Abstract
We report the case of a newborn baby with an unguarded mitral orifice associated with asplenia syndrome, double-outlet right ventricle, dysplastic tricuspid valve, and pulmonary stenosis. This case was accompanied by severe tricuspid regurgitation and severe right ventricular hypertrophy. The patient had a fatal clinical course due to severe hypoxia and congestive heart failure. Unguarded mitral orifice is a rare disease in which there has been no previous report of lethal clinical course during the neonatal period. Prior reports stated that unguarded mitral orifice was a new constellation of defects and that its etiology and embryology could be classified in the same category because of similar associated malformations of double-outlet right ventricle and pulmonary stenosis or atresia. However, the present case was diagnosed on autopsy as also having asplenia syndrome. Therefore, it is possible that the genetic etiology of unguarded mitral orifice in this case was different from cases of non-heterotaxy.
<Learning objective: Unguarded mitral orifice is a rare disease that might be associated with asplenia syndrome and dysplastic tricuspid valve. If unguarded mitral orifice is associated with such defects, the clinical course can be fatal. Therefore, when this diagnosis is recognized, the physician should explain the possibility of neonatal death and plan the treatment of such a case to include grief therapy for the family.>
Keywords: Unguarded mitral orifice, Asplenia syndrome, Dysplastic tricuspid valve, Double outlet right ventricle, Atrioventricular valve regurgitation
Introduction
Unguarded mitral orifice (UMO) is an extremely rare disease characterized by the complete absence of mitral valve leaflets and tensor apparatus (chordae tendineae and papillary muscles) at the mitral annulus and severe thinning of the left ventricular free wall. Only five cases have been reported in the English literature [1], [2], [3], [4]. All cases (except for the most recent case of hypoplastic left heart syndrome) were accompanied by double-outlet right ventricle, atrioventricular discordance, and pulmonary stenosis/atresia [1], [2], [3]. All five cases survived through the neonatal period and infancy. The present report describes the case of a patient with UMO who died on the second day of life due to severe heart failure and hypoxia. This patient had UMO, dysplasia of the tricuspid valve, and hypertrophy of the right ventricle, leading to circulatory collapse and death.
Case report
The family of the patient provided permission to publish the features of this case. The identity of the patient has been protected.
The male fetus was referred to Osaka Medical College Hospital at 36 weeks’ gestation because a cardiac anomaly was suspected. An ultrasound study interpreted the anomaly as double-outlet right ventricle, hypoplastic right ventricle, pulmonary atresia, and complete atrioventricular septal defect (cAVSD). However, UMO was not acknowledged and so we misinterpreted the diagnosis as cAVSD at that time. Severe common atrioventricular valve regurgitation and cardiomegaly with cardiothoracic area ratio of 56% were observed, so he was delivered at 36 weeks’ gestation via emergent Cesarean section with a birth weight of 2398 g. Because of severe hypoxia and bradycardia, he was resuscitated by intubation and oxygen inhalation and was admitted to the neonatal intensive care unit.
On admission, blood pressure was 56/29 mmHg, and oxygen saturation was 70% (with an FiO2 of 100%). A Levine grade II/VI systolic murmur was audible at the right upper sternal border. Blood gas analysis revealed severe hypoxia and lactic acidosis (pH 7.02; lactate 97 mg/dL). Chest radiograph showed a markedly enlarged cardiac silhouette with cardiothoracic ratio of 69% and decreased pulmonary vascular markings.
Postnatal echocardiography revealed ambiguous atrial sidedness and showed that the inferior vena cava and pulmonary vein drained into a left-sided atrium. Both great arteries arose from the right ventricle, and the pulmonary valve and main pulmonary artery were hypoplastic with severe subpulmonary stenosis. Ductus arteriosus was not detected.
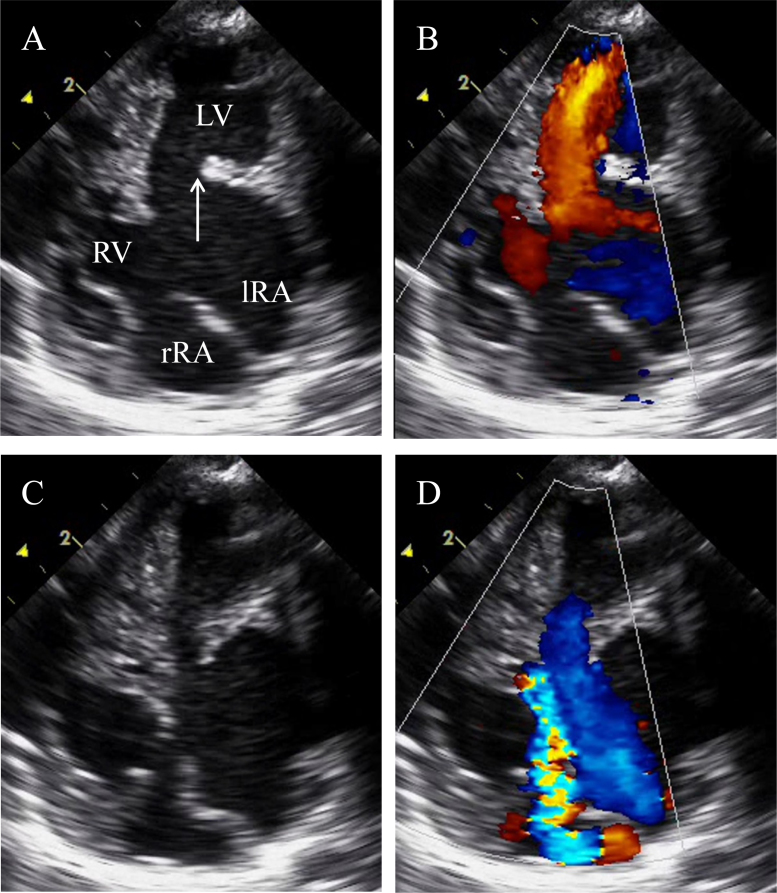
A small secundum and a large primum atrial septal defect were present, but the ventricular septum appeared intact. Only a ridge, but no mitral valve leaflets or tensor apparatus were seen at the left atrioventricular junction (Fig. 1A and B). The left-sided morphologic left ventricle appeared dilated and thin-walled, with poor contractility (Fig. 1C). Doppler examination of the left atrioventricular junction revealed free regurgitation (Fig. 1D). Severe regurgitation of a dysplastic right-sided atrioventricular valve was observed (Fig. 1D). The right ventricular wall was markedly hypertrophied, and the right ventricular chamber volume was diminished (Fig. 1C).
Fig. 1.
Echocardiographic findings. Representative four-chamber views. (A) Transthoracic echocardiography demonstrating the unguarded mitral valve orifice (white arrow), thinned left ventricle walls, and a large primum atrial septal defect. (B) Diastolic frame demonstrating color-flow Doppler through the mitral valve and tricuspid valve. (C) and (D) Systolic frame reveals free regurgitation of the unguarded mitral orifice and severe regurgitation of the tricuspid valve. lRA, left-sided morphologic right atrium; rRA, right-sided morphologic right atrium; RV, right ventricle; LV, left ventricle.
Despite 100% oxygen inhalation, nitric oxide inhalation, and prostaglandin E1 infusion, the hypoxia did not improve. Dopamine infusion was initiated because of hypotension. On the first day of life, prostaglandin E1 infusion was discontinued because of its influence on the systemic blood pressure and because of no observed reopening of the arterial duct. After that, the patient's clinical state was relatively stable, with mild desaturation (75% at room air). On the second day of life, the patient collapsed with sudden hypoxia due to muscular subpulmonary stenosis and hypotension. Volume infusion and inotropic support temporarily improved oxygenation and blood pressure. However, metabolic acidosis continued and the baby died despite maximal intensive care support. Post-mortem autopsy was performed.
Autopsy
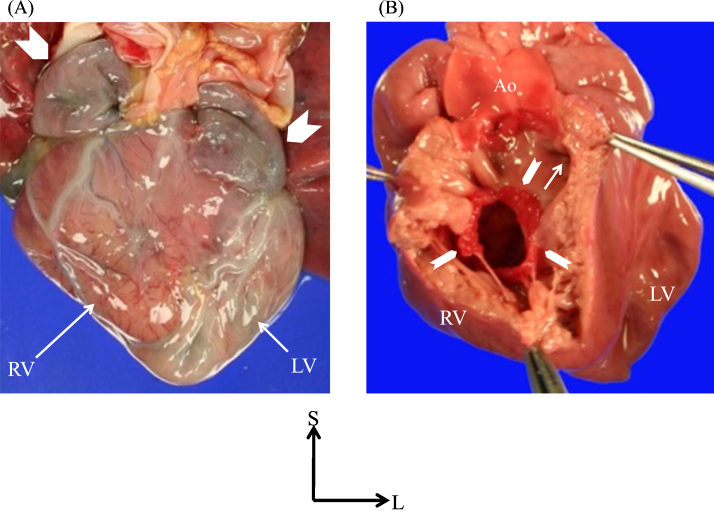
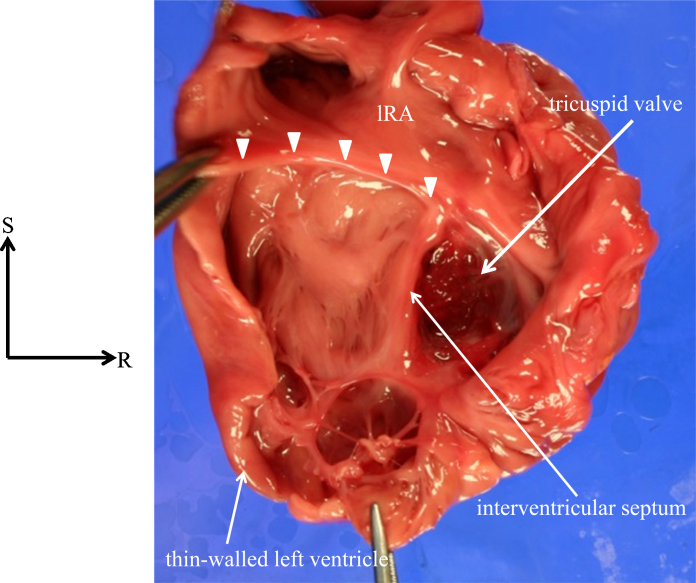
The thoracic and abdominal organs indicated right isomerism without a spleen. The heart was huge and was located in the midline. From the frontal view, the atrial appendages on both sides had the pattern of the morphologically right atrial appendage (Fig. 2A). The left-sided ventricle was remarkably dilated and showed a parchment-like appearance with a very thin wall (1.0 mm) (Fig. 3). Right ventricular hypertrophy was remarkable, such that the right ventricle had a small intraventricular cavity (Fig. 2B). Both great arteries were malpositioned, arising from the right ventricle, with the aortic annulus located rightward and anterior to the pulmonary valve annulus. Of particular significance was the absence of any mitral valve leaflets or apparatus, as well as findings such as the appearance of a ridge formation at the junction between the left-sided right atrium and left-sided left ventricle, and a fibrous network visible in the apex of the left ventricle (Fig. 3). The right atrioventricular valve was dysplastic and myxomatous (Fig. 2B).
Fig. 2.
Autopsy findings of the heart viewed anteriorly. (A) Frontal view of pathological specimen of current case. The frontal view shows the dilated morphologically left ventricle on the left and the thick-walled morphologically right ventricle on the right. The double-sided atrial appendage demonstrated the morphologically right atrial appendage (arrowheads), representing right isomerism of the heart. (B) The right-sided morphologically right ventricle is opened and viewed anteriorly. The right-sided atrioventricular valve (arrowheads) is dysplastic and myxomatous. Both great arteries arise from the morphologically right ventricle, with a remarkably hypertrophied right ventricle with diminished chamber size, producing severe subpulmonary stenosis. The arrow points the ostium of subpulmonary infundibulum. Ao, aorta; RV, right ventricle; LV, left ventricle; S, superior; L, left.
Fig. 3.
Autopsy findings of the heart viewed posteriorly. The left-sided morphologically left ventricle (LV) and the left-sided morphologically right atrium are opened and viewed posteriorly. There are no valve leaflets at the mitral orifice (arrowheads), but some fibrous tissue and a network of fibroelastic thin bundles are present at the apex of the LV. lRA, left-sided morphologic right atrium; S, superior; R, right.
Discussion
UMO is a rare condition. Only five cases have been reported to date [1], [2], [3], [4]. Four of five cases have been reported in hearts associated with situs inversus, double-outlet right ventricle, and pulmonary atresia/stenosis [1], [2], [3]. The current case had morphological similarities, such as double-outlet right ventricle and pulmonary stenosis; moreover, the case was complicated by asplenia syndrome, dysplastic right-sided atrioventricular valve, and valve regurgitation. Owing to severe pulmonary stenosis, hypertrophied right ventricle and valve regurgitation, the patient suffered severe hypoxia and low cardiac output. In contrast to the previously reported five cases, the present case had a fatal clinical course.
The etiology of this rare anomaly of UMO remains unclear. Yasukochi et al. considered that UMO could be the extreme end of the spectrum of Ebstein's anomaly of the mitral valve [1], [5]. These investigators also suggested that a disorder undermining the mitral valve is a possible mechanism and might also be responsible for unguarded tricuspid valve [1], [5]. Moreover, the undermining process can be introduced by either apoptosis of the myocardial cells or maldevelopment of the mesenchymal tissue [6]. Hwang et al. suggested that UMO and double-outlet right ventricle with pulmonary atresia/stenosis may well be etiologically and embryologically interrelated and could be explained by a single cause, because many uncommon abnormalities occur in such strikingly consistent association with one another [3]. However, including the present case, two previous cases have been associated with asplenia syndrome [2]. Therefore, cases with asplenia syndrome should be embryologically distinguished from non-asplenic cases. Asplenic cases and non-asplenic cases have the same phenotypic expressions, such as situ inversus, double-outlet right ventricle, and pulmonary atresia/stenosis, but asplenic and non-asplenic cases may have different genetic origins [7], [8].
Indeed, including the present case, four of five cases had virtually the same cardiovascular pathology. The reason for this can be explained hemodynamically. UMO accompanied by congenital cardiac defects other than double-outlet right ventricle, in which the left ventricle functioned as the main chamber, is fatal during the fetal period, due to massive atrioventricular valve regurgitation in the main ventricular chamber. A patient in the setting of double-outlet right ventricle can survive, because the affected dilated ventricle is not involved with the systemic or pulmonary circulation. Recently, Su et al. reported a case of UMO associated with hypoplastic left heart syndrome [4]. Similarly, in that case, the right ventricle supplied the systemic circulation, and the left ventricle, which was affected by UMO, did not function as a main chamber. Thus that patient survived during the fetal and neonatal period.
Two of the reported five cases subsequently developed severe congestive heart failure and died of congestive heart failure at 17 years and 13 months of age, respectively [1]. UMO is characterized by a markedly dilated left heart and severe congestive left ventricle failure. Left ventricular pump dysfunction, coupled with compression of the right heart by an enlarged left heart, reduces the effective preload on the right ventricle [9]. Therefore, the left ventricular volume overload may reduce systemic output and impair contractile function of a potentially normal right ventricle [9]. However, in contrast to the other five reported cases, the present patient also had tricuspid dysplasia and a hypertrophied right ventricle. These two morphologic features influenced the performance of the right ventricle, which functioned as the main ventricular chamber of the double-outlet right ventricle. Thus, the patient had a fatal clinical course.
In the current case, shunting palliation could be an option of treatment. However, shunting palliation would deteriorate tricuspid valve regurgitation, so tricuspid valve plasty should be performed at the same time. But tricuspid valve plasty for such dysplastic valve performed in the neonatal period would carry a high risk and would not give favorable results. So we did not select surgical interventions.
We have reported a case of a neonate with UMO that was associated with asplenia syndrome, double-outlet right ventricle, dysplastic tricuspid valve, and pulmonary stenosis. This case was accompanied by severe tricuspid regurgitation and severe right ventricular hypertrophy, causing a fatal clinical course.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Yasukochi S., Satomi G., Park I., Ando M., Momma K. Unguarded mitral orifice, mirror-imaged atrial arrangement, and discordant atrioventricular connections. Cardiol Young. 1999;9:478–483. doi: 10.1017/s1047951100005382. [DOI] [PubMed] [Google Scholar]
- 2.Earing M.G., Edwards W.D., Puga F.J., Cabalka A.K. Unguarded mitral orifice associated with discordant atrioventricular connection, double-outlet right ventricle, and pulmonary atresia. Pediatr Cardiol. 2003;24:490–492. doi: 10.1007/s00246-002-0389-8. [DOI] [PubMed] [Google Scholar]
- 3.Hwang M.S., Chang Y.S., Chu J.J., Lin W.S., Su W.J. A potential new constellation of defects: unguarded mitral orifice associated with double-outlet right ventricle {I, D, D} and pulmonary atresia/stenosis. Int J Cardiol. 2011;148:354–357. doi: 10.1016/j.ijcard.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Su J.A., Ho J., Wong P.C. Unguarded mitral orifice associated with hypoplastic left heart syndrome. Cardiol Young. 2015;25:1002–1005. doi: 10.1017/S1047951114001334. [DOI] [PubMed] [Google Scholar]
- 5.Anderson K.R., Zuberbuhler J.R., Anderson R.H., Becker A.E., Lie J.T. Morphologic spectrum of Ebstein's anomaly of the heart: a review. Mayo Clin Proc. 1979;54:174–180. [PubMed] [Google Scholar]
- 6.James T.N., Nichols M.M., Sapire D.W., DiPatre P.L., Lopez S.M. Complete heart block and fatal right ventricular failure in an infant. Circulation. 1996;93:1588–1600. doi: 10.1161/01.cir.93.8.1588. [DOI] [PubMed] [Google Scholar]
- 7.Shiraishi I., Ichikawa H. Human heterotaxy syndrome – from molecular genetics to clinical features, management, and prognosis. Circ J. 2012;76:2066–2075. doi: 10.1253/circj.cj-12-0957. [DOI] [PubMed] [Google Scholar]
- 8.Andersen T.A., Troelsen K.L.L., Larsen L.A. Of mice and men: molecular genetics of congenital heart disease. Cell Mol Life Sci. 2014;71:1327–1352. doi: 10.1007/s00018-013-1430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takagaki M., Ishino K., Kawada M., Ohtsuki S., Hirota M., Tedoriya T., Tanabe Y., Nakai M., Sano S. Total right ventricular exclusion improves left ventricular function in patients with end-stage congestive right ventricular failure. Circulation. 2003;108:II226–II229. doi: 10.1161/01.cir.0000087382.12277.49. [DOI] [PubMed] [Google Scholar]