Abstract
We report a case of fibromuscular dysplasia (FMD). The patient was a 22-year-old female who had received treatment for hypertension for two years. She had also presented with hemorrhage caused by an annular ulcer in the small intestine. In March 2012, she had abdominal pain, was diagnosed with rupture of aneurysms of the gastroepiploic artery, and received embolization. In July 2012, she felt abdominal pain, presented with ruptured aneurysms of the left hepatic artery. She had abdominal pain again and suffered hemorrhagic shock. Contrast-enhanced computed tomography scanning of her abdomen revealed rupture of the left hepatic artery aneurysms and she received emergent coil embolization. Aneurysm expansion was noted, which suggested the necessity of early diagnosis and treatment, but the diagnosis was difficult because a few systemic findings were observed without any typical angiography findings. We decided to perform a small bowel resection for the complication of annular ulcers and reached a diagnosis of FMD according to pathological findings. Differential diagnosis between inflammatory and noninflammatory arteriopathy is difficult in many cases and often largely affects treatment policies. We experienced a rare case where we reached a definite diagnosis of FMD based on pathology of the small intestine ulcer.
<Learning objective: Fibromuscular dysplasia is a type of noninflammatory arteriopathy, but the clinical condition is still unknown. The differentiation between inflammatory and noninflammatory arteriopathy is highly difficult in many cases and largely different treatment policies are applied to these two types of arteriopathy. Rapid decision making regarding the use of immunosuppressant drugs is necessary. We reached a definite diagnosis based on pathological findings of annular ulcers of the small intestine.>
Keywords: Fibromuscular dysplasia, Multiple hepatic aneurysms, Annular ulcer, Coronary aneurysm, Renovascular hypertension
Introduction
Arterial diseases with multiple aneurysms are roughly divided into inflammatory and noninflammatory arteriopathy. Greatly different treatment polices are applied to these two types of arteriopathy while diagnosis is often highly difficult. Fibromuscular dysplasia (FMD) is a type of noninflammatory arteriopathy which predominantly affects women aged from 15 to 50 years and the clinical conditions are still unknown. Arterial lesions of FMD are most commonly found in renal arteries and patients are sometimes diagnosed with intractable hypertension. This time we experienced an FMD patient who presented with abdominal pain as her chief complaint but her contrast-enhanced computed tomography (CT) scanning revealed ruptured hepatic artery aneurysms while her angiogram was notable for multiple aneurysm formation. We had a great difficulty in diagnosing her but reached a definite diagnosis based on pathological findings of annular ulcers of the small intestine which had developed as a complication. We are reporting this case because it is a very rare case even with reference to preceding papers.
Case report
The patient was a 22-year-old woman who had hemorrhaged twice since around May 2010. Annular ulcers were noted in the small intestine in January 2011 by endoscopy, but a wait-and-see approach was taken as it healed naturally. She neither had a smoking history nor had any such an occurrence in her family history. Hypertension became notable. Since her plasma renin activity and aldosterone were elevated to 3.6 ng/ml/hr and 240 pg/ml, respectively, we supposed her hypertension to be caused by renovascular hypertension. However, magnetic resonance (MR) angiography, sonogram, and captopril renogram with the renal artery angiograms did not demonstrate any abnormal appearance in her renal artery. To examine her blood sample again, she started to take oral calcium antagonist (amlodipine). She presented with abdominal pain as the chief complaint in March 2012, was diagnosed with ruptured aneurysms of the gastroepiploic artery (GEA), and received embolization. She visited our hospital again with abdominal pain in July 2012. Contrast-enhanced CT scanning of her abdomen revealed ruptured hepatic artery aneurysms and intra-gallbladder hemorrhage. She was admitted to our cardiovascular medicine unit to receive conservative treatment including blood-pressure control.
Condition on admission: Her height was 161 cm and weight was 40 kg (she had lost 10 kg in the previous year). Her temperature was 36.6 degrees, regular pulse 101 bpm, and blood pressure 177/120 mmHg. She had pain in the right hypochondrium, but no other abnormal neurological and skin findings. She presented with an increased inflammatory response with white blood cells 9300/μl (neutrophils 79.5%; lymphocytes 13.1%) and C-reactive protein 8.6 mg/dL along with progression of anemia with hemoglobin (Hb) 9.7 g/dL. She had negative results with antinuclear antibody, proteinase 3 antineutrophil cytoplasmic antibodies (ANCA), myeloperoxidase-ANCA, and Quanti FERON-TB gold in-tube assay (QFT). Her plasma renin activity was higher than before and plasma aldosterone level was lower than before (33 ng/ml/hr and 25.5 pg/ml, respectively).
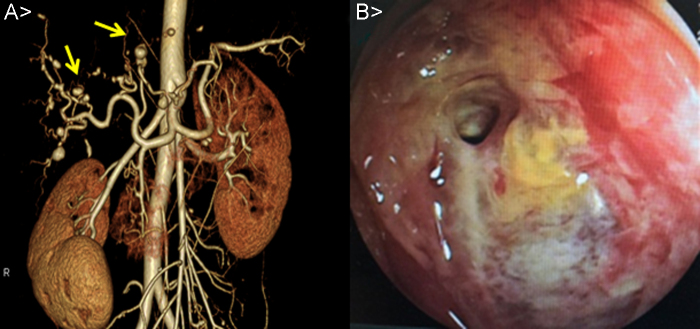
Imaging findings on admission: Her plain chest and abdominal radiograph indicated no abnormal findings while her 12-lead electrocardiogram revealed a high-voltage pattern in the left ventricle under sinus rhythm. Her transthoracic echocardiogram showed good left ventricular systolic performance without valve disease or vegetation. Contrast-enhanced CT scanning of her abdomen at the time of admission was notable for aneurysms in the hepatic left lobe and a low-density area around it as well as intra-gallbladder hemorrhage. Her CT angiograph revealed multiple aneurysms formation in the liver (Fig. 1A).
Fig. 1.
(A) Computed tomography angiography image showing multiple aneurysms in the hepatic artery, and transcatheter coil embolization in the left hepatic artery because of rerupture. (B) Double-balloon enteroscopy showing an all round annular ulcer in a portion of the ileum. Progressive bleeding was not seen.
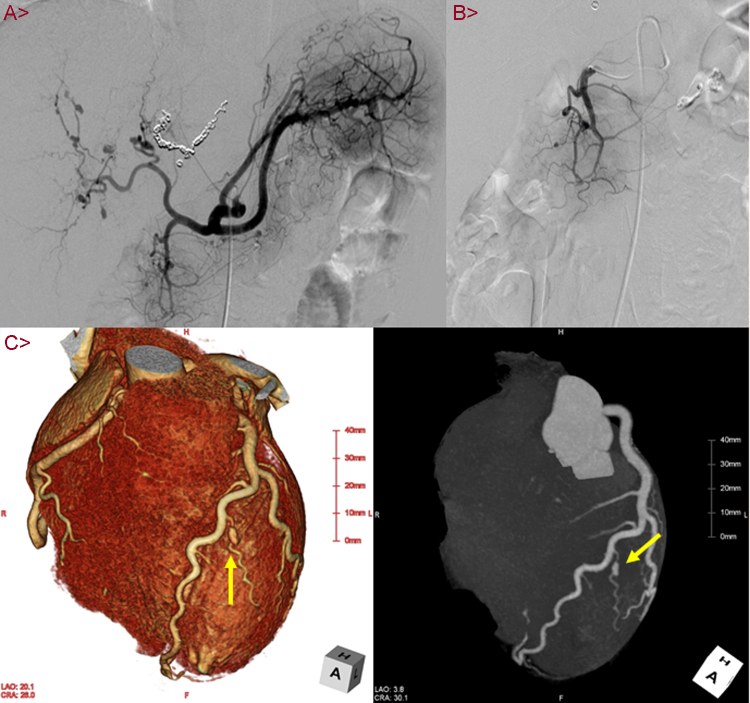
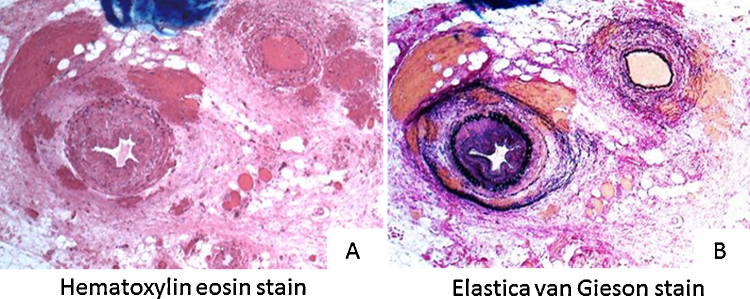
Development after Admission: She was diagnosed with rupture of aneurysms of the left hepatic artery and intra-gallbladder hemorrhage. We decided to provide conservative treatment while exploring the causes because her imaging results indicated subsidence of hemorrhage and no progress of anemia. On day 14, she presented with hemorrhage from the annular ulcers of the small intestine which we had previously observed. We performed endoscopic hemostasis and provided blood transfusion as necessary while observing her condition (Fig. 1B). On day 28, she felt abdominal pain again, the systolic blood pressure fell to 60 mmHg, and she suffered hemorrhagic shock. Contrast-enhanced CT scanning of her abdomen revealed rerupture of the hepatic artery aneurysms. We performed emergent coil embolization on the left hepatic artery (Fig. 2A). Because her aneurysms were found to have progressively aggravated as seen in the recent rerupture, we placed our highest priority in early diagnosis and treatment. Besides, to evaluate the aneurysms and arterial lesions, we performed head and neck magnetic resonance imaging scanning, angiography, and coronary artery CT scanning. No obvious aneurysm formation or stenotic lesions were found in the head and neck. The angiograph of her revealed formation of small aneurysms in the GEA (Fig. 2B) and regional tubular stenosis in the right renal artery (but this differed from a typical string and beads sign of FMD). Her CT scanning of the coronary arteries revealed formation of an aneurysm 5 mm in size on a diagonal branch of the left coronary artery (Fig. 2C). No aneurysm formation was noted in limbs or other parts. It was difficult to differentiate inflammatory arteriopathy (polyarteritis nodosa, etc.) and noninflammatory arteriopathy (FMD, segmental arterial mediolysis, etc.) based on our previous observations. We also found little importance in performing kidney, nerve, or skin biopsy because few physical findings were available from the patient. We considered a liver resection because she presented with multiple hepatic aneurysm formation, but judged it as difficult considering the extent of resection, the risk of recurrence, and the young age of the patient. We decided to perform a small bowel resection not only to cope with the annular ulcers of the small intestine which caused obstruction and hemorrhage but also for the purpose of tissue diagnosis. The pathological findings of the resection specimen were notable for rupture of the internal elastic lamina and two small vessels within an intravascular lumen. It indicated an image of recanalization after vascular obstruction, suggesting exulceration attributable to bowel ischemia. In addition, proliferation of collagen fibers, elastic fibers, and smooth muscles thickened mainly the intima and narrowed the lumen. Invasion of inflammatory cells was not observed (Fig. 3). According to the above pathological findings, we reached a diagnosis of FMD. The post-operational condition of the patient after coil embolization of the hepatic artery aneurysms and the small bowel resection was good without recurrence of abdominal pain or hemorrhage while showing proper improvement of ingestion. She was discharged from the hospital on Day 51. We decided to control her blood pressure and evaluate the aneurysms on an outpatient basis. By taking antihypertensive drugs (amlodipine and candesartan), there were no complications for three years until April 2015.
Fig. 2.
(A) This image is an emergent hepatic artery angiography. Multiple aneurysms are observed in left and right hepatic arteries. Transcatheter coil embolization in the left hepatic artery, because of re-rupture. (B) Selective visceral angiography. One small aneurysm is observed in gastroduodenal artery. (C) Coronary computed tomography angiography image showing one aneurysm (about 5 mm diameter) in the left diagonal branch.
Fig. 3.
(A) Hematoxylin and eosin stain original magnification 160×. The pathological findings of the resection specimen were notable for rupture of the internal elastic lamina and two small vessels within an intravascular lumen. It indicated an image of recanalization after vascular obstruction, suggesting exulceration attributable to bowel ischemia. (B) Elastica van Gieson stain original magnification 160×. In addition, proliferation of collagen fibers, elastic fibers, and smooth muscles thickened mainly the intima and narrowed the lumen. Invasion of inflammatory cells was not obvious.
Discussion
This time we experienced a FMD case where the patient presented with multiple aneurysms formation and we had a great difficulty in differentiation from inflammatory arteriopathy. We consider it as a very rare case because only a few cases have been reported in which the diagnoses were based on pathological findings of annular ulcers in the small intestine. FMD is a vessel anomaly of a moderate degree which develops during arteriolar growth, or vein growth in rare cases. The intima, media, and adventitia of blood vessel walls develop metaplasia, hypoplasia, or hyperplasia of muscle fibers, causing stricture, aneurysm formation, or dissection of the media. FMD is divided into three types. Arterial lesions most commonly appear in renal arteries at a rate of approximately 70%, followed by intracranial lesions (approx. 30%) 1, 2. Approximately 30% of FMD patients are said to present with multiple vascular lesions 1, 2. FMD does not normally develop in coronary arteries or hepatic arteries and the frequency is unknown. No treatment is established and patients mainly receive supportive measures depending on their pathologies. Blood pressure is to be controlled when a complication of hypertension is present. Inflammatory arteriopathy is often accompanied by systemic blood vessel inflammation as well as neurologic findings and/or cutaneous findings reflecting the inflammation, and thus, distinguishable from noninflammatory arteriopathy. The patient we experienced this time presented with no typical angiography findings and few systemic symptoms, and we assume that this resulted while experiencing the difficulty of differentiation between inflammatory and noninflammatory arteriopathy. In addition, compared to polyarteritis nodosa which is an inflammatory arteriopathy, FMD is associated with extremely few cases of annular intestinal ulcers as a complication, and this also contributed to the difficulty with differentiation. We supposed that refractory ulceration was caused by peripheral arterial intestinal ischemia. Because the pathology is progressive, we also considered administration of steroids for early treatment. However, due caution is required for the administration of steroids in cases where differentiation between inflammatory and noninflammatory arteriopathy is difficult because it can increase vascular fragility 3, 4, 5, 6, 7, 8, 9.
Conclusions
We experienced a FMD case where the patient repeated hemorrhage from annular ulcers in the small intestine, presented with ruptured hepatic artery aneurysms and intra-gallbladder hemorrhage, and we had a great difficulty in differentiation from inflammatory arteriopathy. Because differentiation between inflammatory and noninflammatory arteriopathy is highly difficult in many cases and largely different treatment policies are applied to these two types of arteriopathy, tissue diagnosis should be proactively carried out in this type of case.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Slovut D.P., Olin J.W. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–1871. doi: 10.1056/NEJMra032393. [DOI] [PubMed] [Google Scholar]
- 2.Niizuma S., Nakahama H., Inenaga T., Yoshihara F., Nakamura S., Yoshii M., Kamide K., Horio T., Kawano Y. Asymptomatic renal infarction, due to fibromuscular dysplasia, in a young woman with 11 years of follow-up. Clin Exp Nephrol. 2005;9:170–173. doi: 10.1007/s10157-005-0345-z. [DOI] [PubMed] [Google Scholar]
- 3.Paqnoux C., Seror R., Heneqar C., Mahr A., Cohen P., Le Guern V., Bienvenu B., Mouthon L., Guillevin L., French Vasculitis Study Group Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010;62:616–626. doi: 10.1002/art.27240. [DOI] [PubMed] [Google Scholar]
- 4.Chan R.J., Goodman T.A., Aretz T.H., Lie J.T. Segmental mediolytic arteriopathy of the splenic and hepatic arteries mimicking systemic necrotizing vasculitis. Arthritis Rheum. 1998;41:935–938. doi: 10.1002/1529-0131(199805)41:5<935::AID-ART22>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Siegert C.E., Macfarlane J.D., Hollander A.M., van Kemenade F. Systemic fibromuscular dysplasia masquerading as polyarteritis nodosa. Nephrol Dial Transplant. 1996;11:1356–1358. [PubMed] [Google Scholar]
- 6.Daliya P., White T.J., Makhdoomi K.R. Gastric perforation in an adult male following nasogastric intubation. Ann R Coll Surg Engl. 2012;94:e210–e212. doi: 10.1308/003588412X13171221502347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuong P.N., Le Bourgeois P., Houissa-Vuong S., Martin P., Berrod J.L. Intimal muscular fibrodysplasia responsible for an ischemic gastric ulcer in a patient with a von Recklinghausen's disease: a case report. J Mal Vasc. 2001;26:65–68. [PubMed] [Google Scholar]
- 8.Kimura K., Ohtake H., Kato H., Yashiki N., Tomita S., Watanabe G. Multivisceral fibromuscular dysplasia. An unusual case of renal and superior mesenteric involvement. Ann Vasc Dis. 2010;3:152–156. doi: 10.3400/avd.AVDcr01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine S.M., Hellmann D.B., Stone J.H. Gastrointestinal involvement in polyarteritis nodosa (1986–2000): Presentation and outcomes in 24 patients. Am J Med. 2002;112:386–391. doi: 10.1016/s0002-9343(01)01131-7. [DOI] [PubMed] [Google Scholar]