Abstract
Background
The reasons for differences in vaccine effectiveness between live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV) are not clear.
Methods
Blood samples were obtained before vaccination and at days 7 and 21 postvaccination with 2015–2016 quadrivalent IIV or LAIV. Serologic response to the vaccine was measured by hemagglutination inhibition assay. Targeted RNA sequencing and serum cytokine analysis were performed. Paired analyses were used to determine gene expression and were compared between IIV and LAIV recipients. Classification And Regression Trees analysis (CART) identified the strongest associations with vaccine response.
Results
Forty-six enrollees received IIV, and 25 received LAIV. The mean age was 11.5 (±3.7) years. Seroconversion with IIV was associated with changes in expression of PRKRA and IFI6. Nonseroconversion for both IIV and LAIV was characterized by increased interferon-stimulated gene expression. Seroprotection with both vaccines was associated with altered expression of CXCL2 and CD36. For LAIV, CART showed that changes in expression of CD80, CXCL2, and CASP1 were associated with seroprotection. Serum cytokines showed that IIV seroconversion was associated with decreased CCL3. LAIV seroprotection tracked with decreased tumor necrosis factor–α and interferon-γ.
Conclusions
Distinct markers of seroconversion and seroprotection against IIV and LAIV were identified using immunophenotyping and CART analysis.
Keywords: antibodies, cytokines, influenza vaccine, RNA seq
Influenza infection presents a global health challenge with limited therapeutic options. Recommended annual vaccination coverage is suboptimal, and vaccine effectiveness (VE) varies across seasons, virus strains, and vaccine types. Even in seasons with good vaccine strain match to circulating viruses, VE is often around 50%–60%. For example, in children, VE for live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV) during the 2014–2015 season were insignificant against the A/H3N2 strain but were effective against influenza B [1]. LAIV was found to be ineffective when compared with IIV against the A/H1N1/pdm09 virus strain again during the 2015–2016 influenza season [2].
One method used to understand influenza VE is to study immune response to vaccination using standard increases in hemagglutination inhibition (HI) antibody titers to demonstrate the short-term response to vaccination. However, both humoral and cell-mediated immune responses may determine influenza vaccine efficacy [3]. Peripheral blood mononuclear cells (PBMCs) express genes that may correlate with protection from influenza [4], and methods for measuring RNA transcripts in PBMC subsets after influenza vaccination have been described [5]. A positive correlation between HI titers and upregulation of interferon-responsive genes early after IIV vaccination has been reported [6–8]. Elevated antibody titers to IIV have also been associated with upregulation of B-cell-specific transcripts, immunoglobulin genes, and proliferation-associated genes [9] and may be predictors of vaccine-specific antibody and plasmablast responses at 7 days postvaccination [10]. The presence of proinflammatory cytokines correlates with subjective symptoms after influenza vaccination but is not predictive of antibody response [11–13]. Conversely, Fas ligand (sFasL) and interleukin (IL)-12p40 are negative predictors of antibody response to vaccination [14].
Little research has been conducted on correlates of immune protection induced by vaccination in children. Furthermore, decisions on whether to recommend LAIV have been made primarily on data from VE in children, but the immune mechanisms have been less well described. Previous work has demonstrated differential gene expression (targeted RNA seq) between 2015–2016 LAIV and IIV recipients [15] that may account for differences in VE between the 2 vaccine types.
The purpose of this study was to compare changes in gene expression (RNA sequencing from PBMCs and serum cytokines) among children who had received either the LAIV or IIV in the 2015–2016 season, with antibody response to vaccination. We believe that identifying these differences will permit optimal vaccine design for a vaccine with high immunogenicity.
METHODS
Subjects and Eligibility
This study was conducted in the fall of 2015 before influenza was circulating in the region [16]. Intended vaccine recipients aged 3 through 18 years were recruited. Exclusion criteria included body weight under 17 kilograms, having an immunosuppressive disease, taking immunosuppressive medicine or high-dose oral steroids, pregnancy, and having a severe allergy to the influenza vaccine or its components. Parents chose which form of the quadrivalent vaccine—LAIV (FluMist, MedImmune) or IIV (either Fluzone, Sanofi Pasteur, or Fluarix, GlaxoSmithKline, as determined by their health insurance provider)—would be administered to their child. Demographic data were provided by the families and extracted from the electronic medical record (EMR). Prior vaccine history was available for all participants in the EMR or in the state immunization registry.
Specimen Collection and Processing
On day 0, participants provided blood samples using PAXgene tubes (Becton Dickinson) for total RNA and serum separator tubes for HI and cytokines before receiving influenza vaccine. They returned on day 7 (range, 6–10 days) and on day 21 (range, 20–35 days) for additional blood draws. Within 4–6 hours of collection, samples were processed and serum was stored at –70°C until further analysis. Total RNA from day 0 and day 7 was isolated and sequenced for a custom panel of 89 transcripts using the TruSeq Targeted RNA Expression method, as previously described (Supplementary Table 1) [15]. HI titers against all 4 strains in that season’s vaccine—A/California/7/2009 (H1N1)pdm09-like, A/Switzerland/9715293/2013 (H3N2), B/Phuket/3073/2013-like virus, B/Brisbane/60/2008-like virus—from day 0 and day 21 were assayed by the Batelle Laboratory through the Centers for Disease Control and Prevention [17]. Cytokines from day 0 and day 21 were analyzed by cytokine multiplex using the Bioplex platform (Biorad, Hercules, CA) [18].
Statistics
In all analyses, children were grouped by the vaccine received. Demographic characteristics of participants included age group (3–8 years vs 9–17 years), sex, race (black vs nonblack), health insurance (public vs private insurance), parent’s educational level (high school or less vs some college or higher degree), and household smoking status (yes vs no).
Seroprotection was defined as day 21 HI titer ≥1:110. This level is commonly used in pediatric studies as it correlates with protection in 50% of children after influenza vaccination [19]. Seroconversion was defined as a rise in HI titers from day 0 to day 21 of 4-fold or more if day 0 titers were ≥1:10, or a day 21 titer of 1:40 if day 0 titers were <1:10. Cytokine and HI levels were found to be skewed by Shapiro-Wilks tests of normality; therefore, they were log2-transformed. For differential gene analysis, we compared various sample groups with a gene expression filter of >1 counts per million in at least half the samples in a given group.
Analyses included chi-square tests and paired t tests and were performed using SAS software (SAS Institute, Inc., Cary, NC) and SPSS, version 24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY). Paired comparisons for RNA seq differential expression were completed using DESeq2 with nondefault options for sharingMode = “fit-only” and fitType = “local” [20]. Statistical significance was set at P < .05.
CART Analysis
Classification And Regression Trees (CART) [21] software was used to develop recursive partitioning models to determine the set of day 0 to day 7 changes in RNA seq that were most predictive of the vaccine HI response. This nonparametric statistical method for multivariable data creates decision trees with maximum sensitivity and specificity based on a series of dichotomous splits. CART was conducted on 2 equal-sized samples (development and validation) with simple random sampling without replacement. The target variables were seroprotection or seroconversion of HI titers at day 21 by vaccine strain. Predictor variables were day 0 to day 7 RNA seq and cytokine differences and demographics.
The decision trees were pruned to determine the tree with the lowest number of misclassified subjects and highest sensitivity. Receiver operating characteristic (ROC) curves, area under the curve (AUC), sensitivity, specificity, and positive and negative predictive values were estimated using CART. The sensitivity from the CART model was determined using the final seroconversion/protection positive terminal node, and specificity was determined using the previous seroconversion/protection negative terminal nodes.
Study Approval
This study was approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from the participants’ parents with the children’s assent as appropriate.
RESULTS
The study consists of 71 children; 25 had received LAIV, and 46 had received IIV. Vaccine recipients did not differ by any demographic variable (Supplementary Table 2). The majority of the children (59%) did not receive influenza vaccine of any type in the previous season (2014–2015): 13/25 (52%) of the LAIV recipients and 29/46 (63%) of the IIV recipients. Significantly more IIV recipients than LAIV recipients were seroprotected and demonstrated seroconversion at day 21 against all 4 vaccine strains (P < .019 for all) (Table 1).
Table 1.
Proportion of Children Who Were Seroprotected and Seroconverted by Vaccine Type and Strain
| Overall (n = 71), No. (%) |
Vaccine Type | P Valuea | ||
|---|---|---|---|---|
| LAIV (n = 25), No. (%) |
IIV (n = 46), No. (%) |
|||
| Seroprotection (1:110) at day 21 | ||||
| A/H1N1 | 59 (83) | 15 (60) | 44 (96) | <.001 |
| A/H3N2 | 54 (76) | 15 (60) | 39 (85) | .019 |
| B Brisbane | 53 (75) | 14 (56) | 39 (85) | .008 |
| B Phuket | 57 (80) | 15 (60) | 42 (91) | .002 |
| Seroconversion at day 21 | ||||
| A/H1N1 | 29 (41) | 0 (0) | 29 (63) | <.001 |
| A/H3N2 | 27 (38) | 4 (16) | 23 (50) | .005 |
| B Brisbane | 25 (35) | 3 (12) | 22 (48) | .003 |
| B Phuket | 26 (37) | 2 (8) | 24 (52) | <.001 |
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
aChi-square test or Fisher exact test.
Differential Gene Expression from PBMCs After Vaccination With IIV or LAIV
Influenza vaccination resulted in significantly altered gene expression depending upon vaccine type (Supplementary Table 3). Receipt of IIV was associated with decreased expression of IL1B, CCL2, and ICAM1, all pro-inflammatory monocyte and granulocyte genes, and with increased expression of CD24, a known B-cell activator, and DEFA4. LAIV was associated with a significantly increased expression of interferon pathway genes DDX58, IRF7, MX1, IFI6, CXCL10, and CASP1 and decreased expression of BCL2, VEGFA, and IL6, regulators of cell growth and apoptosis. These data indicate a unique PBMC gene response to vaccine type, as was observed with a nearly identical cohort [15].
Differential Gene Expression From PBMCs After Vaccination With IIV or LAIV by Seroconversion Status
Vaccine recipients were stratified by seroconversion status for each vaccine strain (Table 2). In 29 IIV recipients who seroconverted for H1N1, a smaller number of genes was significantly differentially expressed than among 17 patients who did not seroconvert (Table 2). There were 3 genes associated with H1N1 seroconversion, DEFA4 (upregulated), CXCL2, and ICAM1 (down regulated), genes involved in monocyte and neutrophil inflammation. Lack of H1N1 seroconversion following IIV revealed 10 differentially expressed genes, including increases in the interferon-stimulated genes DDX58, IFNAR1, IFIT3, and MX1.
Table 2.
Differentially Expressed Genes Associated With Seroconversion to IIV or LAIV
| Inactivated Influenza Vaccine | |||||||
|---|---|---|---|---|---|---|---|
| H1N1 Seroconverted | H3N2 Seroconverted | B Brisbane Seroconverted | B Phuket Seroconverted | ||||
| Yes (n = 29) | No (n = 17) | Yes (n = 23) | No (n = 23) | Yes (n = 22) | No (n = 24) | Yes (n = 24) | No (n = 22) |
| Upregulated | |||||||
| BAK1 | BAK1 | ||||||
| CD24 | CD24 | CD24 | |||||
| CD36 | CD36 | CD36 | CD36 | ||||
| IFIT3 | |||||||
| IFNAR1 | |||||||
| MX1 | |||||||
| TNFSF10 | TNFSF10 | ||||||
| DEFA4 | DEFA4 | ||||||
| HLA-A | |||||||
| DDX58 | |||||||
| IRF7 | |||||||
| Downregulated | |||||||
| CCL2 | CCL2 | CCL2 | CCL2 | ||||
| CD8A | CD8A | CD8A | CD8A | ||||
| CXCL2 | CXCL2 | CXCL2 | |||||
| ICAM1 | ICAM1 | ||||||
| FASLG | |||||||
| IL1B | IL1B | IL1B | IL1B | ||||
| IL8 | IL8 | ||||||
| TGFB1 | |||||||
| GZMK | |||||||
| Live Attenuated Influenza Vaccine | |||||||
| H1N1 Seroconverted | H3N2 Seroconverted | B Brisbane Seroconverted | B Phuket Seroconverted | ||||
| No (n = 25) | No (n = 21) | No (n = 22) | No (n = 23) | ||||
| Upregulated | |||||||
| CASP1 | CASP1 | CASP1 | CASP1 | ||||
| CD36 | CD36 | CD36 | CD36 | ||||
| DDX58 | DDX58 | DDX58 | DDX58 | ||||
| IRF7 | IRF7 | IRF7 | IRF7 | ||||
| TNFSF10 | TNFSF10 | TNFSF10 | TNFSF10 | ||||
| IFIT3 | IFIT3 | IFIT3 | |||||
| CXCL10 | CXCL10 | ||||||
| IFI6 | IFI6 | ||||||
| MX1 | MX1 | MX1 | |||||
| Downregulated | |||||||
| IL8 | |||||||
| CD8A | CD8A | ||||||
| IL6 | IL6 | ||||||
| VEGFA | |||||||
| CCL2 | |||||||
| BCL2 | BCL2 | BCL2 | BCL2 | ||||
| CXCL2 | CXCL2 | CXCL2 | CXCL2 | ||||
| CXCR4 | CXCR4 | CXCR4 | CXCR4 | ||||
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
One-half of IIV recipients seroconverted to H3N2; there were 6 differentially expressed genes, including decreased expression of inflammatory genes ICAM1, GZMK, IL1B, and CD8A. For H3N2 nonconverters, only CD24 (upregulated) and CCL2 (downregulated) were significantly different, similar to H1N1 nonconverters.
There were 22 patients who seroconverted to B Brisbane, in whom 2 genes were significantly altered, CD8A (downregulated) and TNFSF10 (upregulated). Nine differentially expressed genes correlated with a lack of seroconversion, including CD24 (upregulated) and CCL2 (downregulated).
For the 24 B Phuket seroconverters, 4 genes were differentially expressed: CD8A and IL1B (downregulated) and DEFA4 and HLA-A (upregulated). In 22 nonconverters, 5 genes, including CCL2, CXCL2, IL8, TGFB1 (downregulated), and CD36 (upregulated), were differentially expressed, similar to B Brisbane. Altered inflammatory gene expression (including CD8A) was observed in children who seroconverted to IIV, whereas nonseroconversion was associated with changes in 3 conserved genes, CD24, CD36, and CCL2, for all strains, as well as interferon-stimulated genes for H1N1 nonconversion.
Few children vaccinated with LAIV seroconverted. The bottom of Table 2 shows the differentially expressed genes for nonseroconverters. Alterations in gene expression of interferon-related transcripts were common in participants who were nonconverters, including increases in DDX58, IFIT3, MX1, IRF7, IFI6, and CXCL10. These data illustrate a correlation between interferon gene expression and failure to seroconvert in children following receipt of LAIV.
Differential Gene Expression From PBMCs After Vaccination With IIV or LAIV by Seroprotection Status
These analyses were repeated with seroprotection as the independent variable. IIV induced seroprotection in the majority of participants for all 4 strains (Table 3). Common to all vaccine strains was downregulation of inflammatory genes such as IL8, ICAM1, and CXCL2 among other common transcripts. In the 7 H3N2 nonseroprotected patients, there were 14 unique differential gene expressions, including induction of the interferon genes IFIT3, IRF7, DDX58, IFI6, and MX1. IFIT3 and IFI6 were also identified in 4 B Phuket nonseroprotected patients. These data indicate that IIV-induced interferon gene expression correlates with failed H3N2 and B Phuket seroprotection in a limited number of patients.
Table 3.
Differentially Expressed Genes Associated With Seroprotection to IIV or LAIV
| Inactivated Influenza Vaccine | |||||||
|---|---|---|---|---|---|---|---|
| H1N1 Seroprotected | H3N2 Seroprotected | B Brisbane Seroprotected | B Phuket Seroprotected | ||||
| Yes (n = 44) | No (n = 2) | Yes (n = 39) | No (n = 7) | Yes (n = 39) | No (n = 7) | Yes (n = 42) | No (n = 4) |
| Upregulated | |||||||
| CD36 | CD36 | CD36 | |||||
| DEFA4 | DEFA4 | ||||||
| CASP1 | |||||||
| TNFSF10 | TNFSF10 | ||||||
| CD24 | |||||||
| IFI6 | IFI6 | ||||||
| BAK1 | |||||||
| DDX58 | |||||||
| GTPBP1 | |||||||
| IFIT3 | IFIT3 | IFIT3 | |||||
| IRF7 | |||||||
| MX1 | |||||||
| Downregulated | |||||||
| CCL2 | CCL2 | CCL2 | |||||
| CD8A | CD8A | CD8A | CD8A | ||||
| CXCL2 | CXCL2 | CXCL2 | CXCL2 | ||||
| CXCR4 | CXCR4 | ||||||
| ICAM1 | ICAM1 | ICAM1 | ICAM1 | ||||
| CCL7 | |||||||
| IL8 | IL8 | IL8 | IL8 | ||||
| CD86 | |||||||
| GNLY | |||||||
| GZMA | |||||||
| GZMB | GZMB | ||||||
| IL1A | |||||||
| IL1B | IL1B | IL1B | |||||
| PRF1 | |||||||
| Live Attenuated Influenza Vaccine | |||||||
| H1N1 Seroprotected | H3N2 Seroprotected | B Brisbane Seroprotected | B Phuket Seroprotected | ||||
| Yes (n = 15) | No (n = 10) | Yes (n = 15) | No (n = 10) | Yes (n = 14) | No (n = 11) | Yes (n = 15) | No (n = 10) |
| Upregulated | |||||||
| CASP1 | CASP1 | CASP1 | |||||
| CXCL10 | CXCL10 | ||||||
| IFI6 | IFI6 | ||||||
| MX1 | MX1 | MX1 | |||||
| TNFSF10 | TNFSF10 | TNFSF10 | TNFSF10 | ||||
| IRF7 | IRF7 | IRF7 | |||||
| DDX58 | DDX58 | ||||||
| CD36 | CD36 | CD36 | |||||
| IFIT3 | IFIT3 | ||||||
| Downregulated | |||||||
| CXCL2 | CXCL2 | CXCL2 | CXCL2 | ||||
| CXCR4 | CXCR4 | CXCR4 | |||||
| CCL2 | |||||||
| VEGFA | VEGFA | VEGFA | |||||
| GZMB | |||||||
| IL6 | IL6 | IL6 | IL6 | ||||
| GNLY | |||||||
| TNFA | |||||||
| IL12A | |||||||
| BCL2 | BCL2 | BCL2 | |||||
| TLR3 | |||||||
| IL8 | IL8 | IL8 | |||||
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
Approximately three-fifths of children who received LAIV were seroprotected at day 21 (Table 3, bottom). In H1N1 seroprotected patients, the inflammatory genes IL6 and CXCL2 were significantly decreased. H1N1 nonprotected patients had increased expression of the interferon-related genes IRF7, IFIT3, IFI6, and CXCL10, as well as CASP1. H3N2 seroprotection was associated with differential gene expression of 13 unique genes, including inflammatory and interferon-stimulated genes. Lack of H3N2 protection was associated with increased expression of CASP1, an IL-1β-activating protease critical to pyroptosis. For the B viral strains, increased IFN-stimulated gene expression and CD36 were associated with seroprotection. A lack of protection revealed 3 and 11 differentially expressed genes for B Brisbane and B Phuket, respectively. These included 2 common genes, IL6 (downregulated) and TNFSF10 (upregulated). These data indicate that LAIV-associated interferon-stimulated gene responses dominate the PBMC gene signature.
Classification and Regression Tree Analysis of IIV Seroconversion and LAIV Seroprotection
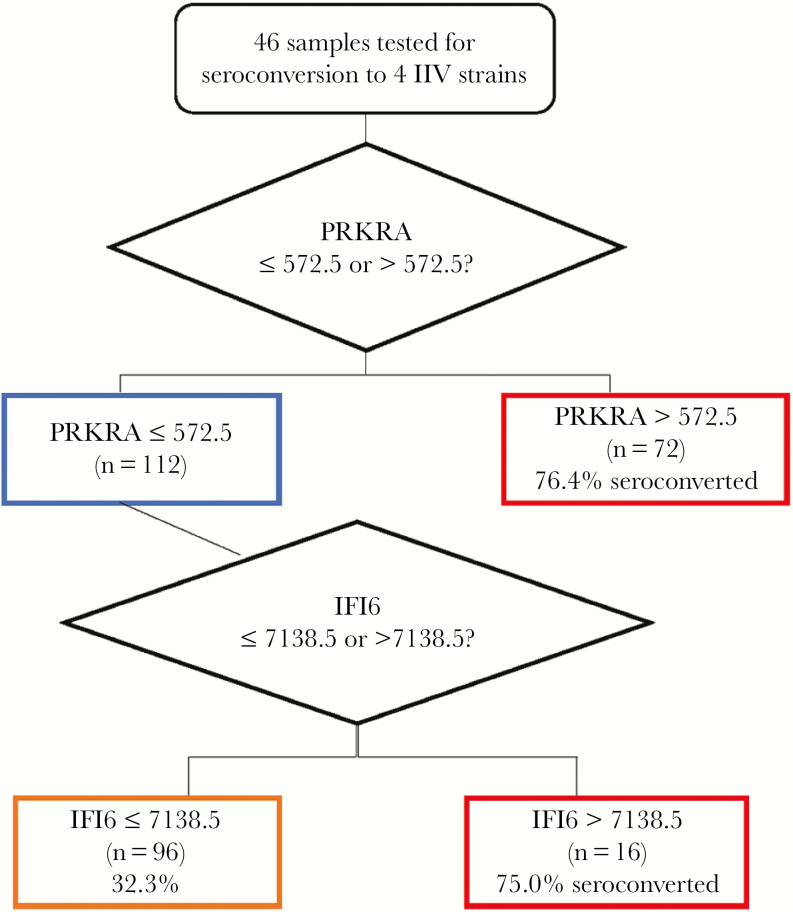
CART analyses were performed to determine if a subset of gene targets could be utilized to predict seroconversion or seroprotection status following vaccination. Seroconversion status for each strain was combined, resulting in an N of 184 (46 patients × 4 strains). IIV seroconversion best fit resulted in the node structure in Figure 1, with an ROC value of 0.72 (specificity, 67.4%; sensitivity, 59.2%) using significant differential expression of 2 IFN-induced genes, PRKRA and IFI6. This finding is consistent with altered IFN-stimulated gene expression in nonseroconverters observed using differential gene expression analysis.
Figure 1.
Gene expression associated with inactivated influenza vaccine (IIV) seroconversion. Classification And Regression Trees analysis was performed on 46 patient samples comparing seroconversion status of each IIV strain with differential gene expression (n = 184). The magnitude of the change in gene expression between day 0 and day 7 was utilized to identify the depicted node structure. IIV seroconversion was fit with a receiver operating characteristic curve value of 0.72 (specificity, 67.4%; sensitivity, 59.2%).
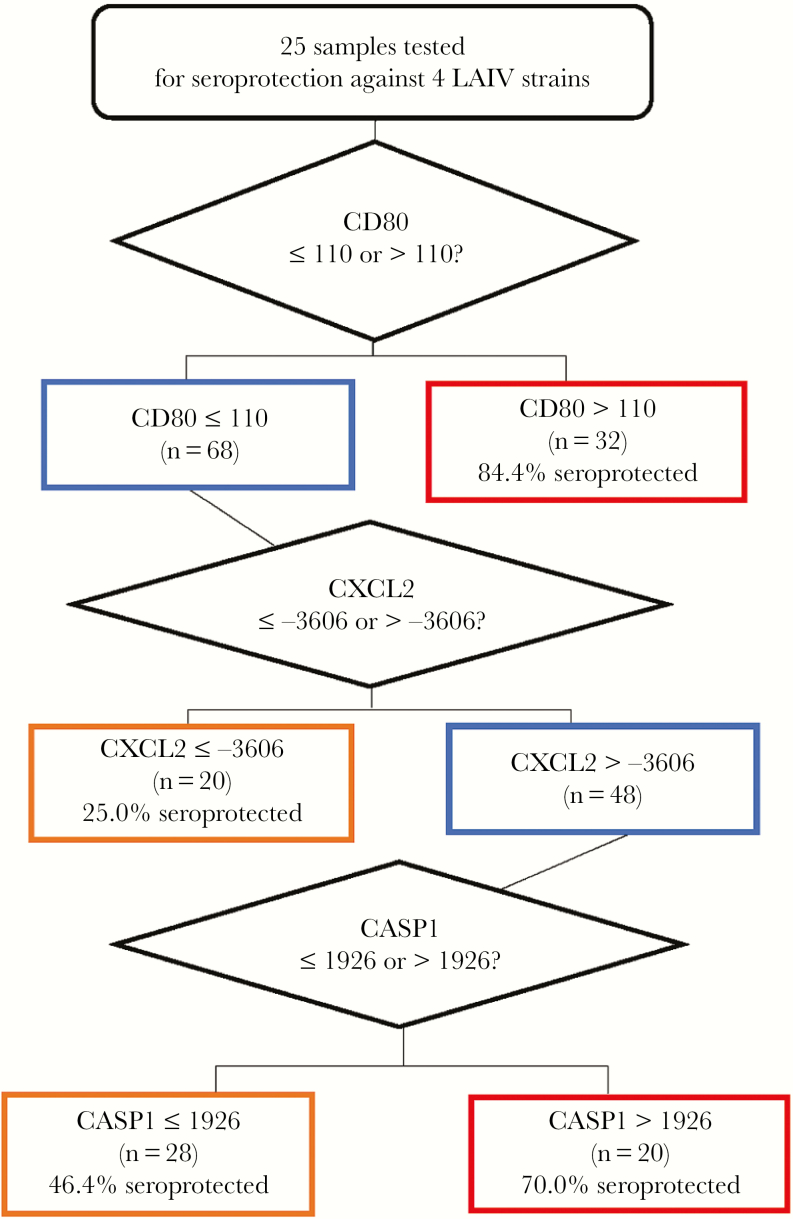
For LAIV, seroprotection was similarly evaluated, with an N of 100 (25 patients × 4 strains). LAIV seroprotection was fit in the node structure shown in Figure 2 with an ROC value of 0.76 (specificity, 53%; sensitivity, 51%) using 3 differentially expressed genes, CD80, CXCL2, and CASP1. These data are consistent with differential gene expression analysis findings that CXCL2 was associated with seroprotection and CASP1 was associated with a lack of seroprotection.
Figure 2.
Gene expression associated with live attenuated influenza vaccine (LAIV) seroprotection. Classification And Regression Trees analysis was performed on 25 patient samples comparing seroconversion status of each inactivated influenza vaccine strain with differential gene expression (n = 100). The magnitude of the change in gene expression between day 0 and day 7 was utilized to identify the depicted node structure. LAIV seroprotection was fit with a receiver operating characteristic curve value of 0.76 (specificity, 53%; sensitivity, 51%).
Cytokine Concentration in Serum After Vaccination With IIV or LAIV by Seroconversion Status
Overall, serum cytokine concentrations decreased significantly between day 0 and day 7 for recipients of either vaccine (data not shown). Seroconversion to the H1N1 strain of the IIV was associated with decreases in CCL4 and IL-9 levels, whereas H3N2, B Brisbane, and B Phuket seroconversion was associated with decreased CCL3 (Table 4). Nonseroconversion was associated with a decrease in several cytokines for each strain. Significant association of cytokines with seroprotection status following receipt of IIV was not observed.
Table 4.
Serum Cytokines Associated With Seroconversion to IIV or LAIV
| Inactivated Influenza Vaccine | |||||||
|---|---|---|---|---|---|---|---|
| H1N1 Seroconverted | H3N2 Seroconverted | B Brisbane Seroconverted | B Phuket Seroconverted | ||||
| Yes (n = 29) | No (n = 17) | Yes (n = 23) | No (n = 23) | Yes (n = 22) | No (n = 24) | Yes (n = 24) | No (n = 22) |
| Downregulated | |||||||
| CCL4 | |||||||
| IL-9 | IL-9 | IL-9 | |||||
| IL-8 | IL-8 | ||||||
| IL-12 | IL-12 | IL-12 | |||||
| IL-13 | IL-13 | IL-13 | IL-13 | ||||
| TNF-A | TNF-A | TNF-A | TNF-A | ||||
| CCL3 | CCL3 | CCL3 | |||||
| VEGF | VEGF | VEGF | |||||
| EOTAXIN | |||||||
| G-CSF | |||||||
| IL-4 | |||||||
| IL-1B | |||||||
| IFNG | |||||||
| Upregulated | |||||||
| IL-7 | |||||||
| Live Attenuated Influenza Vaccine | |||||||
| H1N1 Seroconverted | H3N2 Seroconverted | B Brisbane Seroconverted | B Phuket Seroconverted | ||||
| Yes (n = 0) | No (n = 25) | Yes (n = 4) | No (n = 21) | Yes (n = 3) | No (n = 22) | Yes (n = 2) | No (n = 23) |
| Downregulated | |||||||
| IL-5 | IL-5 | ||||||
| G-CSF | G-CSF | G-CSF | |||||
| CCL4 | CCL4 | CCL4 | |||||
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
LAIV recipients showed significantly decreased G-CSF, CCL4, and IL-5 cytokine concentrations following vaccination. These 3 cytokines also significantly correlated with a lack of seroconversion for H1N1, H3N2, and B Brisbane (Table 4). The number of children who seroconverted to LAIV was small; hence the results were inconclusive. Seroprotection with LAIV was associated with decreased interferon (IFN)-γ and tumor necrosis factor (TNF)–α for 3 of the strains tested (Table 5). These data show differences between LAIV and IIV effects on serum cytokines in the context of vaccine immunogenicity, as measured by HI.
Table 5.
Serum Cytokines Associated With Seroprotection to LAIV
| Live Attenuated Influenza Vaccine | |||||||
|---|---|---|---|---|---|---|---|
| H1N1 Seroconverted | H3N2 Seroconverted | B Brisbane Seroconverted | B Phuket Seroconverted | ||||
| Yes (n = 15) | No (n = 10) | Yes (n = 15) | No (n = 10) | Yes (n = 14) | No (n = 11) | Yes (n = 15) | No (n = 10) |
| Downregulated | |||||||
| IFNG | IFNG | IFNG | |||||
| TNF-A | TNF-A | TNF-A | |||||
| CCL11 | CCL11 | ||||||
| G-CSF | G-CSF | ||||||
| IL-4 | IL-4 | ||||||
| IL-9 | IL-9 | ||||||
| IL-17A | IL1-7A | ||||||
| IL-13 | |||||||
Abbreviations: IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
DISCUSSION
After showing great promise as an alternative needle-sparing influenza vaccine and being preferentially recommended in 2014–2015, LAIV has been shown to have decreased effectiveness during successive seasons [1, 22, 23]. Despite a formulation change in 2015–2016 with a more heat-stable strain of A/H1N1, no significant improvement in VE was found. Therefore, LAIV was not recommended by the Advisory Committee on Immunization Practices (ACIP) or endorsed by the American Academy of Pediatrics (AAP) for the 2016–2017 or 2017–2018 seasons [24, 25]. The reasons why LAIV demonstrated low VE, especially against A strains, have not been elucidated, thus providing rationale for studies of immune response to vaccination. Further, there is limited information available regarding immune correlates of antibody responses to IIV in children. Previous studies have examined peripheral blood responses and reported significant differences between LAIV and IIV [6, 7, 10, 26]. Moreover, in a preliminary study of PBMC transcriptomics following influenza vaccination of children, significant differences in gene expression were reported between LAIV and IIV [15]. This study was designed to refine that analysis by comparing gene expression and cytokines associated with significant HI responses to the 2 vaccines.
Using a targeted panel of 89 transcripts, several differentially expressed genes were identified for each vaccine type with distinct patterns for children who demonstrated seroconversion to the 4 vaccine influenza strains when compared with nonseroconverters.
Several studies support an association between the ability of a vaccine to induce an interferon response and VE [6, 7, 15, 27]. Cao reported that IFN-stimulated genes were increased early postvaccination (day 1) but were elevated later (day 7) following LAIV vaccination. This observation was more dramatic in children younger than 5 years of age when compared with older children [6]. The later IFN-stimulated gene response to LAIV was also shown to be a distinct differentiator with IIV [7, 15]. Our data support the critical role of IFN and illustrate a correlation between interferon gene expression and failure to seroconvert in children following LAIV and IIV. For IIV, increases IFN-stimulated genes were only seen in the context of failed seroconversion to H1N1. However, CART analysis revealed that seroconversion with IIV to all influenza vaccine strains could be indicated by changes in expression levels of the IFN-stimulated genes PRKRA and IFI6. These data suggest that increased IFN-stimulated gene expression 7 days after vaccination may be a strong indicator of failed seroconversion, regardless of vaccine type. The number of seroconverters to LAIV was insufficient to identify markers of immunogenicity in this context; however, the strong IFN-stimulated gene signature induced by LAIV is consistent with failed seroconversion.
Conversely, altered inflammatory gene expression appeared to predict seroconversion and seroprotection. Decreased inflammatory gene expression (including CD8A) was observed in children who seroconverted to IIV, whereas nonseroconversion was associated with 3 conserved genes, CD24, CD36, and CCL2. Although it may be difficult to make direct comparisons with children who received LAIV, these results were supported by a study in adults that demonstrated an inverse age-related association between the presence of inflammatory gene signatures and seroconversion [28].
Seroprotection status for both vaccines was associated with inflammatory gene expression changes. IIV- and LAIV-induced seroprotection was associated with decreased CXCL2 and increased CD36 expression; CXCL2 and CD36 are monocyte-regulating genes. Several other inflammatory genes were associated with specific influenza strains. The current study included a limited number of children who were not seroprotected following IIV, which restricts the conclusions that can be drawn. However, failed seroprotection with LAIV was associated with increased TNFSF10 and CASP1 expression. These genes regulate apoptosis- and pyroptosis-mediated death in immune cells. CART analysis of LAIV seroprotection status further implicated differential CXCL2 and CASP1 expression. Taken together, the current study has identified several conserved candidate markers of seroconversion in PBMCs across responses to the 4 vaccine strains.
Analysis of human cytokine responses to natural infection provides clues to the immune mechanisms that are critical in recovery and in the development of immunity. Serum cytokines have been associated with severe influenza illness in children [29]; thus, cytokine responses to influenza vaccines are expected. However, the timing of the expected cytokine response to influenza vaccination has not been well described. In a study in adults who received IIV, serum cytokines were measured at various time points, and it appears that some changed rapidly after vaccination [30]. Talaat et al. reported elevated responses in IFN-γ and CXCL10 as early as 3 hours after immunization, with many returning to baseline within 2 days. However, IL-8 was found to be lower compared with baseline for up to 2 weeks [30, 31]. A study of adults found that IIV was associated with a higher seroresponse rate than LAIV. Adults who seroconverted to IIV had higher prevaccination levels of IL-8 when compared with IIV nonresponders [13]. Previous studies in children also support the observation that patterns of immunity as measured by mucosal and serum cytokine responses differ in children vaccinated with IIV vs LAIV [12]. In this study, clear differences were observed between 3–18-year-old IIV and LAIV recipients. Specific cytokines that were negatively correlated with IIV-induced seroconversion were CCL3, and cytokines that were negatively correlated with nonconversion were TNF-α and IL-13. Decreased CCL3 associated with IIV seroconversion was also seen in children during the 2014–2015 season (data not shown). CCL4, another monocyte chemokine, and G-CSF were associated with LAIV recipients who failed to seroconvert to the vaccine.
For IIV, no cytokines correlated with seroprotection status. LAIV-induced seroprotection was associated with decreased IFN-γ and TNF-α concentration. These data indicate distinct immunomodulation by LAIV and IIV, with differential cytokine suppression measured at 7 days postvaccination.
This study has several limitations. A targeted panel of 89 immune transcripts based on the experience of the investigators and knowledge of the current literature was used, whereas whole-genome RNA sequencing would likely provide additional insights. The measurement of cytokines and RNA seq were only performed at a single time point after vaccination. It may be useful to obtain serum specimens sooner after vaccination or in a dynamic fashion to best measure the immune response. This study does not address prior vaccination status due to the limited sample size. It is known that prior vaccination influences antibody and B-cell responses to influenza vaccination [32, 33]. A larger study may permit correlating prior vaccination history with cytokine and RNA gene expression. Cao demonstrated different responses between children <5 years of age and older children, whereas another study showed variation in responses between older and younger adults [6]. The patients in this study were between 3 and 18 years of age, with a mean age of 11.5 years. The small sample size also precluded an analysis by age. The children included here were not followed through the respiratory season for the development of influenza infection or disease. Specimens to assess viral shedding after LAIV receipt were not obtained from participants. Finally, this study was conducted during a single influenza season; these results should be validated with an independent cohort. Although LAIV was not recommended by the ACIP or AAP in subsequent seasons, we are currently exploring these relationships in a cohort of children who received IIV during the 2016–2017 season.
This study demonstrates that immune phenotyping of PBMC gene expression and serum cytokine concentrations can be informative in association with antibody responses to IIV and LAIV. Although it is difficult to make comparisons given the lack of seroconversion in the majority of LAIV recipients, it is clear that a lack of antibody seroconversion for both vaccines was associated with induction of IFN-stimulated genes at day 7 postvaccination. This study suggests that a prolonged antiviral response to the vaccine may not be desirable. Changes in inflammatory gene expression were associated with seroprotection responses for both vaccines, including CXCL2 and CASP1 for LAIV. For children who seroconverted to IIV, decreased inflammatory gene expression (including CD8A) was observed; however, no cytokines correlated with seroprotection. Future vaccine development should incorporate these findings for optimal immunogenicity.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank G. K. Balasubramani for assistance with the CART analyses. In addition, we would like to thank Krissy Moehling, PhD, Sean Saul, Sandra Sauereisen, MD, Mary Pat Friedlander, MD, Greg Gallik, MD, Ben Skinker, MD, and Megan Skae for their help.
Author contributions. K.S.C., J.M.M., and R.K.Z. obtained funding. K.S.C., J.M.M., J.F.A., and R.K.Z. designed the study. J.P.N., J.F.A., and W.T.H. performed the research. R.A., U.R.C., and C.J.L. performed the statistical analysis. J.M.M., J.F.A., R.K.Z., and M.P.N. analyzed the data. J.M.M., J.F.A., and M.P.N. wrote the paper with critical input from all co-authors.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC) National Center for Immunization and Respiratory Diseases (NCIRD) through cooperative agreements with the University of Pittsburgh (grant U01 IP000467 and IP001035), by the National Institutes of Health (NIH; grant UL1 TR001857 to the University of Pittsburgh), and by the Clinical Translational Science Institute (CTSI) Translational Research Pilot Awards. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC or the NIH.
Potential conflicts of interest. Drs. Zimmerman and Lin have received research funding from Sanofi Pasteur, Inc., and Merck & Co, Inc. Drs. Zimmerman and Nowalk have received research funding from Pfizer, Inc., and Merck & Co., Inc. Drs. Martin and Alcorn and Ms. Nagg have received research funding from MedImmune, LLC. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zimmerman RK, Nowalk MP, Chung J, et al. ; US Flu VE Investigators 2014-2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016; 63:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015-2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Vries RD, Nieuwkoop NJ, Pronk M, et al. Influenza virus-specific antibody dependent cellular cytoxicity induced by vaccination or natural infection. Vaccine 2017; 35:238–47. [DOI] [PubMed] [Google Scholar]
- 4. Savic M, Dembinski JL, Laake I, et al. Distinct T and NK cell populations may serve as immune correlates of protection against symptomatic pandemic influenza A(H1N1) virus infection during pregnancy. PLoS One 2017; 12:e0188055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoek KL, Samir P, Howard LM, et al. A cell-based systems biology assessment of human blood to monitor immune responses after influenza vaccination. PLoS One 2015; 10:e0118528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao RG, Suarez NM, Obermoser G, et al. Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children. J Infect Dis 2014; 210:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu W, Higgs BW, Morehouse C, et al. A whole genome transcriptional analysis of the early immune response induced by live attenuated and inactivated influenza vaccines in young children. Vaccine 2010; 28:2865–76. [DOI] [PubMed] [Google Scholar]
- 8. Bucasas KL, Franco LM, Shaw CA, et al. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 2011; 203:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan Y, Tamayo P, Nakaya H, et al. Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response. Eur J Immunol 2014; 44:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JH, Mishina M, Chung JR, et al. Cell-mediated immunity against antigenically drifted influenza A(H3N2) viruses in children during a vaccine mismatch season. J Infect Dis 2016; 214:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christian LM, Porter K, Karlsson E, Schultz-Cherry S. Proinflammatory cytokine responses correspond with subjective side effects after influenza virus vaccination. Vaccine 2015; 33:3360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramakrishnan A, Althoff KN, Lopez JA, et al. Differential serum cytokine responses to inactivated and live attenuated seasonal influenza vaccines. Cytokine 2012; 60:661–6. [DOI] [PubMed] [Google Scholar]
- 14. Furman D, Jojic V, Kidd B, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 2013; 9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole KS, Martin JM, Horne WT, et al. Differential gene expression elicited by children in response to the 2015-16 live attenuated versus inactivated influenza vaccine. Vaccine 2017; 35:6893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russell K, Blanton L, Kniss K, et al. Update: influenza activity–United States, October 4, 2015-February 6, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:146–53. [DOI] [PubMed] [Google Scholar]
- 17. WHO Global Influenza Surveillance Network. Serological diagnosis of influenza by haemagglutination inhibition testing. In: Manual for the Laboratory Diagnosis and Virological Surveillanceof Influenza. Geneva, Switzerland: World Health Organization; 2011:59–62. http://apps.who.int/iris/bitstream/handle/10665/44518/9789241548090_eng.pdf;jsessionid=031322A7446BE8FDB6869E5EB37F55D3?sequence=1 [Google Scholar]
- 18. Manni ML, Trudeau JB, Scheller EV, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal Immunol 2014; 7:1186–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081–5. [DOI] [PubMed] [Google Scholar]
- 20. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014; 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breiman L,Friedman J, Stone CJ. Classification and RegressionTrees (Wadsworth Statistics/Probability). Boca Raton, FL: Chapman & Hall; 1984. [Google Scholar]
- 22. Caspard H, Gaglani M, Clipper L, et al. Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2-17 years of age in 2013-2014 in the United States. Vaccine 2016; 34:77–82. [DOI] [PubMed] [Google Scholar]
- 23. Poehling KA, Caspard H, Peters TR, et al. 2015-2016 vaccine effectiveness of live attenuated and inactivated influenza vaccines in children in the United States. Clin Infect Dis 2018; 66:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2017-18 influenza season. MMWR Recomm Rep 2017; 66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. AAP Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2017 - 2018. Pediatrics 2017; 140::e20172550. [DOI] [PubMed] [Google Scholar]
- 26. Levine MZ, Martin JM, Gross FL, et al. Neutralizing antibody responses to antigenically drifted influenza A(H3N2) viruses among children and adolescents following 2014-2015 inactivated and live attenuated influenza vaccination. Clin Vaccine Immunol 2016; 23:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Athale S, Banchereau R, Thompson-Snipes L. Influenza vaccines differentially regulate the interferon response in human dendritic cell subsets. Sci Transl Med 2017; 9:eaaf9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis 1980; 142:844–9. [DOI] [PubMed] [Google Scholar]
- 29. Fiore-Gartland A, Panoskaltsis-Mortari A, Agan AA, et al. ; PALISI PICFlu Investigators Cytokine profiles of severe influenza virus-related complications in children. Front Immunol 2017; 8:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talaat KR, Halsey NA, Cox AB, et al. Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination. Influenza Other Respir Viruses 2018; 12:202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panapasa JA, Cox RJ, Mohn KG, et al. The expression of B & T cell activation markers in children’s tonsils following live attenuated influenza vaccine. Hum Vaccin Immunother 2015; 11:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One 2008; 3:e2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoft DF, Lottenbach KR, Blazevic A. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin Vaccine Immunol 2017; 24:e00414–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.