. The brain changes involve a protein called alpha-synuclein.
C: Mixed dementia: This refers to a diagnosis of two or three types occurring together. For instance, a person may show both Alzheimer’s disease and vascular dementia at the same time.
D: Parkinson’s disease (PD): PD is a neurodegenerative disorder that is characterized by muscular rigidity, resting tremor, akinesia, depression, dementia, olfactory and sleeps disturbances.
E: Huntington’s disease (HD): HD is characterized by specific types of uncontrolled movements but also includes dementia.
Additionally, other disorders leading to symptoms of dementia include: Frontotemporal dementia also known as Pick’s disease. Normal pressure hydrocephalus when excess cerebrospinal fluid accumulates in the brain. Posterior cortical atrophy resembles changes seen in Alzheimer’s disease but in a different part of the brain. Down syndrome increases the likelihood of young-onset Alzheimer’s.
Etiologies of dementia
Many factors are known to be associated with dementia. The most important is the progressive brain cell death and neurodegenerative disease, head injury, a stroke, or a brain tumor, among other causes such as cerebrovascular disease prevention of normal blood flow and less oxygen supply to the brain cells. Additionally, an injury leads to brain cell death resulting into post-traumatic dementia. Traumatic brain injury received by sports player also leads to certain dementias appearing later in life. Additionally, dementia is also caused by pathogenic infections like virus infection, Prion disease, and some other factors like medication interactions, depression, vitamin deficiencies, and thyroid abnormalities.
Role of viruses in neurodegenerative disorders and dementia
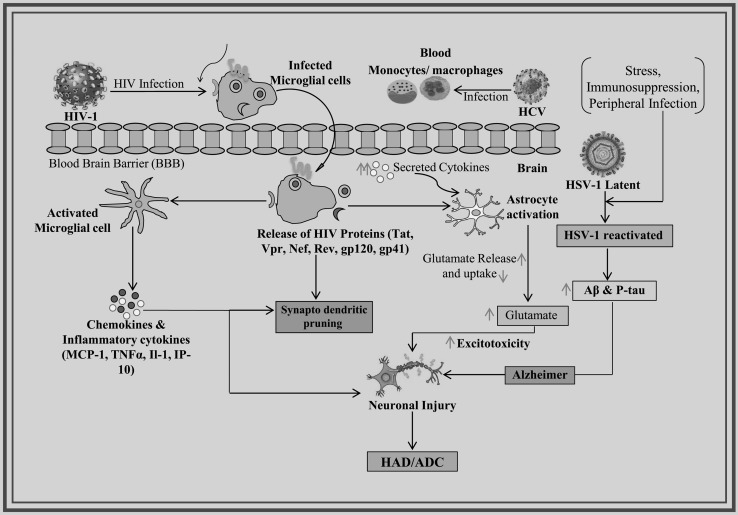
Viruses are known to infect and play a significant role in neurological disorders. The role and linkage of multiple viruses in neurological disorders have been reported and the most important viruses are known as HIV, HSV, Hepatitis virus, ZIKV and Cytomegalovirus (CMV) [2, 3]. The updated information about the most studied viruses like HIV, HSV and Hepatitis C Virus and the role of other viruses in dementia are discussed (Fig. 1). Recently, the evidence for the role of viruses as environmental risk factors and supporting a link between viral infection and motor neuron disease for ALS has been published from a new perspective. Viruses have received longstanding attention as potential ALS triggers. But there is a need for multidisciplinary approaches bridging neurology and infectious diseases research to move the field forward in the future [16]. The role and association of CMV, HSV-1 and Epstein–Barr virus with cognitive functioning and risk of AD and dementia in the general population has been recently reviewed and reported [69, 104].
Fig. 1.
Virus infection leads to development of neurodegeneration and dementia
Human immunodeficiency virus (HIV)
HIV infection has been described well and has the significant role in NDD and the development of dementia, known as HIV-associated dementia (HAD). HAD is a sub-cortical dementia insidious beginning. In the early stage of disease, neurologic symptoms including cognitive slowing, forgetfulness, and concentration difficulties, are mild. Gait disturbance is the most common motor symptom, but the problem with hand coordination is also common, as described by impaired handwriting or typing skills. HAD also known as AIDS dementia Complex (ADC) and HIV-Encephalopathy (HIV-E), identified by neuronal degradation, results in progressive cognitive and motor impairments that might lead to a vegetative/mute condition. The manifestations of neurocognitive impairment vary from asymptomatic impairment to dementia. The HIV infection leads to cognitive impairment [86]. It is observed that virus does not enter directly into the CNS. Blood Brain Barrier (BBB) strictly regulates the HIV-1 molecules entry into the CNS. Monocytes help HIV-1 virus to travel into CNS. They have the freedom to cross the BBB and strive in the brain leading to neurodegeneration and HAD [42].
Surprisingly, virus affects Perivascular macrophages (PM) and microglial cells and replicates and produces viral proteins (neurotoxic or infectious proteins). The neurotoxic viral proteins like Tat, Vpr, Nef, Rev, gp120 and gp41 trigger the activation of astrocyte, which results in decreased glutamate uptake and increased glutamate release. This elevated glutamate level causes neuronal bio-energetic disturbances, which results into abnormal synapto-dendritic pruning and leads to neuronal injury. In addition, systemic inflammation and translocation of microbial products lead to activation of microglial and increased chemokines and cytokines production, contributing to neuronal injury [97]. HIV-associated neurocognitive disorder (HAND) is recorded in 30–50% HIV infected individuals [106]. HAD is the most severe indicator of HAND with advanced HIV disease and low CD4 cell counts [7]. Neuronal injury mechanism in HAND might be caused by HIV-1 secreted neurotoxic viral proteins such as Tat, gp120, and Nef that may lead to the activation of pathways like neuro-inflammatory, promote excitotoxicity, block autophagy, oxidative stress, dysregulation of signaling and mitochondrial dysfunction pathways [30]. HAND is further categorized into 3 sub-disorders: (1) ANI (Asymptomatic neurocognitive impairment): an anomaly in 2 or more cognitive abilities with no functional impairment; (2) MND (Mild neurocognitive disorder): a cognitive injury with mild functional impairment; and (3) HAD: a manifest cognitive injury with manifest functional impairment [28]. It was illustrated that Nef protein and nef mRNA are packaged into exosomes that persist in circulation in patients with HIV-HAD. The expression of Nef in target cells may be induced by mRNA and later increase expression and secretion of Aβ and Aβ peptides. An elevated level of the amyloid peptide could lead to cognitive impairment noticed in HAND [57]. The individuals infected with HIV and mild cognitive impairment are more susceptible to dementia and death [44]. The induction of latency and alteration of steamness in brain NPC occurs by HIV-1 infection in multipotent human neural precursor cells (hNPCs), resulting into changed endogenous neuro-restoration of the CNS and compounds the severity of dementia in adult neuro AIDS cases. In a recent study, it was suggested that a novel molecular cascade involving miR-155 and TRIM32 leading to HIV-1 Tat-induced attenuated proliferation of hNPCs [29].
Herpes simplex virus (HSV)
HSV-1 is a neurotropic dsDNA virus that generally affects oral and nasal epithelial cells where lytic replication of virus takes place, and thus produces new viral particles. This virus infects sensory neurons and moves towards the trigeminal ganglion by axonal transport. In this trigeminal ganglion, virus particles start a latent infection. In this way, the virus moves to CNS and causes serious neurological disorders. It has been observed that virus exists in the latent phase in numerous elderly brains but reactivates sporadically, such as in the peripheral nervous system, under particular conditions, for instance, stress, immuno-suppression, and peripheral infection, causing collective damage and ultimately the development of AD [52, 68, 85]. (Fig. 1).
Hepatitis C virus (HCV)
HCV is a ssRNA virus that causes serious disease. Even though it is a hepatotropic virus, viral RNA has also been identified in PBMC brain samples with neuro-pathological abnormalities. Evidence of HCV neuro-invasion is now accumulating [31, 32]. HCV and HIV co-infected patients with a history of prohibited drug use are rapidly increasing. Though, only a few investigations have analyzed the entry of HCV into the CNS and its clinical and neuro-pathological impacts on HIV-infected individuals. For this objective, the distribution of HCV was investigated in the HIV-infected individual brain. The presence of viral RNA detected in CNS by nested PCR was linked with a history of methamphetamine use, substantial ante mortem cognitive impairment and abundant astrogliosis, and less-acute HIV encephalitis [66]. HCV infection also develops chronic inflammation and results in neuropsychological symptoms accompanied by cognitive impairment in AD individuals. Monocytes/macrophages primarily infected by HCV, cross the BBB and subsequently increase the secretion levels of cytokine which causes the excitoxicity in the central nervous system. HCV infections in the brain tend to increase the risk for AD development. Moreover, viremia and HCV infection are correlated with microglial activation and altered cerebral metabolism. HCV-infected elderly patients are stated to have a higher risk of AD, whereas those who have received antiviral therapy have a lower risk for the AD development [18]. Recently the contribution of Enteroviruses (EVs), including poliovirus, coxsackie virus, echovirus, enterovirus-A71 and, enterovirus-D68, to the development of ALS has been suspected as they can target motor neurons, and patients with prior poliomyelitis show a higher risk of motor neuron disease. In a recently published review, the nature of enteroviral infection, including route of infection, cells targeted, and viral persistence within the central nervous system (CNS) and their molecular mechanism of viral pathogenesis and the molecular and pathological features of ALS and the potential role of enteroviral infection in FTD has been provided [113].
Role of prions in neurodegenerative disorders
The role of prions has been shown in neurodegenerative diseases like AD, PD, HD, ALS and other NDD both in human and animals. Neurodegenerative disorders can be developed with the aggregation of misfolded and aberrant proteins in the CNS and these proteins share prion-like mechanism in the development of NDD [6, 35, 75]. Currently, a strong and dominant hypothesis showed that gain and loss of function is required in prion disease development. Gain-of-function is necessary for disease initiation and spread, but loss-of-function also contributes significantly to the development of NDD [65]. The list of prion diseases in both humans and animals is provided in Table 1. Currently, several studies have indicated that certain misfolded amyloids composed of tau, β-amyloid or α-synuclein can be transferred from cell to cell, suggesting the contribution of mechanisms reminiscent of those by which infective prions spread through the brain. The blocking of a ‘prion-like proteinaceous aggregates spreading in brain cells could be a novel putative therapeutic target towards the management of neurodegenerative diseases. The current knowledge about PrPC as a putative receptor for amyloid proteins and the physiological consequences of these interactions has been reviewed [27].
Table 1.
Types of Prion disease in both human and animals
| Types of disease | Types of host | Pathogenesis |
|---|---|---|
| Kuru | Humans | Infection through ritualistic cannibalism |
| Iatrogenic Creutzfeldt–Jakob disease | Humans | Infection from prion-contaminated HGH, medical equipment, etc. |
| Variant Creutzfeldt–Jakob disease | Humans | Infection from bovine prions |
| Familial Creutzfeldt–Jakob disease | Humans | Germline mutations in the PRNP gene |
| Gerstmann–Sträussler–Scheinker syndrome | Humans | Germline mutations in the PRNP gene |
| Fatal familial insomnia | Humans | Germline mutations in the PRNP gene |
| Sporadic Creutzfeldt–Jakob disease | Humans | Somatic mutation or spontaneous conversion of PrPc to PrPsc |
| Sporadic Fatal insomnia | Humans | Somatic mutation or spontaneous conversion of PrPc to PrPsc |
| Scrapie | Sheep | Infection |
| Bovine spongiform encephalopathy | Cattle | Infection or sporadic |
| Transmissible mink encephalopathy | Mink | Infection with prions from sheep or cattle |
| Chronic wasting disease | Deer, elk | Infection |
| Feline spongiform encephalopathy | Cats | Infection with prion-contaminated bovine tissues or MBM |
| Exotic ungulate encephalopathy | Greater kudu, nyala, oryx | Infection with prion-contaminated MBM |
The prion and prion protein (PrPc)
A prion is a proteinaceous infectious agent which causes neurological disorders in human and animals. Human prion disease is also known as transmissible spongiform encephalopathy (TES). Prion contains only one protein known as PrPc which plays a significant role in NDD in both human and animals. Currently, many proteins like Aβ, α-synuclein, TAR and copper zinc superoxide dismutase are known to behave like prion proteins in NDD like the AD, PD, FTD and motor neuron diseases [6, 53, 87]. The key role of PrPc is to protect and maintain the health of neurons, regulate neurotransmission and prevent excitotoxicity in the brain. The normal protein structure contains only alpha-helices, while pathogenic form PrPsc (sc = scrapie) contains only beta-sheets. Devastating effect can be observed on the CNS after the misfolding of PrPc to PrPsc protein and soluble oligomers formation takes place after the aggregation of abnormal prions in the cells and due to accumulation of these abnormal prions inside neurons, programmed cell death, as well as seizures and seizure-like symptoms in the diseased individuals, occur [13]. The PrPsc can avoid the pathway for their clearance and aggregate into cells and they may propagate from one cell to another, from one individual to another and between species [6, 65].
Prion disease
Prion diseases are incurable neurological disorders that produce a wide range of devastating symptoms in humans and several mammals. The unusual type of self-replicating microbe can initiate prion disease [74, 90]. In prion disease, the normal cellular prion protein (PrPc) gets misfolded and becomes pathogenic and propagates further in the neighboring cells and tissues and can infect other organisms. Currently, three forms of prion disease are reported in human beings and they are designated as sporadic, genetic and acquired [6]. Approximately 1.5 million per year mortality rate has been reported due to the most frequent Sporadic CJD (sCJD) [61]. Globally, only 5–15% cases of genetic prion diseases have been reported which occur due to the mutation in prion protein gene (PRNP) located on human chromosome 20. BSE and Kuru are included in acquired prion diseases with only 2–5% disease incidence rate globally [6]. The symptoms of prion disease (sporadic, genetic and acquired) include cognitive and executive dysfunction, language, and memory impairments [65, 78]. The widespread of amyloidogenesis can be induced by Aβ peptide prion-like aggregates [90, 103]. Recently, it has been reported that prion can serve not only as Aβ receptors but also to spread amyloid neurotoxicity and finally this prion reaches to the brain and plays a significant role in AD-like signaling processes leading to amyloidogenesis followed by neuro-inflammation and synaptic degeneration [17, 46, 90].
Alzheimer’s disease (AD)
The role of prion in the AD is known but the equilibrium of toxic gain of function versus loss-of-function is unclear and it may be involved in different steps in different brain regions [65]. Similar disease pathology has been observed in both AD and prion diseases and many reports documented that the physical, biochemical and genetic interactions of AβPP and PrPc occur during cell and organism physiology and disease development [62, 99]. It has been proposed that PrPc acts as receptor/mediator of Aβ toxicity and contributes significantly to AD [62]. Aβ oligomers and misfolded tau have prion-like properties. The interaction of PrPc occurs in many CNS proteins like AβPP and tau and develops NDDs like an AD, PD and FTD [65]. The interaction of PrPc and AβPP results into loss of normally folded PrPc and disruption of AβPP physiology which finally leads to progression of prion disease. The PrPc can potentially modulate the mechanism of AD pathogenesis and prion disease by regulating the AβPP metabolism. The level of PrPc significantly reduced in AD patients at early stage of prion disease suggests that loss of PrPc plays a significant role in sporadic AD progression [112]. Both the AD and prion disease share many similarities and the interaction study of AβPP and PrPc will provide a valuable information to develop novel therapies for both AD and prion disease [65].
Parkinson’s disease (PD)
PD is a serious disorder of CNS characterized with the accumulation of protein in the form of Lewy bodies and neurites. The formation of Lewy bodies occurs by the autocatalytic conversion of phosphorylated and ubiquitinated forms of α-syn aggregate [70]. Recently, it has been shown that this disease has strong linkage with prion disorder. The Lewy bodies and Lewy neuritis are observed in the substantia nigra pars compacta (SNc) neurons and CNS of PD patients. Point mutations in both the a-synuclein and PrP genes resulted in PD and prion diseases. In both PD and Prion diseases reactive gliosis, protein deposits followed by neuronal death occur [83]. Based on genetic and pathologic research, protein accumulation and cell death in PD could be a result of prion disorder. The prion disease occurs when the normal form of PrPc protein converts into PrPsc. To provide the linkage of prion disease to PD, the transmission of Lewy bodies into grafted SNc cells was performed and results showed that a-synuclein in b-sheet-rich conformation can be transported from one cell to another in PD patients. Based on autopsy results, Lewy bodies were found in the grafted embryonic mesencephalic neurons in the PD patients which further confirm that α-syn has prion-like cell-to-cell transmissibility [67]. In another study, recombinant α-syn fibrils were injected in the substantia nigra, the striatum or the entorhinal cortex of wild-type mice which initiated propagation of phosphorylated α-syn, affecting different brain regions directly or indirectly. These results strongly support the transmission and spread of α-synuclein in neighboring brain cells through axonal transport [77]. The α-synuclein behaves like a prion and PD could be a prion disorder. Due to the mutation in MAPT gene and the tau gene, the formation of filamentous tau inclusions occurs in the brain, causing PD and developing dementia [81]. It is well known that PrPc and a-synuclein adopt an α-helical-rich conformation under physiological conditions and the misfolded protein can covert further and acquire wild type b-sheet configuration and may transmit to neighboring cells and promote neurodegenerative disorders. The pathological form of PrP (PrPsc) displays a predominantly β-sheet conformation at C-terminal region and resistant to proteolytic degradation [6, 41, 83].
Creutzfeldt–Jakob disease (CJD)
CJD was described in 1920 by German neurologist Hans Gerhard Creutzfeldt and the name Creutzfeldt–Jakob was given by Alfons Maria Jakob. This is an incurable, fatal neurodegenerative disorder caused by a prion. This is the most common human prion disease but believed as the rare disorder and found in one out of every one million people/year. The most important symptoms include loss of memory, paranoia, psychosis anxiety, and depression, changes in personality, hallucinations and finally progressive dementia. The normal prion protein folds into infectious protein and accumulates in a larger amount in the affected cells and can spread to neighboring cells, leading to disruption of neuronal cell function and cell death [8]. CJD has two forms designated as familial (fCJD) and (sporadic form: sCJD). The rapid brain tissue degeneration occurs in CJD and brain develops holes and forms kitchen sponge-like texture. Recently, it has been reported that some hormones get contaminated with PrP prion, resulting in iatrogenic CJD [94]. Additionally, it is known that the transplantation of PrP prion contaminated dura mater in CJD patients significantly increased the Ab plaques and cerebral amyloid angiopathy (CAA) [34].
Amyotrophic lateral sclerosis (ALS)
ALS is a serious neuromuscular disease in which central and peripheral motor neurons are affected by the prion-like spread of misfolded proteins. The symptoms include weakness, muscle paralysis and finally, death occurs due to respiratory failure [43, 55]. The important mutations observed in the superoxide dismutase-1 (SOD1), TARDBP, C9ORF72, FUS genes, and bone morphogenetic protein modifier genes resulted in increased susceptibility. Approximately, 150 mutations were observed in SOD1 related to the development of familial ALS [107]. In ALS, the substantial loss of functions and prion-like propagation of SOD1 misfolding occurs [65].
Huntington disease (HD)
HD was initially described by Charles Oscar Waters in 1841, later detailed description was given by George Huntington in 1872, and finally, it was designated as Huntington’s disease (HD). This is an inherited brain disorder causing cell death in both men and women equally. As the disease progresses, the body movement becomes jerkier and the mental ability declines, resulting in dementia [24, 33]. Unlike other neurodegenerative diseases, HD is inherited. HD has recently been classified as a prion-like disease [90]. In the wild-type huntingtin protein, ~ 35 glutamine residues are found at N-terminal region. The spontaneous aggregation occurs in the expanded polyglutamine repeats of the huntingtin in cultured cells and this shows that it could be the prion [92].
Role of miRNAs in neurodegenerative disorders
MicroRNAs (miRNAs) bind to the 3′-UTR of mRNA and lead to gene expression and suppression. MicroRNAs (miRNAs) play a key role in modifying the physiological and pathophysiogical progressions of neurodegenerative disorders. The significant up-regulation of miR-21 was observed in the human and monkey brain with HIV associated dementia and SIV encephalitis. The novel role of miR-21 was identified and reported as potential signature and crucial effector of HIV induced neuronal dysfunction and neurodegeneration. In a recent study, the expression levels of miR-196a were increased in a mouse model of spinal and bulbar muscular atrophy (SBMA) [101, 114]. Recently, the role of mir30-hSNCA was examined in hSNCA gene silencing in vivo and positive effects with AAV-mir30-hSNCA on forelimb behaviour and SNDA neurons were observed [58]. There are abundant miRNAs in CNS where they show specific expression platform and perform biologically essential functions like synaptic plasticity and neural plasticity. They are involved in the indirect regulation of neurodegeneration by controlling the proliferation and autonomous rejuvenation of neural stem cells. Several neurodegenerative disorders lead to dysregulation of miRNAs which finally culminates in neuronal cell death. Recently, dysregulated miRNAs and their role in several neurological disorders explored in many neurological disorders like AD, PD, ALS and HD opened a new avenue for the development of new treatment and management strategy. The miRNA expression profiling leads to the recognition of signature molecules related to detection, prognosis, staging and progress of reaction for the treatment of NDD. Most of the research efforts have been undertaken in the last few years using the invertebrate model system, but they still require further verification by using an animal model. Last decade has shown progression in the establishment of circular miRNAs as a potential biomarker in the diagnosis of NDD. The types of miRNAs and their profiling in many NDD are listed in Table 2. Recently a review has been published mainly focusing on the role of MicroRNAs as novel drug targets and biomarkers for neurodegenerative disorders like AD, PD and HD and their role in other neurological disorders including traumatic brain injury and status epilepticus [51, 91].
Table 2.
Circulating miRNAs for various neurodegenerative disorders
| miRNAs | Sample | Approach | Method | NDD |
|---|---|---|---|---|
| Increased expression of miR-34a and miR-181b | PBMC | miRNA profiling | Microarray | AD |
| Reduced expression of miR-27a-3p | CSF | miRNA profiling | qRT-PCR | AD |
| 60 miRNAs differentially regulated between the different Braak stages, including Let-7 family members | CSF | miRNA profiling | qRT-PCR | AD |
| Elevated levels of miR-146a and miR-155 | CSF | Candidate miRNA | Microarray | AD |
| Significant increase in miR-9, miR-125b, miR-146a, miR-155 | CSF | Candidate miRNA | Microarray | AD |
| 12-miRNA signature: | Peripheral blood | miRNA profiling | NGS | AD |
| 7-miRNA signature: | Plasma | miRNA profiling | Nanostring | AD |
| Elevated levels of let-7b | CSF | Candidate miRNA | qRT-PCR | AD |
| miR-15a associated with amyloid plaque score | Plasma | Candidate miRNA | Microarray | AD |
| miR-29a/b, miR-181c, and miR-9 down-regulated | Serum | Candidate miRNA | qRT-PCR | AD |
| Increased levels of miR-34c | PBMC | Candidate miRNA | qRT-PCR | AD |
| Circulating brain-enriched miRNAs: “miR‐132 family” and “miR‐134 family” | Plasma | Candidate miRNA | qRT-PCR | AD |
| 18 significantly under-expressed miRNAs | PBMC | miRNA profiling | Microarray | PD |
| miR-1, miR-22-5p and miR-29 distinguish non-treated PD from healthy subjects | Total Peripheral Blood | miRNA profiling | qRT-PCR | PD |
| miR-16-2-3p, miR-26a-2-3p, miR30a differentiate treated from untreated patients | Total peripheral blood | miRNA profiling | qRT-PCR | PD |
| miR-331-5p upregulated | Plasma | miRNA profiling | qRT-PCR | PD |
| miR-1826, miR-450b-3p, miR-626, and miR-505 | Plasma | miRNA profiling | Microarray | PD |
| 16 miRNAs (including miR-16, miR-20a and miR-320) modified in patients pre-DBS | Leukocytes | miRNA profiling | NGS | PD |
| 8 miRNAs (miR-451, miR-1275, miR-328, miR-638, miR-149, miR-665 and miR-338-3) significantly up- or downregulated | Leukocytes | miRNA profiling | Microarray | ALS |
| Expression of ALS-specific miRNA inflammatory signature | Monocytes | miRNA profiling | Microarray | ALS |
Alzheimer’s disease (AD)
AD is the most general stage of prime degenerative dementia. Clinico-pathological examinations indicated a long preclinical stage of the disease. In the last few years, the report about the altered expression and regulation of miRNAs in AD has been published. Altered miRNAs profiling has been widely observed in brain tissue samples or cell cultures but little information is available regarding circulating miRNA in AD [37, 45, 47, 110]. Schipper et al. in 2007, studied the possibility of miRNAs as a biomarker in AD. By using microarray technology, the higher expression of miRNAs was observed in peripheral blood mononuclear cells (PBMCs) taken from AD individuals [98]. A recent study based on next generation sequencing (NGS) observed that 12 miRNAs signature could differentiate between AD with 93% accuracy as compared to control group [64]. Using Nanostring technology, Kumar et al. [60] observed a unique 7 miRNAs signature sequence in plasma which can differentiate AD patient with normal control with 95% accuracy. In a separate study, miRNAs in cerebro spinal fluid (CSF) by qRT-PCR observed lower expression of hsa-miR-27a-3p in AD patients [95]. Cogswell et al. [22] also conducted the miRNAs study from CSF by qRT-PCR and same expression pattern were observed in both CSF and brain tissue for miRNAs of AD individuals. Lukiw et al. [4, 72] identified miRNAs associated with inflammatory signaling pathway and reported that miR-146a and miR-155 are plentiful in AD CSF samples. Another group study identified miR let-7b which activates TLR 7 and causes neurodegeneration from CSF samples of AD patient [63]. Circulatory miRNA (miR-15a) were obtained from plasma and found to be significantly associated with amyloid plaques serum for the pathological diagnosis of AD [10], and down-regulation of miR29a/b, mir-181 c and miR-9 in serum was also observed [38]. It is well reported that amyloid β (Aβ) level is directly regulated by SPT by post transcriptional regulation of various miRNAs known as miR-137, miR-181c, miR-9 and miR-29a/b 39 and it is believed that SPT could be an important target for AD diagnosis. The miR-34 family is another important miRNA implicated in the AD and affects two signalling pathways: Bcl-2 for cell survival/apoptosis and SIRTI de-acetylation for p53 or neuro-protective signalling. The increased level of miR-34c in both PBMC and plasma in the AD as compared to control was observed [11]. Plasma miRNAs biomolecules were also reported for mild cognitive impairment, an intermediate stage between normal aging and AD (dementia) [26, 100]. Two sets of miRNAs (miR-132 family consisting of miR-128 and miR-134) have 79% and 100% specificity. Their study also matched with the separate longitudinal study which identified miRNAs and MCI in many infected individuals at asymptomatic conditions 1–5 years before diagnosis. The miR-132 family contains miR-128/miR-491-5p, miR-132/miR-491-5p and miR-874/miR-491-5p, while miR-134 family contains miR-134/miR-370, miR-323-3pmiR-370 and miR-382/miR-370 [100]. Recently, the role of miRNA in the AD and their therapeutic potential have been presented on the activities of ten miRNAs in biological pathways involved in the AD pathogenesis [80].
Parkinson’s disease (PD)
PD is a neurodegenerative disorder affecting millions of people globally. PD leads to defective motor function as a reduction of dopamine producing brain cells. The symptom includes stiffness, tremors, slow and impaired balance, anxiety, depression, and dementia. Environmental acquired risk factors and genetic factors are established as a risk factor for this disease [82]. Mutations in the α-synclein gene (SNCA) and leucine-rich repeat kinase 2 (LRRK2) are responsible for the late onset, while Parkin (PARK2) and PTEN induced putative kinase (PINK1) are oncogenes DJ1 (DJ1) responsible for early onset [23]. The neuropathological features including cellular inclusions known as Lewy bodies, identified by protein biomolecules, provided conflicting results, therefore, the identification of circulatory biomarkers like miRNAs has immense remedial prospective; however, it is still in infancy and open research opportunities are available to explore new miRNAs and their role in PD [50]. In a study based on the application of microarray approaches, researchers determined the expression profile in PMBC of 19 patients with 13 controls; and total 18 miRNAs were identified with significantly lower expression [76]. In another study, qRT-PCR results showed that the expression of miR-1, miR-22-5p, and miR-29 in peripheral blood differs in PD from healthy individuals. While expression of miR-16-2-3p, miR-26a-2-3p and miR30a also differs among treated and control groups [50]. The profiling of miRNA from the plasma of PD patients has also been conducted by another group using qRT-PCR and miR-331-5p was identified as up-regulated significantly [15]. Additionally, miR-1826, miR-450-3p and miR-505 were identified from 32 patients and 32 controls conducted by another group [15, 59]. By using next generation sequencing analysis in total leukocytes, 16 miRNAs were observed to be changed significantly in PD patients containing miR16, miR20a, and miR-320 as compared to control group [102].
Amyotrophic lateral sclerosis (ALS)
ALS is another serious neurological disorder with symptoms like muscular weakness, atrophy, paralysis and respiratory failure affecting neurons responsible for voluntary movements. Various types of studies are being conducted to identify biomarkers for ALS using CSF and blood samples up to protein level [105], but due to lack of satisfied results, more studies are also being conducted to explore miRNA as a possible biomarker for ALS. Recently, the profiling of total 911miRNAs from leukocytes of ALS patients using microarray technology was performed and only 8 miRNAs were observed to be significantly up and down regulated as compared to control [25]. One parallel study identified a profile containing an inflammatory signature of around 56 miRNAs which was considerably affected in CD14 + CD16 monocytes and they can be used as ALS biomarker [14]. Although these two studies were executed by similar technical methodologies, the number of dysregulated miRNAs found in leukocytes and monocytes is not overlapped and comparable [14, 25].
For the other neurological disorders like HD and FTD, little information is available based on circulating miRNAs in plasma or serum. Interestingly only one miR-34b was observed to be up-regulated in response to Huntingtin (mHTT) mutant indicating the role of miRNA-34b as a biomarker of HD [36]. There is a little concordance between these miRNAs both for precise disease as compared to various NDD. There is an urgent need for the more detailed investigation to be considered for the miRNA as a diagnostic biomarker. The therapeutic potential against HD was investigated by reducing the expression of HTT using RNA interference (RNAi), and approximately 45% reduction of rhesus HTT expression was observed in the mid- and caudal putamen and the partial suppression of wild-type HTT expression is well tolerated in the primate putamen. Based on these results, it is possible that RNAi could be a novel strategy for HD therapy [79]. The level of virus-specific RNAs (vsRNAs) or miRNAs was found to be very high in the exterior of cells of their origin CSF and blood serum [4].
Concluding remarks
Based on the latest information, now it is known that the viruses, prions and microRNAs are associated with neurological disorders leads to emergence of dementia stages. Currently, no treatment is available to control the progressive neurodegenerative diseases. Discovery of miRNAs elaborated the knowledge and understanding of post transcriptional gene regulation in NDD [5, 93]. Data available so far indicate that miRNAs profiling study in AD and other NDD suffer from poor consensus, which is the main concern. Many difficulties are still present in considering these miRNAs as biomarkers for neurological disorders. There is an urgent need of more detailed investigation to consider these miRNAs as a diagnostic biomarker. There is an urgent need to understand the role of other RNA or DNA based helper viruses in promoting and intensifying the action of miRNA. Most importantly, there is an urgent need to understand the potential spread of miRNA information from cell to cell, tissue to tissue, and between species [12, 56, 84, 96]. The generated additional information during the last few years will provide new perspectives for designing and development of an effective therapeutic and disease management strategy. The understanding about the prion and NDD has reached an advanced stage. In-vitro studies about the prion and associated NDDs have elucidated multiple aspects of neurological disorders which are found to be very useful in designing and development of an effective therapeutics and disease management strategies. The complexity of gain-versus loss-of PrPc functions in prion diseases should be further examined and requires strong support based on detailed research work [65]. The role and linkage of prion in NDDs have been reported and the generated information contributed significantly towards the understanding of neurological disorders. More detailed studies are urgently required about the progression of prion diseases and this information will play a strong and valuable role in the designing and drug development strategies for NDD. The recent information showed that prion-like propagation of misfolded protein states is not limited to the prion protein [54, 108]. It is well known that amyloidogenic proteins or peptides, like Aβ, α-synuclein, tau, huntingtin, can also spread from cell to cell or can be transmitted from animal to animal or human to animal in a prion-like fashion to cause NDD [1, 9, 103, 108, 111]. Both AD and prion disease share many similarities and the interaction study of AβPP and PrPc will provide valuable information in developing novel therapies for both AD and prion disease [65]. Based on the above and recent literature, there is still more detailed information required about the role of viruses, prions and miRNAs in neurological disorder. The identification of specific miRNAs can be used as candidate diagnostics biomarker and development of future therapeutics. The detailed understanding of involved mechanism of virus infection, prions and microRNAs in neurological disorders and dementia disease development will help in management and design and development of an effective treatment plan.
Acknowledgements
The authors are grateful to Special Infectious Agents Unit (SIAU), King Fahd Medical Research Center (KFMRC), King Abdulaziz University (Jeddah, Saudi Arabia) for the facilities provided.
References
- 1.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64(6):783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad F, Siddiqui A, Kamal MA, Sohrab SS. Inhibition of Neurogenesis by Zika virus infection. CNS Neurol Disord Drug Targets. 2018;17(2):78–86. doi: 10.2174/1871527317666180202115114. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad F. ZIKV leads to microcephaly. EC Microbiol. 2016;4:12–13. [Google Scholar]
- 4.Alexandrov PN, Dua P, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF) Int J Biochem Mol Biol. 2012;3(4):365–373. [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandrov PN, Dua P, Lukiw WJ. Up-regulation of miRNA-146a in progressive, age-related inflammatory neurodegenerative disorders of the human CNS. Front Neurol. 2014;5:181. doi: 10.3389/fneur.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annus A, Csati A, Vecsei L. Prion diseases: new considerations. Clin Neurol Neurosurg. 2016;150:125–132. doi: 10.1016/j.clineuro.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armitage WJ, Tullo AB, Ironside JW. Risk of Creutzfeldt–Jakob disease transmission by ocular surgery and tissue transplantation. Eye. 2009;23(10):1926–1930. doi: 10.1038/eye.2008.381. [DOI] [PubMed] [Google Scholar]
- 9.Baskakov IV, Katorcha E. Multifaceted role of sialylation in prion diseases. Front Neurosci. 2016;10:358. doi: 10.3389/fnins.2016.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekris LM, Lutz F, Montine TJ, Yu CE, Tsuang D, Peskind ER, et al. MicroRNA in Alzheimer’s disease: an exploratory study in brain, cerebrospinal fluid and plasma. Biomark Biochem Indic Expo Response Susceptibility Chem. 2013;18(5):455–466. doi: 10.3109/1354750X.2013.814073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatnagar S, Chertkow H, Schipper HM, Yuan Z, Shetty V, Jenkins S, et al. Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front Mol Neurosci. 2014;7:2. doi: 10.3389/fnmol.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Front Cell Neurosci. 2013;7:153. doi: 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11(4):301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Investig. 2012;122(9):3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardo LF, Coto E, de Mena L, Ribacoba R, Moris G, Menendez M, et al. Profile of microRNAs in the plasma of Parkinson’s disease patients and healthy controls. J Neurol. 2013;260(5):1420–1422. doi: 10.1007/s00415-013-6900-8. [DOI] [PubMed] [Google Scholar]
- 16.Celeste DB, Miller MS. Reviewing the evidence for viruses as environmental risk factors for ALS: a new perspective. Cytokine. 2018;108:173–178. doi: 10.1016/j.cyto.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Chen B, Soto C, Morales R. Peripherally administrated prions reach the brain at sub-infectious quantities in experimental hamsters. FEBS Lett. 2014;588(5):795–800. doi: 10.1016/j.febslet.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu S-L, Chen C-M, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clement C, Hill JM, Dua P, Culicchia F, Lukiw WJ. Analysis of RNA from Alzheimer’s disease post-mortem brain tissues. Mol Neurobiol. 2016;53(2):1322–1328. doi: 10.1007/s12035-015-9105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Codocedo JF, Rios JA, Godoy JA, Inestrosa NC. Are microRNAs the molecular link between metabolic syndrome and Alzheimer’s disease? Mol Neurobiol. 2016;53(4):2320–2338. doi: 10.1007/s12035-015-9201-7. [DOI] [PubMed] [Google Scholar]
- 21.Cogoni C, Ruberti F, Barbato C. MicroRNA landscape in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2015;14(2):168–175. doi: 10.2174/1871527314666150116123305. [DOI] [PubMed] [Google Scholar]
- 22.Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis JAD. 2008;14(1):27–41. doi: 10.3233/JAD-2008-14103. [DOI] [PubMed] [Google Scholar]
- 23.Coppede F. Genetics and epigenetics of Parkinson’s disease. Sci World J. 2012;2012:489830. doi: 10.1100/2012/489830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayalu P, Albin RL. Huntington disease: pathogenesis and treatment. Neurol Clin. 2015;33(1):101–114. doi: 10.1016/j.ncl.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 25.De Felice B, Guida M, Guida M, Coppola C, De Mieri G, Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene. 2012;508(1):35–40. doi: 10.1016/j.gene.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 26.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2(1):15–21. doi: 10.1016/S1474-4422(03)00262-X. [DOI] [PubMed] [Google Scholar]
- 27.Del Río JA, Ferrer I, Gavín R. Role of cellular prion protein in interneuronal amyloid transmission. Prog Neurobiol. 2018 doi: 10.1016/j.pneurobio.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Elbirt D, Mahlab-Guri K, Bezalel-Rosenberg S, Gill H, Attali M, Asher I. HIV-associated neurocognitive disorders (HAND) Isr Med Assoc J. 2015;17(1):54–59. [PubMed] [Google Scholar]
- 29.Fatima M, Kumari R, Schwamborn JC, Mahadevan A, Shankar SK, Raja R, et al. Tripartite containing motif 32 modulates proliferation of human neural precursor cells in HIV-1 neurodegeneration. Cell Death Differ. 2016;23(5):776–786. doi: 10.1038/cdd.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fields JA, Dumaop W, Crews L, Adame A, Spencer B, Metcalf J, et al. Mechanisms of HIV-1 tat neurotoxicity via CDK5 translocation and hyper-activation: role in HIV-associated neurocognitive disorders. Curr HIV Res. 2015;13(1):43–54. doi: 10.2174/1570162X13666150311164201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishman SL, Murray JM, Eng FJ, Walewski JL, Morgello S, Branch AD. Molecular and bioinformatic evidence of hepatitis C virus evolution in brain. J Infect Dis. 2008;197(4):597–607. doi: 10.1086/526519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher NF, McKeating JA. Hepatitis C virus and the brain. J Viral Hepat. 2012;19(5):301–306. doi: 10.1111/j.1365-2893.2012.01591.x. [DOI] [PubMed] [Google Scholar]
- 33.Frank S. Treatment of Huntington’s disease. Neurother J Am Soc Exp NeuroTher. 2014;11(1):153–160. doi: 10.1007/s13311-013-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frontzek K, Lutz MI, Aguzzi A, Kovacs GG, Budka H. Amyloid-beta pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt–Jakob disease after dural grafting. Swiss Med Wkly. 2016;146:w14287. doi: 10.4414/smw.2016.14287. [DOI] [PubMed] [Google Scholar]
- 35.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11(3):155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaughwin PM, Ciesla M, Lahiri N, Tabrizi SJ, Brundin P, Bjorkqvist M. Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntington’s disease. Hum Mol Genet. 2011;20(11):2225–2237. doi: 10.1093/hmg/ddr111. [DOI] [PubMed] [Google Scholar]
- 37.Geekiyanage H, Chan C. MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer’s disease. J Neurosci Off J Soc Neurosci. 2011;31(41):14820–14830. doi: 10.1523/JNEUROSCI.3883-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol. 2012;235(2):491–496. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goedert M. Neurodegeneration. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled abeta, tau, and alpha-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 40.Goedert M, Falcon B, Clavaguera F, Tolnay M. Prion-like mechanisms in the pathogenesis of tauopathies and synucleinopathies. Curr Neurol Neurosci Rep. 2014;14(11):495. doi: 10.1007/s11910-014-0495-z. [DOI] [PubMed] [Google Scholar]
- 41.Goedert M, Masuda-Suzukake M, Falcon B. Like prions: the propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain J Neurol. 2016 doi: 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- 42.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 43.Grad LI, Fernando SM, Cashman NR. From molecule to molecule and cell to cell: prion-like mechanisms in amyotrophic lateral sclerosis. Neurobiol Dis. 2015;77:257–265. doi: 10.1016/j.nbd.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez-Rapp J, Martin-Lanneree S, Hirsch TZ, Launay JM, Mouillet-Richard S. Hijacking PrP(c)-dependent signal transduction: when prions impair Abeta clearance. Front Aging Neurosci. 2014;6:25. doi: 10.3389/fnagi.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill JM, Lukiw WJ. MicroRNA (miRNA)-mediated pathogenetic signaling in Alzheimer’s disease (AD) Neurochem Res. 2016;41(1–2):96–100. doi: 10.1007/s11064-015-1734-7. [DOI] [PubMed] [Google Scholar]
- 48.Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD) Front Aging Neurosci. 2014;6:127. doi: 10.3389/fnagi.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hock EM, Polymenidou M. Prion-like propagation as a pathogenic principle in frontotemporal dementia. J Neurochem. 2016;138(Suppl 1):163–183. doi: 10.1111/jnc.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain J Neurol. 2010;133(Pt 3):713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Idda ML, Munk R, Abdelmohsen K, Gorospe M. Noncoding RNAs in Alzheimer’s disease. Wiley Interdiscip Rev RNA. 2018 doi: 10.1002/wrna.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itzhaki RF. Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Front Aging Neurosci. 2014;6:202. doi: 10.3389/fnagi.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarosz-Griffiths HH, Noble E, Rushworth JV, Hooper NM. Amyloid-beta receptors: the good, the bad, and the prion protein. J Biol Chem. 2016;291(7):3174–3183. doi: 10.1074/jbc.R115.702704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70(4):532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karim S, Mirza Z, Kamal MA, Abuzenadah AM, Azhar EI, Al-Qahtani MH, et al. The role of viruses in neurodegenerative and neurobehavioral diseases. CNS Neurol Disord Drug Targets. 2014;13(7):1213–1223. doi: 10.2174/187152731307141015122638. [DOI] [PubMed] [Google Scholar]
- 56.Katsarou K, Rao AL, Tsagris M, Kalantidis K. Infectious long non-coding RNAs. Biochimie. 2015;117:37–47. doi: 10.1016/j.biochi.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Khan MB, Lang MJ, Huang M-B, Raymond A, Bond VC, Shiramizu B, et al. Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Aβ(1-42) secretion in SH-SY5Y neural cells. J Neurovirol. 2016;22(2):179–190. doi: 10.1007/s13365-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khodr CE, Becerra A, Han Y, Bohn MC. Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects. Brain Res. 2014;1550:47–60. doi: 10.1016/j.brainres.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khoo SK, Petillo D, Kang UJ, Resau JH, Berryhill B, Linder J, et al. Plasma-based circulating MicroRNA biomarkers for Parkinson’s disease. J Parkinsons Dis. 2012;2(4):321–331. doi: 10.3233/JPD-012144. [DOI] [PubMed] [Google Scholar]
- 60.Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M, et al. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS ONE. 2013;8(7):e69807. doi: 10.1371/journal.pone.0069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ladogana A, Puopolo M, Croes EA, Budka H, Jarius C, Collins S, et al. Mortality from Creutzfeldt–Jakob disease and related disorders in Europe, Australia, and Canada. Neurology. 2005;64(9):1586–1591. doi: 10.1212/01.WNL.0000160117.56690.B2. [DOI] [PubMed] [Google Scholar]
- 62.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15(6):827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 64.Leidinger P, Backes C, Deutscher S, Schmitt K, Mueller SC, Frese K, et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013;14(7):R78. doi: 10.1186/gb-2013-14-7-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leighton PL, Allison WT. Protein misfolding in prion and prion-like diseases: reconsidering a required role for protein loss-of-function. J Alzheimers Dis JAD. 2016;54(1):3–29. doi: 10.3233/JAD-160361. [DOI] [PubMed] [Google Scholar]
- 66.Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, et al. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196(3):361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- 67.Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2010;25(8):1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- 68.Licastro F, Carbone I, Raschi E, Porcellini E. The 21st century epidemic: infections as inductors of neuro-degeneration associated with Alzheimer’s disease. Immun Ageing. 2014;11(1):22. doi: 10.1186/s12979-014-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lövheim H, Olsson J, Weidung B, Johansson A, Eriksson S, Hallmans G, et al. Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer’s disease development. J Alzheimers Dis. 2018;61(3):939–945. doi: 10.3233/JAD-161305. [DOI] [PubMed] [Google Scholar]
- 70.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. NeuroReport. 2007;18(3):297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 72.Lukiw WJ. Evolution and complexity of micro RNA in the human brain. Front Genet. 2012;3:166. doi: 10.3389/fgene.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lukiw WJ. Variability in micro RNA (miRNA) abundance, speciation and complexity amongst different human populations and potential relevance to Alzheimer’s disease (AD) Front Cell Neurosci. 2013;7:133. doi: 10.3389/fncel.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lukiw WJ, Dua P, Pogue AI, Eicken C, Hill JM. Upregulation of micro RNA-146a (miRNA-146a), a marker for inflammatory neurodegeneration, in sporadic Creutzfeldt–Jakob disease (sCJD) and Gerstmann–Straussler–Scheinker (GSS) syndrome. J Toxicol Environ Health Part A. 2011;74(22–24):1460–1468. doi: 10.1080/15287394.2011.618973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marciniuk K, Taschuk R, Napper S. Evidence for prion-like mechanisms in several neurodegenerative diseases: potential implications for immunotherapy. Clin Dev Immunol. 2013;2013:473706. doi: 10.1155/2013/473706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martins M, Rosa A, Guedes LC, Fonseca BV, Gotovac K, Violante S, et al. Convergence of miRNA expression profiling, alpha-synuclein interacton and GWAS in Parkinson’s disease. PLoS ONE. 2011;6(10):e25443. doi: 10.1371/journal.pone.0025443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, et al. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol Commun. 2014;2:88. doi: 10.1186/s40478-014-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mays CE, Kim C, Haldiman T, van der Merwe J, Lau A, Yang J, et al. Prion disease tempo determined by host-dependent substrate reduction. J Clin Investig. 2014;124(2):847–858. doi: 10.1172/JCI72241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, et al. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther. 2011;19(12):2152–2162. doi: 10.1038/mt.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miya Shaik M, Tamargo IA, Abubakar MB, Kamal MA, Greig NH, Gan SH. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes Basel. 2018 doi: 10.3390/genes9040174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamed NV, Herrou T, Plouffe V, Piperno N, Leclerc N. Spreading of tau pathology in Alzheimer’s disease by cell-to-cell transmission. Eur J Neurosci. 2013;37(12):1939–1948. doi: 10.1111/ejn.12229. [DOI] [PubMed] [Google Scholar]
- 82.Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat. 2010;31(7):763–780. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olanow CW, McNaught K. Parkinson’s disease, proteins, and prions: milestones. Mov Disord Off J Mov Disord Soc. 2011;26(6):1056–1071. doi: 10.1002/mds.23767. [DOI] [PubMed] [Google Scholar]
- 84.Perkel JM. Assume nothing: the tale of circular RNA. Biotechniques. 2013;55(2):55–57. doi: 10.2144/000114061. [DOI] [PubMed] [Google Scholar]
- 85.Piacentini R, De Chiara G, Li Puma DD, Ripoli C, Marcocci ME, Garaci E, et al. HSV-1 and Alzheimer’s disease: more than a hypothesis. Front Pharmacol. 2014;5:97. doi: 10.3389/fphar.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinheiro CAT, Souza LDM, Motta JVS, Kelbert EF, Souza MS, Martins CSR, et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz J Med Biol Res. 2016;49(10):e5344. doi: 10.1590/1414-431x20165344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poggiolini I, Saverioni D, Parchi P. Prion protein misfolding, strains, and neurotoxicity: an update from studies on mammalian prions. Int J Cell Biol. 2013;2013:910314. doi: 10.1155/2013/910314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 89.Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336(6088):1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quinlan S, Kenny A, Medina M, Engel T, Jimenez-Mateos EM. MicroRNAs in Neurodegenerative diseases. Int Rev Cell Mol Biol. 2017;334:309–343. doi: 10.1016/bs.ircmb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11(2):219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roth W, Hecker D, Fava E. Systems biology approaches to the study of biological networks underlying Alzheimer’s disease: role of miRNAs. Methods Mol Biol. 2016;1303:349–377. doi: 10.1007/978-1-4939-2627-5_21. [DOI] [PubMed] [Google Scholar]
- 94.Rudge P, Jaunmuktane Z, Adlard P, Bjurstrom N, Caine D, Lowe J, et al. Iatrogenic CJD due to pituitary-derived growth hormone with genetically determined incubation times of up to 40 years. Brain J Neurol. 2015;138(Pt 11):3386–3399. doi: 10.1093/brain/awv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sala Frigerio C, Lau P, Salta E, Tournoy J, Bossers K, Vandenberghe R, et al. Reduced expression of hsa-miR-27a-3p in CSF of patients with Alzheimer disease. Neurology. 2013;81(24):2103–2106. doi: 10.1212/01.wnl.0000437306.37850.22. [DOI] [PubMed] [Google Scholar]
- 96.Sarkies P, Miska EA. Molecular biology. Is there social RNA? Science. 2013;341(6145):467–468. doi: 10.1126/science.1243175. [DOI] [PubMed] [Google Scholar]
- 97.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder-pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(5):309. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schipper HM, Maes OC, Chertkow HM, Wang E. MicroRNA expression in Alzheimer blood mononuclear cells. Gene Regul Syst Bio. 2007;1:263–274. doi: 10.4137/grsb.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmitz M, Wulf K, Signore SC, Schulz-Schaeffer WJ, Kermer P, Bahr M, et al. Impact of the cellular prion protein on amyloid-beta and 3PO-tau processing. J Alzheimers Dis JAD. 2014;38(3):551–565. doi: 10.3233/JAD-130566. [DOI] [PubMed] [Google Scholar]
- 100.Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging. 2012;4(9):590–605. doi: 10.18632/aging.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sobue G. MicroRNA in neurodegenerative disorders. Rinsho Shinkeigaku. 2013;53(11):942–944. doi: 10.5692/clinicalneurol.53.942. [DOI] [PubMed] [Google Scholar]
- 102.Soreq L, Salomonis N, Bronstein M, Greenberg DS, Israel Z, Bergman H, et al. Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Front Mol Neurosci. 2013;6:10. doi: 10.3389/fnmol.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stohr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, DeArmond SJ, et al. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc Natl Acad Sci USA. 2012;109(27):11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torniainen-Holm M, Suvisaari J, Lindgren M, Härkänen T, Dickerson F, Yolken RH. Association of cytomegalovirus and Epstein–Barr virus with cognitive functioning and risk of dementia in the general population: 11-year follow-up study. Brain Behav Immun. 2018;69:480–485. doi: 10.1016/j.bbi.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8(1):94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- 106.Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst) 2016;4:1–5. doi: 10.1016/j.dadm.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valentine JS, Doucette PA, Zittin Potter S. Copper–zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 108.Walker LC, Jucker M. Neurodegenerative diseases: expanding the prion concept. Annu Rev Neurosci. 2015;38:87–103. doi: 10.1146/annurev-neuro-071714-033828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walker LC, Schelle J, Jucker M. The prion-like properties of amyloid-beta assemblies: implications for Alzheimer’s disease. Cold Spring Harb Perspect Med. 2016 doi: 10.1101/cshperspect.a024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, et al. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci Off J Soc Neurosci. 2008;28(5):1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watts JC, Condello C, Stohr J, Oehler A, Lee J, DeArmond SJ, et al. Serial propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2014;111(28):10323–10328. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whitehouse IJ, Miners JS, Glennon EB, Kehoe PG, Love S, Kellett KA, et al. Prion protein is decreased in Alzheimer’s brain and inversely correlates with BACE1 activity, amyloid-beta levels and Braak stage. PLoS ONE. 2013;8(4):e59554. doi: 10.1371/journal.pone.0059554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xue YC, Feuer R, Cashman N, Luo H. Enteroviral infection: the forgotten link to amyotrophic lateral sclerosis? Front Mol Neurosci. 2018;11:63. doi: 10.3389/fnmol.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis. 2010;1:e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao Y, Bhattacharjee S, Dua P, Alexandrov PN, Lukiw WJ. microRNA-based biomarkers and the diagnosis of Alzheimer’s disease. Front Neurol. 2015;6:162. doi: 10.3389/fneur.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]