Abstract
Background
Cervical dystonia is a hyperkinetic movement disorder of unknown cause. Symptoms of cervical dystonia have been induced in animals in which the integrity of the nigro‐tectal pathway is disrupted, resulting in reduced inhibition of the deep layers of the superior colliculus. This same pathway is believed to play a critical role in saccade generation, particularly visually guided, express saccades. It was hypothesized that individuals with cervical dystonia would present with a higher frequency of express saccades and more directional errors.
Methods
Eight individuals with cervical dystonia and 11 age‐ and sex‐matched control participants performed three saccadic paradigms: pro‐saccade, gap, and anti‐saccade (120 trials per task). Eye movements were recorded using electro‐oculography.
Results
Mean saccadic reaction times were slower in the cervical dystonia group (only statistically significant in the anti‐saccade task, F(1, 35) = 4.76, p = 0.036); participants with cervical dystonia produced fewer directional errors (mean 14% vs. 22%) in the anti‐saccade task; and had similar frequencies of express saccades in the gap task relative to our control population (chi‐square = 1.13, p = 0.287). All cervical dystonia participants had lower frequencies of express saccades ipsilateral to their dystonic side (the side to which their head turns), (chi‐square = 3.57, p = 0.059).
Discussion
The finding of slower saccadic reaction times in cervical dystonia does not support the concept of reduced inhibition in the nigro‐tectal pathway. Further research is required to confirm the observed relationship between the lateralization of lower frequencies of express saccades and direction of head rotation in cervical dystonia.
Keywords: Cervical dystonia, express saccades, saccadic reaction time, SRT, nigro‐tectal pathway
Introduction
Dystonia
Dystonia is a hyperkinetic movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both.1 Cervical dystonia, affecting the muscles of the head, neck and shoulders is the most common form of adult‐onset isolated focal dystonia.2,3 Increasingly non‐motor symptoms are also being recognized, including sensory abnormalities: in particular, visual and tactile perception,4–8 pain, and psychiatric disorders.9–13
The etiology of cervical dystonia is not yet fully understood; both genetic and environmental factors are believed to play a role in its development. Cervical dystonia has long been considered a disorder of the basal ganglia. However, more recent research points to a network model, with additional brain areas being implicated including the cerebral cortex, cerebellum, thalamus, and brainstem.14–20 Whatever the model, consensus exists that impaired gamma‐aminobutyric acid (GABA)‐mediated inhibition is a key feature of dystonia.14,15,21,22
The superior colliculus (SC) is a multilayered structure in the midbrain. Each layer contains a retinotopic map of the surrounding environment23 such that activation of neurons at a specific location in the map evokes a response directed toward the corresponding location in space. The SC is involved in the control of visually triggered saccadic eye movements and orienting behaviors,23–27 including eye, head, neck, and arm movement.28,29 It is also considered to be a critical node in the pathogenesis of cervical dystonia.30 Superior collicular activity is modulated by GABA from both intrinsic and extrinsic sources (with up to 45% of SC neurons being GABAergic in the cat31). The substantia nigra pars reticulata (SNpr), of the basal ganglia, tonically inhibits the deep layers of the SC via the nigro‐tectal pathway.32,33 An animal model of cervical dystonia is produced when this GABAergic projection is disrupted in non‐human primates34,35 and cats.36 Chemical disruption of this tonic inhibitory projection, releasing the deep layers of the SC from inhibition, has also been shown to stimulate saccadic behavior in non‐human primates.32,33 Saccadic eye movements involving the nigro‐tectal pathway may potentially manifest differently in individuals with cervical dystonia relative to healthy controls.
Saccades and the superior colliculus
The superficial layer of the SC receives visual input from the retina and primary visual cortex.37 The intermediate and deep layers receive somatosensory and auditory information and project directly to the brain stem reticular formation and the spinal cord to drive saccades and reflexive responses.28,38 While many brain regions are involved in the control of saccadic eye movements and visual fixation,39 the SC appears to exert critical control over a subclass of visually triggered saccades, express saccades.40–42
The saccadic reaction time (SRT) is the time taken to initiate a saccade, i.e. time from presentation of the target/“go” stimulus to onset of saccadic movement. Express saccades are typically identified as saccades with an SRT within a predefined range. Sample ranges from studies in non‐human primates include 80–100 ms,43 50–105 ms,44 60–110 ms,45 and 70–120 ms, 46 whereas ranges quoted in human studies include 70–110 ms,47 75–110 ms,48 80–130 ms,49 85–135 ms,50 and 90–140 ms.39 Some subjects (both human and non‐human primates) display no, or very few, express saccades,39,40 while others have been classified as “express saccade makers”.49–51 There are inherent limitations to defining express saccades on the basis of an absolute SRT value, particularly in the context of the variability across studies in the methods of recording eye movements (scleral search coils, video‐oculography, electro‐oculography) and in the computation of saccade onset.46,48,52 Marino et al.53 proposed an expanded definition of express saccades, which avoids predefined SRT limits. They allude to the reaction time of a saccade (express or regular) comprising two independent time components: the time for a visual response in the superficial layer (call this τs) and the time to trigger a visual and motor response in the visuomotor neurons in the intermediate layer (call this τD, direct or τID, indirect). There is electrophysiological evidence for the existence of direct excitatory connections from the superficial to the intermediate layers of the SC.54,55 When a visuomotor neuron in the intermediate layers has a high level of background activity, at the time of visual response from a superficial visual neuron, it can rapidly (τD) fire saccade‐related bursts, triggering an express saccade,53,54 (SRT = τs + τD). However, if the activity level of the visuomotor neuron is low, the interlaminar excitation only results in a weak response, insufficient to trigger a saccade. Additional excitation via the cortical pathway (τID) is required to fire the visuomotor neuron, resulting in a regular saccade (SRT = τs + τID). If the intermediate or deep layer visuomotor neuron is subject to the inhibitory projection of the SNpr, its background excitation will be suppressed. However, if this projection is impaired or reduced, there will be an increased likelihood of express saccades. Marino et al.53 recorded from cell populations in primates and could therefore distinguish regular and express saccades based on the timing of the visuomotor neuron excitations, e.g. τD or τID. In light of their work, and the limitations of taking a predefined range regardless of method of eye movement recording or definition of saccade onset, we propose a means of analyzing saccadic behavior based on subject‐specific thresholds derived from their “baseline”, pro‐saccade performance. This approach enables a subject‐specific assessment of saccades, allowing for individual variation in the time of the visual response of the superficial neurons (τs).
The likelihood of inducing express saccades increases under certain experimental settings including study protocol,56 increased training,48 and stimulus properties (luminance,53 predictability44,57). In the gap paradigm protocol first described by Saslow,56 a temporal gap (typically 200 ms) is introduced between the disappearance of the fixation point and target appearance. This gap gives rise to higher frequencies of express saccades (either as a result of release from the inhibitory effects of the SC fixation neurons58 or as the more recent “equilibrium hypothesis” suggests, the lack of foveal fixation results in faster equilibrium between the right and left SC, allowing more rapid saccade generation23).
Individuals with cervical dystonia have normal eye movements on clinical assessment. There are few studies in cervical dystonia in which saccadic eye movements are specifically recorded. In one study, Stell and colleagues reported that individuals with cervical dystonia were able to make random, predictive, remembered, and self‐paced saccades equally well as control subjects.60 They concluded that it was unlikely that the striato(caudato)‐nigro‐collicular pathway is involved in the pathomechanism of cervical dystonia. However, they did not employ the gap or anti‐saccade paradigms, which arguably enable a more pure assessment of nigro‐tectal pathway.
Hypothesis
If reduced nigro‐tectal inhibition of the intermediate and deep layers of SC is involved in the pathogenesis of cervical dystonia, then we hypothesize that individuals with cervical dystonia will demonstrate an increased proportion of both express saccades (in the pro and gap task) and reflexive saccades (directional errors) during the anti‐saccade task; representing an impaired ability to suppress saccades towards the target.
Study aim
The present study aimed to assess saccadic behavior, particularly express saccades, in individuals with cervical dystonia compared with age‐ and sex‐matched controls, as a means of potentially shedding light on alterations in GABAergic activity of the nigro‐tectal pathway.
Methods
Participants
A total of 19 people participated in this study: 8 with cervical dystonia (three men, aged 54 ± 5 years, and five women aged 61 ± 1.6 years), and 11 healthy controls (four men, aged 49 ± 5.9 years, and seven women 56 ± 7.7 years). Participants with cervical dystonia were recruited through the movement disorders clinic at St. Vincent’s University Hospital (Table 1). A ninth individual with cervical dystonia also participated, but his data, which displayed abnormal saccadic behavior, were not included in this study as he had taken 2 mg of diazepam in the hours prior to participation. Controls were recruited through notices in Trinity College Dublin and personal contacts of the authors. Aside from cervical dystonia, none of the participants had any other known neurological disorder or visual impairment (other than that correctable by lenses). None of the participants were taking medication that might affect eye movements for at least 48 hours prior to the study. The average time since the last botulinum toxin injection was 83 days, with the exception of CD1, who participated 4 days after her treatment. All participants gave signed informed consent. The study was approved by the Ethics Committee of St. Vincent’s University Hospital, Dublin. The experiments were performed at the Trinity College Institute of Neuroscience, Dublin.
Table 1. Summary of Participants with CD.
| Code | Side of CD | Age | Gender |
|---|---|---|---|
| CD1 | Left | 61 | F |
| CD2 | Left | 59 | M |
| CD3 | Right | 49 | M |
| CD4 | Right | 60 | F |
| CD5 | Right | 64 | F |
| CD6 | Right | 61 | F |
| CD7 | Right | 60 | F |
| CD8 | Left | 54 | M |
Abbreviations: CD, cervical dystonia; F, Female; M, Male.
The table provides a summary profile of our participants with CD, noting side of head turn/torticollis, age (years), and gender.
Protocol
Three saccade tasks were undertaken in separate blocks. Subjects performed pro‐saccades (looking towards the target, #trials = 120), anti‐saccades (looking in the opposite direction to the target, #trials = 60 × 2), and gap tasks (looking towards the target, following a temporal gap of 200 ms between fixation offset and target onset, #trials = 120), in that order. Please note that the gap task is essentially a pro‐saccade task with a temporal gap; however, for ease of distinguishing between tasks, the tasks will be referred to as pro‐saccade, anti‐saccade, and gap throughout this paper. Given the additional concentration required to perform the anti‐saccade paradigm, this task was split into two blocks of 60 trials. All paradigms were run in a single session. A block of 120 trials lasted just under 8 minutes. However, with set‐up, instruction, and practice runs, a single session lasted approximately 1 hour. Subjects were invited to rest in between blocks of trials. Juice and a small snack were made available to ward off fatigue and maximize concentration.
Stimuli were displayed on ViewSonic G90fB Graphics Series 19″ CRT Monitor with a display area of 18″ (refresh rate 75 Hz, resolution 1,024 × 768). Luminance was measured directly in front of the monitor using a Vernier Light Sensor; target stimuli and fixation cross, 237.395 lux; gray background, 4.26 lux; and black background, 0.13 lux. The computer screen was positioned 65 cm in front of the subject in a windowless dark room. Participants were asked to seat themselves such that the computer monitor was directly in front of their central gaze and to maintain the same seated position and head position throughout each block of trials. In order to prevent head movement and provide consistency, subjects rested their chin on a chin rest fixed to the edge of the desk, throughout all tasks. The visual paradigm was custom developed using Presentation software (NeuroBehavioral Systems). The display monitor was turned on at least 30 minutes prior to commencing recordings, to allow it to warm up and for its brightness to settle.
The protocol for each trial ran as follows: the screen was black for 250 ms; a white fixation cross (dimensions 0.5° × 0.5°, degrees of visual angle) was displayed for 1,000–3,500 ms (mean duration 1,500 ms); in the case of the gap paradigm only, the screen was black again for 200 ms; the target stimulus (a white square 0.5° × 0.5°) was displayed for 1,000 ms, 10° to the left or right of center; then the screen went gray with the word “BLINK” displayed for 1,000 ms. To aid concentration, three brief rest periods (6 seconds each) were spaced evenly in each task, during which time the screen turned purple and displayed a countdown from three to one. Variability of the fixation period was introduced to reduce pre‐emptive movements. The gray “BLINK” screen, presented following each saccade, served two purposes: the lighter hue was a break from the black background to prevent dark adaptation, and the command to blink encouraged participants to blink at the end of each trial so as to minimize blink artifact contaminating the saccade zone. Targets were located ±10° from center, ensuring full visibility from eye movement alone,59 while the head remained stationary on the chin rest.
Eye movements were measured using electro‐oculography (EOG) via ActiveTwo (BioSemi); 1,024 Hz sampling rate and 115 dB dynamic range. Seven Ag–AgCl electrodes were used and attached to the left and right mastoid bones (BioSemi references, DRL/CMS), left and right outer canthi (for horizontal EOG), above and below the right eye (for blink detection) and left of center on the forehead (reference electrode). Before placement, the electrodes and skin were cleaned with alcohol wipes, and a small amount of conduction gel was placed on each electrode. Electrodes were attached, using self‐adhesive sticker and medical tape, at least 10 minutes prior to commencing recording to allow impedances to settle. To ensure consistency during the experiment a script and a set of instructions were prepared and used for all participants. Prior to starting each task, subjects performed a practice run of 10 trials and were asked to repeat back the instructions they had been given for that task.
Data processing and analysis
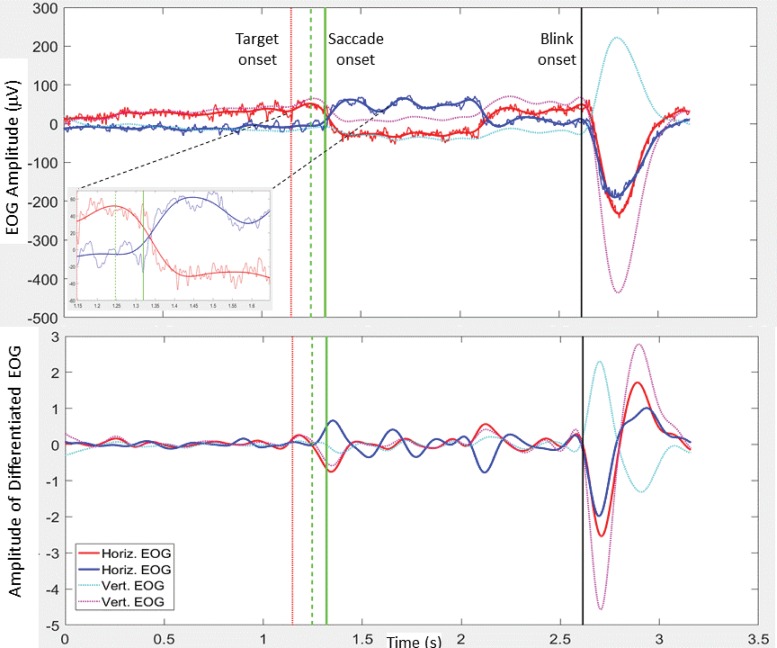
Custom algorithms, written in MATLAB (MathWorks, Version 2016b), were developed to process the EOG data. The raw EOG data, referenced to the electrode on the upper forehead, were de‐trended, and zero‐phase bandpass filtered. Two bandwidths were employed giving rise to two versions of processed EOG data: the smoothed (BPF 0.2–6 Hz) and the higher resolution (BPF 0.2–125 Hz) data. Unless otherwise stated the processing steps below used the smoothed EOG data. Trigger codes were outputted from Presentation® to the Biosemi system indicating the onset of fixation cross, left/right target, “blink” command, etc. These triggers were used to epoch the data into individual trials. The following steps were taken for each trial: 1) blink detection: both vertical and horizontal EOG data were used. Blink detection is based on finding local minima of the differentiated EOG from the electrodes on the left and right side and below the eye, and a corresponding maximum in the EOG signal from above the right eye. Blink onset is defined as the local turning point in the EOG, preceding the above minima/maximum (Figure 1). If a blink occurred within 500 ms of the appearance of the target, i.e. in the expected saccade zone, blink interference was registered and no further analysis was carried out on that trial. 2) Saccade epoch: an epoch from target onset to the end of trial was analyzed for saccade onset detection. Note, if a blink onset was detected before the end of the trial, the trial end was reset to be the blink onset. 3) Initial saccade onset detection: pairs of peaks from left and right differentiated EOG (each normalized to its own absolute maximum) were identified. One peak from each pair was required to be at least 0.4, i.e. 40% of normalized and differentiated EOG signal. The first pair of peaks satisfying these conditions was selected and the algorithm stepped backwards until the point of intersection of the left and right differentiated EOG data (Figure 1B). This was defined as the initial saccade onset. 4) Saccade onset: a subsection (from the initial saccade onset to the subsequent positive turning point) of the higher resolution EOG data (0.2–125 Hz) was employed to fine‐tune saccade onset (local minimum before peak) (inset Figure 1). 5) Parameters: the SRT was defined as the time interval from target onset to saccade onset. The saccadic direction was recorded, enabling analysis of right and left movements. If the saccade was in the opposite direction to the target for the pro‐saccade or gap tasks, or towards the target for the anti‐tasks, the saccade was marked as having a directional error. The EOG amplitude at the point of reaching the target was also recorded.
Figure 1. Electro‐oculography (EOG) Data from a Sample Leftward Trial. (A) The smoothed (0.2–6 Hz) and higher resolution (0.2–125 Hz) EOG is shown for the right (red) and left (blue) signals. Smoothed vertical EOG is also shown (dotted). The onset of the fixation cross occurs at zero (start of trial). The red dotted vertical lines represent target onset and the black vertical line represents blink onset/trial end. The solid vertical green line marks saccade onset. The dashed vertical green line just prior to saccade onset marks the initial estimate of saccade onset. The inset shows magnified horizontal EOG (both smoothed and high definition) from target onset to 0.5 seconds after target onset. Again saccade onset and initial saccade onset are indicated by vertical lines. (B) The differentiated horizontal and vertical smoothed EOG, with vertical lines as above. See explanations in text.
A velocity thresholding approach is often employed as a means of defining saccade onset, e.g. the first point at which velocity exceeds 30° per second. In order to compare our approach to a more traditional velocity threshold approach, EOG data and velocity profiles for horizontal EOG (superimposed with the above automatically detected saccade onsets) were viewed manually from several hundred individual trials. Velocity thresholds at ±30° per second were delineated in the velocity plots. Comparisons were made when the EOG data to be processed by the velocity‐thresholding approach were bandpass filtered at 0.2–6 Hz, 0.2–25 Hz, and 0.2–50 Hz. Stringent filtering characteristics provide smooth, clean signals (enabling reliable detection of events) but with associated loss of timing information (reduced precision in SRT estimates). A more generous passband gives higher definition (more precise SRT, when the correct point on the velocity profile is identified); however, the wider passband leads to significantly more noise in the data; greatly increasing susceptibility to false positives (wrongly identified saccade onsets) in the velocity threshold approach. Saccade onset by our approach performed reliably, even in the presence of noise, and was found to closely align with threshold crossings (corresponding to correct EOG saccade onset) in higher resolution velocity signals (0.2–50 Hz).
The gap effect and anti‐effect were calculated for each subject and defined as: gap effect = median(SRTPRO) – median(SRTGAP) anti‐effect = median(SRTANTI) – median(SRTPRO).
In line with the approach taken by others,39,48 results from leftward and rightward movements were analyzed separately. Saccades were accepted if the SRT was greater than 50 ms and less than 600 ms. SRTs from pro‐saccade and gap tasks of all subjects were analyzed (n = 4,739). Although the percentage of SRTs from correct movements outnumbered SRTs from erroneous movements throughout, 50 ms was chosen as the lower cutoff for acceptable saccades as above this point there was a distinct reduction in SRTs from erroneous movements. Only 1.4% of SRTs (n = 67) were below this cutoff. This cutoff aligned with the upper limit of a distinct sub‐population of SRTs from “early saccades” in the anti‐saccade task. The upper cutoff of 600 ms was selected as SRTs above this point (0.74%; n = 35) had a near‐equal chance of being correct or erroneous. Unless otherwise stated, all SRT values reported below are for saccades made in the correct direction. The median SRT for leftward and rightward movements was calculated for each subject. The 10th percentile of the pro‐saccade distribution was chosen as a threshold for the classification of express saccades, as it was both subject specific and would distinguish between “express” and “regular” SRT populations, where distinction exists.
Mixed one‐way analysis of variance (ANOVA), with the factors of side (left, right) and group (cervical dystonia cohort [CD] and healthy controls [HC]) were performed to compare the reaction times of the side between CD and controls. The Kruskal–Wallis test was performed on the percentile data to test for between group differences. Details are outlined in the results section.
Sample size calculation
Owing to the paucity of similar studies, the expected variance of the data was unknown. However, the sample size for this pilot study was deemed appropriate with reference to existing saccade studies in healthy controls,39,47,48,56 and those in which eye movements are compared between controls and individuals with movement disorders.60–63
Results
SRT values: task, side, and group
Group means and standard deviations for the CD and HC cohorts under each condition are presented in Table 2. Mean SRTs (pro‐saccade task) in the CD group were (left, right) 225 ms (SD = 62 ms), 214 ms (SD = 50 ms), and in HC (left, right), 199 ms (SD = 33 ms), 206 ms (SD = 47 ms). As expected, the gap paradigm produced faster SRTs such that the mean and standard deviations of SRTs of the CD group were (left, right), 177 ms (SD = 44 ms), 159 ms (SD = 31 ms) and in HC (left, right), 156 ms (SD = 25 ms), 151 ms (SD = 18 ms). The SRTs of saccades in the anti‐saccade task were slower than pro‐saccade reaction times, in line with expectations: CD (left, right) 313 (SD = 46), 318 (SD = 45); HC (left, right), 284 (SD = 35), 292 (SD = 32). The effect of group (CD vs. HC) and side (left vs. right) were assessed using mixed one‐way ANOVA. Neither of the factors of group or side had a significant impact for the pro‐saccade task (group: F(1, 35) = 1.13, p = 0.295, side: F(1, 35) = 0, p = 0.98), or the gap task (group: F(1, 35) = 2.09, p = 0.157, side: F(1, 35) = 1.25, p = 0.271). However, a statistically significant difference in SRT was detected between the CD and HC cohorts for the anti‐saccade task (F(1, 35) = 4.76, p = 0.036). There was no effect of side for the anti‐saccade task (F(1, 35) = 0.3, p = 0.589). As anticipated from the above reported changes in group mean SRT, a multiway ANOVA of left and right median SRT values, with group and task as factors, revealed a highly significant effect of task (F(2, 110) = 129, p < 10–28). The gap effect and anti‐effect were calculated for each individual (as defined above). Group means and standard deviations for the gap effect were CD 56 ms (SD = 45 ms); HC 51 ms (SD = 37 ms). The anti‐effect was larger; mean SRT difference was CD 97 ms (SD = 30 ms) and 84 ms (SD = 38 ms) for HC.
Table 2. Summary of Saccadic Reaction Times and Percentage of Express Saccades.
| SRT | % Saccades SRT< 140 ms | %Saccades SRT < subject 10th %tile | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||||||||
| Pro (ms) | Gap (ms) | Anti (ms) | Pro (ms) | Gap (ms) | Anti (ms) | Gap (%) | Gap (%) | 10th %tile (ms) | Gap (%) | 10th %tile (ms) | Gap (%) | ||
| CD | μ | 225 | 177 | 313 | 214 | 159 | 318 | 22 | 29 | 174 | 50 | 167 | 55 |
| SD | 62 | 44 | 46 | 50 | 31 | 45 | 18 | 27 | 35 | 13 | 34 | 19 | |
| HC | μ | 199 | 156 | 284 | 206 | 151 | 292 | 37 | 37 | 152 | 47 | 156 | 53 |
| SD | 33 | 25 | 35 | 47 | 18 | 32 | 26 | 22 | 16 | 21 | 21 | 22 | |
Abbreviations: CD, cervical dystonia; HC, healthy controls; μ, Mean; SD, Standard deviation; SRT, Saccadic Reaction Time; 10th %tile, 10th percentile of the pro‐saccade distribution (see text).
Summary results for participants CD (n = 8) and HC (n = 11). Shown are the group μ and SD values for each parameter. % Saccades in the gap task with an SRT below a threshold (140 ms, or a subject‐specific threshold). Values shown for leftward and rightward movements.
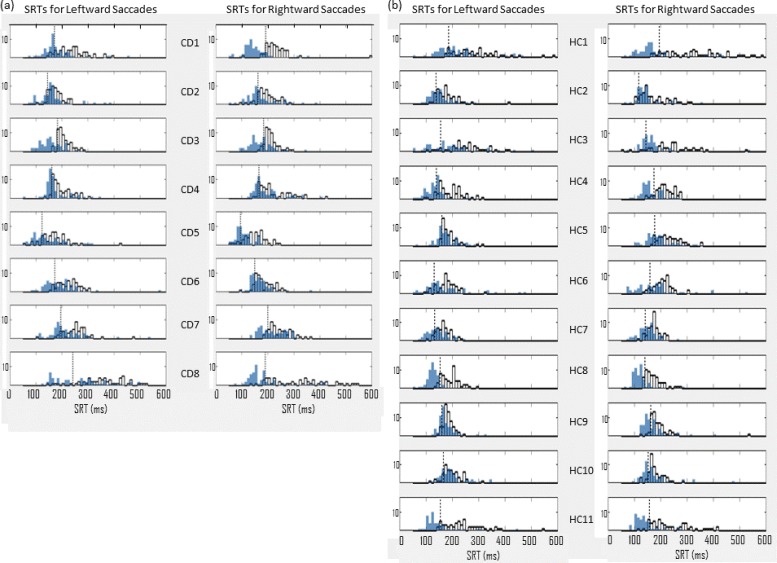
Figure 2 illustrates the distributions for leftward and rightward saccades under pro‐saccade and gap conditions. The effect of task is clearly apparent (SRTs becoming faster with the gap paradigm).
Figure 2. Distributions of Saccadic Reaction Times (SRTs). SRT distributions for leftward and rightward saccades under pro‐saccade (white) and gap (blue) conditions. Each row represents the left and right SRTs for one subject. (A) Cervical dystonia (CD), n = 8. (b) Health controls (HC), n = 11. Dotted black vertical lines mark the 10th percentile of the pro‐saccade distribution in each case. This is used as the subject‐specific threshold for the identification of express saccades. Compare this to the more traditional, predefined threshold of 140 ms.
The use of the chin rest appeared to assist CD participants to stabilize their head position. Only one participant reported that she felt her head turn during the experiment (CD5, Table 1). Inspection of her results reveals SRTs are slower for leftward than for rightward saccades. It is tempting to interpret this as being related to the side of torticollis (right). However, further inspection of both CD and HC data (Table 1 and Figure 2) reveals variation in SRTs of leftward and rightward saccades, but no consistent relationship to side of dystonic head rotation. In addition the statistical analysis reported above demonstrates that side does not have a statistical impact on SRT.
Express saccades
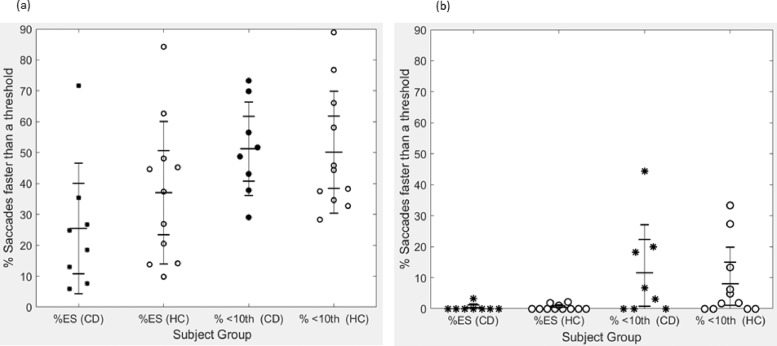
For comparative purposes, we applied both a standard predefined hard threshold of 140 ms,39 and a subject‐specific threshold when classifying saccades as express or otherwise. The subject‐specific threshold is defined as the 10th percentile of the pro‐saccade SRT distribution (for the corresponding direction) for each subject; see demarcation on each subjects’ pro‐saccade data (Figure 2). The mean and standard deviation of the subject‐specific thresholds for left and right saccades were 174 ms (SD = 35 ms) and 167 ms (SD = 34 ms) for CD and 152 ms (SD = 16 ms) and 156 ms (SD = 14 ms) respectively for HC (Table 2). Occasionally the 140 ms demarcation for express saccade aligns with the 10th percentile (see Figure 2; HC8 and HC11) and marks a clear distinction between express and regular saccades. However, the subject‐specific demarcation often better represents this distinction, e.g. CD1, CD3, CD7, CD8, HC1, HC4, HC9 in Figure 2. The percentage of express saccades under both thresholding approaches is plotted for individuals with cervical dystonia and controls for the gap and anti‐saccade tasks in Figure 3. The subject‐specific threshold results in higher percentages of express saccades being detected in both conditions; however, these percentages were not statistically different between CD and HC (results from Kruskal–Wallis one‐way ANOVA for the gap task, chi‐square = 1.13, p = 0.287; and chi‐square = 0.37, p = 0.54, for the anti‐saccade task).
Figure 3. Percentage of Express Saccades. Percentage of saccades below threshold (express saccades) in the gap task (A) and anti‐saccade task (B). Results are shown for percentage of saccades below each of the “hard” threshold of 140 ms (%ES, express saccades), and the dynamic, subject‐specific threshold (% < 10th, 10th percentile of the saccadic reaction time distribution for the pro‐saccade task for that individual). Data from individuals with cervical dystonia (CD) black asterisks (n = 8), and healthy controls (HC) ‘o’ (n = 11).
Asymmetrically reduced express saccades and direction of head turn in cervical dystonia
The side of visual stimulation that produced the lower percentage of express saccades in the gap task (as defined by the subject‐specific threshold) corresponded to the direction of torticollis in each of the eight cervical dystonia patients (see Table 1 and Figure 2). The mean frequency of express saccades ipsilateral to the direction of torticollis was 45% (SD = 11%) compared to the frequency of express saccades in the opposite direction (60%, SD = 17%), producing a borderline statistically significant difference according to a Kruskal–Wallis one‐way ANOVA, chi‐square = 3.57, p = 0.059.
Directional errors
The percentage of directional errors was examined in all tasks, with the highest levels occurring in the anti‐saccade paradigm, as expected. However, contrary to expectation, the cervical dystonia group displayed a lower level of directional errors in all tasks: pro‐saccade (mean = 1%, SD = 1.6%), gap task (mean 1%, SD = 0.8%), and anti‐saccade task (mean = 14%, SD = 10%). The corresponding values for controls were pro‐saccade (mean = 2%, SD = 2%), gap task (mean = 2%, SD = 1.5%), and anti‐saccade (mean = 22%, SD = 18.4%). Three control subjects displayed particularly high levels of directional error, between 47% and 51%, whereas the highest level of directional error in the cervical dystonia cohort was 28%. Kruskal–Wallis one‐way ANOVA were carried out to determine the effect of group on the percentage of directional errors for each task (movements to left and right sides were combined in each task for this analysis). The percentage of directional errors were not significantly different between the CD and HC groups in any of the tasks: pro‐saccade task, chi‐square = 0.72, p = 0.397; gap task, chi‐square = 2.82, p = 0.093; and anti‐saccade task, chi‐square = 0.44, p = 0.509.
Discussion
In this study we sought to assess saccadic behavior, particularly express saccades, in individuals with cervical dystonia and healthy controls, as a means of potentially gaining insight into the GABAergic behavior of the nigro‐tectal pathway. Contrary to expectations, mean SRT values tended to be slower in the CD group across all tasks (only reaching statistical significance in the anti‐saccade task, (F(1, 35) = 4.76, p = 0.036)); individuals with cervical dystonia produced fewer errors (mean 14% vs. 22%) in the anti‐saccade task and had similar frequencies of express saccades in the gap task relative to our control population (chi‐square = 1.13, p = 0.287).
The analysis of saccadic behavior in this study strongly relies on SRT. It is acknowledged that SRT may vary significantly in an individual on a trial‐by‐trial basis, even in circumstances where both stimuli and task remain constant,59 hence the distributions obtained for each individual for the same task (Figure 2). It is worth noting not only the spread of the distributions for an individual, but also the variability in ranges of SRTs in the pro‐saccade task across subjects. Note also the SRT distributions for the gap paradigm present with a bimodal form for some, though not all, subjects. The bimodality of the gap distribution is often relied upon as a means of defining the SRT cutoff for express saccades.45 This may be valid in studies on non‐human primates who have undergone months of training on the task. However, in this study on human subjects, bimodality is not always present; a finding which concurs with others,47,48,53 particularly in older cohorts,39 similar to our own. These observations, in addition to the variation in saccade onset detection, highlight a weakness in relying on a predefined cutoff value for SRTs. Marino et al.53 recorded both slow and fast express saccades, where the resultant SRT (τS + τD) is influenced by the qualities (timing, magnitude) of the visual response in the superficial layer of the SC (τS), which in turn is affected by the luminance of the stimulus. Such a finding points to a degree of subjectivity in SRTs of express saccades. The combination of the above considerations supports our approach of using a subject‐specific threshold for the identification of express saccades. This approach enabled an individualized assessment of performance for each participant. The side that produced the lower percentage of express saccades (as defined by the subject‐specific threshold), corresponded to the side affected by dystonia for each patient. The sample size in this study (n = 8) is too small to determine the significance of this result. However, this trait warrants further research.
Saccadic behavior in cervical dystonia vs. controls
Many studies beautifully examine saccade‐related behavior of single neurons in the SC33,46,53,64–67 and SNpr.32,53,68–73 In this human behavioral study, we cannot know such minutia of detail, and to some extent a simplified view needs to be adopted. Our protocol was designed to minimize variation of input signals to the SC and by consequence, stabilize the underlying physiological behavior. Reduced GABAergic activity in the nigro‐tectal pathway (likely affecting the “pauser” rather than the “burster” neurons, see Handel and Glimcher73) has been shown to induce symptoms of cervical dystonia34–36 and stimulate saccades32,72 in animal models. We postulated that a dystonia‐related reduction in GABAergic activity would manifest as altered saccadic behavior (reduced SRT generally, more express saccades in the gap task and more directional errors in the anti‐saccade task). That this was not the case raises many questions. Could there be compensatory activity, higher than normal “burster” activity perhaps? Is the nigro‐tectal projection not affected by altered GABAergic activity associated with cervical dystonia? Could reduced GABAergic activity occur upstream of the SNpr–SC connection,14 such that the SNpr receives less striatal inhibition? That being the case, the SNpr would exert stronger inhibitory control over the deep layers of the SC, slowing all saccadic activity and minimizing directional errors. Or, might reduced pre‐saccadic activity in the visuomotor neurons in our CD group, relate to defective initial processing in the visual sensory neurons in the superficial layers of the SC?30,74
It may be that a targeted reduction in GABAergic activity at the striato‐nigral or nigro‐tectal interconnections would not occur in isolation,15 and that this expectation is too simplistic a view for what is a more complex disorder. Liu and Basso68 suggest that rather than acting simply as a gate for saccade initiation, the influence of SNpr inhibition on visually guided saccades is more subtle, shaping the balance of excitation and inhibition across the SC. This is supported by research indicating a more complex role in saccade regulation arising from interactions among SNpr axons and inhibitory and excitatory neurons in the intermediate layer of the SC.75 In their study of GABA levels in the brains of individuals with focal hand dystonia, Levy and Hallett14 report that GABAergic neurons are non‐uniformly affected in patients. Other studies highlight the impact of GABAergic activity in the cerebellum and cortex in cervical dystonia.15 The implications of involvement of additional brain areas on the behavior of the striato‐nigral or nigro‐tectal interconnections and saccade generation, or indeed the manifestation of cervical dystonia symptoms, are not straightforward.
Conclusion
Our results do not support our hypothesized increase in express saccades and directional errors arising from reduced SNpr–SC inhibition in cervical dystonia. The finding that the side affected by cervical dystonia corresponded to the direction of eye movements that produced fewer express saccades warrants further investigation.
Acknowledgments
We thank each of our participants for volunteering to take part in this study. We also thank Dr. John Butler for his advice on our statistical analysis and for proofreading this paper. Thanks also to Dr. Owen Killian for his input in proofing the manuscript. We would like to thank the anonymous reviewers for their constructive and thought‐provoking comments.
Footnotes
Funding: This study was made possible by a grant from the Health Research Board of Ireland (CSA‐2012‐5).
Financial Disclosures: Prof. Michael Hutchinson receives research grants from Dystonia Ireland, the Health Research Board of Ireland (CSA‐2012‐5), Foundation for Dystonia Research (Belgium) and the Irish Institute of Clinical Neuroscience. Dr. Sean O’Riordan reports receiving a speaker’s honorarium from Abbvie. Prof. Richard Reilly receives funding from Science Foundation Ireland, Enterprise Ireland, and the Health Research Board of Ireland.
Conflicts of Interest: The authors report no conflict of interest.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent.
References
- 1.Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. 10.1002/mds.25475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asgeirsson H, Jakobsson F, Hjaltason H, Jonsdottir H, Sveinbjornsdottir S. Prevalence study of primary dystonia in Iceland. Mov Disord. 2006;21:293–298. doi: 10.1002/mds.20674. 10.1002/mds.20674 [DOI] [PubMed] [Google Scholar]
- 3.Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. Lancet Neurol. 2004;3:673–678. doi: 10.1016/S1474-4422(04)00907-X. 10.1016/S1474‐4422(04)00907‐X [DOI] [PubMed] [Google Scholar]
- 4.Beck RB, McGovern EM, Butler JS, Birsanu D, Quinlivan B, Beiser I, et al. Measurement & analysis of the temporal discrimination threshold applied to cervical dystonia. J Vis Exp. 2018;131 doi: 10.3791/56310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley D, Whelan R, Kimmich O, O'Riordan S, Mulrooney N, Brady P, et al. Temporal discrimination thresholds in adult-onset primary torsion dystonia: an analysis by task type and by dystonia phenotype. J Neurol. 2012;259:77–82. doi: 10.1007/s00415-011-6125-7. 10.1007/s00415‐011‐6125‐7 [DOI] [PubMed] [Google Scholar]
- 6.Butler JS, Beiser IM, Williams L, McGovern E, Molloy F, Lynch T, et al. Age-related sexual dimorphism in temporal discrimination and in adult-onset dystonia suggests GABAergic mechanisms. Front Neurol. 2015;6:258. doi: 10.3389/fneur.2015.00258. 10.3389/fneur.2015.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conte A, McGovern EM, Narasimham S, Beck R, Killian O, O'Riordan S, et al. Temporal discrimination: mechanisms and relevance to adult-onset dystonia. Front Neurol. 2017;8:625. doi: 10.3389/fneur.2017.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson M, McGovern EM, Narasimham S, Beck R, Reilly RB, Walsh CD, et al. The premotor syndrome of cervical dystonia: disordered processing of salient environmental stimuli. Mov Disord. 2018;33:232–237. doi: 10.1002/mds.27229. 10.1002/mds.27229 [DOI] [PubMed] [Google Scholar]
- 9.Contarino MF, Smit M, van den Dool J, Volkmann J, Tijssen MA. unmet needs in the management of cervical dystonia. Front Neurol. 2016;7:165. doi: 10.3389/fneur.2016.00165. 10.3389/fneur.2016.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundel H, Wolf A, Xidara V, Busch R, Ladwig KH, Jacobi F, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191:465–473. doi: 10.1097/01.NMD.0000081667.02656.21. 10.1097/01.NMD.0000081667.02656.21 [DOI] [PubMed] [Google Scholar]
- 11.LeDoux MS, Vemula SR, Xiao J, Thompson MM, Perlmutter JS, Wright LJ, et al. Clinical and genetic features of cervical dystonia in a large multicenter cohort. Neurol Genet. 2016;2:e69. doi: 10.1212/NXG.0000000000000069. 10.1212/NXG.0000000000000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lencer R, Steinlechner S, Stahlberg J, Rehling H, Orth M, Baeumer T, et al. Primary focal dystonia: evidence for distinct neuropsychiatric and personality profiles. J Neurol Neurosurg Psychiatry. 2009;80:1176–1179. doi: 10.1136/jnnp.2008.170191. 10.1136/jnnp.2008.170191 [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson M, McGovern EM, Narasimham S, Beck R, Reilly RB, Walsh CD, et al. The premotor syndrome of cervical dystonia: Disordered processing of salient environmental stimuli. Mov Disord. 2018;33:232–237. doi: 10.1002/mds.27229. 10.1002/mds.27229 [DOI] [PubMed] [Google Scholar]
- 14.Levy LM, Hallett M. Impaired brain GABA in focal dystonia. Ann Neurol. 2002;51:93–101. 10.1002/ana.10073 [PubMed] [Google Scholar]
- 15.Berman BD, Pollard RT, Shelton E, Karki R, Smith‐Jones PM, Miao Y. GABAA receptor availability changes underlie symptoms in isolated cervical dystonia. Front Neurol. 2018;9:188. doi: 10.3389/fneur.2018.00188. 10.3389/fneur.2018.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix CM, Vitek JL. Toward a network model of dystonia. Ann NY Acad Sci. 2012;1265:46–55. doi: 10.1111/j.1749-6632.2012.06692.x. 10.1111/j.1749‐6632.2012.06692.x [DOI] [PubMed] [Google Scholar]
- 17.Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 2013;28:944–957. doi: 10.1002/mds.25527. 10.1002/mds.25527 [DOI] [PubMed] [Google Scholar]
- 18.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. 10.1016/j.nbd.2011.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakkottai VG, Batla A, Bhatia K, Dauer WT, Dresel C, Niethammer M, et al. Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum. 2017;16:577–594. doi: 10.1007/s12311-016-0825-6. 10.1007/s12311‐016‐0825‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. 10.1016/j.neuroscience.2013.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garibotto V, Romito LM, Elia AE, Soliveri P, Panzacchi A, Carpinelli A, et al. In vivo evidence for GABA(A) receptor changes in the sensorimotor system in primary dystonia. Mov Disord. 2011;26:852–857. doi: 10.1002/mds.23553. 10.1002/mds.23553 [DOI] [PubMed] [Google Scholar]
- 22.Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. 10.1016/j.nbd.2010.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauzlis RJ, Goffart L, Hafed ZM. Neuronal control of fixation and fixational eye movements. Phil Trans R Soc Lond B Biol Sci. 2017;372:20160205. doi: 10.1098/rstb.2016.0205. 10.1098/rstb.2016.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. 10.1073/pnas.0408311101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares SC, Maior RS, Isbell LA, Tomaz C, Nishijo H. Fast detector/first responder: interactions between the superior colliculus-pulvinar pathway and stimuli relevant to primates. Front Neurosci. 2017;11:67. doi: 10.3389/fnins.2017.00067. 10.3389/fnins.2017.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafed ZM, Lovejoy LP, Krauzlis RJ. Superior colliculus inactivation alters the relationship between covert visual attention and microsaccades. Eur J Neurosci. 2013;37:1169–1181. doi: 10.1111/ejn.12127. 10.1111/ejn.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CY, Hafed ZM. Orientation and contrast tuning properties and temporal flicker fusion characteristics of primate superior colliculus neurons. Front Neural Circuits. 2018;12:58. doi: 10.3389/fncir.2018.00058. 10.3389/fncir.2018.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. 10.1152/physrev.1986.66.1.118 [DOI] [PubMed] [Google Scholar]
- 29.Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol. 1996;76:927–952. doi: 10.1152/jn.1996.76.2.927. 10.1152/jn.1996.76.2.927 [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson M, Isa T, Molloy A, Kimmich O, Williams L, Molloy F, et al. Cervical dystonia: a disorder of the midbrain network for covert attentional orienting. Front Neurol. 2014;5:54. doi: 10.3389/fneur.2014.00054. 10.3389/fneur.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mize RR. Immunocytochemical localization of gamma-aminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol. 1988;276:169–187. doi: 10.1002/cne.902760203. 10.1002/cne.902760203 [DOI] [PubMed] [Google Scholar]
- 32.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. 10.1152/jn.1985.53.1.292 [DOI] [PubMed] [Google Scholar]
- 33.Hikosaka O, Wurtz RH. Effects on eye movements of a GABA agonist and antagonist injected into monkey superior colliculus. Brain Res. 1983;272:368–372. doi: 10.1016/0006-8993(83)90586-3. 10.1016/0006‐8993(83)90586‐3 [DOI] [PubMed] [Google Scholar]
- 34.Burbaud P, Bonnet B, Guehl D, Lagueny A, Bioulac B. Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey. Brain Res. 1998;780:102–107. doi: 10.1016/s0006-8993(97)01158-x. 10.1016/S0006‐8993(97)01158‐X [DOI] [PubMed] [Google Scholar]
- 35.Holmes AL, Forcelli PA, DesJardin JT, Decker AL, Teferra M, West EA, et al. Superior colliculus mediates cervical dystonia evoked by inhibition of the substantia nigra pars reticulata. J Neurosci. 2012;32:13326–13332. doi: 10.1523/JNEUROSCI.2295-12.2012. 10.1523/JNEUROSCI.2295‐12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malouin F, Bedard PJ. Evaluation of head motility and posture in cats with horizontal torticollis. Exp Neurol. 1983;81:559–570. doi: 10.1016/0014-4886(83)90326-6. 10.1016/0014‐4886(83)90326‐6 [DOI] [PubMed] [Google Scholar]
- 37.Altman J. Some Fiber Projections to the Superior Colliculus in the Cat. J Comp Neurol. 1962;119:77–95. doi: 10.1002/cne.901160206. 10.1002/cne.901190107 [DOI] [PubMed] [Google Scholar]
- 38.Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol. 2008;18:544–551. doi: 10.1016/j.conb.2008.11.004. 10.1016/j.conb.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. 10.1007/s002210050473 [DOI] [PubMed] [Google Scholar]
- 40.Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. 10.1152/jn.1987.57.4.1033 [DOI] [PubMed] [Google Scholar]
- 41.Pierrot‐Deseilligny C, Rosa A, Masmoudi K, Rivaud S, Gaymard B. Saccade deficits after a unilateral lesion affecting the superior colliculus. J Neurol Neurosurg Psychiatry. 1991;54:1106–1109. doi: 10.1136/jnnp.54.12.1106. 10.1136/jnnp.54.12.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol. 1996;76:908–926. doi: 10.1152/jn.1996.76.2.908. 10.1152/jn.1996.76.2.908 [DOI] [PubMed] [Google Scholar]
- 43.Munoz DP, Wurtz RH. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J Neurophysiol. 1992;67:1000–1002. doi: 10.1152/jn.1992.67.4.1000. 10.1152/jn.1992.67.4.1000 [DOI] [PubMed] [Google Scholar]
- 44.Schiller PH, Haushofer J, Kendall G. How do target predictability and precueing affect the production of express saccades in monkeys? Eur J Neurosci. 2004;19:1963–1968. doi: 10.1111/j.1460-9568.2004.03299.x. 10.1111/j.1460‐9568.2004.03299.x [DOI] [PubMed] [Google Scholar]
- 45.Schiller PH, Slocum WM, Carvey C, Tolias AS. Are express saccades generated under natural viewing conditions? Eur J Neurosci. 2004;20:2467–2473. doi: 10.1111/j.1460-9568.2004.03663.x. 10.1111/j.1460‐9568.2004.03663.x [DOI] [PubMed] [Google Scholar]
- 46.Aizawa H, Kobayashi Y, Yamamoto M, Isa T. Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J Neurophysiol. 1999;82:1642–1646. doi: 10.1152/jn.1999.82.3.1642. 10.1152/jn.1999.82.3.1642 [DOI] [PubMed] [Google Scholar]
- 47.Edelman JA, Kristjansson A, Nakayama K. The influence of object-relative visuomotor set on express saccades. J Vis. 2007;7:12. doi: 10.1167/7.6.12. 10.1167/7.6.12 [DOI] [PubMed] [Google Scholar]
- 48.Bibi R, Edelman JA. The influence of motor training on human express saccade production. J Neurophysiol. 2009;102:3101–3110. doi: 10.1152/jn.90710.2008. 10.1152/jn.90710.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knox PC, Wolohan FD. Temporal stability and the effects of training on saccade latency in “express saccade makers”. PLoS One. 2015;10:e0120437. doi: 10.1371/journal.pone.0120437. 10.1371/journal.pone.0120437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biscaldi M, Fischer B, Stuhr V. Human express saccade makers are impaired at suppressing visually evoked saccades. J Neurophysiol. 1996;76:199–214. doi: 10.1152/jn.1996.76.1.199. 10.1152/jn.1996.76.1.199 [DOI] [PubMed] [Google Scholar]
- 51.Cavegn D, Biscaldi M. Fixation and saccade control in an express-saccade maker. Exp Brain Res. 1996;109:101–116. doi: 10.1007/BF00228631. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Hirai N. The role of fixation point and subjects' readiness in the occurrence of express saccades as revealed by the self-initiation paradigm. Neurosci Res. 2000;36:235–244. doi: 10.1016/s0168-0102(99)00127-3. 10.1016/S0168‐0102(99)00127‐3 [DOI] [PubMed] [Google Scholar]
- 53.Marino RA, Levy R, Munoz DP. Linking express saccade occurrence to stimulus properties and sensorimotor integration in the superior colliculus. J Neurophysiol. 2015;114:879–892. doi: 10.1152/jn.00047.2015. 10.1152/jn.00047.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isa T. Intrinsic processing in the mammalian superior colliculus. Curr Opin Neurobiol. 2002;12:668–677. doi: 10.1016/s0959-4388(02)00387-2. 10.1016/S0959‐4388(02)00387‐2 [DOI] [PubMed] [Google Scholar]
- 55.Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102:2581–2593. doi: 10.1152/jn.00498.2009. 10.1152/jn.00498.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am. 1967;57:1024–1049. doi: 10.1364/josa.57.001024. 10.1364/JOSA.57.001024 [DOI] [PubMed] [Google Scholar]
- 57.Pare M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76:3666–3681. doi: 10.1152/jn.1996.76.6.3666. 10.1152/jn.1996.76.6.3666 [DOI] [PubMed] [Google Scholar]
- 58.Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol. 1995;73:2558–62. doi: 10.1152/jn.1995.73.6.2558. 10.1152/jn.1995.73.6.2558 [DOI] [PubMed] [Google Scholar]
- 59.Antoniades C, Ettinger U, Gaymard B, Gilchrist I, Kristjansson A, Kennard C, et al. An internationally standardised antisaccade protocol. Vision Res. 2013;84:1–5. doi: 10.1016/j.visres.2013.02.007. 10.1016/j.visres.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 60.Stell R, Bronstein AM, Gresty M, Buckwell D, Marsden CD. Saccadic function in spasmodic torticollis. J Neurol Neurosurg Psychiatry. 1990;53:496–501. doi: 10.1136/jnnp.53.6.496. 10.1136/jnnp.53.6.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lueck CJ, Tanyeri S, Crawford TJ, Henderson L, Kennard C. Antisaccades and remembered saccades in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1990;53:284–288. doi: 10.1136/jnnp.53.4.284. 10.1136/jnnp.53.4.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaunak S, O'Sullivan E, Blunt S, Lawden M, Crawford T, Henderson L, et al. Remembered saccades with variable delay in Parkinson’s disease. Mov Disord. 1999;14:80–86. doi: 10.1002/1531-8257(199901)14:1<80::aid-mds1014>3.0.co;2-m. 10.1002/1531‐8257(199901)14:1<80::AID‐MDS1014>3.0.CO;2‐M [DOI] [PubMed] [Google Scholar]
- 63.Maurer C, Mergner T, Lucking CH, Becker W. Adaptive changes of saccadic eye-head coordination resulting from altered head posture in torticollis spasmodicus. Brain. 2001;124((Pt 2)):413–26. doi: 10.1093/brain/124.2.413. 10.1093/brain/124.2.413 [DOI] [PubMed] [Google Scholar]
- 64.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. 10.1152/jn.1985.53.1.266 [DOI] [PubMed] [Google Scholar]
- 65.Marino RA, Trappenberg TP, Dorris M, Munoz DP. Spatial interactions in the superior colliculus predict saccade behavior in a neural field model. J Cogn Neurosci. 2012;24:315–336. doi: 10.1162/jocn_a_00139. 10.1162/jocn_a_00139 [DOI] [PubMed] [Google Scholar]
- 66.Phongphanphanee P, Kaneda K, Isa T. Spatiotemporal profiles of field potentials in mouse superior colliculus analyzed by multichannel recording. J Neurosci. 2008;28:9309–9318. doi: 10.1523/JNEUROSCI.1905-08.2008. 10.1523/JNEUROSCI.1905‐08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaneda K, Phongphanphanee P, Katoh T, Isa K, Yanagawa Y, Obata K, et al. Regulation of burst activity through presynaptic and postsynaptic GABA(B) receptors in mouse superior colliculus. J Neurosci. 2008;28:816–827. doi: 10.1523/JNEUROSCI.4666-07.2008. 10.1523/JNEUROSCI.4666‐07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol. 2008;100:1098–1112. doi: 10.1152/jn.01043.2007. 10.1152/jn.01043.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. 10.1152/jn.1983.49.5.1268 [DOI] [PubMed] [Google Scholar]
- 70.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. II. Visual responses related to fixation of gaze. J Neurophysiol. 1983;49:1254–1267. doi: 10.1152/jn.1983.49.5.1254. 10.1152/jn.1983.49.5.1254 [DOI] [PubMed] [Google Scholar]
- 71.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. 10.1152/jn.1983.49.5.1230 [DOI] [PubMed] [Google Scholar]
- 72.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. 10.1152/jn.1983.49.5.1285 [DOI] [PubMed] [Google Scholar]
- 73.Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol. 1999;82:3458–3475. doi: 10.1152/jn.1999.82.6.3458. 10.1152/jn.1999.82.6.3458 [DOI] [PubMed] [Google Scholar]
- 74.Hutchinson M, Kimmich O, Molloy A, Whelan R, Molloy F, Lynch T, et al. The endophenotype and the phenotype: temporal discrimination and adult-onset dystonia. Mov Disord. 2013;28:1766–1774. doi: 10.1002/mds.25676. 10.1002/mds.25676 [DOI] [PubMed] [Google Scholar]
- 75.Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci. 2008;28:11071–11078. doi: 10.1523/JNEUROSCI.3263-08.2008. 10.1523/JNEUROSCI.3263‐08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]