Abstract
The present study aimed to investigate the role of adenylyl cyclase activator in preventing diabetic nephropathy in rats. Renal function parameters, renal hypertrophy, lipid profile, markers of oxidative stress and free radical scavenging activities were assessed. Histopathology was performed to confirm Streptozotocin induced renal morphological changes in diabetic rats. Male Wistar rats were used in the present study to reduce the effect of estrogen. Rats were subjected to high fat diet (HFD) for two weeks followed by low dose of Streptozotocin (STZ) [35 mg/kg, i.p.] to develop experimental diabetic nephropathy in eight weeks. Two weeks treatment with low dose of Forskolin (10 mg/kg) reduced the level of diabetic nephropathy markers but results observed were not significant. Whereas, Forskolin intermediate dose (20 mg/kg) and high dose (30 mg/kg) treated rats significantly attenuated diabetes induced elevated renal function parameters and endogenous antioxidants enzymatic activities. High dose of Forskolin was found to be more effective in attenuating the renal structural and functional abnormalities. Forskolin prevented renal structural and functional abnormalities in diabetic rats. Histopathological evaluation revealed that Forskolin (20 mg/kg and 30 mg/kg) treated diabetic rats demonstrated reduced vacuolar degeneration of tubules and glomerulosclerosis. In the present study, Glibenclamide (0.6 mg/kg) and Atorvastatin (0.5 mg/kg) were used as standard drugs. Our results demonstrated synergistic effects, when high dose of Forskolin was co-administered with standard drugs. In conclusion, treatment with adenylyl cyclase activator, Forskolin in diabetic rats reduced the elevated serum glucose level, biomarkers of renal morphological dysfunction, renal hypertrophy, dyslipidaemia, oxidative stress and improved renal structure, function and enhanced level of endogenous antioxidants. Forskolin has a potential to prevent nephropathy without showing any effect on body weight in diabetic rats.
Keywords: Dyslipidaemia, hypertrophy, thiobarbituric acid reactive substances, glutathione, superoxide dismutase and histopathology
Introduction
As per the World health organization reports currently diabetes affects more than 170 million people worldwide and the number will increase up to 370 million by 2030. India is known as the diabetes capital of the world. Diabetes mellitus (DM) is increasing worldwide and particularly type 2 DM and the major complication of DM include diabetic nephropathy (DN). Chronic hyperglycaemia can affects kidneys, heart, eyes and nerves. The affected organs in DN are kidneys and it is the major cause of end stage renal disease worldwide and also the major cause of morbidity and mortality in DM [1].
Worldwide many pharmacological drugs are available to treat the DN, but it is still a matter of concern today, because these pharmacological drugs are less efficacious and are associated with side effects. There is still a need of new therapeutic drugs which not only prevent the development of DN by various metabolic and inflammatory pathways but on the other side are side effects free. The popularity of the complementary medicines has increased nowadays. Hyperglycaemia leads to increased oxidative stress and activation of polyol pathway, which may cause inflammation and renal damage.
Mitochondrial dysfunction, advanced glycation end processes and others are believed to be the probable sources [2]. Increasing evidence indicates that the disruption of mitochondrial bioenergetics may be important in the development and progression of DN [3].
Forskolin (FSK) a diterpene isolated from the plant Coleus forskohlii, rapidly activate adenylyl cyclase (AC) which increases the cyclic adenosine monophosphate (cAMP) level by converting the adenosine triphosphate (ATP) into cAMP [4]. Previous studies have reported that FSK possesses several biological properties including antioxidant and anti-inflammatory activities [5]. The present study aimed to investigate the role of adenylyl cyclase activator in controlling experimental diabetic nephropathy in rats.
Materials and methods
Experimental animals
Seventy, male Wistar rats, with an initial body weight of 180-220 g were used in this study. All experimental procedures used in this study were approved by Institutional Animal Ethical Committee (IAEC) (RITS/IAEC/2016/08/08) as per the instructions of CPCSEA, Government of India (888/PO/Re/S/05/CPCSEA). All animals were housed in standard light/dark cycle with free access to standard high-fat diet and water ad libitum. The animals were housed in metabolic cages. A 24-h urine collection was obtained from each rat for laboratory investigations. The study was carried out for 12 weeks.
Drugs and chemicals
Streptozotocin (STZ) 35 mg/kg was procured from Sigma Chemicals, St. Louis, USA. FSK (10, 20 and 30 mg/kg, p.o.) was obtained from Bangladesh Petroleum Exploration and Production Company Ltd., Rajasthan, India. Glibenclamide (0.6 mg/kg, p.o.) and Atorvastatin (0.5 mg/kg/day, p.o.) were obtained from Sigma Aldrich [P] Ltd., Bangalore, was dissolved in distilled water. All other chemicals used in the present study were of analytical grade. FSK was dissolved in distilled water and then administered orally to the animals for 2 weeks after 8 weeks STZ administration. The three doses of FSK were selected on the basis of the acute oral toxicity studies reported in addition to the previous studies carried out on the FSK [6].
Induction and assessment of diabetes mellitus
DM was induced by feeding high fat diet (HFD) to rats for 2 weeks. Component of HFD (g/kg) were powdered normal pellet diet 365 g, lard 310 g, casein 250 g, cholesterol 10 g, vitamin and mineral mix 60 g, DL-methionine 0.3 g, followed by single low dose of STZ 35 mg/kg, i.p. further followed by HFD for next 8 weeks [7]. Diabetic rats having blood glucose of > 200 mg/dl after 1 week of STZ administration were used in the present study. Blood samples were obtained from retero-orbital sinus and serum glucose level was determined by glucose oxidase-peroxidase (GOD-POD) method.
Experimental protocol (n = 7)
Ten groups were employed and each group consisting of seven rats. Glibenclamide and Atorvastatin have been reported to have anti-diabetic and lipid lowering compounds [8,9]. Therefore, Glibenclamide and Atorvastatin have been employed as standard drugs in the present study. All test and standard drugs were administered for two weeks in diabetic rats.
Group-I (Normal control): Rats were maintained on standard food and water regimen and no treatment was given.
Group II (FSK per se): Normal rats were administered FSK [30 mg/kg, p.o.].
Group III (Diabetic Control): Normal rats were fed HFD for 2 weeks, followed by single dose of STZ [35 mg/kg, i.p.] and further followed by HFD for another 10 weeks.
Group IV: Rats were administered FSK [10 mg/kg, p.o.], 8 weeks after STZ administration.
Group V: Rats were administered FSK [20 mg/kg, p.o.], 8 weeks after STZ administration.
Group VI: Rats were administered FSK [30 mg/kg, p.o.], 8 weeks after STZ administration.
Group VII: Rats were administered Glibenclamide [0.6 mg/kg, p.o.], 8 weeks after STZ administration.
Group VIII: Rats were administered Atorvastatin [0.5 mg/kg/day, p.o.], 8 weeks after STZ administration.
Group IX: Rats were administered Glibenclamide [0.6 mg/kg, p.o.] and FSK [30 mg/kg, p.o], 8 weeks after STZ administration.
Group X: Rats were administered Atorvastatin [0.5 mg/kg, p.o.] and FSK [30 mg/kg, p.o], 8 weeks after STZ administration.
Evaluation of renal parameters
Serum creatinine, blood urea nitrogen (BUN) and protein in urine level were determined in all serum samples by using standard diagnostic kits (Transasia Bio-Medicals Ltd., Baddi, India).
Assessment of renal hypertrophy
Estimation of absolute kidney weight, kidney weight/body weight ratio and total renal collagen content.
Assessment of lipid profile
In addition, diabetes mellitus induced lipid alterations were assessed by measuring serum total cholesterol, low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL) and high-density lipoprotein (HDL).
Assessment of renal markers of oxidative stress and free radical scavenging activity
Diabetes induced renal oxidative stress were assessed by measuring thiobarbituric acid reactive substances (TBARS) level using Ohkawa, 1979 method [10].
Assessment of kidney antioxidant parameters
Estimation of reduced glutathione (GSH)
The reduced GSH was estimated by method described by Ellman (1959) [11]. A 1 mL of supernatant was precipitated with 1 mL of 4% sulfosalicylic acid and cold digested at 4°C for one hour. After centrifugation at 1,200 × g for 15 minutes, 0.1 mL supernatant, 2.7 mL of 0.1 M phosphate buffer solution (pH 8) and 0.2 mL of DTNB were added in the solution mixture. Due to reaction, yellow colour appeared first at absorbance at 412 nm was measured using UV-VIS spectrophotometer (Labindia) and GSH level was expressed in µmol/mg protein.
Estimation of superoxide dismutase (SOD)
SOD activity was determined by the method of Misra and Fradovich (1972) [12]. Epinephrine underwent auto-oxidation at pH 10.2. 0.2 mL supernantant of rat kidney homogenate, 0.8 mL of 50 mM glycine buffer (pH 10.4) and 0.2 mL epinephrine were mixed together. Five minutes later, absorbance at 480 nm was measured using a UV-VIS spectrophotometer (Labindia). SOD activity was expressed as µmol/min/mL.
Estimation of catalase (CAT)
Catalase activity was measured according to procedure of Aebi (1984) [13] at room temperature. 0.1 mL supernatant was mixed in a cuvette in which 1.9 mL of 50 mM phosphate buffer solution (pH 7.0) was added. Reaction was initiated by adding 1.0 mL of freshly prepared 30 mM H2O2. Absorbance at 240 nm was measured using a UV-VIS spectrophotometer (Labindia). Catalase activity was expressed as nmol H2O2 consumed/min/mL.
Renal histopathological examination
Immediately after sacrificing the rats, kidney was isolated and rinsed with ice cold 0.9% saline and kept in 10% formalin solution. After fixation with 10% formalin renal tissue was dehydrated through graded alcohol sequence and then embedded into paraffin wax. Paraffin blocks were sectioned and stained with haematoxylin and eosin (H&E). After fixation with DPX-292, kidney sections were observed under light microscope 400 × (Nikon Japan) and photographed using highly digital zoomed camera.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Results obtained from various groups were statistically analysed by One Way - ANOVA followed by Post hoc Tukey’s test. P < 0.05 was considered statistically significant.
Results
All drugs were dissolved in drinking water and administered orally in normal and diabetic rats for two weeks after eight weeks of STZ administration.
Effect of FSK on body weight
Diabetic rats subjected to HFD significantly increased the body weight when compared to normal control group. Two weeks treatment with FSK (10, 20 and 30 mg/kg), Glibenclamide (0.6 mg/kg) and Atorvastatin (0.5 mg/kg) reduced the body weight, however the results were not significant when compared with diabetic control group. Body weight was observed unaltered in treated rats and diabetic control group (Table 1).
Table 1.
Effect of FSK, Glibenclamide and Atorvastatin on body weight, serum glucose, BUN, protein in urine and urine output
| Groups | Body Weight (g) | Serum Glucose (mg/dL) | BUN (mg/dL) | Protein in Urine (mg/24 hrs.) | Urine output (mL/24 hrs.) |
|---|---|---|---|---|---|
| Normal Control | 210.1 ± 14.80 | 105.8 ± 9.95 | 19.12 ± 7.47 | 11.66 ± 2.83 | 8.42 ± 2.63 |
| FSK per se | 215.0 ± 13.80 | 106.9 ± 10.23 | 19.93 ± 5.18 | 11.94 ± 2.31 | 8.43 ± 2.50 |
| Diabetic Control | 328.0 ± 19.03a | 360.8 ± 16.90a | 102.90 ± 7.39a | 85.52 ± 3.33a | 76.57 ± 4.61a |
| FSK-10 in Diabetic group | 312.1 ± 18.68a | 336.6 ± 17.00a | 94.57 ± 6.79a | 78.43 ± 4.46a | 69.29 ± 4.64a |
| FSK-20 in Diabetic group | 321.0 ± 18.85a | 278.6 ± 13.22b | 76.89 ± 6.29b | 67.69 ± 3.73b | 62.29 ± 4.53b |
| FSK-30 in Diabetic group | 315.0 ± 16.83a | 203.9 ± 15.03b,c | 60.41 ± 6.90b,c | 54.16 ± 4.30b,c | 50.43 ± 4.72b,c |
| Glibenclamide in Diabetic group | 311.3 ± 19.64a | 169.4 ± 12.62b,d | 46.84 ± 6.68b,d | 43.69 ± 5.42b,d | 36.00 ± 3.87b,d |
| Atorvastatin in Diabetic group | 310.6 ± 14.23a | 345.0 ± 13.71a | 87.85 ± 5.89b | 72.53 ± 4.97b | 67.14 ± 4.94b |
| Glibenclamide + FSK-30 in Diabetic group | 307.6 ± 12.34a | 131.6 ± 9.67b,e | 32.08 ± 4.59b,e | 32.28 ± 3.96b,e | 23.71 ± 4.60b,e |
| Atorvastatin + FSK-30 in Diabetic group | 308.4 ± 12.73a | 234.7 ± 17.26b,f | 56.63 ± 8.11b,f | 56.63 ± 4.94b,f | 47.71 ± 3.94b,f |
Values are expressed as Mean ± SD (n = 7 per group).
P < 0.05 vs normal control, FSK per se group;
P < 0.05 vs diabetic control & FSK-10 mg/kg treated group;
P < 0.05 vs FSK-20 mg/kg treated group;
P < 0.05 vs FSK-30 mg/kg treated group;
P < 0.05 vs GB-0.6 mg/kg treated group;
P < 0.05 vs Atorvastatin-0.5 mg/kg treated group.
Effect of FSK on serum glucose, urine output, serum creatinine, BUN and protein in urine in diabetic rats
The concentration of serum glucose, urine output, serum creatinine, BUN and protein in urine were noted to be significantly increased (P < 0.05) in HFD and low dose of STZ administered rats when compared with normal rats. Treatment with low dose of FSK-10 mg/kg slightly reduced the biomarkers of nephropathy but the results were not statistically significant. However, treatment with intermediate and high dose of FSK (20 mg/kg and 30 mg/kg) significantly attenuated the levels of these markers in dose dependent manner.
Moreover, treatment with Glibenclamide-0.6 mg/kg significantly attenuated diabetes induced elevated biomarkers when compared with FSK-30 mg/kg treated group. Further, treatment with the combination of FSK-30 mg/kg and Glibenclamide-0.6 mg/kg treated group exhibited marked reduction in renal dysfunction parameters in treated rats when compared with Glibenclamide-0.6 mg/kg treated group.
Furthermore, treatment with Atorvastatin-0.5 mg/kg alone did not affect the serum glucose level. However, Atorvastatin-0.5 mg/kg in combination with FSK-30 mg/kg significantly reduced the serum glucose level and other renal dysfunction parameters when compared with diabetic control and Atorvastatin-0.5 mg/kg alone treated group (Tables 1 and 2).
Table 2.
Effect of FSK, Glibenclamide and Atorvastatin on serum creatinine, absolute kidney weight, kidney weight/body weight %, total renal collagen content and serum total cholesterol
| Groups | Serum Creatinine (mg/dL) | Absolute Kidney Weight (g) | Kidney weight/Body weight % | Total Renal Collagen Content (mg/g) | Serum Total Cholesterol (mg/dL) |
|---|---|---|---|---|---|
| Normal Control | 0.60 ± 0.12 | 0.42 ± 0.06 | 0.61 ± 0.04 | 2.32 ± 0.21 | 62.51 ± 6.79 |
| FSK per se | 0.58 ± 0.11 | 0.45 ± 0.11 | 0.58 ± 0.04 | 2.48 ± 0.24 | 60.18 ± 6.12 |
| Diabetic Control | 1.83 ± 0.07a | 1.35 ± 0.09a | 1.15 ± 0.06a | 5.95 ± 0.35a | 165.7 ± 6.77a |
| FSK-10 in Diabetic group | 1.72 ± 0.07a | 1.25 ± 0.06a | 1.08 ± 0.06a | 5.46 ± 0.27a | 154.3 ± 8.93a |
| FSK-20 in Diabetic group | 1.42 ± 0.06b | 1.11 ±0.09b | 1.01 ± 0.05b | 4.82 ± 0.24b | 127.9 ± 4.88b |
| FSK-30 in Diabetic group | 1.05 ± 0.08b,c | 0.93 ± 0.06b,c | 0.90 ± 0.04b,c | 3.94 ± 0.27b,c | 108.0 ± 7.09b,c |
| Glibenclamide in Diabetic group | 0.86 ± 0.06b,d | 0.77 ± 0.07b,d | 0.79 ± 0.05b,d | 3.21 ± 0.30b,d | 151.1 ± 7.73b |
| Atorvastatin in Diabetic group | 1.61 ± 0.11b | 1.13 ± 0.10b | 0.99 ± 0.04b | 4.55 ± 0.28b | 84.55 ± 8.16b,d |
| Glibenclamide + FSK-30 in Diabetic group | 0.69 ± 0.04b,e | 0.61 ± 0.06b,e | 0.69 ± 0.04b,e | 2.74 ± 0.27b,e | 103.1 ± 4.22b,e |
| Atorvastatin + FSK-30 in Diabetic group | 0.98 ± 0.07b,f | 0.80 ± 0.55b,f | 0.81 ± 0.05b,f | 4.08 ± 0.34b,f | 69.78 ± 8.38b,f |
Values are expressed as Mean ± SD (n = 7 per group).
P < 0.05 vs normal control, FSK per se group;
P < 0.05 vs diabetic control & FSK-10 mg/kg treated group;
P < 0.05 vs FSK-20 mg/kg treated group;
P < 0.05 vs FSK-30 mg/kg treated group;
P < 0.05 vs GB-0.6 mg/kg treated group;
P < 0.05 vs Atorvastatin-0.5 mg/kg treated group.
Effects of FSK on renal hypertrophy parameters
The level of renal hypertrophy (absolute kidney weight, kidney weight/body weight ratio and renal collagen content) were noted to be markedly increased (P < 0.05) in diabetic rats after eight weeks of STZ administration when compared with normal rats. Treatment with low dose of FSK-10 mg/kg for two weeks did not produce any significant effect on renal hypertrophy in treated rats when compared with diabetic control group.
However, treatment with FSK-20 mg/kg and FSK-30 mg/kg produced significant effects in dose dependent manner. Moreover, treatment with Glibenclamide-0.6 mg/kg significantly attenuated diabetes induced elevated renal hypertrophy parameters when compared with diabetic control group and FSK-30 mg/kg treated group. Further, treatment with the combination of FSK-30mg/kg and Glibenclamide-0.6 mg/kg treated group exhibited marked reduction in renal hypertrophy parameters when compared with Glibenclamide-0.6 mg/kg treated group.
However, treatment with Atorvastatin-0.5 mg/kg significantly reduced the renal hypertrophy when compared diabetic control group. Treatment with the combination of Atorvastatin-0.5 mg/kg and FSK-30 mg/kg exhibited marked reduction in absolute kidney weight, kidney weight/body weight and renal collagen content when compared with diabetic control group and Atorvastatin-0.5 mg/kg alone treated group (Table 2).
Effects of FSK on lipid profile
Diabetic rats subjected to HFD significantly (P < 0.05) increased the serum total cholesterol, LDL and VLDL level whereas HDL level was found to be significantly reduced. Treatment with FSK-10 mg/kg did not produce any significant effects on dyslipidaemia. However, two weeks treatment with FSK-20 mg/kg and FSK-30 mg/kg significantly reduced the elevated level of total cholesterol, LDL and VLDL and increased the level of HDL in treated rats when compared with diabetic control group.
Treatment with Glibenclamide-0.6 mg/kg significantly improved the lipid profile when compared with diabetic control group. Moreover, treatment with combination of Glibenclamide-0.6 mg/kg with FSK-30 mg/kg markedly improved the lipid profile when compared to Glibenclamide-0.6 mg/kg treated group.
However, treatment with Atorvastatin-0.5 mg/kg significantly attenuated diabetes induced dyslipidaemia when compared with diabetic control group and FSK-30 mg/kg treated group. Further, co-administration of Atorvastatin-0.5 mg/kg with FSK-30 mg/kg group exhibited marked reduction in total cholesterol, LDL and VLDL and increased HDL level when compared with Atorvastatin-0.5 mg/kg treated group (Tables 2 and 3).
Table 3.
Effect of FSK, Glibenclamide and Atorvastatin on HDL, LDL and VLDL
| Groups | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) |
|---|---|---|---|
| Normal Control | 8.64 ± 0.37 | 37.54 ± 6.12 | 15.76 ± 2.27 |
| FSK per se | 8.24 ± 0.50 | 37.71 ± 5.71 | 14.99 ± 1.99 |
| Diabetic Control | 3.25 ± 0.42a | 170.8 ± 5.23a | 43.87 ± 3.12a |
| FSK-10 in Diabetic group | 3.85 ± 0.42a | 156.1 ± 8.31a | 40.29 ± 2.68a |
| FSK-20 in Diabetic group | 4.83 ± 0.47b | 129.0 ± 9.62b | 35.15 ± 1.92b |
| FSK-30 in Diabetic group | 6.03 ± 0.40b,c | 107.1 ± 7.55b,c | 29.88 ± 2.44b,c |
| Glibenclamide in Diabetic group | 4.31 ± 0.54b | 147.7 ± 12.46b | 37.75 ± 2.67b |
| Atorvastatin in Diabetic group | 6.78 ± 0.45b,d | 70.41 ± 6.20b,d | 22.57 ± 2.07b,d |
| Glibenclamide + FSK-30 in Diabetic group | 6.55 ± 0.31b,e | 101.2 ± 8.81b,e | 27.88 ± 2.25b,e |
| Atorvastatin + FSK-30 in Diabetic group | 7.90 ± 0.43b,f | 39.58 ± 6.23b,f | 17.74 ± 1.62b,f |
Values are expressed as Mean ± SD (n = 7 per group).
P < 0.05 vs normal control, FSK per se group;
P < 0.05 vs diabetic control & FSK-10 mg/kg treated group;
P < 0.05 vs FSK-20 mg/kg treated group;
P < 0.05 vs FSK-30 mg/kg treated group;
P < 0.05 vs GB-0.6 mg/kg treated group;
P < 0.05 vs Atorvastatin-0.5 mg/kg treated group.
Effects of FSK on renal TBARS level
TBARS level was significantly increased in diabetic control group (P < 0.05) as compared to normal control group. Treatment with FSK-10 mg/kg did not produce any significant effects on TBARS level. However, two weeks treatment with FSK-20 mg/kg and FSK-30 mg/kg significantly reduced the elevated level of TBARS level in dose dependent manner when compared with diabetic control group.
Treatment with Glibenclamide-0.6 mg/kg significantly reduced the elevated TBARS level in treated rats. Moreover, treatment with combination of Glibenclamide-0.6 mg/kg with FSK-30 mg/kg markedly reduced the diabetes induced elevated TBARS when compared to Glibenclamide-0.6 mg/kg treated group.
However, treatment with Atorvastatin-0.5 mg/kg significantly attenuated diabetes induced elevated TBARS when compared with diabetic control group. Further, co-administration of Atorvastatin-0.5 mg/kg with FSK-30 mg/kg group exhibited marked reduction in TBARS when compared with Atorvastatin-0.5 mg/kg treated group (Table 4).
Table 4.
Effect of FSK, Glibenclamide and Atorvastatin on TBARS, glutathione, SOD and catalase
| Groups | TBARS (nmol/mg) | GSH (μM/mg protein) | SOD (µmol/min/mL) | Catalase (nmol H2O2 consumed/min/mL) |
|---|---|---|---|---|
| Normal Control | 0.60 ± 0.09 | 232.6 ± 11.83 | 14.22 ± 0.85 | 7.61 ± 0.57 |
| FSK per se | 0.62 ± 0.06 | 232.9 ± 10.75 | 13.29 ± 1.11 | 7.17 ± 0.51 |
| Diabetic Control | 1.77 ± 0.12a | 97.71 ± 10.95a | 3.64 ± 0.94a | 2.51 ± 0.44a |
| FSK-10 in Diabetic group | 1.66 ± 0.09a | 106.6 ± 10.39a | 4.62 ± 0.63a | 3.02 ± 0.29a |
| FSK-20 in Diabetic group | 1.47 ± 0.08b | 136.4 ± 9.88b | 9.14 ± 0.69b | 4.37 ± 0.39b |
| FSK-30 in Diabetic group | 1.17 ± 0.07b,c | 162.90 ± 12.54b,c | 10.71 ± 0.78b,c | 5.24 ± 0.33b,c |
| Glibenclamide in Diabetic group | 0.99 ± 0.09b,d | 197.0 ± 12.94b,d | 12.25 ± 0.94b,d | 6.14 ± 0.36b,d |
| Atorvastatin in Diabetic group | 1.57 ± 0.08b | 139.3 ± 13.05b | 9.00 ± 0.81b | 4.31 ± 0.56b |
| Glibenclamide + FSK-30 in Diabetic group | 0.75 ± 0.09b,e | 230.6 ± 16.25b,e | 13.79 ± 0.69b,e | 6.98 ± 0.40b,e |
| Atorvastatin + FSK-30 in Diabetic group | 1.02 ± 0.10b,f | 175.90 ± 10.88b,f | 12.00 ± 1.00b,f | 5.58 ± 0.29b,f |
Values are expressed as Mean ± SD (n = 7 per group).
P < 0.05 vs normal control, FSK per se group;
P < 0.05 vs diabetic control & FSK-10 mg/kg treated group;
P < 0.05 vs FSK-20 mg/kg treated group;
P < 0.05 vs FSK-30 mg/kg treated group;
P < 0.05 vs GB-0.6 mg/kg treated group;
P < 0.05 vs Atorvastatin-0.5 mg/kg treated group.
Effects of FSK on antioxidants enzymes activities (GSH, SOD and Catalase)
A marked decrease (P < 0.05) in antioxidant enzymes (GSH, SOD and Catalase) activity was noted in diabetic rats when compared with normal rats. Treatment with FSK-10 mg/kg improved the enzymatic activities but results obtained were not statistically significant. Treatment with intermediate and high dose of FSK (20 mg/kg and 30 mg/kg) significantly improved the levels of these markers when compared with diabetic control group.
However, treatment with Glibenclamide-0.6 mg/kg significantly improved the level of these markers in treated rats. Moreover, treatment with combination of Glibenclamide-0.6 mg/kg with FSK-30 mg/kg markedly enhanced the diabetes induced reduced antioxidants activities when compared with Glibenclamide-0.6 mg/kg treated group.
However, treatment with Atorvastatin-0.5 mg/kg significantly raised the activities when compared with diabetic control group.
Further, co-administration of Atorvastatin-0.5 mg/kg with FSK-30 mg/kg group exhibited marked increased in levels of these antioxidant enzymes when compared with Atorvastatin-0.5 mg/kg treated group (Table 4).
Effects of FSK on renal histology
Kidney sections stained with H&E clearly indicates normal glomeruli and tubules of normal control rats as compared to diabetic rats. However, glomerulosclerosis, vacuolar degeneration of tubules, mesangial cell expansion, thick wall and narrow lumen were observed in diabetic control rats. These pathological changes were significantly reduced when diabetic rats were treated with FSK (20 mg/kg and 30 mg/kg) and combination of FSK-30 mg/kg with Glibenclamide-0.6 mg/kg and Atorvastatin-0.5 mg/kg (Figure 1).
Figure 1.
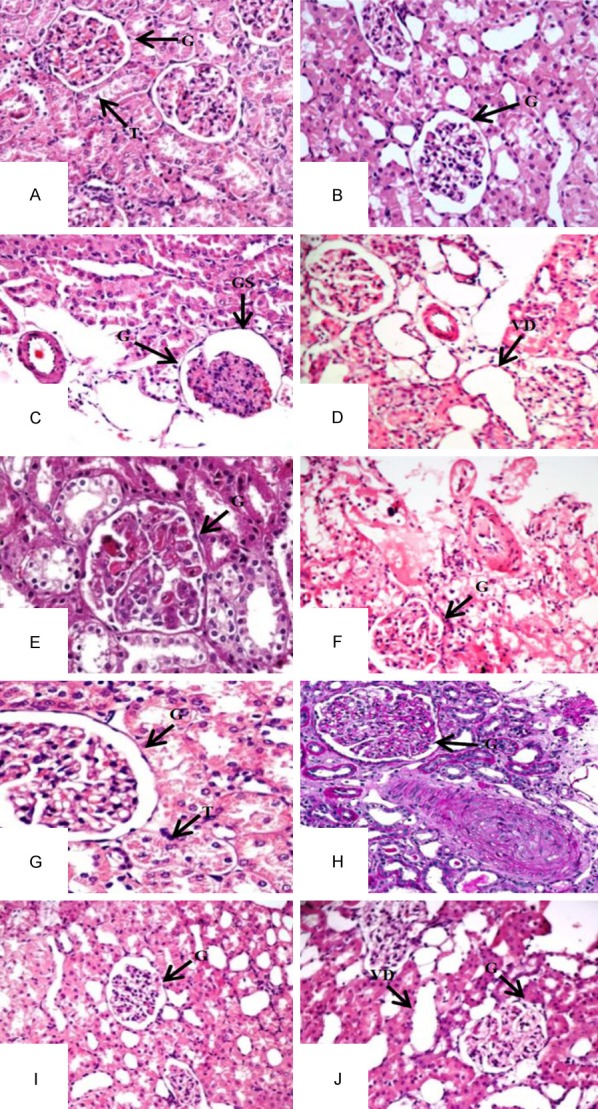
Kidney photomicrographs using Haematoxylin and Eosin Staining. A: Normal Control; B: FSK per se; C: Diabetic Control; D: FSK-10 mg/kg; E: FSK-20 mg/kg; F: FSK-30 mg/kg; G: Glibenclamide-0.6 mg/kg; H: Atorvastatin-0.5 mg/kg; I: Glibenclamide-0.6 mg/kg + FSK-30 mg/kg; J: Atorvastatin-0.5 mg/kg + FSK-30 mg/kg. G: Glomeruli, T: Tubules, GS: Glomerular Space, VD: Vacuolar Degeneration.
Discussion
Metabolic disturbance, oxidative stress, and podocyte injury play important role in the progression of DN. Meanwhile, FSK was first demonstrated to be a hypotensive agent with anti-spasmolytic and cardiotonic activity [14]. In the present study, we investigated the effect of FSK on DN. Our results indicated that FSK can be a potential drug that can prevent renal dysfunction, dyslipidaemia, oxidative stress and DN in HFD and STZ administered wistar rats.
The various studies in the DN shows that there is a reduction in the body weight of the diabetic control animals due to the increased muscle wasting and loss of tissue proteins in diabetes [15]. In our study, the body weight was significantly increased in the diabetic rats when compared with normal rats due to free access to HFD. It has been reported that rats fed with HFD followed by STZ-low dose develops Type-II DM [16]. No significant effect on body weight was observed when diabetic rats were treated with different dose of FSK alone or in combination with Glibenclamide and Atorvastatin for two weeks after eight weeks of STZ administration.
Altered glomerular filtration rate is the main indicator of DN and many studies have reported that the administration of STZ in the rats elevate the serum renal markers [17,18]. Elevated level of serum creatinine and proteinuria is an indication of renal dysfunction [19,20]. To evaluate the renal functions, various biochemical parameters were performed in blood serum, urine and in tissue homogenate like serum glucose, serum creatinine, BUN, protein in urine, absolute kidney weight, renal hypertrophy, total renal collagen content were performed.
Eight weeks after STZ administration, significantly increased concentration of biochemical parameters were observed which indicated development of DN [21]. Reversed effects were observed when diabetic rats were treated with intermediate and high dose of adenylyl cyclase activator, FSK (20 mg/kg and 30 mg/kg). Moreover, FSK treatment improved the structure and functions of kidney and prevented the development of DN and concurrently reduced the glucose level.
Moreover, absolute kidney weight and ratio of kidney weight/body weight was noted to be significantly elevated when compared with normal rats. These changes confirmed the development of renal structural abnormalities and nephropathy in diabetic kidney after eight weeks of STZ administration. Structural and functional changes were significantly prevented in diabetic rats when treated with FSK 30 mg/kg. On the basis of above discussion it may be concluded that FSK 30 mg/kg without affecting the body weight, prevented the development of DN in rats subjected to HFD.
There is a strong correlation between insulin deficiency and insulin resistance with dyslipidaemia. Dyslipidaemia has been reported to play critical role in the development and progression of DN in rats [22]. Studies have reported that the increased level of lipids in diabetes increases the risk of DN [23]. Elevated level of lipids leads to glomerular and tubular dysfunction in diabetes due to increased intracellular concentration of fatty acids [24]. According to the survey by the National Health and Nutrition Examination Survey, 61% of patients with type 2 diabetes were obese and 46% of diabetic patients had lipid abnormalities [25].
Lipid profile was estimated by measuring serum total cholesterol, LDL, HDL and VLDL level. HDL concentration was noted to be significantly reduced and level of serum total cholesterol, LDL and VLDL were noted be increased in diabetic rats. Treatment with FSK (20 mg/kg and 30 mg/kg) significantly reversed the level of HDL and total cholesterol, LDL and VLDL. Combinatorial effects of Atorvastatin 0.5 mg/kg with FSK 30 mg/kg presented synergistic results when compared with diabetic rats treated with Atorvastatin 0.5 mg/kg or FSK 30 mg/kg alone.
Kidneys are the most susceptible organs to oxidative damage caused by the free radicals. Hyperglycaemic conditions promote the oxidative stress resulting from the increased generation of reactive oxygen species, plays important role in pathophysiology of DN [26]. In DN patients, there is an increased production of free radicals which leads to lipid peroxidation. It has also been reported that free radical generation also decrease the activity of antioxidant enzymes due to auto oxidation and non-enzymatic glycosylation [27]. GSH, SOD and Catalase are the enzymes which scavenge the free radicals by destroying the peroxides and provide the antioxidant defence mechanism [28,29].
Oxidative stress was assessed by measuring the level of TBARS and level of endogenous antioxidant enzymes were analysed by evaluating the activity of GSH, SOD and CAT in diabetic rats. Two weeks treatment with FSK decreased TBARS level and increased the level of endogenous antioxidants activities (GSH, SOD and CAT) in diabetic rats in dose dependent manner when compared with untreated rats.
FSK directly activates AC, which increases intracellular cAMP levels [30]. Further, it has been reported that Adenosine monophosphate Activated Protein Kinase (AMPK) may be a key factor that regulate lipid metabolism, antioxidant, anti-inflammatory activity and inhibits Tumor Necrosis Factor (TNF)-α, 1β, 6 and 8 [31]. The activation of the cAMP-dependent protein kinase (PKA) may inhibit TNF-α and nuclear factor-kappa B (NF-κB) which is implicated in inflammation & oxidative stress. NF-κB is produced by almost all cell types and is activated by a wide variety of cell-stress stimuli including hyperglycaemia, obesity, increased plasma free fatty acids, oxidative stress, hypertension, proteinuria and renal fibrosis etc. [32,33].
To further elucidate the renoprotective action of FSK we have done histopathological analysis using H&E staining revealed the occurrence of glomerulosclerosis, vacuolar degeneration of tubules, mesangial cell expansion with extracellular matrix accumulation in glomerular structure in diabetic kidney.
In the present study, we analysed the effect of FSK on biomarkers of DN, oxidative stress and concluded that FSK employed renoprotective effects by improving kidney functions partly by reducing hyperglycaemia and partly by increasing intracellular cAMP level by enhancing the PKA and CREB phosphorylation activation in diabetic rats.
Conclusion
On the basis of our study, it may be concluded that in diabetic condition marked increase in the biomarkers of renal hypertrophy, oxidative stress and dyslipidaemia play pathologic role in the development of nephropathy in rats. Our study demonstrated that Forskolin has potential to prevent diabetes induced renal hypertrophy, oxidative stress and dyslipidaemia. Treatment with Forskolin reduced the progression of DN by preventing kidney functional and structural abnormalities. Moreover, FSK has a potential to enhance the effect of lipid lowering and anti-diabetic activity of standard drugs.
Acknowledgements
We would like to thanks I. K. Gujral Punjab Technical University Jalandhar (India) for the support and encouragement.
Disclosure of conflict of interest
None.
References
- 1.Xue R, Gui D, Zheng L, Zhai R, Wang F, Wang N. Mechanistic insight and management of diabetic nephropathy: recent progress and future perspective. J Diabetes Res. 2017;2017:1839809. doi: 10.1155/2017/1839809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kikkawa R. Chronic complications in diabetes mellitus. Br J Nutr. 2000;84:S183–5. doi: 10.1079/096582197388653. [DOI] [PubMed] [Google Scholar]
- 3.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–12. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elisabeth S, Veronika W, Jens S. Cyclic nucleotide signalling in kidney fibrosis. Int J Mol Sci. 2015;16:2320–2351. doi: 10.3390/ijms16022320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva MR, Trujillo X, Hernandez BT, Pastor ES, Urzua Z, Mancilla E, Urzúa Z, Mancilla E, Huerta M. Effect of chronic administration of forskolin on glycemia and oxidative stress in rats with and without experimental diabetes. Int J Med Sci. 2014;11:448–52. doi: 10.7150/ijms.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehan S, Parveen S, Kalra S. Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Regen Res. 2017;12:290–300. doi: 10.4103/1673-5374.200812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arya A, Yadav HN, Sharma PL. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Mol Cell Biochem. 2011;354:57–66. doi: 10.1007/s11010-011-0805-6. [DOI] [PubMed] [Google Scholar]
- 8.Owolabi OJ, Omogbai EK. Co-administration of glibenclamide and amlodipine induces resistance to hyperglycemic treatment in streptozotocin induced adapted/non adapted diabetic rats. Clin Exp Pharmacol. 2011;1:102. [Google Scholar]
- 9.Sun H, Yuan Y, Sun Z. Cholesterol contributes to diabetic nephropathy through SCAP-SREBP-2 pathway. Int J Endocrinol. 2013;2013:592576. doi: 10.1155/2013/592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 11.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 13.Aebi H. Catalase in vitro. Method Enzym. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 14.Lmdner E, Dohadwalla AN, Bhartacharya BK. Positive inotropic and blood pressure lowering activity of a diterpene derivative isolated from Coleus forskohli: forskolin. Arzneimittelforschung. 1978;28:284–9. [PubMed] [Google Scholar]
- 15.Swanston-Fiatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes: studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–4. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan K, Viswanad B, Lydia A, Kaul CL, Ramarao P. Combination of high-fat diet-fed, low-dose streptozotocin treated rat. A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–20. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Alderson NL, Chachich ME, Frizzell N, Canning P, Metz TO, Januszewski AS, Youssef NN, Stitt AW, Baynes JW, Thorpe SR. Effect of antioxidants and ACE inhibition on chemical modification of proteins and progression of nephropathy in the streptozotocin diabetic rat. Diabetologia. 2004;47:1385–95. doi: 10.1007/s00125-004-1474-8. [DOI] [PubMed] [Google Scholar]
- 18.Mauer SM, Steffes MW, Brown DM. The kidney in diabetes. Am J Med. 1981;70:603–12. doi: 10.1016/0002-9343(81)90582-9. [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Budhiraja S, Lal H, Arora BR. Renoprotection by telmisartan versus benazepril in streptozotocin induced diabetic nephropathy. Iranian J Pharmacol Ther. 2006;5:135–9. [Google Scholar]
- 20.Kadian S, Mahadevan N, Balakumar P. Differential effects of low-dose fenofibrate treatment in diabetic rats with early onset nephropathy and established nephropathy. Eur J Pharmacol. 2013;698:388–96. doi: 10.1016/j.ejphar.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Campion CG, Sanchez-Ferras O, Batchu SN. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis. 2017;4:1–18. doi: 10.1177/2054358117705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawanami D, Matoba K, Utsunomiya K. Dyslipidemia in diabetic nephropathy. Renal Replacement Therapy. 2016;2:16. [Google Scholar]
- 23.Shivanand KG, Manjunath ML, Jeganathan PS. Lipid profile and its complications in diabetes mellitus. Int J Biomed Adv Res. 2012;3:775–80. [Google Scholar]
- 24.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5:2373–9. doi: 10.2215/CJN.08160910. [DOI] [PubMed] [Google Scholar]
- 25.Suh DC, Choi IS, Plauschinat C, Kwon J, Baron M. Impact of comorbid conditions and race/ethnicity on glycemic control among the US population with type 2 diabetes, 1988-1994 to 1999-2004. J Diabetes Complications. 2010;24:382–91. doi: 10.1016/j.jdiacomp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Alhaider AA, Korashy HM, Ahmed MS, Mobark M, Kfoury H, Mansour MA. Metormin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192:233–42. doi: 10.1016/j.cbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Ayalasomayajula SP, Kompella UB. Subconjunctivally administered celecoxib-PLGA microparticles sustain retinal drug levels and alleviate diabetes-induced oxidative stress in a rat model. Eur J Pharmacol. 2005;511:191–8. doi: 10.1016/j.ejphar.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Bolzán AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512:121–34. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh T, Maity TK, Sengupta P, Dash DK, Bose A. Antidiabetic and in vivo antioxidant activity of ethanolic extract of Bacopamonnieri L. Aerial parts: a possible mechanism of action. Iran J Pharm Res. 2008;7:61–8. [Google Scholar]
- 30.Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signalling. Cell Mol Neurobiol. 2003;23:305–14. doi: 10.1023/A:1023684503883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashida N, Chihara S, Tayama E, Takaseya T, Enomoto N, Kawara T, Enomoto N, Kawara T, Aoyagi S. Antiinflammatory effects of colforsindaropate hydrochloride, a novel water-soluble forskolin derivative. Ann Thorac Surg. 2001;71:1931–8. doi: 10.1016/s0003-4975(01)02531-0. [DOI] [PubMed] [Google Scholar]
- 32.Soetikno V, Sari F, Veeraveedu P, Thandavarayan R, Harima M, Sukumaran V, Lakshmanan AP, Suzuki K, Kawachi H, Watanabe K. Curcumin ameliorates macrophage infiltration by inhibiting NF-kappaB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab. 2011;8:35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124:139–52. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]


