Abstract
The central nervous system controls the innate immunity by modulating efferent neuronal networks. Recently, we have reported that central brain stimulation inhibits inflammatory responses. In the present study, we investigate whether spinal p38 mitogen-activated protein kinase (MAPK) affects joint inflammation in experimental arthritis. Firstly, we observed that intra-articular administration of zymosan in mice induces the phosphorylation of the spinal cord p38 MAPK. In addition, we demonstrated that spinal p38 MAPK inhibition with intrathecal injection of SB203580, a conventional and well-characterized inhibitor, prevents knee joint neutrophil recruitment, edema formation, experimental score and cytokine production. This local anti-inflammatory effect was completely abolished with chemical sympathectomy (guanethidine) and beta-adrenergic receptors blockade (nadolol). In conclusion, our results suggest that pharmacological strategies involving the modulation of spinal p38 MAPK circuit can prevent joint inflammation via sympathetic networks and beta-adrenoceptors activation.
Keywords: arthritis, beta-adrenergic receptors, neuroimmunomodulation, neutrophil, p38 MAPK, sympathetic nervous system
INTRODUCTION
Neutrophils are critical for the immune system to eliminate microorganisms during infection by producing microbicide mediators including reactive oxygen species, neutrophil extracellular traps (NETs) and proteases. However, neutrophils do not discriminate between microorganisms and host cells, and when unregulated, neutrophils can cause cellular damage and tissue injury as observed in the joints of patients with rheumatoid arthritis [1]. Rheumatoid arthritis is a chronic autoimmune disease of unknown etiology causing joint inflammation and affecting approximately 0.5–1% of the adult population worldwide [2]. Many experimental and clinical studies reveal an autonomic dysfunction in the arthritis pathophysiology [3]. These results suggest that these autonomic dysfunctions can contribute to arthritis. Thus, modulation of the autonomic nervous system may provide a therapeutic mean for treating arthritis [4–6].
We have reported that electrical stimulation of peripheral sympathetic networks prevents neutrophil infiltration into the arthritic knee joints and described the central neuronal components modulating arthritis [6–8]. Recently, we demonstrated that pharmacological activation of the spinal GABA-B receptors increases neutrophil migration into the femora-tibial cavity through a mechanism dependent on the p38 mitogen-activated protein kinases (MAPK) [6]. P38 MAPK is a key intracellular pathway contributing to chronic inflammation and extensively investigated as a potential therapeutic target for arthritis [9]. Previous studies suggest that spinal cord p38 MAPK participates in the neuronal nociceptive networks [10]. Furthermore, peripheral tumor necrosis factor (TNF) can activate nociceptive networks leading to spinal cord p38 MAPK activation in rat adjuvant arthritis [11]. However, it is unknown whether inhibition of spinal cord p38 MAPK affects neutrophil joint infiltration, and what neuronal components mediate this effect. In this study, we investigated (i) the potential of spinal cord p38 MAPK to control knee joint inflammation in murine zymosan-induced arthritis and (ii) whether this effect is mediated by the sympathetic networks and beta-adrenoceptors.
MATERIAL AND METHODS
Chemicals
Zymosan, SB203580, guanethidine and nadolol were purchased from Sigma Chemical Company (St. Louis, MO, USA). The reagents were dissolved or suspended in sterile saline, with exception of SB203580, which was dissolved in DMSO 5%. Control animals received equal volumes of vehicles by the same route.
Animals
Adult male Balb/c mice (22–26 g) were obtained from the breeding facility of the University of São Paulo at Ribeirão Preto. Animals were housed in a temperature-controlled (22 ± 1 °C) room and maintained on a 12-h light/dark cycle with lights on at 7:00 a.m. with free access to food and water. All the experiments were conducted following the National Institute of Health guidelines for the welfare of experimental animals after approval by the Ethics Committee of the Ribeirão Preto Medical School (COBEA Protocol 137/2013).
Zymosan-induced arthritis
Briefly, zymosan suspension (30 μg) in sterile saline was injected into the mice femoral–tibial joint (intra-articular; i.a.) of right knee under light isoflurane anesthesia (1.5%) [6,12]. Joint swelling (edema): Firstly, the knee joint thicknesses were measured by caliper and the results were expressed as the mean ± SEM of the knee joint diameter 6 h after zymosan injection in millimeters (mm). Joint’s experimental score: This inflammatory parameter was also evaluated 6 h after zymosan injection by a blinded researcher as follows: 0 = no evidence of inflammation; 1 = edema of the femorotibial cavity (slight edema); 2 = edema involving all joint capsule surrounding the knee (large edema); 3 = the same as 2 plus small hemorrhagic spots along the synovial bursa; 4 = the same as 2 plus large hemorrhagic spots or blood/pus leakage [7]. Knee neutrophil recruitment: After knee edema measurement and experimental score determination, mice were euthanized by cervical displacement under isoflurane anesthesia, and then, the knee joint was opened and washed with saline solution containing EDTA (1 mm). Aliquots of the resulting washes were diluted in Turk solution, and then, leukocytes were counted with the aid of a Neubauer chamber and a light microscope. Differential cell counts were stained with hematoxylin–eosin and also counted under a light microscope. The number of differentiated cells was calculated by the percentage found in the total number of cells (100 cells in total). Results were expressed as the mean ± SEM of the number of neutrophils per knee joint. Chemokine/cytokine measurement: In another set of experiments, tumor necrosis factor (TNF), interleukin (IL)-1β and macrophage inflammatory protein (MIP)-2 levels in the articular joints of control and experimental animals were determined 1.5 h after i.a. injection of zymosan. In brief, knee joints were dissected out, frozen with liquid nitrogen, crushed in a mortar and pestle, and then solubilized in PBS containing anti-proteases. Cytokine/chemokine levels were evaluated using a commercially available enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (Duo-Set kits; R&D Systems, Minneapolis, MN, USA), and the results were expressed as the mean ± SEM of cytokine/chemokine levels in pg/mg of joint tissue.
Intrathecal SB203580 administration
Intrathecal (i.t.; 5 μL) injections were performed under light isoflurane anesthesia (1.5%). The dorsal fur of each mouse was shaved, the lumbar spinal column was manually arched, and a 29-gauge needle was directly inserted into the L4–L5 subarachnoid space [13]. Tail-flick response confirmed that the needle was correctly positioned in the subarachnoid space. SB203580 and vehicle administered into the subarachnoid space can diffuse into the cerebrospinal fluid and bathe all the neural components of the spinal cord.
Chronological drug administration
Nadolol (subcutaneous; s.c.; 5 mg/kg) was administered 30 min before the SB203580 injection [6]. Guanethidine (s.c.; 50 mg/kg) was administered 48 and 24 h before the SB203580 injection. Finally, SB203580 (i.t.; 0.1–3 μg) was injected 5 min before i.a. zymosan administration.
Western blots
One hour and half and 3 h after zymosan administration, the animals were euthanized by cervical dislocation and the synovial tissue surrounding the knee joint was cut and immediately frozen in liquid nitrogen and stored at −70 °C. On the day of the assay, the samples were immersed in liquid nitrogen, pulverized by a blunt impact, homogenized in a lysis buffer (Sigma; Saint Louis, MO, USA) containing protease inhibitors (Cell Signaling, Danvers, MA, USA) and centrifuged for 10 min at 400 g (4 °C) to collect the supernatant. The protein concentration of the lysate was determined by Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The protein samples were separated through an SDS/PAGE gel trough a Mini-PROTEAN Tetra Cell (Bio-Rad, Hercules, CA, EUA) and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Little Chalfont, UK) followed by overnight incubation at 2–8 °C with primary anti-p-p38 MAPK (1 : 500) and anti-p38 MAPK (1 : 500) (Cell Signaling Technology, Danvers, MA, USA) dissolved in filtered TBS-T buffer containing 5% BSA. On the next day, the membrane was then incubated for 1 h at room temperature with an HRP-conjugated secondary antibody (1 : 10 000; Jackson ImmunoResearch, West Grove, PA, USA). ECL solution (Amersham Pharmacia Biotech) was used for the visualization of the membranes’ blot in a ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Knee joint neutrophil recruitment, cytokines and p38 MAPK phosphorylation were statistically analyzed by one-way analysis of variance (anova) followed by the Tukey’s multiple comparison post hoc test, with the aid of the GraphPad Prism Software (GraphPad Software Inc., San Diego, CA, USA). The joint diameter (edema) and experimental score were analyzed with the two-way anova for repeated measures followed by the Bonferroni’s post hoc test when indicated. The analysis of the difference between two groups was performed by Student’s t test. The experimental sample n refers to the number of animals. Differences were considered statistically significant when P < 0.05.
RESULTS
Inhibition of spinal cord p38 MAPK prevents zymosan-induced arthritis
We first analyzed whether zymosan-induced local inflammation in the joint activates spinal cord p38 MAPK. Intra-articular injection of zymosan into adult male Balb/c mice induces phosphorylation of spinal cord p38 MAPK at 1.5 and 3 h as shown by Western blot (Figure 1a). Zymosan did not affect p38 MAPK protein concentration, and p38 MAPK phosphorylation was normalized according to p38 MAPK protein expression. These results suggest that spinal cord p38 MAPK may contribute arthritis progression. Then, we analyzed whether inhibition of spinal p38 MAPK can prevent knee joint inflammation in arthritis. Intrathecal administration of the p38 MAPK inhibitor SB203580 prevents neutrophil infiltration into the knee synovial fluid in a dose-dependent manner (Figure 2a). Therefore, the inhibition of spinal p38 MAPK also prevents joint edema formation (Figure 2b) and improves the arthritic experimental score (Figure 2c). Finally, we observed that i.t. SB203580 injection also decreases the synovial levels of TNF and IL-1β, but not MIP-2 (Figure 3a-c).
Figure 1.
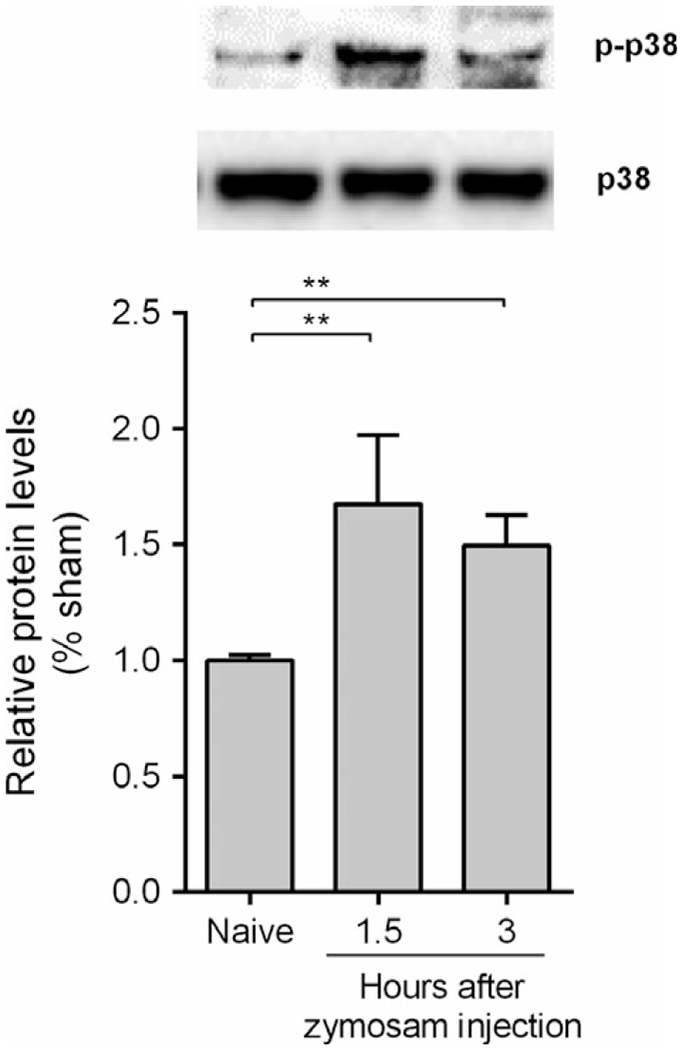
Spinal cord p38 MAPK phosphorylation increases in knee joint inflammation. P38 MAPK phosphorylation (p-p38) was measured 1.5 and 3 h after zymosan injection (i.a.; 30 μg) by Western blotting. Data are expressed as the mean ± SEM (n = 3 animals/group). **P < 0.01; ***P < 0.001 vs. naive group. (One-way anova followed by Tukey’s post hoc test).
Figure 2.
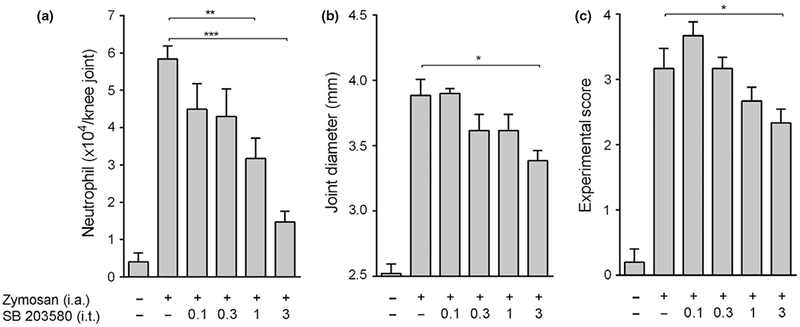
Spinal cord p38 MAPK inhibition improves knee joint inflammation. Knee joint (a) joint neutrophil migration, (b) edema formation and (c) experimental score were measured 6 h after zymosan injection (i.a.; 30 μg) in mice pretreated with SB203580 (i.t.; 0.1–3 μg). Data are expressed as the mean ± SEM (n = 5–10 mice/group).*P < 0.05; **P < 0.01; ***P < 0.001 vs. control group (zymosan). (One-way anova followed by Tukey’s post hoc test and Two-way anova for repeated measures followed by Bonferroni’s post hoc test).
Figure 3.
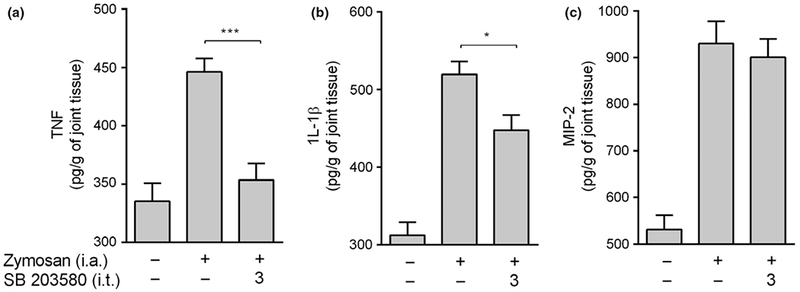
Spinal cord p38 MAPK inhibition reduces joint cytokine production. Knee joint (a) TNF-α, (b) IL-1β and (c) MIP-2 levels were measured 1.5 h after zymosan injection (i.a.; 30 μg) in mice pretreated with SB203580 (i.t.; 3 μg) by ELISA. Data are expressed as the mean ± SEM (n = 4–8 mice/group). *P < 0.05, **P < 0.01 and ***P < 0.001 vs. control group (zymosan). (One-way anova followed by Tukey’s post hoc test).
Inhibition of spinal cord p38 MAPK prevents arthritis via the sympathetic networks and beta-adrenoceptors
Next we analyzed the neuronal mechanisms mediating the effects of inhibiting spinal p38 MAPK in arthritis. We recently reported that electrical stimulation of the peripheral sympathetic networks prevents neutrophil infiltration into the arthritic knee joint [6–8]. Thus, we analyzed whether chemical sympathectomy abolishes the improvement in arthritic induced by pharmacological inhibition of p38 MAPK. Chemical sympathectomy with guanethidine (s.c.; 50 mg/kg) blocks the production of catecholamines at 24 and 48 h. Chemical sympathectomy reverts the improvement in arthritis induced by inhibiting spinal p38 MAPK (Figure 4a). Given that the sympathetic networks produce catecholamines that act via beta-adrenoceptors, we also analyzed whether these receptors contribute to the improvement in arthritis induced by i.t. SB203580 administration. Beta-adrenoceptor inhibitor nadolol prevents the improvement in arthritic induced by inhibiting spinal p38 MAPK (Figure 4b). Thus, i.t. administration of SB203580 prevents zymosan-induced arthritis via sympathetic networks and beta-adrenoceptors.
Figure 4.
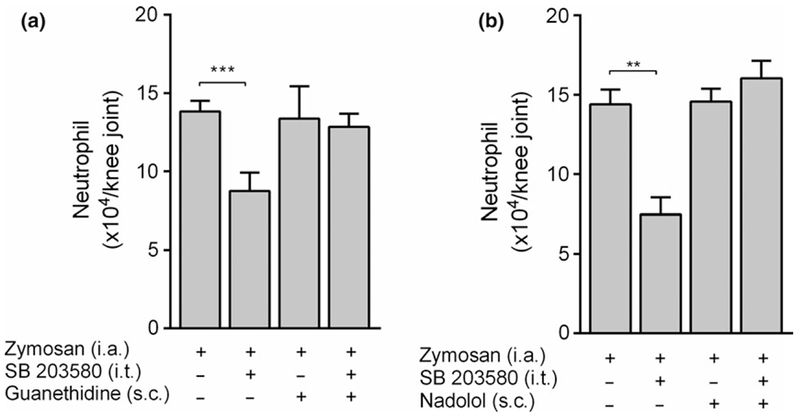
The anti-inflammatory of spinal p38 MAPK inhibition is dependent on participation of sympathetic nervous system and beta-adrenergic receptors activation. (a) Guanethidine (s.c.; 50 mg/kg; −48 and −24 h) and (b) nadolol (s.c.; 5 mg/kg;−30 min) were previously administrated in mice injected with SB203580 (i.t.; 3 μg). Neutrophil recruitment was measured 6 h after zymosan injection (i.a.; 30 μg). Data are expressed as the mean ± SEM (n = 4–8 mice/group). **P < 0.01 and ***p < 0.001 vs. control group (zymosan). (One-way anova followed by Tukey’s post hoc test)
DISCUSSION
Currently, the best available treatment for arthritis has focused on delaying disease progression and is based on the use of biological disease-modifying anti-rheumatic drugs (DMARDs), which can act by neutralizing TNF and preventing neutrophil activation [14]. However, these pharmacological therapies are still expensive and showed various systemic side effects including the risk of serious infection and cancer [15,16], and therefore, experimental efforts have emphasized on innovative treatments for local regulation of joint inflammation. Recent studies have indicated that the nervous system surveys peripheral inflammation controlling reflexively the innate immunity to maintain physiological homeostasis by activating efferent neural networks [17–19]. Previous studies showed that peripheral inflammatory factors such as TNF trigger nociceptive networks activating spinal cord p38 MAPK [10,11]. On the other hand, pharmacological blockade of spinal p38 MAPK suppressed synovial inflammation and joint destruction in chronic arthritic rats [11]. Given that our previous data demonstrated that spinal GABAergic circuits amplify neutrophil migration in experimental arthritis and that these circuits signal via p38 MAPK [6], we analyzed whether inhibition of spinal p38 MAPK affects knee joint inflammation. Our present study shows that inhibition of spinal p38 MAPK prevents arthritic knee joint inflammation via sympathetic networks and beta-adrenoceptors activation inhibiting neutrophil infiltration and cytokine production.
Early studies in several models of inflammation have indicated that the vagus nerve controls innate immunity via efferent signals. These neural anti-inflammatory mechanisms converge in a systemic response based on the release of norepinephrine and dopamine from either the spleen or the adrenal glands, respectively [20,21]. However, we have recently reported that vagal signaling can regulate local inflammation through an alternative afferent vagal mechanism, which is independent of the classical efferent vagal components (e.g., spleen, adrenal glands or acetylcholine-producing lymphocytes). These immunomodulatory signals originated from the central nervous system are dependent on sympathetic networks connecting to the inflamed tissue [6–8]. For example, we reported that activation of the baroreflex (the neuronal network keeping cardiovascular homeostasis) inhibits neutrophil migration in arthritic joints via autonomic reflex [7]. We also showed that afferent vagal signals activate specific sympatho-excitatory brain structures, such as locus coerulus and paraventricular nucleus, inhibiting knee joint inflammation via peripheral sympathetic innervations and beta-adrenoceptors activating [8]. In agreement with our data, other studies demonstrated the role of sympathetic nervous system [22–25] and beta-adrenergic receptors [26–28] in the neural control of immunity.
In addition, our present study shows that inhibition of spinal p38 MAPK prevents the production of inflammatory factors such as TNF and IL-1β in the knee synovial fluid. This effect was selective for these factors because MIP-2 chemokine levels were not affected. TNF and IL-1β are inflammatory cytokines contributing to rheumatoid arthritis by recruiting and activating neutrophils [29]. We previously reported that central stimulation of the sympathetic system inhibits the local production of inflammatory cytokines in the arthritic knee joint by inducing local norepinephrine release [6–8]. Moreover, norepinephrine could also act on synoviocytes, macrophage-like cells considered the primary source of inflammatory factors in zymosan-induced arthritis [30].
The present study shows a limitation that needs to be discussed. The potential use of this neuroimmune pathway was investigated only in zymosan-induced arthritis, in which it has been observed only local inflammation and innate immune response. However, this classical model has allowed the investigation of a specific neural network involved in the modulation of joint inflammation [6–8] and, together with previous experimental data, reinforces the potential role of central p38 MAPK inhibition in joint inflammatory diseases [11]. Further studies in other experimental models, as collagen-induced arthritis, are needed to validate this therapeutic approach in rheumatoid arthritis treatment.
The spinal cord is an critical connection between the central nervous system and the peripheral sympathetic networks innervating the arthritic knee joints [6,31]. Thus, pharmacological or electrical (bioelectronics) strategies affecting the spinal neurons can represent a therapeutic approach to control joint inflammation in arthritis [6,11,32,33]. Although inhibitors of p38 MAPK have been investigated as a pharmacological therapy for the treatment of inflammatory diseases and cancer, many adverse effects have been considered because this class of drugs interferes in biological processes as cellular development, differentiation and proliferation [34]. Spinal drug administration requires lower concentrations of drug treatment than those required for systemic administration and therefore could be a promising strategy to avoid the undesired side effects preventing, for example, the systemic immunosuppression, a clinical condition that can lead to opportunistic infections. However, in this case, the potential side effects of p38 MAPK inhibition in the central and peripheral nervous systems cannot be neglected [34].
The present data complement our previous studies demonstrating the existence of an elaborate neuroimmune route constituted by peripheral and central components [6–8] adding the spinal p38 MAPK as pro-inflammatory component involved in the progression of joint inflammation. Finally, our study also suggests the design of innovative strategies that could show clinical advantages as compared to the current pharmacological therapy.
ACKNOWLEDGEMENTS
The research leading to these results received funding from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 2011/20343-4, 2013/08216-2 and 2012/04237-2), from the National Council for Scientific and Technological Development (CNPq, grant 150718/2010-1, 478504/2010-1 and 142068/2012-8). The authors thank Ieda R. Santos, Sérgio R. Rosa and Giuliana Bertozi for technical assistance.
Footnotes
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
REFERENCES
- 1.Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun. Rev (2010) 9 531–535. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS Evolving concepts of rheumatoid arthritis. Nature (2003) 423 356–361. [DOI] [PubMed] [Google Scholar]
- 3.Adlan AM, Paton JFR, Lip GYH, Kitas GD, Fisher JP Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J. Physiol (2017) 595 967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine YA, Koopman FA, Faltys M et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE (2014) 9 e104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koopman FA, Chavan SS, Miljko S et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci (2016) 113 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassi GS, do C Malvar D, Cunha TM, Cunha FQ, Kanashiro A. Spinal GABA-B receptor modulates neutrophil recruitment to the knee joint in zymosan-induced arthritis. Naunyn Schmiedebergs Arch. Pharmacol (2016) 389 851–861. [DOI] [PubMed] [Google Scholar]
- 7.Bassi GS, Brognara F, Castania JA et al. Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Brain Behav. Immun (2015) 49 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassi GS, Dias DPM, Franchin M et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav. Immun (2017) 64 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schett G, Zwerina J, Firestein G The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis (2008) 67 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson CI, Marsala M, Westerlund A et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J. Neurochem (2003) 86 1534–1544. [DOI] [PubMed] [Google Scholar]
- 11.Boyle DL, Jones TL, Hammaker D et al. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med (2006) 3 e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keystone EC, Schorlemmer HU, Pope C, Allison AC Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum (1977) 20 1396–1401. [DOI] [PubMed] [Google Scholar]
- 13.Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods (1994) 32 197–200. [DOI] [PubMed] [Google Scholar]
- 14.Colmegna I, Ohata BR, Menard HA Current understanding of rheumatoid arthritis therapy. Clin. Pharmacol. Ther (2012) 91 607–620. [DOI] [PubMed] [Google Scholar]
- 15.Minozzi S, Bonovas S, Lytras T et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin. Drug Saf (2016) 15 11–34. [DOI] [PubMed] [Google Scholar]
- 16.Saliba L, Moulis G, Abou Taam M et al. Tumor necrosis factor inhibitors added to nonbiological immunosuppressants vs. nonbiological immunosuppressants alone: a different signal of cancer risk according to the condition. A disproportionality analysis in a nationwide pharmacovigilance database. Fundam. Clin. Pharmacol (2016) 30 162–171. [DOI] [PubMed] [Google Scholar]
- 17.Kanashiro A, Sônego F, Ferreira RG. et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol. Res (2017) 117 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulloa L The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discovery (2005) 4 673–684. [DOI] [PubMed] [Google Scholar]
- 19.Chavan SS, Pavlov VA, Tracey KJ Mechanisms and therapeutic relevance of neuro-immune communication. Immunity (2017) 46 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosas-Ballina M, Olofsson PS, Ochani M et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science (2011) 334 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres-Rosas R, Yehia G, Pena G et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med (2014) 20 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kox M, van Eijk LT, Zwaag J et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl Acad. Sci (2014) 111 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martelli D, Yao ST, McKinley MJ, McAllen RM Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol (2014) 592 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosas-Ballina M, Ochani M, Parrish WR et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci (2008) 105 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pongratz G, Melzer M, Straub RH The sympathetic nervous system stimulates anti-inflammatory B cells in collagen-type II-induced arthritis. Ann. Rheum. Dis (2012) 71 432–439. [DOI] [PubMed] [Google Scholar]
- 26.Vida G, Peña G, Kanashiro A. et al. β2-adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J (2011) 25 4476–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva RL, Castanheira FV, Figueiredo JG et al. Pharmacological beta-adrenergic receptor activation attenuates neutrophil recruitment by a mechanism dependent on nicotinic receptor and the spleen. Inflammation (2016) 39 1405–1413. [DOI] [PubMed] [Google Scholar]
- 28.Elenkov IJ, Haskó G, Kovács KJ, Vizi ES Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J. Neuroimmunol (1995) 61 123–131. [DOI] [PubMed] [Google Scholar]
- 29.Bolon B, Campagnuolo G, Zhu L, Duryea D, Zack D, Feige U Interleukin-1beta and tumor necrosis factor-alpha produce distinct, time-dependent patterns of acute arthritis in the rat knee. Vet. Pathol (2004) 41 235–243. [DOI] [PubMed] [Google Scholar]
- 30.Pettipher ER, Salter ED Resident joint tissues, rather than infiltrating neutrophils and monocytes, are the predominant sources of TNF-alpha in zymosan-induced arthritis. Cytokine (1996) 8 130–133. [DOI] [PubMed] [Google Scholar]
- 31.Schaible H-G, Straub RH Function of the sympathetic supply in acute and chronic experimental joint inflammation. Auton. Neurosci (2014) 182 55–64. [DOI] [PubMed] [Google Scholar]
- 32.Bressan E, Mitkovski M, Tonussi CR LPS-induced knee-joint reactive arthritis and spinal cord glial activation were reduced after intrathecal thalidomide injection in rats. Life Sci (2010) 87 481–489. [DOI] [PubMed] [Google Scholar]
- 33.Ueno M, Ueno-nakamura Y, Niehaus J Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci (2016) 19 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dambach DM Potential adverse effects associated with inhibition of p38alpha/beta MAP kinases. Curr. Top. Med Chem (2005) 5 929–939. [DOI] [PubMed] [Google Scholar]


