Abstract
We previously reported that activation of the baroreflex, a critical physiological mechanism controlling cardiovascular homeostasis, through electrical stimulation of the aortic depressor nerve attenuates joint inflammation in experimental arthritis. However, it is unknown whether baroreflex activation can control systemic inflammation. Here, we investigate whether baroreflex activation controls systemic inflammation in conscious endotoxemic rats. Animals underwent sham or electrical aortic depressor nerve stimulation initiated 10 min prior to a lipopolysaccharide (LPS) challenge, while inflammatory cytokine levels were measured in the blood, spleen, heart and hypothalamus 90 min after LPS treatment. Baroreflex activation did not affect LPS-induced levels of pro-inflammatory (tumor necrosis factor, interleukin 1β and interleukin 6) or anti-inflammatory (interleukin 10) cytokines in the periphery (heart, spleen and blood). However, baroreflex stimulation attenuated LPS-induced levels of all these cytokines in the hypothalamus. Notably, these results indicate that the central anti-inflammatory mechanism induced by baroreflex stimulation is independent of cardiovascular alterations, since aortic depressor nerve stimulation that failed to induce hemodynamic changes was also efficient at inhibiting inflammatory cytokines in the hypothalamus. Thus, aortic depressor nerve stimulation might represent a novel therapeutic strategy for neuroprotection, modulating inflammation in the central nervous system.
Keywords: Baroreflex, Aortic depressor nerve, Neuroimmunomodulation, Inflammation, Lipopolysaccharide, Hypothalamus
1. Introduction
The nervous system has been selected through evolution to control physiological homeostasis. One of the most critical processes of neuromodulation is the ability of the nervous system to regulate innate immunity and modulate inflammation. There are three major pathways of neuromodulation: the hypothalamic–pit uitaryadrenal axis (Sternberg et al., 1989), the cholinergic vagal anti-inflammatory pathway (Tracey, 2002; Ulloa, 2005), and the sympathetic splanchnic anti-inflammatory pathway (Martelli et al., 2014b). We recently reported that baroreflex activation through electrical stimulation of the aortic depressor nerve (ADN) attenuates, via sympathetic innervation, joint inflammation in a rat model of arthritis. Our results depicted the baroreflex antiinflammatory pathway as a new physiological mechanism of neuromodulation of inflammation and the innate immune system (Bassi et al., 2015).
Baroreceptors are sensory neurons monitoring the arterial pressure in the aortic arch, carotid sinuses and major blood vessels (Chapleau et al., 1988; Krieger et al., 1982). These mechanoreceptors induce reflex responses increasing parasympathetic and decreasing sympathetic drive to maintain the blood pressure at nearly constant levels (Chapleau et al., 1988; Krieger et al., 1982). Baroreflex activation is considered a promising therapeutic approach for patients with resistant hypertension (Alnima et al., 2014; Bisognano et al., 2011; Halbach et al., 2015; Hoppe et al., 2012). Clinical studies showed that hypertensive patients have attenuated baroreflex function (Ding et al., 2011; Huang et al., 2017; Subha et al., 2016) associated with a high inflammatory profile (Harrison et al., 2011; Mattace-Raso et al., 2010; Sesso et al., 2007; Solak et al., 2016). These results suggest that baroreflex dysfunction may facilitate inflammation in hypertensive subjects.
We recently reported that baroreflex activation attenuates local inflammation in arthritic knee joints (Bassi et al., 2015). This mechanism significantly inhibits neutrophil recruitment, articular edema and inflammatory cytokine levels in the synovial fluid (Bassi et al., 2015). This anti-inflammatory mechanism involves sympathetic modulation (Bassi et al., 2015); however, it is unknown whether baroreflex activation can control systemic inflammation. To expand knowledge about the anti-inflammatory potential of electrical stimulation of the baroreflex, we analyze, in the present study, whether baroreflex activation by electrical ADN stimulation controls systemic inflammation in endotoxemic rats.
2. Results
2.1. Time course of cardiovascular responses to bacterial endotoxin
Intraperitoneal administration of either saline or lipopolysaccharide (LPS) did not affect the mean arterial pressure (MAP) (Fig. 1). However, LPS induced tachycardia 45 min after its administration, which lasted up to 90 min compared to the saline treatment (Fig. 1).
Fig. 1.
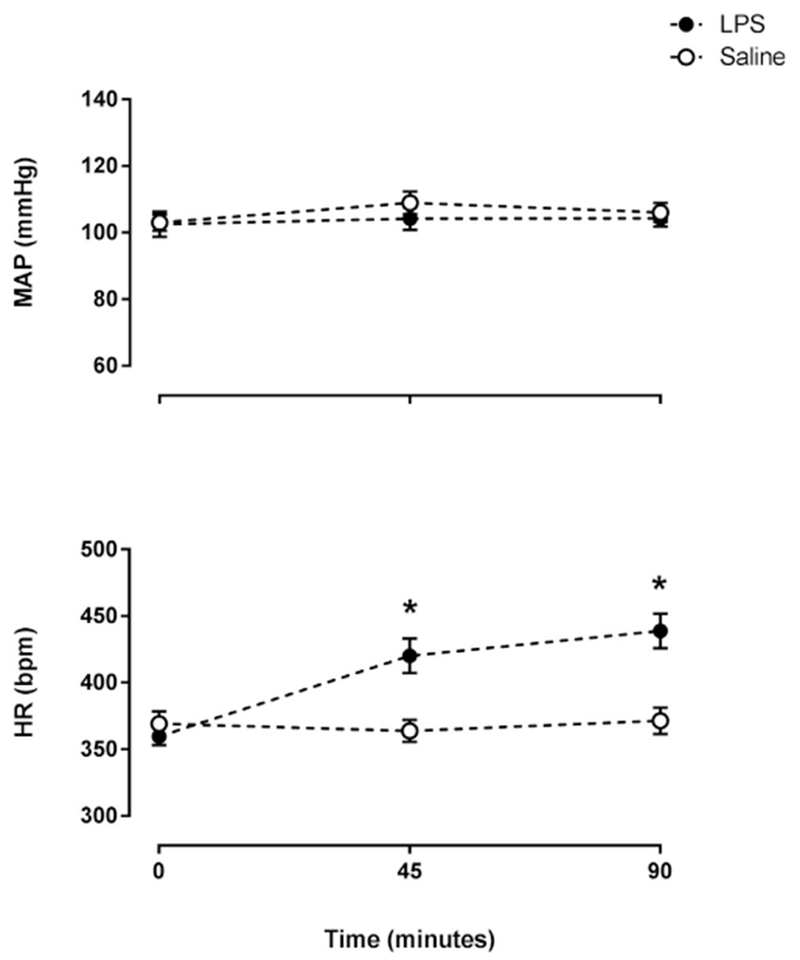
LPS increased heart rate but did not affect the mean arterial pressure. Time course of mean arterial pressure (MAP) and heart rate (HR) at baseline (time zero), 45 and 90 min after the administration of LPS (n = 13) or saline (n = 12). *P < .05 vs. Saline.
2.2. Baroreflex activation and hemodynamic responses
Electrical ADN stimulation quickly (within the first 5 s) decreased MAP and heart rate (HR) (Fig. 2A and B). These effects were transient because the hemodynamic parameters had returned to baseline levels at the 20th min of electrical ADN stimulation. However, electrical ADN stimulation did not affect cardio-circulatory hemodynamics in 12 of 25 animals. Even with electrical stimulation, these animals did not present a decrease in MAP and HR (Fig. 2C and D). Moreover, the animals in the LPS + ADNs (–) group showed a statistical increase of 9% in HR at the 20th min of electrical ADN stimulation (10 min after LPS injection) without affecting MAP (Fig. 2D). Nevertheless, all endotoxemic animals exhibited an increased HR by the end of the experimental protocol at the 90th min after the LPS challenge (Fig. 3).
Fig. 2.
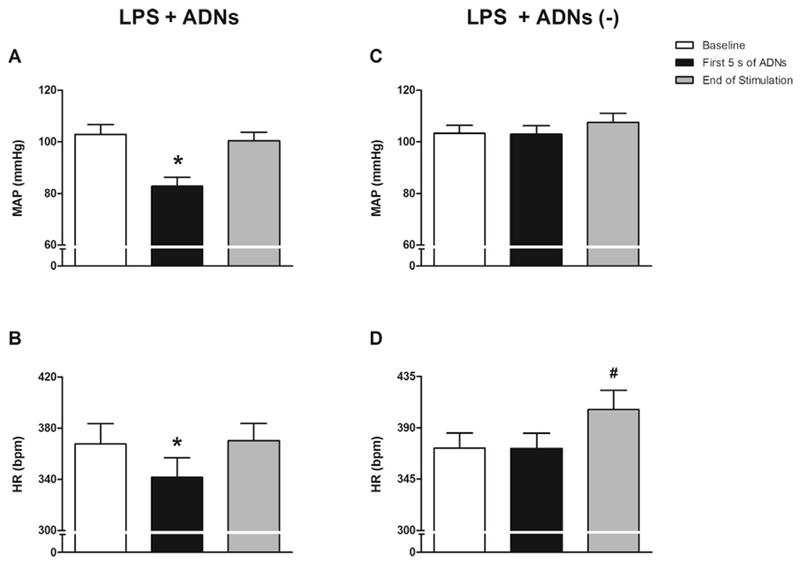
Hemodynamic responses to electrical stimulation of the aortic depressor nerve. Bar graphs represent the mean arterial pressure (MAP; A and C) and heart rate (HR; B and D) at baseline; at 5 s after initiating electrical stimulation of the aortic depressor nerve (ADNs), with (LPS + ADNs) or without [LPS + ADNs (–)] hemodynamic changes; and at the end of electrical stimulation (5 s). LPS + ADNs: n = 13; LPS + ADNs (–): n = 12. Bars represent mean ± standard error. *P < .05 vs. baseline; #P < .05 vs. baseline and first 5 s of ADNs.
Fig. 3.
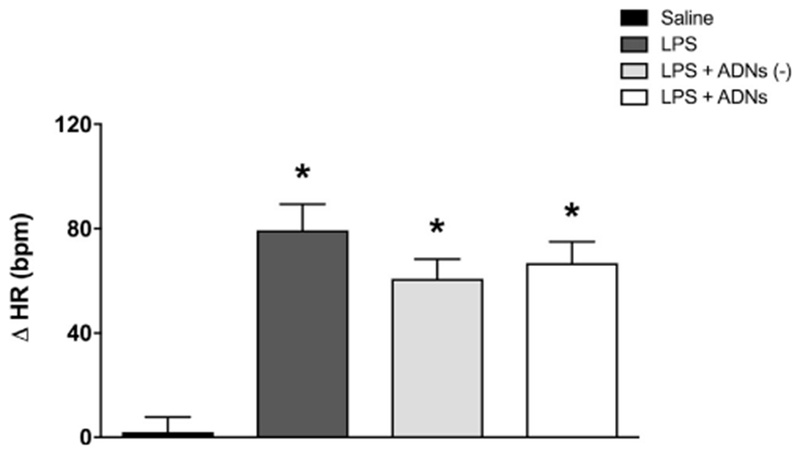
Ninety minutes after the LPS injection, aortic depressor nerve stimulation did not attenuate the increase in heart rate induced by LPS. Bar graphs show the changes in heart rate (△HR) 90 min after the administration of saline or LPS, or LPS combined or not combined with previous aortic depressor nerve stimulation (ADNs), with or without hemodynamic changes. Bars represent mean ± standard error, n = 12 to 13. *P < .05 vs. saline.
2.3. Baroreflex stimulation did not affect peripheral cytokine levels in endotoxemic rats
Intraperitoneal injection of bacterial LPS increased the plasma levels of all cytokines analyzed, including pro-inflammatory cytokines [tumor necrosis factor (TNF), interleukin 1β (IL-1β) and interleukin 6 (IL-6)] and anti-inflammatory cytokine [interleukin 10 (IL-10)] (Fig. 4A–D). Baroreflex stimulation did not affect the levels of any of these cytokines in the blood (Fig. 4A–D). LPS also increased the levels of TNF and IL-6 in the heart and spleen (Fig. 4E, G, I and K). In addition, LPS specifically increased IL-1β levels in the heart but not in the spleen (Fig. 4F and J). LPS also induced anti-inflammatory cytokine IL-10 in the blood, but not in the heart or the spleen (Fig. 4D, H, L). Thus, ADN stimulation did not change the peripheral levels of any of these cytokines in the blood, spleen or heart. These results indicate that baroreflex stimulation did not regulate peripheral cytokine levels in endotoxemia. It is important to highlight that despite the short recovery time from surgery, the subjects did not exhibit any sign of sickness behavior until the beginning of the experiment.
Fig. 4.
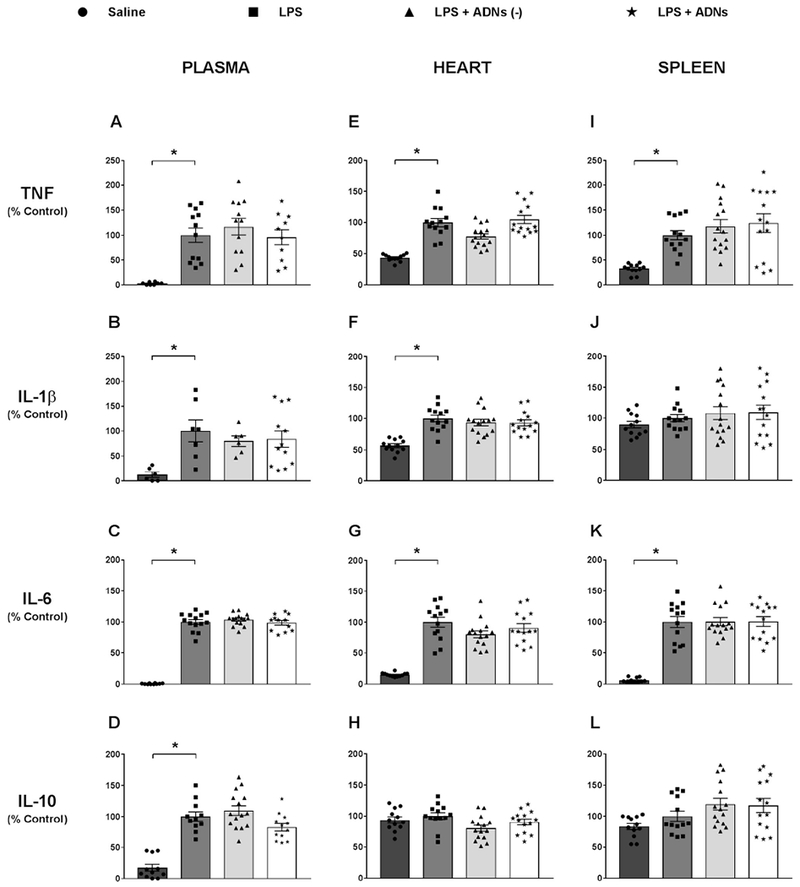
Aortic depressor nerve stimulation did not affect plasma and peripheral organ (heart and spleen) levels of cytokines in response to LPS administration. Plasma (A, B, C and D) and tissue (heart: E, F, G, H and spleen: I, J, K and L) levels of pro-inflammatory and anti-inflammatory cytokines 90 min after the administration of saline or LPS, or LPS combined or not combined with electrical stimulation of the aortic depressor nerve (ADNs), with or without hemodynamic changes. Tumor necrosis factor (TNF): plasma (A), heart (E) and spleen (I); interleukin 1β (IL-1β): plasma (B), heart (F) and spleen (J); interleukin 6 (IL-6): plasma (C), heart (G) and spleen (K); interleukin 10 (IL-10): plasma (D), heart (H) and spleen (L). Bars represent mean ± standard error, n = 6–15. *P < .05.
2.4. Baroreflex stimulation attenuated central cytokine levels in the hypothalamus
Intraperitoneal LPS administration also increased the levels of all the cytokines in the hypothalamus, including inflammatory (TNF, IL-1β, IL-6) and anti-inflammatory (IL-10) cytokines (Fig. 5A–D). Baroreflex activation attenuated the LPS-induced levels of all the cytokines in the hypothalamus (Fig. 5A–D) independent of the cardiovascular effects.
Fig. 5.
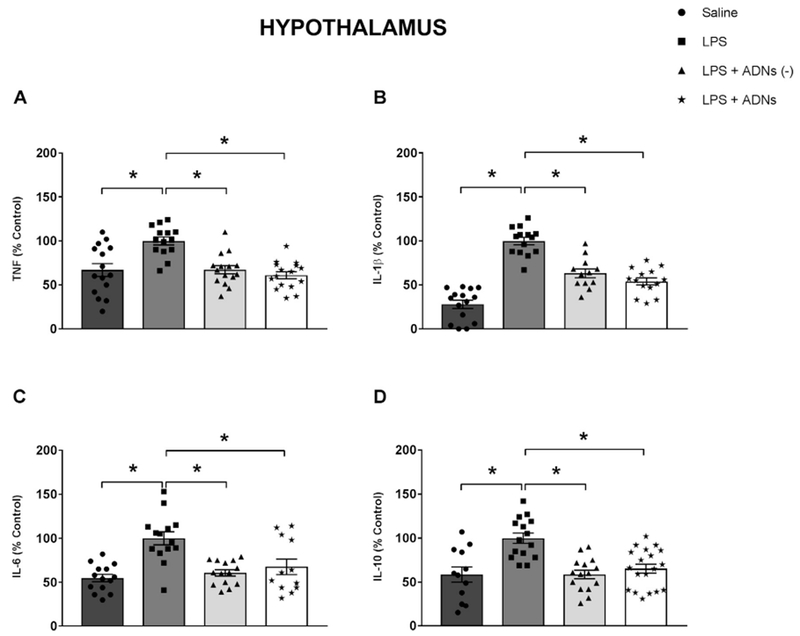
Aortic depressor nerve stimulation decreases the LPS-induced production of cytokines in the hypothalamus. Hypothalamic levels of tumor necrosis factor (TNF, A), interleukin 1β (IL-1β, B), interleukin 6 (IL-6, C) and interleukin 10 (IL-10, D) 90 min after the administration of saline or LPS, or LPS combined or not combined with stimulation of the aortic depressor nerve (ADNs), with or without hemodynamic changes. Bars represent mean ± standard error, n = 12 to 19. *P < .05.
3. Discussion
The present results show that electrical stimulation of the ADN, a known afferent nerve responsible for controlling cardiovascular homeostasis, induced central anti-inflammatory effects inhibiting LPS-induced hypothalamic levels of all cytokines analyzed. This effect is due to a direct central, but not peripheral, mechanism because ADN stimulation did not affect the levels of any of these cytokines in the peripheral blood or organs. In the present study, we injected LPS intraperitoneally and studied whether baroreflex stimulation can control systemic inflammation. This experimental model of systemic inflammation has been widely used in the literature to investigate new therapeutic strategies for sepsis (Cai et al., 2010).
It is well-established that cytokines control inflammation and induce cellular damage and organ failure contributing to the pathogenesis of multiple acute and chronic disorders such as sepsis and autoimmune diseases (Akiyama et al., 2000; Allan and Rothwell, 2001; Mennicken et al., 1999). Inflammatory cytokines mediate communication between the immune and other systems inducing particular biological activities after binding to the receptors of the targeted cells (Wyss-Coray and Mucke, 2002). Neurons also have cytokine receptors, suggesting an active bidirectional crosstalk between the immune and nervous systems (Wyss-Coray and Mucke, 2002). Additionally, cytokines have different functions in the central nervous system and can induce neurotoxicity, which appears to contribute to the physiopathology of psychiatric [depression, schizophrenia] and neurodegenerative diseases [Parkinson’s and Alzheimer’s diseases] (Allan and Rothwell, 2003; Corsi-Zuelli et al., 2017; Dantzer et al., 2008). Moreover, since inflammation in the central nervous system, particularly in the hypothalamus, has recently been shown to be intricately involved in the development and maintenance of hypertension (Khor and Cai, 2017), we suggest that the selective and central anti-inflammatory potential of ADN stimulation could be an additional mechanism involved in the baroreflex anti-hypertensive effects.
Current studies highlight the need to develop innovative therapies based on electrical neural stimulation for treating diseases with non-pharmacological approaches (De Ferrari et al., 2011; Famm et al., 2013; Schwartz, 2013). “Electroceuticals” have been considered a promising future for treating a number of diseases including central inflammatory and neurological disorders (Famm et al., 2013). Recent studies showed that electrical stimulation of peripheral nerves (including vagus) or direct brain stimulation, induces protective effects in ischemic stroke, traumatic brain injury, as well as in experimental cerebral ischemia and reperfusion (Jiang et al., 2014; Meneses et al., 2016; Notturno et al., 2014; Schweighöfer et al., 2016).
Our study shows that baroreflex stimulation inhibits LPS-induced inflammatory cytokines in the brain (hypothalamus). Surprisingly, electrical ADN stimulation that failed to induce hemodynamic changes was also efficient at inhibiting inflammatory cytokines in the hypothalamus. These findings suggest that the central baroreflex anti-inflammatory mechanism is independent of cardiovascular alterations. In line with our results, vagal stimulation at high intensity produces hemodynamic alterations but not anti-inflammatory effects, while low intensity vagal stimulation induces immunomodulatory properties without promoting hemodynamic alterations (Bassi et al., 2017). Thus, our results indicate that during parasympathetic activation, it is not essential for changes in the hemodynamic parameters to occur in order for an anti-inflammatory effect to occur.
Although baroreflex stimulation attenuated central cytokine levels, it did not affect peripheral cytokines in endotoxemic animals. In fact, electrical ADN stimulation did not change the levels of inflammatory cytokines (TNF, IL-1β and IL-6) and antiinflammatory cytokine IL-10 in the plasma, spleen or heart. Of note, the baroreflex acts by reducing sympathetic and increasing parasympathetic drive (Chapleau et al., 1988; Krieger et al., 1982), a well-known autonomic network controlling inflammation (Huston et al., 2006; Martelli et al., 2016; Tracey, 2002). It is well-established that the spleen is the main source of peripheral TNF, which is released into the bloodstream during endotoxemia (Huston et al., 2006). However, taking into account that the spleen is also the targeted organ of the “inflammatory reflex” (Huston et al., 2006) and that the sympathetic splanchnic nerve inhibits the inflammatory response in the spleen (Martelli et al., 2016), it is surprising that baroreflex stimulation did not affect systemic cytokine levels. However, it is possible that simultaneous activation of the parasympathetic nervous system (anti-inflammatory) and inhibition of the sympathetic nervous system (pro-inflammatory) following electrical activation of the baroreflex accounted for the lack of effect on systemic inflammation. Nevertheless, our present results concur with previous studies of our group showing that baroreflex activation via ADN stimulation inhibits joint cytokine levels in experimental arthritis by sympathetic modulation, but, again, through a mechanism independent of the spleen (Bassi et al., 2015).
An important aspect of our study is that the experiments were performed in unanesthetized animals, mimicking clinical settings and physiological conditions (De Paula et al., 1999; Salgado et al., 2006). Previous studies investigating neural electrical stimulation were conducted primarily in anesthetized rodents. A significant exception can be attributed to Martelli and colleagues (Martelli et al., 2014a) who demonstrated, in conscious rats, that the splanchnic anti-inflammatory pathway has a powerful and sustained inhibitory influence on inflammatory processes. It is well known that anesthetics interfere particularly with the neural mechanisms modulating the immune system (Picq et al., 2013). For instance, several studies showed that ketamine and isoflurane exhibit potent anti-inflammatory properties (Flondor et al., 2008; Hofstetter et al., 2005; Qin et al., 2015). Notably, our laboratory studies of neural cardiocirculatory control in conscious rodents has provided significant information concerning the neural regulation of inflammation (Bassi et al., 2015), particularly under physiological conditions without the interference of anesthesia, as conducted in the present study.
4. Conclusions
Baroreflex activation could be used as a novel therapeutic strategy for treating inflammatory diseases involving the central nervous system, such as stroke, brain trauma, amyotrophic lateral sclerosis, Parkinson’s and Alzheimer’s disease and even hypertension. However, future studies are required to investigate the neural pathways and molecular mechanisms mediating these neuroprotective properties, as well as the optimal parameters for activating the ADN under different inflammatory conditions.
5. Experimental procedure
5.1. Experimental animals
The experiments were performed on male Wistar rats weighing 250–300 g obtained from the Main Animal Facility of the University of São Paulo (Campus of Ribeirão Preto; Ribeirão Preto, SP, Brazil), maintained under controlled temperature (22 °C) and constant 12 h light–dark cycle and provided with food and water ad libitum. All procedures were reviewed and approved by the Committee of Ethics in Animal Research of the Ribeirão Preto Medical School – University of São Paulo (Protocol number 161/2016).
5.2. Surgical procedures
Animals were anesthetized with a cocktail of ketamine and xylazine (50 mg/kg and 10 mg/kg, i.p.) and then subjected to surgical procedures to isolate the left ADN for the implantation of electrodes, catheterization of the femoral artery and insertion of a peritoneal catheter. The catheter implanted into the femoral artery was filled with 100 IU/ml heparin in saline. The ADN group was implanted with a bipolar stainless-steel electrode with an interleads distance of 2 mm. The electrodes were constructed by attaching two 40 mm long stainless-steel wires (0.008 in. bare, 0.011 in. Teflon coated; model 791,400; A-M Systems, Sequim, WA, USA) to a small plug (GF-6; Microtech, Boothwyn, PA, USA). The bared tips of the electrodes consisted of 2 mm lengths, forming hooks that were implanted around the ADN. First, the electrode was tunneled through the sternocleidomastoid muscle and the small plug was exteriorized in the nape of the neck. Next, the short segment of the ADN that was implanted with the bipolar stainless-steel electrodes was carefully covered with silicone impression material (Kwik-Sil silicone elastomer; World Precision Instruments, Sarasota, FL, USA). A few minutes were allowed for complete polymerization of the silicone impression material. In the “sham surgery” groups, the animals were subjected to the same surgical procedures, but the electrodes were not implanted around the nerve.
Under the same anesthesia, the left femoral artery was catheterized with polyethylene tubing (PE-50 soldered to PE-10 polyethylene tube; Intramedic, Clay Adams, Parsippany, NJ, USA) for arterial pressure recording. Additionally, a catheter (PE-50 polyethylene tube; Intramedic, Clay Adams, Parsippany, NJ, USA) was inserted into the abdominal cavity for the administration of LPS from Escherichia coli 0111: B4 (Sigma-Aldrich, St. Louis, MO, USA) or sterile saline (vehicle). The catheters were pulled up through a subcutaneous track to the animal’s neck and exteriorized in the back of the nape; next, the surgical incisions were sutured. Flunixin meglumine (Banamine, 2.5 mg/kg, i.m.; Schering-Plough, Cotia, SP, Brazil) was injected immediately after the end of surgery.
5.3. Assessment of the hemodynamic parameters and electrical stimulation of the aortic depressor nerve
Twenty-four hours after the surgical procedures, arterial pressure and HR from conscious rats were recorded. Briefly, the arterial catheter was connected to a pressure transducer (MLT844; ADInstruments, Bella Vista, Australia) and the signal was amplified (ML224; ADInstruments, Bella Vista, Australia) and sampled by an IBM/PC computer (Core 2 Duo, 2.2 GHz, 4 GB RAM) attached to an analog-to-digital interface (PowerLab, ADInstruments, Bella Vista, Australia). The experiment was conducted with the animals moving freely in their own cage (one rat per cage) and silence was maintained to minimize environment stress. Only rats that showed no signs of distress during electrical stimulation of the ADN were assigned to the study. The electrodes were connected to an external square pulse generator to activate the ADN (0.5 mA, 0.25 ms, 15 Hz). Arterial pressure recordings were processed with computer software (LabChart 7.0, ADInstruments, Bella Vista, Australia) capable of detecting inflection points and systolic, diastolic and mean arterial pressure, as well as HR beat-by-beat time series.
5.4. Experimental procedures
The rats were assigned into four groups:
-
1)
Saline: Sham surgery of electrode implantation around the ADN and vehicle administration;
-
2)
LPS: Sham surgery of electrode implantation around the ADN and LPS administration;
-
3)
LPS + ADNs (—): Electrode implantation around the ADN, electrical stimulation combined with failure to promote hemodynamic effects and LPS administration;
-
4)
LPS + ADNs: Electrode implantation around the ADN, electrical stimulation showing hemodynamic effects and LPS administration.
The experimental protocol consisted of basal recordings of arterial pressure and HR for 30 min, followed by electrical stimulation of the ADN for 10 min before and 10 min after intraperitoneal administration of LPS (5 mg/kg, i.p.) or vehicle (5 mL/kg, i.p.). LPS was sonicated for 30 min before the injection. The hemodynamic parameters were recorded throughout 90 min after LPS injection, and then the blood samples were collected through the catheter from the femoral artery before the rats were killed by decapitation for rapid collection of the spleen, heart and hypothalamus. The entire hypothalamus was dissected from the brain taking into account the following limits: the anterior border of the optic chiasma, the anterior border of the mammillary bodies, and the lateral hypothalamic sulci with a depth of 2 mm. The samples of spleen, heart and hypothalamus were immediately frozen in liquid nitrogen. Blood samples were maintained on ice until centrifugation at 3500 rpm for 15 min at 4 °C. The plasma was then collected, and all biological material was frozen at −80 °C and stored until analysis.
5.5. Cytokine measurements
On the day of the assay, the samples were thawed and maintained on ice. The hypothalamus, spleen, and heart were homogenized in 0.5 mLofPBSusingaPolitron-PT-3100(Evisa; Kinematica, Luzern, Switzerland) and then centrifuged at 1.000 rpm for 10 min at 4 °C. The plasma and tissue supernatant samples were used to measure the cytokine (TNF, IL-1β, 1L-6, and 1L-10) levels by an immune-enzymatic ELISA method using Duo set kits from R&D Systems (Minneapolis, MN, USA) according to the manufacturer’s instructions. Plasma and tissue cytokine levels are expressed as percentages compared to the control group (LPS).
5.6. Statistical analysis
The hemodynamic parameters were analyzed by one-way analysis of variance (ANOVA) for repeated measures followed by Student-Newman-Keuls post hoc test when indicated, two-way ANOVA for repeated measures followed by Student-Newman-Keuls post hoc test, or one-way ANOVA followed by Dunn’s post hoc test. The data obtained from the tissues and plasma were analyzed either by one-way ANOVA with Student-Newman-Keuls post hoc test or by Kruskal-Wallis – a nonparametric statistical test – followed by Dunn’s post hoc test when the data did not pass the Shapiro-Wilk test of normality. Differences were considered statistically significant if P < .05. The results are shown as the mean ± s tandard error of the mean.
Acknowledgements
FB held a Scholarship for Scientific Initiation from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo – Brazil), Process # 2014/15386-4; and now holds a PhD Scholarship from FAPESP, Process # 2017/05163-6. This work received financial support from FAPESP, Process # 2013/20549-7 and # 2011/20343-4.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ, 2003. Inflammation in central nervous system injury. Philos. Trans. R. Soc. Lond., B Biol. Sci 358,1669–1677. https://doi.org/10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ, 2001. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci 2, 734–744. https://doi.org/10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Alnima T, Kroon AA, de Leeuw PW, 2014. Baroreflex activation therapy for patients with drug-resistant hypertension. Expert Rev. Cardiovasc. Ther 12, 955–962. https://doi.org/10.1586/14779072.2014.931226. [DOI] [PubMed] [Google Scholar]
- Bassi GS, Brognara F, Castania JA, Talbot J, Cunha TM, Cunha FQ, Ulloa L, Kanashiro A, Dias DPM, Salgado HC, 2015. Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Immun. Brain Behav https://doi.org/10.1016/j.bbi.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi GS, Dias DPM, Franchin M,Talbot J, Reis DG, Menezes GB, Castania JA, Garcia-Cairasco N, Resstel LBM, Salgado HC, Cunha FQ, Cunha TM, Ulloa L, Kanashiro A, 2017. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav. Immun 64, 330–343. https://doi.org/10.1016/j.bbi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA, 2011. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J. Am. Coll. Cardiol 58, 765–773. https://doi.org/10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Cai B, Deitch EA, Ulloa L, 2010. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm. 2010, 642462 https://doi.org/10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau MW, Hajduczok G, Abboud FM, 1988. Mechanisms of resetting of arterial baroreceptors: an overview. Am. J. Med. Sci 295, 327–334. [DOI] [PubMed] [Google Scholar]
- Corsi-Zuelli das FM, Brognara G, Quirino F, da GF, Hiroki S, Fais CH, Del-Ben RS, Ulloa CM, Salgado L, Kanashiro HC, Loureiro A, 2017. Neuroimmune interactions in Schizophrenia: focus on vagus nerve stimulation and activation of the alpha-7 nicotinic acetylcholine receptor. Front. Immunol 8 https://doi.org/10.3389/fimmu.2017.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. https://doi.org/10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GM, Crijns HJGM, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ, 2011. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J 32, 847–855. https://doi.org/10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- De Paula PM, Castania JA, Bonagamba LG, Salgado HC, Machado BH, 1999. Hemodynamic responses to electrical stimulation of the aortic depressor nerve in awake rats. Am. J. Physiol 277, R31–R38. [DOI] [PubMed] [Google Scholar]
- Ding W, Zhou L, Bao Y, Zhou L, Yang Y, Lu B, Wu X, Hu R, 2011. Autonomic nervous function and baroreflex sensitivity in hypertensive diabetic patients. Acta Cardiol. 66, 465–470. https://doi.org/10.2143/AC.66.4.2126595. [DOI] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M, 2013. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161. https://doi.org/10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flondor M, Hofstetter C, Boost KA, Betz C, Homann M, Zwissler B, 2008. Isoflurane inhalation after induction of endotoxemia in rats attenuates the systemic cytokine response. Eur. Surg. Res 40, 1–6. https://doi.org/10.1159/000107614. [DOI] [PubMed] [Google Scholar]
- Halbach M, Hickethier T, Madershahian N, Reuter H, Brandt MC, Hoppe UC, Muller-Ehmsen J, 2015. Acute on/off effects and chronic blood pressure reduction after long-term baroreflex activation therapy in resistant hypertension. J. Hypertens 33, 1697–1703. https://doi.org/10.1097/HJH.0000000000000586. [DOI] [PubMed] [Google Scholar]
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM, 2011. Inflammation, immunity, and hypertension. Hypertension 57, 132–140. https://doi.org/10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter C, Flondor M, Boost KA, Koehler P, Bosmann M, Pfeilschifter J, Zwissler B, Muhl H, 2005. A brief exposure to isoflurane (50 s) significantly impacts on plasma cytokine levels in endotoxemic rats. Int. Immunopharmacol 5, 1519–1522. https://doi.org/10.1016/j.intimp.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Brandt M-C, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovett EG, Haller H, 2012. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J. Am. Soc. Hypertens 6, 270–276. https://doi.org/10.1016/j.jash.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Huang D, Zhou J, Su D, Yu W, Chen J, 2017. Variations of perioperative baroreflex sensitivity in hypertensive and normotensive patients. Clin. Exp. Hypertens 39, 74–79. https://doi.org/10.1080/10641963.2016.1210624. [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L, 2006. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med 203, 1623–1628. https://doi.org/10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C, 2014. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS ONE 9, e102342 https://doi.org/10.1371/journal.pone.0102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor S, Cai D, 2017. Hypothalamic and inflammatory basis of hypertension. Clin. Sci 131, 211–223. https://doi.org/10.1042/CS20160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger EM, Salgado HC, Michelini LC, 1982. Resetting of the baroreceptors. Int. Rev. Physiol 26, 119–146. [PubMed] [Google Scholar]
- Martelli D, Farmer DGS, Yao ST, 2016. The splanchnic anti-inflammatory pathway: could it be the efferent arm of the inflammatory reflex? Exp. Physiol 101, 1245–1252. https://doi.org/10.1113/EP085559. [DOI] [PubMed] [Google Scholar]
- Martelli D, Yao ST, Mancera J, McKinley MJ, McAllen RM, 2014a. Reflex control of inflammation by the splanchnic anti-inflammatory pathway is sustained and independent of anesthesia. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R1085–R1091. https://doi.org/10.1152/ajpregu.00259.2014. [DOI] [PubMed] [Google Scholar]
- Martelli D, Yao ST, McKinley MJ, McAllen RM, 2014b. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol 592, 1677–1686. https://doi.org/10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FUS, Verwoert GC, Hofman A, Witteman JCM, 2010. Inflammation and incident-isolated systolic hypertension in older adults: the Rotterdam study. J. Hypertens 28, 892–895. https://doi.org/10.1097/HJH.0b013e328336ed26. [DOI] [PubMed] [Google Scholar]
- Meneses G, Bautista M, Florentino A, Díaz G, Acero G, Besedovsky H, Meneses D, Fleury A, Del Rey A, Gevorkian G, Fragoso G, Sciutto E, 2016. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J. Inflamm. (Lond.) 13, 33 https://doi.org/10.1186/s12950-016-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennicken F, Maki R, de Souza EB, Quirion R, 1999. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol. Sci 20, 73–78. [DOI] [PubMed] [Google Scholar]
- Notturno F, Pace M, Zappasodi F, Cam E, Bassetti CL, Uncini A, 2014. Neuroprotective effect of cathodal transcranial direct current stimulation in a rat stroke model. J. Neurol. Sci 342, 146–151. https://doi.org/10.1016/j.jns.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Picq CA, Clarenςon D, Sinniger VE, Bonaz BL, Mayol J-FS, 2013. Impact of anesthetics on immune functions in a rat model of vagus nerve stimulation. PLoS ONE 8, e67086 https://doi.org/10.1371/journal.pone.0067086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M-Z, Gu Q-H, Tao J, Song X-Y, Gan G-S, Luo Z-B, Li B-X, 2015. Ketamine effect on HMGB1 and TLR4 expression in rats with acute lung injury. Int. J. Clin. Exp. Pathol 8, 12943–12948. [PMC free article] [PubMed] [Google Scholar]
- Salgado MCO, Justo SVS, Joaquim LF, Fazan R Jr, Salgado HC, 2006. Role of nitric oxide and prostanoids in attenuation of rapid baroreceptor resetting. Am. J. Physiol. Heart Circ. Physiol 290, H1059–H1063. https://doi.org/10.1152/ajpheart.00219.2005. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, 2013. Vagal stimulation for heart diseases: from animals to men. An example of translational cardiology. Neth. Heart J 21, 82–84. https://doi.org/10.1007/s12471-012-0368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighöfer H, Rummel C, Roth J, Rosengarten B, 2016. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Int. Care Med. Exp 4, 19 https://doi.org/10.1186/s40635-016-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM, 2007. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension 49, 304–310. https://doi.org/10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M, 2016. Hypertension as an autoimmune and inflammatory disease. Hypertens. Res 39, 567–573. https://doi.org/10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL, 1989. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc. Natl. Acad. Sci. U.S.A 86, 2374–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subha M, Pal P, Pal GK, Habeebullah S, Adithan C, Sridhar MG, 2016. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, low-grade inflammation, and oxidative stress in pregnancy-induced hypertension. Clin. Exp. Hypertens 38, 666–672. https://doi.org/10.1080/10641963.2016.1200596. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, 2002. The inflammatory reflex. Nature 420, 853–859. https://doi.org/10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Ulloa L, 2005. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov 4, 673–684. https://doi.org/10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L, 2002. Inflammation in neurodegenerative disease-a double-edged sword. Neuron 35, 419–432. [DOI] [PubMed] [Google Scholar]


