Abstract
Sex is a biological variable that affects immune responses to bacterial and other types of infectious agents. Males and females are known to have differential oral bacterial disease burden in periodontal and endodontic disease. Understanding that there is a contribution from both sex and gender to these oral diseases, we discuss in this review recent sex-based findings that provide a pathobiological basis for differences observed between males and females. Sexual dimorphism of immune responses with respect to neutrophil trafficking and osteoclast differentiation and formation is presented as a plausible mechanism to explain the sexual differences. We also emphasize that sex, as a biological variable, should be considered in these types of oral immunologic studies.
Keywords: sex characteristics, bone and bones, neutrophils, osteoclasts, bacterial infections, chemokines
Introduction
Sex is a biological variable that affects immune responses to foreign antigens, including bacterial challenges. The sex of an individual is defined by the differential organization of sexual chromosomes, reproductive organs, and sex steroid levels and is distinct from gender, which includes behaviors and activities that are determined by societal or cultural cues among humans. Male and female differences in immunologic responses may be influenced by both sex and gender, with sex contributing to physiologic and anatomic differences that influence exposure, recognition, clearance, and transmission of bacteria and viruses (Rettew et al. 2010; Klein and Flanagan 2016). In contrast, gender can reflect behaviors that influence exposure to bacteria, access to health care, or health-seeking behaviors that affect the course of infection. Although we acknowledge that sex and gender both influence the immune response, this review focuses on the sex-based biological factors that influence immunologic differences as they affect oral bacterial diseases. Despite a growing body of literature illustrating sex-based differences in immune responses, immunology ranks the lowest of 10 biological disciplines for reporting the sex of animal or human subjects in published papers, with <10% of articles analyzing data by sex (Klein and Flanagan 2016). The field of sex-based biology has been going through a revolution since 2015, when research funding agencies and periodicals initiated new policies to promote greater consideration, reporting, and analyses of sex and gender in the biomedical sciences in an effort to improve rigor and reproducibility (Collins and Tabak 2014; Clayton 2016).
It is increasingly important to acknowledge sex differences in immune responses in the impact of alveolar bone homeostasis when we consider the marked differences seen between males and females in oral bacterial diseases. In this review, we explore the current knowledge regarding immunologic differences between the sexes as reflected by hormonal, genetic, and environmental impacts on the immune system that can change throughout life and alter susceptibility to common oral infections. Specifically, we review how sex differences affect 2 key components of immunity that regulate alveolar bone homeostasis: chemokine biology associated with neutrophil trafficking and osteoimmunology during oral bacterial challenge.
General Aspects of Sex Differences in Innate Immune Responses
In most mammalian species, innate immune responses differ dramatically between males and females, which implies that some sex differences may be germline encoded (Klein and Flanagan 2016). In general, men mount a more aggressive acute inflammatory response to microbial pathogens than do women. Consistent with this concept, women have diminished proinflammatory responses as compared with men in standardized endotoxemia models (Shiau and Reynolds 2010a, 2010b). Moreover, a review of sex-dependent response to acute cardiac injury showed that women, via estrogen, had a dampened acute inflammatory response as compared with men (Kher et al. 2005). As an example, transcriptional analyses revealed sex differences in the expression of several innate immune genes along toll-like receptor (TLR) pathways and induction of type I interferon responses (Klein and Flanagan 2016). Following bacterial or viral challenges, the expression of TLR-pathway genes and many proinflammatory signaling genes—including myeloid differentiation primary response gene 88, Janus kinase 2, signal transducer and activator of transcription 3, nuclear factor–κB, interferon γ, and tumor necrosis factor α (TNF-α)—are differentially expressed between sexes (Hannah et al. 2008; Klein et al. 2010). Additionally, putative androgen response elements and estrogen response elements are present in the promoter regions of several genes that regulate innate immunity, which suggests that sex steroids may directly cause dimorphic innate immune responses (Hannah et al. 2008; Rettew et al. 2010).
These differences in innate immune system component gene expression between sexes alter the production of cytokines and chemokines by innate immune cells. Point in fact, peripheral blood mononuclear cells (PBMCs) from human males have long been known to produce more TNF-α than that of PBMCs from females following bacterial lipopolysaccharide (LPS) stimulation (Asai et al. 2001; Moxley et al. 2002). Indeed, neutrophils in men express higher levels of TLR-4 and produce more TNF-α than do their female counterparts—both constitutive and LPS-inducible expression (Aomatsu et al. 2013). Because TLR-4 expression is greater on immune cells from males than females, stimulation with LPS results in greater proinflammatory cytokine production by male immune cells, which can be reversed by removal of androgens in male rodents (Rettew et al. 2008). The sex differential expression of pattern recognition receptors, such as TLRs, is crucial for interpreting sex-specific activity of innate immune cells following stimulation. However, females have the ability to clear bacterial infections more efficiently than do their male counterparts (Fischer et al. 2015). In part, this is due to the increased phagocytic activity of neutrophils and macrophages in females versus males (Spitzer 1999).
Since the immune response has many aspects that sex differences were shown to affect, see the most recent comprehensive review on this topic (Klein and Flanagan 2016). Herein, we focus on 2 aspects where current evidence supports sexual dimorphism during oral bacterial infectious disease: neutrophil trafficking and osteoclastogenesis. Sex differences of other inflammatory mediators during oral infections are described in the Appendix. Here, we include recent information on hormonal control of interleukin 1β (IL-1β) in oral infections, with sex differences in the complement system. We highlight information on sex differences in eicosanoid expression and sex-dependent gene regulation through genomic imprinting and research opportunities relative to sex differences with many of these mediators in oral disease.
Sex Differences in Chemokine Biology Associated with Neutrophil Trafficking
In general, oral infectious diseases, including periodontal diseases and endodontic infections, lead to the destruction of the alveolar bone and surrounding tissues that support the dentition. The current paradigm of periodontal and endodontic bone loss supports the predominate role of host inflammatory response for tissue destruction (Kirkwood et al. 2007; Graves et al. 2011). Large epidemiologic studies indicate that males have a higher degree of periodontal disease susceptibility and progression/severity than do females after controlling for all major covariates (Shiau and Reynolds 2010a). During periodontal and periapical disease initiation, neutrophils are critical meditators of inflammation and are the first cells to migrate to the local site of infection (Graves et al. 2011; Mantovani et al. 2011). Once at the infected site, neutrophils function to secret proinflammatory cytokines and reactive oxygen species to clear the pathogen and perpetuate the host immune response (Mantovani et al. 2011). Moreover, resolution of inflammation via downregulation of neutrophils can be a key determinant between a normal immune response and pathogenicity. One of the key aspects of bacterial-driven bone loss is persistent inflammation driven by infiltrating leukocytes in response to infection.
Sex differences in the neutrophil recruitment and chemokine expression between male and female mice were observed in periodontal and periapical infection models (Scotland et al. 2011; Valerio et al. 2017). Figure 1 shows that in response to oral commensal bacterial infection, a large number of infiltrating cells were positive for a neutrophil-specific marker (Ly6G) in a sex-dependent manner (Valerio et al. 2017). Numerous chemoattractants at the site of inflammation in vivo suggested that overlapping signals govern neutrophil recruitment. However, studies from mouse models indicated that these signals collaborate temporally and spatially to ensure that innate immune cells faithfully respond and reach the site of infection (Sadik et al. 2011). Neutrophil release from the bone marrow is a rapid way to increase the number of circulating neutrophils to be recruited into tissues in response to infection. Therefore, neutrophil egress represents a critical step in response to periodontal pathogens and periodontal disease progression. Chemokines control the retention and egress from the bone marrow. The CXC chemokine receptor 4 (CXCR4) and its ligand SDF-1 (stromal cell–derived factor 1; or CXCL12) are involved in the trafficking of leukocytes into and out of extravascular tissues. As shown in Figure 2, CXCR4 retains neutrophils, while CXCR2 promotes their egress (Sadik et al. 2011). SDF-1/CXCL12 is the ligand for CXCR4, while KC (CXCL1) and macrophage inflammatory protein 2 (CXCL2) are the CXCR2 ligands. Both these murine CXC-chemokines are genetically similar in function to human IL-8 (Tekamp-Olson et al. 1990). In this system, there is a permanent tug-of-war between CXCR4 and CXCR2 ligands, with the CXCR4 ligands usually winning to promote neutrophil retention in the marrow. During inflammation, granulocyte colony-stimulating factor mobilizes neutrophils by shifting the balance between CXCR4 and CXCR2 ligands (Bajrami et al. 2016). Under the current model of neutrophil egress from the bone marrow reserve, CXCL12 functions as the retention signaling, and upon stimulation, the ratio of CXCR4 to CXCL12 increases in favor of CXCR4, thus promoting egress (Sadik et al. 2011). Patients with periodontal disease had higher levels of CXCL12 in GCF as compared with healthy subjects (Havens et al. 2008). Indeed, the same study found that patient who underwent mechanical therapy demonstrated decreased levels of CXCL12. Immunohistochemical staining showed that CXCL12 and CXCR4 levels were elevated in periodontal disease tissues in humans and in a preclinical model of periodontitis. Scotland et al. (2011) investigated the role of sex and immune cell infiltration in response to acute infection using a peritonitis model. In that study, they noted that the “female” acute inflammatory response was blunted due to reduced neutrophil recruitment as compared with that of matched male mice. Specifically, the authors found that CXCL12 and CXCR4 were significantly increased in female mice versus males, thus preventing egress. Neutrophils associated with oral periapical bacterial infection in mice were evaluated for CXCL12 and CXCR4 expression as well (Valerio et al. 2017). While Cxcl12 mRNA was not different between sexes, Cxcr4 mRNA was reduced by nearly half in males, supporting egress as a reasonable explanation of the observed greater neutrophil infiltration in males in response to oral infection.
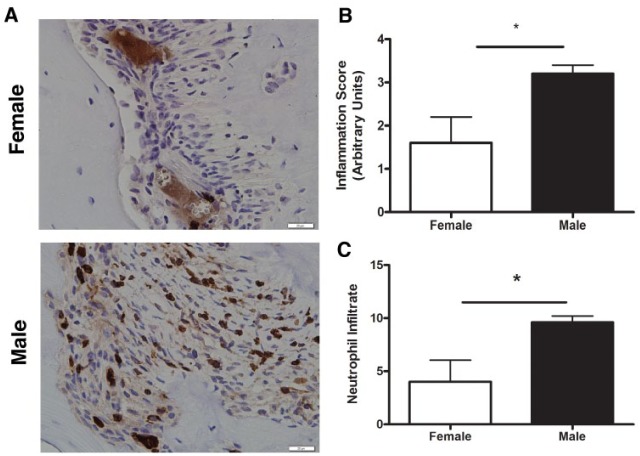
Figure 1.
Neutrophil infiltration and inflammation are greater in males in response to infection. Male and female mice (n = 5 per group) were subject to endodontic surgery by root canal exposure, allowing for colonization of oral bacteria into the periapical region. (A) Inflammatory infiltration was measured with hematoxylin and eosin staining. Neutrophil-specific infiltration was measured with a Ly6G-specific antibody and subsequent immunohistochemical staining. Quantitation of (B) inflammatory score and (C) neutrophil infiltration relative to inflammatory score. Pictures are representative independent observations, and graphic analysis is depicted as mean ± SD of an ordinal pathologic scaling system (i.e., staining scale). B, alveolar bone adjacent to root apex; PA, periapical connective tissue. *P < 0.05. Used with permission (Valerio et al. 2017).
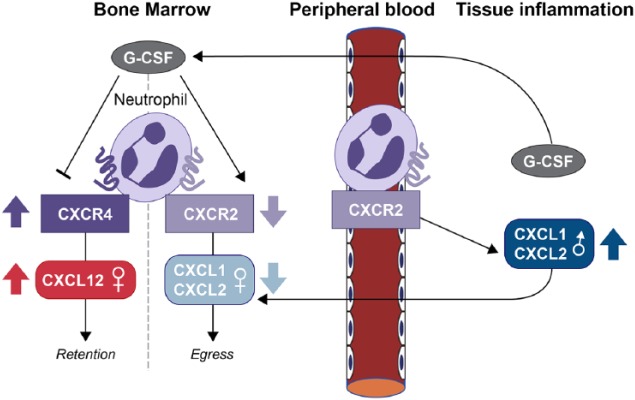
Figure 2.
Regulation of neutrophil egress from the bone marrow by CXCR4 and CXCR2 ligands. CXCR4 ligand SDF-1 (stromal cell–derived factor 1; or CXCL12) acts to retain neutrophils in the bone marrow, whereas CXCR2 ligands KC (CXCL1) and MIP-2 (CXCL2) promote neutrophil egress. Granulocyte colony-stimulating factor (G-CSF) mobilizes neutrophils by altering CXCR4:CXCR2 ratios. Local CXCL1 and G-CSF also increase neutrophil egress into tissues. Sex differences in chemokines are depicted by arrows.
A crucial component of periodontal health is the recruitment of neutrophils to periodontal tissue to maintain homeostasis. In most studies, use of mice for various inflammatory or infectious models of disease highlight the selectivity of chemokine ligands that operate to facilitate and orchestrate a highly specific temporal and tissue-selective neutrophil-homing response (Sadik et al. 2011). However, these studies were primarily under microbial-replete conditions where the immune system functioned to maintain homeostasis simply because it carried a massive microbial burden. Between germ-free mice and specific pathogen–free mice, gingival tissues from CXCR2-deficient mice failed to properly recruit neutrophils to the junctional epithelium, thereby revealing this receptor as the major mechanism of neutrophil transmigration into periodontal tissue. Thus, CXCR2 is required for periodontal homeostasis through neutrophil homing to healthy periodontal tissue (Zenobia et al. 2013). Under inflammatory conditions, chemokines CXCL1 and CXCL2, expressed by tissue cells and other activated neutrophils, engage their receptor CXCR2 and activate neutrophils in an autocrine manner (Sasmono et al. 2007; Tester et al. 2007). In a murine model of Aggregatibacter actinomycetemcomitans oral infection, CXCL1 was the first chemokine to be upregulated and maintained throughout infection (Garlet et al. 2005).
Once recruited to infected tissues, many cytokines and chemokines are released locally to activate these cells, leading to a subsiding infection. Sex-based differential expression of common cytokines and chemokines that regulate neutrophils in response to infection characterizes the cellular infiltrate in response to oral bacterial infection, as explored with gene arrays. Studies with a periapical model of endodontic infection showed that male mice exhibited enhanced bone loss and inflammatory infiltration as compared with females (McAbee et al. 2012). The Table summarizes the key findings from Ly6G+ cells isolated and stimulated with bacterial components following short-term exposures (Valerio et al. 2017). Here, female and male mRNA is expressed in treated cells relative to their own unstimulated controls. These results illustrate that males exhibited a significant increase in chemokines Cxcl1, Cxcl2, Cxcl3, and Cxcl10 (3.26-, 9.8-, 6.5-, and 82.07-fold, respectively). Conversely, males expressed significantly less Cxcr2, Cxcr3, and Cxcr4 (−2.69-, −38.09-, and −3.74-fold, respectively; Valerio et al. 2017). The Table also shows that male neutrophils have enhanced expression of mRNAs encoding IL-1β, IL-6, and TNF-α (5.2-, 3.86-, and 8.54-fold, respectively). Additional data indicate that male neutrophils express significantly more IL-10 (>11-fold) than that of matched females, with iNOS mRNA expression significantly higher in males (>10-fold) than in females (Valerio et al. 2017).
Similar findings regarding sex-based differences in autoimmune and other disease states support that these data are not unique to oral infections (Fan et al. 2014; Wang et al. 2017). Based on these findings, it is likely that the disparity in bone loss observed between sexes may result in part from neutrophil number, egress, or recruitment pools to the site of infection. Additional studies showed sex-specific induction of chemokine CXCL5/CXCL6 that contributes toward sexual dimorphism in neutrophil recruitment in diverse acute inflammatory responses due to increased stimulation and trafficking of bone marrow neutrophils in males (Madalli et al. 2015). Other investigators indicated that neutrophils from the spleen, not the bone marrow, may be a source of sex dimorphism (Kay et al. 2015). Male mice have greater splenic stores of neutrophils, despite similar spleen sizes, thereby signifying another source of potential pathogenic leukocytes. This work demonstrates that males and females have distinct leukocyte-trafficking profiles in inflammation, suggesting that the spleen, not the bone marrow, plays a role in determining sex differences in the available pool of immune cells. Yet another proposed mechanism highlighting the differences in the inflammatory response between the sexes is the presence of proresolving factors in females relative to males (Rathod et al. 2017). These factors were shown to reduce neutrophil activity and thus overall inflammatory burden. To more fully understand how sex differences in neutrophil responses occur, additional studies focused on sex hormones, specific genes, and chromosomes need to be conducted to address regulation of chemokine production and neutrophil trafficking.
Sex Differences in Osteoclastogenesis during Oral Inflammation and Infection
Bone is a dynamic tissue where homeostasis is currently defined as the balance of osteoclast-mediated bone resorption that is tightly coupled with osteoblast-mediated bone formation in the presence of commensal bacteria (Novince et al. 2017). In the absence of a clear bacterial infection, sex differences in skeletal mass and structure are evident; however, the mechanisms governing this process are not completely appreciated. Clearly, sex hormones can regulate bone remodeling in the adult, but there is evidence that sex differences occur before sexual maturity (Callewaert et al. 2010). In addition, the genetic determinants that regulate bone mass acquisition, and the levels of androgens and estrogens do not appear to account for all aspects of skeletal sexual dimorphism (Xiong et al. 2009).
A number of bone-related diseases are characterized by excessive osteolysis, such as osteoporosis and Paget’s disease, where there is an uncoupling of bone resorption and bone formation (Jevon et al. 2002). Although both these diseases are common among the elderly, they have a significant difference in prevalence between the sexes, where osteoporosis is more common in females and Paget’s disease in males. Relative to oral bacterial diseases, males have a higher degree of periodontal disease susceptibility and progression/severity than do females (Shiau and Reynolds 2010a); males also exhibit a higher degree of radiographic signs of periapical infection (Marquis et al. 2006). Indeed, systematic reviews of sex-related differences emerged in relation to periodontitis across regions and nations, which diminishes the possibility that specific extrinsic or environmental factors alone account for differences in disease risk (Shiau and Reynolds 2010a). However, recent data from the from the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions indicated that sex and age differences did not meet significance when clinical attachment loss is used as the key endpoint of measure (Needleman et al. 2018). Thus, sex differences in oral bone loss have been addressed over the past few years, and a brief review of current data is presented here.
While necessary for immune cell recruitment and clearance of the pathogen, the prolonged expression of inflammatory cytokines and chemokines in the oral infectious microenvironment leads to collateral tissue damage and subsequent alveolar bone loss. In fact, multiple studies showed that these same cytokines and chemokines can influence pathogen-driven osteoclastogenesis (Ha et al. 2011; Valerio et al. 2015). Osteoclasts are bone-resorptive cells that form in response to differentiating cytokines, including macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor–κB ligand (RANKL; Boyle et al. 2003; Fig. 3). Osteoclasts are of hematopoietic origin and share the same lineage as macrophages. The phenotype of osteoclast progenitor populations in murine bone marrow was characterized by analyzing their cell surface markers. Bone marrow–derived osteoclasts arise from hematopoietic stem cell progenitor populations late in monocyte differentiation and were distinctly defined as CD45R−GR-1−CD11blo/−CD115+ in mice (Jacquin et al. 2006). The inflammatory periodontal microenvironment is rich in cytokines and chemokines necessary to support bacterial-induced osteoclast formation, resulting in pathogenic bone loss (Lee and Lorenzo 2006). Indeed, it was established that the chemokines CXCL1 and CXCL2 are required to drive LPS-induced osteoclast formation and that blocking their common receptor (CXCR2) blunts pathogenic osteoclast formation (Valerio et al. 2015).
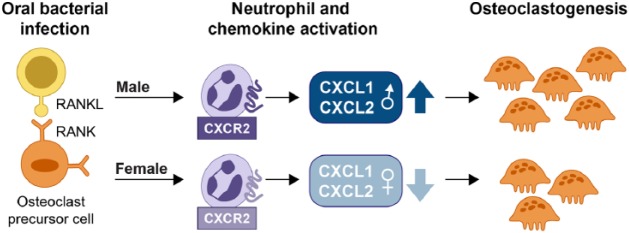
Figure 3.
Sexual dimorphism in osteoclastogenesis. In response to bacterial infection, osteoclast precursor cell populations, initially primed with RANKL/M-CSF, can differentiate into mature osteoclasts in the presence of chemokines, including CXCL1 and CXCL2, as depicted here. Sex-dependent regulation of CXCL1 and CXCL2 contribute toward the differential expansion of osteoclasts. M-CSF, macrophage-colony stimulating factor; RANKL, receptor activator of nuclear factor–κB ligand.
Data support sex differences in osteoclastogenesis and bone-resorptive activity. Osteoclast precursors from PBMCs in males showed a significant enhancement of bone lacunar resorption activity despite having the same numbers of circulating osteoclast precursors as females, based on in vitro assays (Jevon et al. 2002). Osteoclast-specific markers were increased in males versus age-matched females. There were also differences found between sexes depending on the reagents used to generate osteoclasts. Interestingly, male osteoclasts were phenotypically much smaller than female osteoclasts when exposed to LPS; however, they were greater in overall number and enzymatic activity. PBMCs from males were more sensitive to vitamin D exposure than were those of females, and osteoclasts formed in cultures from male PBMCs were more active in response to RANKL exposure (Jevon et al. 2002). These data are consistent with the role of estrogens in females exerting an antiresorptive effect. A more recent study addressed the nature of lacunar resorptive pits formed by osteoclasts from male and female PBMC osteoclast precursors (Merrild et al. 2015). When osteoclasts are seeded onto bone slices to measure form-resorption pits, they either form round pits or long trenches. Pits are thought to correspond to intermittent resorption, whereas trenches represent a faster rate of resorption that requires distinct assembly of the resorption apparatus. In this study, female osteoclasts had more round pits, whereas male osteoclasts formed trenches and had higher collagenolytic activity and expression of osteoclast maturation markers, consistent with deeper demineralization (Merrild et al. 2015). Our group also observed more osteoclasts in male versus female mice in a periapical model of bacteria-induced bone loss (McAbee et al. 2012; Valerio et al. 2017; Fig. 4).
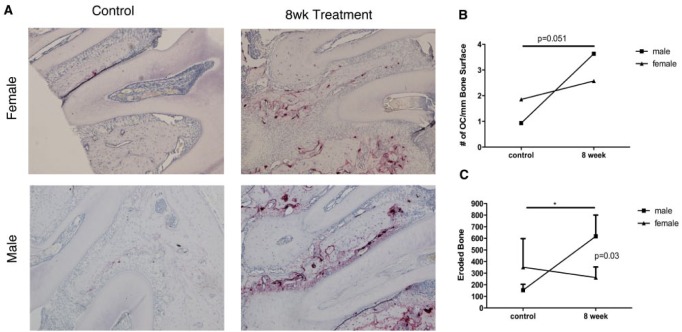
Figure 4.
Osteoclastogenesis and bone degradation are differentially regulated by sex following exposure to bacterial infection. Based on the endodontic model of bacterial infection, male- and female-derived samples were subjected to tartrate-resistant acid phosphatase staining for osteoclast enumeration (arrows) and measurements of eroded bone. (A) Histologic samples from control and mice exposed to bacterial infection for 8 wk. (B) Enumeration of osteoclast lining surface of bone. (C) Quantification of eroded alveolar bone. Data represent at least 5 independent observations per group. B, alveolar bone; R, root. *P < 0.05. Used with permission (Valerio et al. 2017).
Consistent with these in vitro observations, male mice were reported by our group to have significantly more bone loss than that of females in response to preclinical oral bacterial periapical infection (McAbee et al. 2012). In this model, males had an enhanced inflammatory profile and more neutrophil infiltration as compared with females, suggesting sex differences in bacterial-driven osteoclastogenesis. Subsequently, these observations were explored further to understand the role of bacterial-driven neutrophil activity and subsequent osteoclastogenesis between sexes with in vitro models of bacterial-driven osteoclast formation, and they address potential molecular mechanisms that govern sex-dependent susceptibility in oral disease (Valerio et al. 2017).
Initially, it was demonstrated by immunophenotyping that the murine osteoclast progenitor population CD3−CD45R−Gr1− CD11blo/−CD115+ was reduced nearly 50% in males versus females. Interestingly, no other progenitor populations showed any differences. Osteoclastogenesis with defined osteoclast precursor populations (dOCPs) derived from male and female mice was determined via a protocol to mimic the periodontal microenvironment (Valerio et al. 2015). Here, dOCPs were first primed with RANKL/M-CSF and then stimulated with LPS derived from A. actinomycetemcomitans, a periodontal organism associated with an aggressive form of periodontitis. All male populations of dOCPs yielded significantly more osteoclasts than matched female dOCPs. To determine if the increased number of osteoclasts observed in males and increased size observed in females were the result of differences in genes involved in osteoclast formation and fusion, dOCPs were analyzed for expression of specific genes associated with osteoclast differentiation. Nfatc1 (NFATc1) and Tm7sf4 (DCSTAMP) mRNAs were expressed significantly more in males than their female counterparts. Earlier studies with vitamin D and RANKL suggested that other osteoclast-specific markers, including tartrate-resistant acid phosphatase, were increased in male versus female PBMC precursors (Jevon et al. 2002). These studies support the notion of a sexual dimorphism in response to pathogenic infection and indicate that specific osteoclast genes have sexual dimorphic gene expression to explain to phenotypic differences between males and females in oral infectious bone loss. The inherent capacity of dOCPs to differentiate into mature osteoclasts may reside, in part, to sex differences in osteoclast fusion mechanisms. However, additional factors in the inflammatory microenvironment are likely influencing the differences in the size and number of osteoclasts formed between sexes and require further exploration.
Intersection of the Neutrophil and Osteoclast Pathobiology
Sex differences in numerous chemokines and their receptors with cytokines in the bacterial-laden oral bone microenvironment likely contribute to the sex differences observed in periodontal and endodontic infection–related bone loss. Neutrophils differentially produce significantly more chemokines (Table) in males versus females. Given that inherent osteoclast differentiation potential and response to specific chemokines can induce osteoclastogenesis, an intersection between neutrophils and osteoclasts potentially explains the sexual dimorphism in bacterial-based oral diseases that result in oral bone loss.
Table.
Male versus Female Inflammatory Polymerase Chain Reaction Array Summary from Isolated Neutrophils.
| Treatment vs. Control |
|||
|---|---|---|---|
| Gene ID | Function | Female | Male |
| C5ar1 | Neutrophil migration and recruitment | 1.10 | 1.02 |
| Ccl4 | Neutrophil-derived inflammation chemoattractant | −1.53 | 3.06 |
| Ccl5 | Neutrophil activation, increased association with infiltration | 1.34 | 18.40 |
| Ccr1 | Involvement in neutrophil migration | 1.41 | −5.10 |
| Cxcl10 | Neutrophil chemotaxis, binds with Cxcr3 | 1.47 | 82.07 |
| Cxcl11 | Neutrophil chemotaxis, binds with Cxcr3 | 1.15 | 2.08 |
| Cxcl12 | Negative regulation of neutrophil chemotaxis | −1.82 | −3.54 |
| Cxcl1 | Neutrophil chemoattractant, binds to CXCR2 | −2.53 | 3.26 |
| Cxcl2 | Neutrophil chemoattractant, binds to CXCR2 | −3.39 | 9.80 |
| Cxcl3 | Neutrophil chemoattractant | 1.03 | 6.50 |
| Cxcl9 | Associated with CXCL1 function, activated by TLR ligand | 2.18 | 1.68 |
| Cxcr2 | Neutrophil activation, chemotaxis | −1.02 | −2.69 |
| Cxcr3 | Interaction with CXCL10 for chemotaxis | −1.18 | −38.09 |
| Cxcr4 | Negative regulation of neutrophil chemotaxis | 1.11 | −3.74 |
| Cxcr7 | Associated with CXCR4 | −1.89 | 3.96 |
| Il1b | Encodes IL-1β, inflammation cytokine | 1.66 | 5.20 |
| Il6 | Encodes IL-6, inflammation cytokine | 1.30 | 3.86 |
| Itgam | Encodes for CD11b, migration/adhesion | 1.51 | −3.75 |
| Itgb2 | Encodes for CD11c, migration/adhesion | 1.02 | −1.72 |
| Mapk14 | Encodes for p38 MAPK, inflammation signaling | −1.28 | −1.93 |
| Tnf | Encodes for TNF-α, inflammation cytokine | −2.34 | 8.54 |
| Xcl1 | Positive regulator of neutrophil chemotaxis | −1.16 | 2.84 |
mRNA gene expression from male and female murine neutrophils (L6G+) stimulated with 100 ng/mL of Actinobacillus actinomycetemcomitans lipopolysaccharide, 4 h postisolation. Values represent fold change relative to the untreated control from each sex. Increased gene expression >2-fold is presented and is positive. Reduced gene expression <2-fold is presented and is negative.
All the dOCP precursor populations express CXCL1, CXC2, and their cognate receptor CXCR2. LPS induction of these chemokines following priming with M-CSF and RANKL was shown to induced osteoclast formation (Valerio et al. 2015). The involvement of CXCL1 and CXCL2 ligands was verified through the use of the cognate receptor CXCR2 with an antibody to block osteoclastogenesis, as well as with use of individual recombinant chemokine ligands CXCL1 and CXCL2 to induce osteoclast formation. Also, while CXCL1 and CXCL2 were effective in inducing osteoclast formation, CXCL2 was more effective and generated larger osteoclasts. Previous work showed that in females, estrogen reduced CXCL2 expression in monocytes treated with LPS (Rettew et al. 2010). Interestingly, CXCL1 was upregulated in response to LPS but was not differentially regulated by sex. Another study showed that even in response to commensal bacteria, CXCL2 expression was increased, leading to increased neutrophil recruitment even during periodontal homeostasis in health (Zenobia et al. 2013). However, these investigators did not report which sex of mice was used in their studies. Taken together, these data indicate that CXCL2 produced by neutrophils may be key to understanding differences in osteoclast formation and bone loss between sexes.
With respect to observed differences in sexual dimorphism, CXCL10 and CXCR3 expression is most striking (Table). Female neutrophils treated with LPS saw a modest increase in Cxcl10 (6-fold) and decrease in Cxcr3 (3-fold) mRNA levels. In stark contrast, male neutrophils had a sharp reduction in Cxcr3 (−38-fold) and a large increase in Cxcl10 (82-fold) relative to control. These findings are of interest, as the CXCR3-CXCL10 signaling axis was implicated in neutrophil mediated pathogenesis. Importantly, excessive CXCL10 can contribute to osteoclast-driven bone loss by upregulating RANKL (Lee et al. 2011). These data—with previous findings that LPS induces CXCL10, which in turn induces osteoclast formation—suggest that excessive CXCL10 secreted by neutrophils into the inflammatory microenvironment may be a contributor to the excessive bone loss seen in males (Silva et al. 2007). As highlighted, Cxcl10 mRNA is elevated over 82-fold in male versus female neutrophils. Peritoneal macrophages from male mice express higher levels of TLR4 and produce more CXCL10 following LPS stimulation than do macrophages from females (Rettew et al. 2008). Data indicate that CXCR3 and CXCL10 are highly expressed in diseased versus healthy periodontal tissues (Kabashima et al. 2002; Garlet et al. 2003) and associated with higher levels of IFN-γ in these tissues. IFN-γ-producing TH1 lymphocytes are well established in macrophage activation during periodontal disease progression. Indeed, adoptive transfer of Th1 lymphocytes is sufficient for induction of alveolar bone loss (Kawai et al. 2000). In addition, Th1 is the predominate phenotype in aggressive periodontitis and in experimental models of periodontal disease (Garlet et al. 2003; Garlet et al. 2004; Garlet et al. 2005; Garlet et al. 2006; Silva et al. 2007). In contrast, Th2 lymphocytes producing anti-inflammatory cytokines IL-4, IL-10, and IL-13 can attenuate periodontal disease destruction (Onoe et al. 1996; Sasaki et al. 2000; Pestka et al. 2004).
In conclusion, we provide a collection of recent evidence in this review to support the concept that in response to oral infection, male neutrophils can more easily egress to the sight of infection and produce greater amounts of inflammatory cytokines and chemokines to preferentially activate infiltration of specific osteoclast precursor populations, present or as they enter the microenvironment, thereby creating more osteoclasts when compared with those of females. Together, these findings highlight that key chemokine ligands are differentially regulated by sex and could be studied further for targeted therapies to reduce inflammation and inflammatory-driven bone loss in oral bacterial diseases.
Author Contributions
M.S. Valerio, contributed to conception, design, and data analysis, critically revised the manuscript; K.L. Kirkwood, contributed to conception, design, and data analysis, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518798825 for Sexual Dimorphism in Immunity to Oral Bacterial Diseases: Intersection of Neutrophil and Osteoclast Pathobiology by M.S. Valerio and K.L. Kirkwood in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported in part by the National Institutes of Health (grant 1R21 DE027017-01 to K.L.K.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aomatsu M, Kato T, Kasahara E, Kitagawa S. 2013. Gender difference in tumor necrosis factor-alpha production in human neutrophils stimulated by lipopolysaccharide and interferon-gamma. Biochem Biophys Res Commun. 441(1):220–225. [DOI] [PubMed] [Google Scholar]
- Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. 2001. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 16(5):340–343. [DOI] [PubMed] [Google Scholar]
- Bajrami B, Zhu H, Kwak HJ, Mondal S, Hou Q, Geng G, Karatepe K, Zhang YC, Nombela-Arrieta C, Park SY, et al. 2016. G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling. J Exp Med. 213(10):1999–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. 2003. Osteoclast differentiation and activation. Nature. 423(6937):337–342. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. 2010. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol. 207(2):127–134. [DOI] [PubMed] [Google Scholar]
- Clayton JA. 2016. Studying both sexes: a guiding principle for biomedicine. FASEB J. 30(2):519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Tabak LA. 2014. NIH plans to enhance reproducibility. Nature. 505(7485):612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Dong G, Zhao G, Liu F, Yao G, Zhu Y, Hou Y. 2014. Gender differences of B cell signature in healthy subjects underlie disparities in incidence and course of sle related to estrogen. J Immunol Res. 2014:814598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Jung N, Robinson N, Lehmann C. 2015. Sex differences in immune responses to infectious diseases. Infection. 43(4):399–403. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Avila-Campos MJ, Milanezi CM, Ferreira BR, Silva JS. 2005. Actinobacillus actinomycetemcomitans–induced periodontal disease in mice: patterns of cytokine, chemokine, and chemokine receptor expression and leukocyte migration. Microbes Infect. 7(4):738–747. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, Silva JS. 2006. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 21(1):12–20. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Martins W, Jr, Fonseca BA, Ferreira BR, Silva JS. 2004. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 31(8):671–679. [DOI] [PubMed] [Google Scholar]
- Garlet GP, Martins W, Jr, Ferreira BR, Milanezi CM, Silva JS. 2003. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 38(2):210–217. [DOI] [PubMed] [Google Scholar]
- Graves DT, Oates T, Garlet GP. 2011. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J Oral Microbiol. 3. doi: 10.3402/jom.v3i0.5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Lee Y, Kim HH. 2011. CXCL2 mediates lipopolysaccharide-induced osteoclastogenesis in RANKL-primed precursors. Cytokine. 55(1):48–55. [DOI] [PubMed] [Google Scholar]
- Hannah MF, Bajic VB, Klein SL. 2008. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav Immun. 22(4):503-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens AM, Chiu E, Taba M, Wang J, Shiozawa Y, Jung Y, Taichman LS, D’Silva NJ, Gopalakrishnan R, Wang C, et al. 2008. Stromal-derived factor-1alpha (CXCL12) levels increase in periodontal disease. J Periodontol. 79(5):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin C, Gran DE, Lee SK, Lorenzo JA, Aguila HL. 2006. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone Miner Res. 21(1):67–77. [DOI] [PubMed] [Google Scholar]
- Jevon M, Sabokbar A, Fujikawa Y, Hirayama T, Neale SD, Wass J, Athanasou NA. 2002. Gender- and age-related differences in osteoclast formation from circulating precursors. J Endocrinol. 172(3):673–681. [DOI] [PubMed] [Google Scholar]
- Kabashima H, Yoneda M, Nagata K, Hirofuji T, Maeda K. 2002. The presence of chemokine (MCP-1, MIP-1alpha, MIP-1beta, IP-10, RANTES)–positive cells and chemokine receptor (CCR5, CXCR3)–positive cells in inflamed human gingival tissues. Cytokine. 20(2):70–77. [DOI] [PubMed] [Google Scholar]
- Kawai T, Eisen-Lev R, Seki M, Eastcott JW, Wilson ME, Taubman MA. 2000. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 164(4):2102–2109. [DOI] [PubMed] [Google Scholar]
- Kay E, Gomez-Garcia L, Woodfin A, Scotland RS, Whiteford JR. 2015. Sexual dimorphisms in leukocyte trafficking in a mouse peritonitis model. J Leukoc Biol. 98(5):805–817. [DOI] [PubMed] [Google Scholar]
- Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. 2005. Sex differences in the myocardial inflammatory response to acute injury. Shock. 23(1):1–10. [DOI] [PubMed] [Google Scholar]
- Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. 2007. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 43:294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol. 16(10):626–638. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. 2010. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 10(5):338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Seo M, Juhnn YS, Kim JY, Hong YJ, Lee YJ, Lee EB, Song YW. 2011. Potential role and mechanism of IFN-gamma inducible protein-10 on receptor activator of nuclear factor kappa-B ligand (RANKL) expression in rheumatoid arthritis. Arthritis Res Ther. 13(3):R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Lorenzo J. 2006. Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol. 18(4):411–418. [DOI] [PubMed] [Google Scholar]
- Madalli S, Beyrau M, Whiteford J, Duchene J, Singh Nandhra I, Patel NS, Motwani MP, Gilroy DW, Thiemermann C, Nourshargh S, et al. 2015. Sex-specific regulation of chemokine CXCL5/6 controls neutrophil recruitment and tissue injury in acute inflammatory states. Biol Sex Differ. 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 11(8):519–531. [DOI] [PubMed] [Google Scholar]
- Marquis VL, Dao T, Farzaneh M, Abitbol S, Friedman S. 2006. Treatment outcome in endodontics: the Toronto study. Phase III: initial treatment. J Endod. 32(4):299–306. [DOI] [PubMed] [Google Scholar]
- McAbee J, Li Q, Yu H, Kirkwood KL. 2012. Sexual dimorphism in periapical inflammation and bone loss from mitogen-activated protein kinase phosphatase-1 deficient mice. J Endod. 38(8):1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrild DM, Pirapaharan DC, Andreasen CM, Kjærsgaard-Andersen P, Møller AM, Ding M, Delaissé JM, Søe K. 2015. Pit- and trench-forming osteoclasts: a distinction that matters. Bone Res. 3:15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC. 2002. Sexual dimorphism in innate immunity. Arthritis Rheum. 46(1):250–258. [DOI] [PubMed] [Google Scholar]
- Needleman I, Garcia R, Gkranias N, Kirkwood KL, Kocher T, Iorio AD, Moreno F, Petrie A. 2018. Mean annual attachment, bone level, and tooth loss: a systematic review. J Periodontol. 89 Suppl 1:S120–S139. [DOI] [PubMed] [Google Scholar]
- Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, Steinkamp HM, Kirkwood KA, Huang E, Westwater C, et al. 2017. Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci Rep. 7(1):5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen QR, Seo H, Ohta H, Nozawa S, Kudo I, et al. 1996. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase-2-dependent prostaglandin synthesis in osteoblasts. J Immunol. 156(2):758–764. [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. 2004. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 22:929–979. [DOI] [PubMed] [Google Scholar]
- Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, Van Eijl S, Gee LC, Bansal J, Pitrola K, et al. 2017. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. 127(1):169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettew JA, Huet-Hudson YM, Marriott I. 2008. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 78(3):432–437. [DOI] [PubMed] [Google Scholar]
- Rettew JA, Marriot I, Huet YM. 2010. Sex differences in innate immune responses to bacterial pathogens. In: Roberts C, Klein SL. editors. Sex hormones and immunity to infection.New York (NY): Springer; p. 123–146. [Google Scholar]
- Sadik CD, Kim ND, Luster AD. 2011. Neutrophils cascading their way to inflammation. Trends Immunol. 32(10):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, Stashenko P. 2000. Il-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 165(7):3626–3630. [DOI] [PubMed] [Google Scholar]
- Sasmono RT, Ehrnsperger A, Cronau SL, Ravasi T, Kandane R, Hickey MJ, Cook AD, Himes SR, Hamilton JA, Hume DA. 2007. Mouse neutrophilic granulocytes express mrna encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol. 82(1):111–123. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. 2011. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 118(22):5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau HJ, Reynolds MA. 2010. a. Sex differences in destructive periodontal disease: a systematic review. J Periodontol. 81(10):1379–1389. [DOI] [PubMed] [Google Scholar]
- Shiau HJ, Reynolds MA. 2010. b. Sex differences in destructive periodontal disease: exploring the biologic basis. J Periodontol. 81(11):1505–1517. [DOI] [PubMed] [Google Scholar]
- Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. 2007. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 86(4):306–319. [DOI] [PubMed] [Google Scholar]
- Spitzer JA. 1999. Gender differences in some host defense mechanisms. Lupus. 8(5):380–383. [DOI] [PubMed] [Google Scholar]
- Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. 1990. Cloning and characterization of cdnas for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 172(3):911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester AM, Cox JH, Connor AR, Starr AE, Dean RA, Puente XS, Lopez-Otin C, Overall CM. 2007. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PloS One. 2(3):e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio MS, Basilakos DS, Kirkpatrick JE, Chavez M, Hathaway-Schrader J, Herbert BA, Kirkwood KL. 2017. Sex-based differential regulation of bacterial-induced bone resorption. J Periodontal Res. 52(3):377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio MS, Herbert BA, Basilakos DS, Browne C, Yu H, Kirkwood KL. 2015. Critical role of MKP-1 in lipopolysaccharide-induced osteoclast formation through CXCL1 and CXCL2. Cytokine. 71(1):71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Ellis JJ, Pennisi DJ, Song X, Batra J, Hollis K, Bradbury LA, Li Z, Kenna TJ, Brown MA. 2017. Transcriptome analysis of ankylosing spondylitis patients before and after TNF-alpha inhibitor therapy reveals the pathways affected. Genes Immun. 18(3):184–190. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Jiao Y, Hasty KA, Canale ST, Stuart JM, Beamer WG, Deng HW, Baylink D, Gu W. 2009. Quantitative trait loci, genes, and polymorphisms that regulate bone mineral density in mouse. Genomics. 93(5):401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenobia C, Luo XL, Hashim A, Abe T, Jin L, Chang Y, Jin ZC, Sun JX, Hajishengallis G, Curtis MA, et al. 2013. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 15(8):1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518798825 for Sexual Dimorphism in Immunity to Oral Bacterial Diseases: Intersection of Neutrophil and Osteoclast Pathobiology by M.S. Valerio and K.L. Kirkwood in Journal of Dental Research