Abstract
Background
Active inflammatory bowel disease increases the risk of adverse pregnancy outcomes. Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). As a small molecule, tofacitinib is likely to cross the placental barrier; however, information on the effects of tofacitinib on pregnancy outcomes is limited. We report pregnancy and newborn outcomes among patients in UC clinical studies with prenatal (maternal/paternal) exposure to tofacitinib.
Methods
Pregnancies with maternal/paternal exposure to tofacitinib were identified and outcomes reported in 5 tofacitinib UC interventional studies (up to March 2017). Outcomes from tofacitinib rheumatoid arthritis (RA), psoriasis, and psoriatic arthritis interventional studies, and RA noninterventional postapproval safety studies, spontaneous adverse event reporting, and registry data are also reported.
Results
Of 1157 patients enrolled in the UC interventional studies, 301 were women of childbearing age. Eleven cases of maternal exposure and 14 cases of paternal exposure to tofacitinib (doses of 5 mg or 10 mg twice daily) before/at the time of conception or during pregnancy were identified. Outcomes included 15 healthy newborns, no fetal deaths, no neonatal deaths, no congenital malformations, 2 spontaneous abortions, and 2 medical terminations. Outcomes across other tofacitinib studies and postmarketing cases were consistent, with a healthy newborn being the most common outcome and no fetal deaths.
Conclusions
Based on the limited data available, pregnancy and newborn outcomes among patients with prenatal (maternal/paternal) exposure to tofacitinib in UC studies appear similar to those reported for other tofacitinib clinical study populations and the general population.
Keywords: pregnancy/women’s issues, inflammation, inflammatory bowel diseases, ulcerative colitis
INTRODUCTION
Patients with ulcerative colitis (UC) have a significantly higher risk of adverse birth outcomes compared with controls, including low birth weight, preterm delivery, and neonatal death.1, 2 In addition, active disease at the time of conception has been associated with a higher risk of disease relapse during pregnancy.3 These findings highlight a need for careful management of UC during pregnancy while minimizing the potential effects of therapy on the developing fetus. Consensus statements for the management of inflammatory bowel disease (IBD) during pregnancy recommend that most medical therapies should be continued if possible, both around the time of conception and throughout the pregnancy, to maintain remission.4, 5 However, there is a lack of clinical study and real-world evidence on pregnancy outcomes among patients with IBD who have been exposed to therapies before and during pregnancy.4, 5
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), and UC, and was previously investigated for psoriasis (PsO).
Although placental disposition has not been assessed, tofacitinib is a small molecule (free-base form 312.4 Dalton); it is therefore reasonable to assume that tofacitinib can cross the placenta. Other therapies used for UC, including monoclonal antibodies and thiopurines, have also been documented to cross the placenta, and, in the case of monoclonal antibodies, concentrations in cord blood may exceed maternal levels.6, 7
The early phase of the clinical program for tofacitinib in RA, PsO, UC, and PsA studied doses ranging from 0.5 mg to 50 mg twice daily (BID) and 20 mg to 60 mg once daily (QD). Tofacitinib 5 mg and 10 mg BID were brought forward to phase III for RA,8–13 PsO,14–16
In preclinical animal studies, tofacitinib was feticidal and teratogenic in rats and rabbits at exposures 146 times and 13 times greater, respectively, than the human dose of 5 mg BID approved in RA and at exposures 73 times and 6.3 times greater, respectively, than the human dose of 10 mg BID.20 Teratogenic effects included external and soft tissue malformations of anasarca and membranous ventricular septal defects, respectively, and skeletal malformations or variations. In addition, in female rats, there was reduction in fertility due to an increase in postimplantation loss at exposures 17 times and 8.3 times greater, respectively, than the human doses of 5 mg BID and 10 mg BID. In a peri- and postnatal study in rats, reductions in litter size, postnatal survival, and pup body weights were observed at exposure levels 73 times the human dose of 5 mg BID and 36 times the human dose of 10 mg BID. Tofacitinib had no effects on male fertility, sperm motility, or sperm concentration in male rats at exposures 133 times and 67 times greater, respectively, than the human doses of 5 mg BID and 10 mg BID.20 The link between animal studies and human risk, however, is variable, and it is challenging to predict the impact of tofacitinib on human pregnancy or fertility based on these data. There are no controlled clinical studies designed to determine the effects of tofacitinib in pregnant women or breastfeeding women, and prospectively collected registry data remain limited.
Prescribers and female patients of reproductive potential taking tofacitinib for the approved indication should be advised to review guidance on contraception and use of tofacitinib during pregnancy in country-specific product labeling. Women of childbearing potential enrolled in tofacitinib clinical development program studies are required to take appropriate contraceptive precautions (ie, methods that either alone or in combination result in a failure rate of less than 1% per year when used consistently and correctly) and are monitored for pregnancy. In clinical studies, tofacitinib was required to be discontinued in female patients who became pregnant.
A previous publication reported pregnancy outcomes from RA and PsO safety databases up to April 2014, based on the reporting of adverse events (AEs) in clinical studies, postapproval safety studies (RA only), and spontaneous AE reporting (RA only).21 The aims of this analysis were to report pregnancy outcomes from UC patients in the tofacitinib safety database up to March 2017 and to provide an update on outcomes in patients being treated with tofacitinib for other indications for comparison.
METHODS
Ethical Considerations
The studies were conducted in compliance with the Declaration of Helsinki, the Good Clinical Practice guidelines of the International Conference on Harmonisation, and relevant local country regulations. All patients enrolled in the tofacitinib UC clinical studies provided informed consent, and all participating institutions provided institutional review board approval before participation.
Analysis Cohorts
Cases of maternal and paternal exposure to tofacitinib (defined as maternal or paternal exposure to tofacitinib before or at the time of conception and/or during the course of pregnancy) were identified in the Pfizer safety databases up to March 7, 2017, which includes 5 UC interventional studies from the tofacitinib clinical development program. The studies included a phase II induction study (NCT00787202), 2 phase III induction studies (OCTAVE Induction 1 [NCT01465763] and OCTAVE Induction 2 [NCT01458951]), a 52-week phase III maintenance study (OCTAVE Sustain, NCT01458574), and an ongoing, open-label, long-term extension study (OCTAVE Open, NCT01470612) (Table 1).
Table 1:
Interventional Studies in Patients With UC From the Tofacitinib Development Program
| Study | Design | Tofacitinib Dose | Exposure Time |
|---|---|---|---|
| Phase II | |||
| NCT0078720222 | Double-blind, placebo-controlled RCT in patients with moderate to severe UC (n = 194) | 0.5, 3, 10, 15 mg BID | Up to 8 wk |
| Phase III | |||
| NCT01465763 (OCTAVE Induction 1)17 | Double-blind, placebo-controlled RCT in patients with moderate to severe UC (n = 598) | 10 mg BID | Up to 8 wk |
| NCT01458951 (OCTAVE Induction 2)17 | Double-blind, placebo-controlled RCT in patients with moderate to severe UC (n = 541) | 10 mg BID | Up to 8 wk |
| NCT01458574 (OCTAVE Sustain)17 | Double-blind, placebo-controlled RCT of maintenance therapy in respondersa from OCTAVE Induction 1 and 2 (n = 593) | 5 mg or 10 mg BID | Up to 52 wk |
| NCT01470612 (OCTAVE Open; ongoing) | Long-term extension study including patients who completed OCTAVE Induction 1 or 2, or OCTAVE Sustain (n = 944) | 5 mg or 10 mg BID | More than 4 y |
Abbreviation: RCT, randomized controlled trial.
aResponders were defined as patients with ≥3 points and ≥30% decrease from baseline total Mayo score and a decrease in rectal bleeding subscore ≥1 point or an absolute bleeding subscore of 0 or 1 at week 8 of OCTAVE Induction 1 and 2.
Patients in the UC interventional studies were required to have moderate to severe UC. They received placebo or tofacitinib at doses ranging from 0.5 to 15 mg BID (Table 1). Permitted concomitant UC medications were oral 5-aminosalicylates or oral corticosteroids (tapering of corticosteroids was mandatory in the maintenance and long-term extension studies). Patients who received tofacitinib 5 mg or 10 mg BID as the predominant dose were included in the analysis cohort.
Interventional studies in patients with RA, PsO, and PsA identified in the Pfizer safety database up to March 7, 2017, are summarized in Supplementary Table 1. Pregnancy cases from noninterventional, postapproval safety studies (RA only) and spontaneous AE reporting (reported use on- and off-label from all countries around the world in which tofacitinib is marketed) were also identified (up to March 2017), and included cases referred or self-referred to the Organization of Teratology Information Specialists Registry (OTIS; a nonprofit organization monitoring medications, chemicals, and other exposures during pregnancy).23
Outcomes
Cases were reviewed for any pregnancy-related outcomes and were categorized as healthy newborn (including preterm births and otherwise healthy newborns with low birth weight), spontaneous abortion, medical termination (including elective termination), fetal demise (defined as death after 20 weeks’ gestation), congenital malformation, neonatal death, or pending or lost to follow-up (including refusal of consent to follow-up).
RESULTS
Pregnancy Outcomes Identified in the Tofacitinib UC Clinical Program
A total of 1157 patients (including 301 women of childbearing age) with 1612.77 patient-years of tofacitinib exposure were included in the tofacitinib UC interventional studies (Table 2). A total of 25 cases of pregnancy were reported: 11 cases of maternal exposure were reported among the 301 women of childbearing age) and 14 cases of paternal exposure. All cases involved patients who participated in the phase III OCTAVE studies.
Table 2:
Number of Pregnancies With Maternal or Paternal Exposure to Tofacitinib Identified in the UC Interventional Studiesa
| Characteristic | All Tofacitinib |
|---|---|
| Patients enrolled, No. | 1157 |
| Tofacitinib exposure, PY | 1612.77 |
| No. women of childbearing ageb | 301 |
| No. pregnancy reportsc | 25 |
| No. pregnancy reports of maternal exposure (% of women of childbearing age) | 11 (3.7) |
| Median aged (range), y | 30 (24–41) |
| No. pregnancy reports of paternal exposure | 14 |
Abbreviation: PY, patient-years.
aData as of December 16, 2016 for Study NCT01470612.
bAge 18–44 years for patients enrolled in UC clinical development program studies.
cIncluding both cases of maternal and paternal exposure.
dOf women who reported cases of maternal exposure during pregnancy.
Of the 11 maternal cases, tofacitinib exposure began within the first trimester in all cases. The most common outcome (Table 3) was healthy newborn (total, n = 4; including 1 preterm birth at 36 weeks). Two spontaneous abortions were reported. There were 2 medical terminations. Outcome is pending in 2 cases, and 1 case had an unknown outcome.
Table 3:
Pregnancy Outcomes in Cases of Maternal or Paternal Exposure Identified in the Tofacitinib UC Intervention Studies
| Cases of Exposure | Maternal Exposure to Tofacitinib (n = 11), No. (% of Identified Cases) | Paternal Exposure to Tofacitinib (n = 14), No. (% of Identified Cases) |
|---|---|---|
| Healthy newborna | 4 (36.4) | 11 (78.6) |
| Medical terminationb | 2 (18.2) | 0 (0.0) |
| Neonatal death | 0 (0.0) | 0 (0.0) |
| Fetal death | 0 (0.0) | 0 (0.0) |
| Congenital malformation | 0 (0.0) | 0 (0.0) |
| Spontaneous abortion | 2 (18.2) | 0 (0.0) |
| Pending or lost to follow-up | 3 (27.3) | 3 (21.4) |
aIncludes 1 preterm birth (36 weeks, 2.92 kg).
bCase #1: the patient decided to terminate the pregnancy based on the potential risks of tofacitinib. Case #2: reason unknown.
Of the 14 paternal cases (Table 3), tofacitinib exposure began within the first trimester in all cases. There were 11 healthy newborns. Outcome is pending in 1 case, whereas in the remaining 2 cases, consent for follow-up was not provided.
Pregnancy Outcomes Identified in the Tofacitinib Clinical Program in Patients With RA, PsO, and PsA
The numbers of patients included in the analysis cohorts for RA, PsO, and PsA and the overall cohort (UC, RA, PsO, and PsA) are shown in Table 4.
Table 4:
Number of Pregnancies With Maternal or Paternal Exposure to Tofacitinib Identified in the RA, PsO, and PsA Intervention Studies and Across All Indications (Including UC)
| Characteristic | RA | PsO | PsA | Alla |
|---|---|---|---|---|
| No. patients enrolled | 7061 | 3663 | 783 | 12,664 |
| Tofacitinib exposure, PY | 22,874.52 | 8950.16 | 1237.89 | 34,675.34 |
| No. women of childbearing ageb | 1663 | 519 | 146 | 2629 |
| No. pregnancy reportsc | 46 | 80 | 7 | 158 |
| No. pregnancy reports of maternal exposure (% of women of childbearing age) | 39 (2.3) | 20 (3.9) | 4 (2.7) | 74 (2.8) |
| Median aged (range), y | 31 (22–40) | 29 (19–43) | 32 (27–37) | 30 (19–43) |
| No. pregnancy reports of paternal exposure | 7 | 60 | 3 | 84 |
Abbreviation: PY, patient-years.
aIncludes UC data.
bAge 18–44 years for patients enrolled in PsO and PsA clinical development program studies; age 18–45 years for patients enrolled in RA clinical development program studies.
cIncluding both cases of maternal and paternal exposure.
dOf women who reported cases of maternal exposure during pregnancy (based on n = 38 for RA).
The number of cases of maternal exposure identified in the RA, PsO, and PsA cohorts was 39, 20, and 4, respectively, and the number of cases of paternal exposure was 7, 60, and 3, respectively.
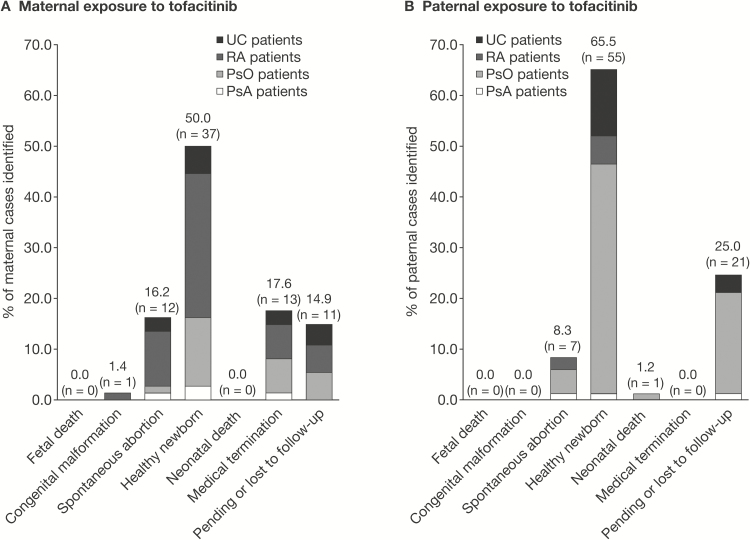
In all cases, maternal exposure began within the first trimester. The most common outcome was healthy newborn (RA, n = 21; PsO, n = 10; PsA, n = 2), including 3 preterm births at 35 weeks (RA), 37 weeks (RA, low birth weight, 2.1 kg; [10th percentile at 37 weeks, 2.5 kg24]), and 37 weeks (PsA). There was 1 congenital malformation (pulmonary valve stenosis) in the newborn of a 32-year-old RA patient with hypertension treated with angiotensin II receptor antagonist losartan (50 mg QD), with diet-controlled gestational diabetes, and who received tofacitinib 5 mg BID. Ten spontaneous abortions (RA, n = 8; PsO, n = 1; PsA, n = 1) were reported. There were 11 medical terminations (RA, n = 5; PsO, n = 5; PsA, n = 1). Eight maternal exposure cases (RA, n = 4; PsO, n = 4) were pending or lost to follow-up. An overview of outcomes of pregnancies with maternal tofacitinib exposure across UC, RA, PsO, and PsA is shown in Figure 1A.
FIGURE 1.
Overview of pregnancy outcomes following maternal (A) and paternal (B) exposure to tofacitinib in the UC, RA, PsO, and PsA clinical studies. Outcomes are categorized as fetal death, congenital malformation, spontaneous abortion, healthy newborn, medical termination, and pending or lost to follow-up.
Paternal exposure in cases from the RA, PsO, and PsA cohorts began in the first trimester in all except 5 cases, for which timing of exposure in relationship to the estimated date of conception could not be determined. The most common pregnancy outcome was healthy newborn, with 44 cases reported (RA, n = 5; PsO, n = 38; PsA, n = 1). Of these 44 healthy newborns, 2 were born preterm at 36 weeks (RA) and 35 weeks (PsO). Other outcomes included 7 spontaneous abortions (RA, n = 2; PsO, n = 4; PsA, n = 1) and 1 neonatal death (PsO, the newborn developed cardiac arrest just after delivery at 36 weeks and had other unspecified clinical factors that may have contributed to the death). The remaining 18 cases (PsO, n = 17; PsA, n = 1) were pending or lost to follow-up. An overview of outcomes of pregnancies with paternal tofacitinib exposure across UC, RA, PsO, and PsA is shown in Figure 1B.
Pregnancy Outcomes Identified in Noninterventional Safety Studies and Spontaneous AE Reporting
There were 45 postmarketing cases of tofacitinib exposure during pregnancy reported in the safety database. Of these, 28 cases were reported in patients with RA (23 spontaneous cases and 5 noninterventional study cases), and there were 17 cases for which the indication was not provided (Table 5).
Table 5:
Pregnancy Outcomes in Cases Identified in Noninterventional Safety Studies and Spontaneous AE Reporting
| Cases of Exposure, No. (% of Identified Cases) | RA | Indication Not Reported | All |
|---|---|---|---|
| n = 27 Maternal Cases | n = 15 Maternal Cases | n = 42 Maternal Cases | |
| n = 1 Paternal Case | n = 2 Paternal Cases | n = 3 Paternal Cases | |
| Healthy newborna | 5 (17.9) | 2 (11.8)b | 7 (15.6)b |
| Medical termination | 1 (3.6) | 0 (0.0) | 1 (2.2) |
| Fetal death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neonatal death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Congenital malformation | 1 (3.6) | 0 (0.0) | 1 (2.2) |
| Spontaneous abortion | 3 (10.7) | 0 (0.0) | 3 (6.7) |
| Pending or lost to follow-up | 18 (64.3)b | 15 (88.2)b | 33 (73.3)c |
aIncludes preterm births (n = 3).
bCases of maternal exposure except for 1 paternal case.
cCases of maternal exposure except for 2 paternal cases.
There were 27 cases of maternal exposure and 1 case of paternal exposure within the 28 cases reported in RA patients. In all 28 cases, exposure to tofacitinib began in the first trimester. Outcomes for the 27 cases of maternal exposure are shown in Table 5. Three healthy newborns were born preterm at 37 weeks (n = 1, born with a short umbilical cord) and at 34 weeks (n = 2). There was 1 congenital malformation of ventricular septal defect, in association with concurrent reports of viral meningitis and gastroenteritis. Concomitant medication during this pregnancy included paracetamol tablets (oral 1 g BID), citalopram tablets (oral 20 mg QD), and clorazepate dipotassium tablets (oral 10 mg QD).
Of the 17 pregnancies without a reported indication, 15 were maternal cases and 2 were cases of paternal exposure. Among the 15 maternal cases, exposure to tofacitinib began in the first trimester (n = 6) and was unknown in 9 cases. Pregnancy outcomes are shown in Table 5.
DISCUSSION
A previous publication reported 91 pregnancies in patients, or partners of patients, receiving tofacitinib in the RA and PsO tofacitinib clinical development program studies, as of April 2014 (maternal exposure, n = 47; paternal exposure, n = 44).21 In this updated report, analysis of 5 tofacitinib UC clinical development program studies has identified 25 pregnancies (maternal exposure, n = 11; paternal exposure, n = 14). No fetal deaths and no congenital malformations were reported.
There are limited data on pregnancy outcomes among women with UC. Studies have reported a higher risk of adverse outcomes, which include low birth weight, small for gestational age, spontaneous abortion, preterm delivery, and neonatal death.1, 2 Patients in the tofacitinib UC clinical studies were permitted to continue use of 5-aminosalicylic acid. One preterm birth was reported in a case of paternal exposure.
The findings from the UC studies were generally consistent with the updated analysis across the entire tofacitinib program in UC, RA, PsO, and PsA. A total of 158 pregnancies were reported (maternal exposure, n = 74; paternal exposure, n = 84). There were no reports of fetal demise. One case of congenital malformation (pulmonary valve stenosis) and 19 spontaneous abortions were reported. The remaining known outcomes were healthy newborns (n = 93) or medical terminations (n = 13), and 32 cases were pending or lost to follow-up. In addition to the pregnancy cases reported in the tofacitinib program, 45 pregnancies (including 42 cases of maternal exposure) were identified in noninterventional safety studies and spontaneous AE reporting (up to March 7, 2017). Outcomes included 7 healthy newborns, a congenital malformation of ventricular septal defect, 3 spontaneous abortions, 1 medical termination, and 33 cases pending or lost to follow-up.
The observed frequencies of congenital malformations and spontaneous abortions (1.0% and 10.8%, respectively) reported in the tofacitinib clinical development program studies, noninterventional safety studies, and spontaneous AE reporting appeared to be consistent with the background risks in the general population. In the general population of the United States, the background risk was ~3% for major birth defects and ~10% for spontaneous abortions, respectively.25, 26 Reported background risks for spontaneous abortion in other regions were generally similar to those in the United States: United Kingdom, ~20%27; Denmark, 10.9%28; China, 7.9%29; and Brazil, 14.0%.30
A key limitation of this analysis was that it was not prospective; cases were identified as AEs in the Pfizer safety database. As contraception was required and pregnancy was a discontinuation criterion in female patients in the tofacitinib clinical development program, outcomes may have been under-reported or lost to follow-up after discontinuation of the study drug. In addition, limited information is available on the exact date of conception and gestational age, and, at the time of the report, information on disease activity in each patient was not prospectively collected. Furthermore, with only 1 patient receiving 5 mg BID at the time of conception (a case of spontaneous abortion) among the 11 UC maternal cases, meaningful comparisons of outcomes between the 5 mg and 10 mg BID doses could not be made. Finally, there are not enough data to create a control group, as the number of placebo patients and exposure to placebo were limited compared with tofacitinib among patients reporting pregnancies, due to the clinical study designs. Although the observed frequencies of congenital malformations and spontaneous abortions appeared to be consistent with the background risks in the general population in the United States, given the findings from preclinical animal studies and the lack of controlled clinical studies designed to determine the effects of tofacitinib in pregnant or breastfeeding women and limited prospectively collected registry data, prescribers and female patients of reproductive potential should be advised to review guidance on contraception and use of tofacitinib during pregnancy in country-specific product labeling.
Given the relatively few cases of pregnancy reported with tofacitinib, definitive conclusions cannot be drawn regarding the effect of tofacitinib on pregnancy and newborn outcomes. Pregnancy outcomes in patients receiving tofacitinib will continue to be monitored in clinical studies through routine pharmacovigilance and via postapproval safety studies.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the patients, investigators, and study teams involved in the studies reported in this analysis, and John D. Isaacs, Alexandra B. Kimball, Vibeke Strand, Richard B. Warren, and Daniel Xibille for their contributions to the previous manuscript on this topic, which underpins the updated analyses reported here.
Conflicts of interest: Uma Mahadevan was a consultant for AbbVie, Celgene, Janssen, Pfizer Inc, and Takeda. Marla C. Dubinsky was a consultant for AbbVie, BMS, Celgene, Gilead, Janssen, Pfizer Inc, Takeda, and UCB. Chinyu Su, Nervin Lawendy, Thomas V. Jones, Amy Marren, Haiying Zhang, and Daniela Graham are employees and shareholders of Pfizer Inc. Megan E. B. Clowse was a consultant for UCB. Steven R. Feldman was a consultant for and received research support from Pfizer Inc, and received support from AbbVie, Celgene, Janssen, Lilly, Merck, Ortho, and Samsung. Daniel C. Baumgart was a consultant for AbbVie, Biogen, BMS, Celgene, Ferring, Forward Pharma, Genentech (Roche Group), Gilead, Janssen, Pfizer Inc, Shield Therapeutics, Takeda, and TiGenix.
Supported by: The studies reported in this manuscript were sponsored by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Sandrine M. Dupré, PhD, of CMC Connect, a division of Complete Medical Communications Ltd, Manchester, UK and was funded by Pfizer Inc, New York, NY, USA in accordance with the Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Author contributions: U. Mahadevan: data interpretation and critical review of the manuscript. M. C. Dubinsky: data interpretation and critical review of the manuscript. C. Su: data interpretation and critical review of the manuscript. N. Lawendy: data collection, analysis, and interpretation and critical review of the manuscript. T. V. Jones: data collection, analysis, and interpretation and critical review of the manuscript. A. Marren: data interpretation and critical review of the manuscript. H. Zhang: data interpretation and critical review of the manuscript. D. Graham: data collection, analysis, and interpretation and critical review of the manuscript. S. R. Feldman: data interpretation and critical review of the manuscript. M. E. B. Clowse: data interpretation and critical review of the manuscript. D. C. Baumgart: data interpretation and critical review of the manuscript. All authors approved the final submitted version of the manuscript. U. Mahadevan is the guarantor of the article.
REFERENCES
- 1. Cornish J, Tan E, Teare J, et al. . A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stephansson O, Larsson H, Pedersen L, et al. . Congenital abnormalities and other birth outcomes in children born to women with ulcerative colitis in Denmark and Sweden. Inflamm Bowel Dis. 2011;17:795–801. [DOI] [PubMed] [Google Scholar]
- 3. de Lima-Karagiannis A, Zelinkova-Detkova Z, van der Woude CJ. The effects of active IBD during pregnancy in the era of novel IBD therapies. Am J Gastroenterol. 2016;111:1305–12. [DOI] [PubMed] [Google Scholar]
- 4. van der Woude CJ, Kolacek S, Dotan I, et al. ; European Crohn’s Colitis Organisation (ECCO) European evidenced-based consensus on reproduction in inflammatory bowel disease. J Crohns Colitis. 2010;4:493–510. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734–57.e1. [DOI] [PubMed] [Google Scholar]
- 6. Mahadevan U, Wolf DC, Dubinsky M, et al. . Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286–92; quiz e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Julsgaard M, Christensen LA, Gibson PR, et al. . Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110–9. [DOI] [PubMed] [Google Scholar]
- 8. Burmester GR, Blanco R, Charles-Schoeman C, et al. ; ORAL Step investigators Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 9. Fleischmann R, Kremer J, Cush J, et al. ; ORAL Solo Investigators Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 10. Kremer J, Li ZG, Hall S, et al. . Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 11. van der Heijde D, Tanaka Y, Fleischmann R, et al. . ORAL Scan Investigators Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 12. van Vollenhoven RF, Fleischmann R, Cohen S, et al. . ORAL Standard Investigators Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 13. Lee EB, Fleischmann R, Hall S, et al. . ORAL Start Investigators Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 14. Bachelez H, van de Kerkhof PC, Strohal R, et al. . OPT Compare Investigators Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–61. [DOI] [PubMed] [Google Scholar]
- 15. Papp KA, Menter MA, Abe M, et al. . OPT Pivotal 1 and OPT Pivotal 2 investigators Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949–61. [DOI] [PubMed] [Google Scholar]
- 16. Bissonnette R, Iversen L, Sofen H, et al. . Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2015;172:1395–406. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Su C, Sands BE, et al. . OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 18. Gladman D, Rigby W, Azevedo VF, et al. . Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 19. Mease P, Hall S, FitzGerald O, et al. . Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 20. Pfizer Inc. Xeljanz prescribing information. February 2016. http://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed August 11, 2017. [Google Scholar]
- 21. Clowse ME, Feldman SR, Isaacs JD, et al. . Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Ghosh S, Panes J, et al. . Study A3921063 Investigators Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24. [DOI] [PubMed] [Google Scholar]
- 23. Organization of Teratology Information Specialists (OTIS) registry. 2017. http://parthenonmanagementgroup.com/portfolio/organization-of-teratology-information-specialists-otis/. Accessed August 11, 2017.
- 24. Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention (CDC). Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978– . MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 26. The American College of Obstetricians and Gynecologists. Frequently Asked Questions. Early Pregnancy Loss. 2015. https://www.acog.org/Patients/FAQs/Early-Pregnancy-Loss. Accessed June 11, 2018. [Google Scholar]
- 27. Royal College of Obstetricians and Gynaecologists. NICE clinical guideline: ectopic pregnancy and miscarriage: diagnosis and initial management in early pregnancy of ectopic pregnancy and miscarriage. December 2012. http://www.nice.org.uk/guidance/cg154/evidence/cg154-ectopic-pregnancy-and-miscarriage-full-guideline3. Accessed August 11, 2017. [Google Scholar]
- 28. Nybo Andersen AM, Wohlfahrt J, Christens P, et al. . Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Chen C, Wang L, et al. . Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–84. [DOI] [PubMed] [Google Scholar]
- 30. Cecatti JG, Guerra GV, Sousa MH, et al. . Abortion in Brazil: a demographic approach. Rev Bras Ginecol Obstet. 2010;32:105–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.