To the Editor,
Thank you for the opportunity to respond to the letter from Galazis et al. (Galazis et al., 2018). The authors correctly state that women newly diagnosed with endometrial cancer are not routinely screened for cardiovascular risk factors in the UK. This is a missed opportunity for primary prevention; our data point to a very high prevalence of unrecognized and undertreated hypercholesterolemia, hypertension and hyperglyaemia in these women (Kitson et al., 2018). This is somewhat unsurprising given that nearly two-thirds of our patients are obese (BMI > 30 kg/m2), and obesity is a known aetiological driver for both endometrial cancer and cardiovascular disease. Adipose-derived estrogen unopposed by progesterone in postmenopausal women is the most accepted theory linking obesity to endometrial carcinogenesis; however, the concept of metabolically unhealthy obesity, rather than excess adiposity per se, is gaining popularity, and goes some way towards explaining why some but not all obese women develop endometrial cancer.
Defining metabolically unhealthy obesity is a contentious issue that has yet to be resolved, but most published studies have utilised the closely related concept of the metabolic syndrome. This is ‘a constellation of interrelated risk factors of metabolic origin’ that collectively predispose an individual to cardiovascular disease (Grundy et al., 2005). Individuals meeting the diagnostic criteria for the metabolic syndrome are considered metabolically unhealthy. Several indices have been used to characterise the metabolic syndrome, but the National Cholesterol Education Program's Adult Treatment Panel III (NECP ATP III) is most widely adopted.
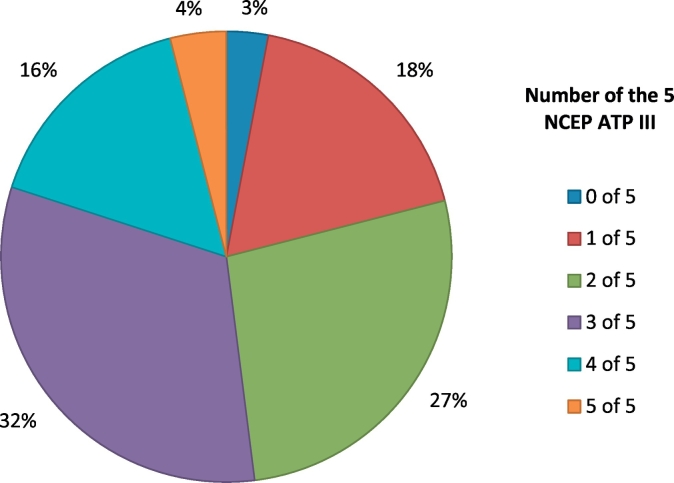
Using these criteria, we metabolically phenotyped a subset of our endometrial cancer patients. We measured waist circumference and blood pressure, and checked fasting blood glucose, lipid levels and liver enzymes. Those with elevated alanine transaminase (ALT) or alkaline phosphatase levels were referred for an ultrasound scan of their liver. In total, 52% (95% confidence intervals 42.2–61.8%) met the diagnostic criteria for metabolic syndrome (Table 1), with ≥3 of 5 indicative features (Fig. 1). In addition, 14 women were found to have previously unrecognized hepatic steatosis and non-alcoholic fatty liver disease. By contrast, 23.1% of the general US population meet the diagnostic criteria for the metabolic syndrome (Ford et al., 2004). There are no comparable data for a UK population; in our age and ethnicity-matched Health Survey for England (HSE) control women, HbA1c was measured instead of fasting blood glucose, and there were insufficient data to diagnose metabolic syndrome in 15.5%. Nonetheless, just 9.2% (95% confidence intervals 7.3–11.2) of control women met the criteria for metabolic syndrome, more than five-fold fewer than our endometrial cancer patients, a comparison that was highly significant (p < .0001), and mirrors previous work (Rosato et al., 2011). Endometrial cancer patients who met the diagnostic criteria for metabolic syndrome were more likely to be obese than those who did not (82.7% vs. 37.5%, p = .0001). However, the presence of metabolic syndrome was not associated with age, grade or stage of endometrial cancer (data not shown).
Table 1.
Proportion of women meeting each criterion of the metabolic syndrome according to the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP-III).
| Indicative feature/ criterion | % of study population meeting criterion (n = 100) |
|---|---|
|
Waist circumference ≥88 cm in women |
77 |
|
Elevated triglycerides ≥150 mg/dL (1.7 mmol/L) OR on drug treatment for elevated triglycerides |
19 |
|
Reduced HDL-Cholesterol <50 mg/dL (1.3 mmol/L) in women OR on drug treatment for reduced HDL-C |
26 |
|
Elevated blood pressure ≥130 mmHg systolic blood pressure OR ≥ 85 mmHg diastolic blood pressure OR on antihypertensive drug treatment in a patient with a history of hypertension |
86 |
|
Elevated fasting glucose ≥100 mg/dL (5.6 mmol/L) OR on drug treatment for elevated glucose |
44 |
Fig. 1.
Proportion of endometrial cancer patients with 1–5 indicative features of the metabolic syndrome, according to the National Cholesterol Education Program's Adult Treatment Panel III criteria (NCEP ATP-III).
Whilst these findings lend strong support to cardiovascular risk factor screening and optimization in endometrial cancer survivors, they also hint at common biological mechanisms underpinning the pathogenesis of the two conditions. Further, they offer the very exciting possibility that simple, safe, non toxic drugs with proven activity against elements of the metabolic syndrome may offer women protection against both cardiovascular disease and endometrial cancer. Interventions that reduce the risk of endometrial cancer are urgently needed to limit escalating incidence rates and consequent deaths from the disease (Crosbie and Morrison, 2014).
Accumulating evidence highlights the potential anti-cancer effect of aspirin (Takiuchi et al., 2018), statins (Arima et al., 2017) and metformin, an insulin sensitising drug long used in the treatment of type 2 diabetes (Sivalingam et al., 2014). Data supporting an anti-cancer activity of the beta-blocker antihypertensive drugs have been less persuasive (Sanni et al., 2017). It is tantalising to think that adequately controlled features of the metabolic syndrome, through treatment with aspirin, metformin and a statin may shift women from metabolically unhealthy to healthy obese. Equally appealing is the concept that so doing reduces their risk of endometrial cancer. Such a strategy would benefit women who are unwilling or unable to lose weight through dietary intervention and exercise programs. Most interesting of all is the possibility that being identified as metabolically unhealthy, and at risk of both cardiovascular disease and endometrial cancer, creates the perfect ‘teachable moment’ for motivating obese women to lose weight through diet and exercise (van den Brekel-Dijkstra et al., 2015).
Conflict of interest
The authors report no conflict of interest.
Author contributions
SJK, VNS, MKR and EJC devised the study. SJK, VNS, JL and EJC conducted the study. SJK, JL, MKR and EJC analysed the data. SJK and EJC wrote the first draft of the manuscript. EJC obtained funding for the study and is its Principal Investigator and guarantor. All authors provided critical comment and approved the final version of the manuscript.
Funding statement
EJC was funded by a National Institute for Health Research (NIHR) Clinician Scientist award (NIHR-CS-012-009) and this article presents independent research funded by the NIHR, supported by the NIHR Manchester Biomedical Research Centre and facilitated by the Greater Manchester Local Clinical Research Network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Arima R., Marttila M., Hautakoski A., Arffman M., Sund R., Ilanne-Parikka P. Antidiabetic medication, statins and the risk and prognosis of endometrioid endometrial cancer in patients with type 2 diabetes. Gynecol. Oncol. 2017;146(3):636–641. doi: 10.1016/j.ygyno.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Crosbie E.J., Morrison J. The emerging epidemic of endometrial cancer: Time to take action. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.ED000095. (Dec 22;12:ED000095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E.S., Giles W.H., Mokdad A.H. Increasing Prevalence of the Metabolic Syndrome among U.S. adults. Diabetes Care. 2004;27(10):2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- Galazis N., Mappouridou S., Mavrou A. 2018. Cardiovascular Disease in Women Diagnosed with Endometrial Cancer. (Gynecologic Reports) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Kitson S.J., Lindsay J., Sivalingam V.N. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: a prospective case control study. Gynecol. Oncol. 2018;148(1):154–160. doi: 10.1016/j.ygyno.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato V., Zucchetto A., Bosetti C., Dal Maso L., Montella M., Pelucchi C. Metabolic syndrome and endometrial cancer risk. Ann. Oncol. 2011;22(4):884–889. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- Sanni O., McMenamin U.C., Cardwell C.R., Sharp L., Murray L.J., Coleman H.G. Commonly used medications and endometrial cancer survival: a population-based cohort study. Br. J. Cancer. 2017;117(3):432–438. doi: 10.1038/bjc.2017.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivalingam V.N., Myers J., Nicholas S., Balen A.H., Crosbie E.J. Metformin in reproductive health, pregnancy and gynaecological cancer: emerging and established indications. Hum. Reprod. Update. 2014;20:853–868. doi: 10.1093/humupd/dmu037. [DOI] [PubMed] [Google Scholar]
- Takiuchi T., Blake E.A., Matsuo K., Sood A.K., Brasky T.M. Aspirin use and endometrial cancer risk and survival. Gynecol. Oncol. 2018;148(1):222–232. doi: 10.1016/j.ygyno.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brekel-Dijkstra K., Rengers A.H., Niessen M.A., de Wit N.J., Kraaijenhagen R.A. Personalized prevention approach with use of web-based cardiovascular risk assessment with tailored lifestyle follow up in primary care practice – a pilot study. Eur. J. Prev. Cardiol. 2015;23(5):544–551. doi: 10.1177/2047487315591441. [DOI] [PubMed] [Google Scholar]