Abstract
The research objectives of this study are to analyse the volatile compositions of different basil types available in Thai markets and to descriptively determine their aromatic qualities. Essential oils were hydro-distillated from fresh leaves of two Holy basil (Ocimum sanctum) varieties namely, white and red and other basil species, including Tree basil (O. gratissimum), Thai basil (O. basilicum var. thyrsiflorum), and Lemon basil (O. citriodorum). Oil physiochemical characteristics and volatile chromatograms from Gas Chromatography–Mass Spectrometry (GC-MS) were used to qualitatively and quantitatively describe the chemical compositions. Estragole, eugenol, and methyl eugenol were among the major volatiles found in the essential oils of these basil types. Classification by Principal Component Analysis (PCA) advised that these Ocimum spp. samples are grouped based on either the distinctive anise, citrus aroma (estragole, geranial and neral), or spice-like aroma (methyl eugenol, β-caryophyllene, and α-cubebene). The essential oils were also used for descriptive sensorial determination by five semi-trained panellists, using the following developed terms: anise, citrus, herb, spice, sweet, and woody. The panellists were able to differentiate essential oils of white Holy basil from red Holy basil based on the intensity of the anisic attribute, while the anise and citrus scents were detected as dominant in the Lemon basil, Tree basil, and Thai basil essential oils. The overall benefit from this research was the elucidation of aromatic qualities from Thai common Ocimum species in order to assess their potential as the raw materials for new food products.
Keywords: Ocimum spp., essential oil, aromatic profiles, Thai food
1. Introduction
The genus Ocimum (belonging to the Lamiaceae which is recognised as the richest essential oil-bearing plant family) is represented by more than 150 species that are grown widely and distributed throughout tropical and temperate regions [1]. They are collectively known as the “basils” which are in commercial demand for their nutritional, aromatic, ornamental, culinary, religious, and medicinal importance [2]. Among those, Holy basil (O. sanctum), Sweet or Thai basil (O. basilicum), Lemon basil (O. citriodorum), and Tree basil (O. gratissimum) are frequently cultivated in several countries of South and South-East Asia including Thailand as culinary herbs [3,4]. There has been an increasing concern on liable health problems associated with synthetic food flavouring agents. Therefore, food researchers are focusing on the search for natural products that could replace chemically synthetised food additives [5,6].
Essential oil, as well as any other plant based natural ingredients, have been the beneficiary of legal, regulatory, and consumer preference as the result of a shared opinion on food safety [6,7]. Recent demands are for their essential oils, not only for medicinal industries but also for food industries [1,8,9,10]. Thus, it has come as no surprise that food suppliers of ethnic food ingredients quest for authentic Thai basils essential oil. However, the diversity of basil varieties, available as raw material for essential oil extraction, may lead to the confusion of basil aroma for Thai food. Varietal variations could also affect levels of volatile ingredients and profiles, thereby illustrating their distinctively sensorial aroma. Thai basils of different genotypes could be well differentiated on the basis of both essential oil compositions and sensory properties [11,12,13]. Within the genus Ocimum, the major constituents of essential oils are also diverse. From plants grown under the same condition, O. basilicum oil consisted of linalool with other constituents being camphor and humulene which gave the sweet-subtle aroma to its essential oil, while O. sanctum comprised a higher content of chavicol, eugenol, and eucalyptol [14]. None the less, there is limited data on aromatic profiles particularly of descriptive sensory analysis among five basil types available in Thailand viz., white and red Holy basil, Thai basil, Lemon basil, and Tree basil. The aim of this study is therefore to describe the aromatic identities of common Thai basils by chemical and sensory analyses.
2. Materials and Methods
2.1. Plant Material
To obtain reliable sources of material, the aerial parts of different stages were collected from five Ocimum spp. types viz., O. sanctum var. Rama (red), O. sanctum var. Shyama (white), O. citriodorum, O. basilicum var. thyrsiflorum, and O. gratissimum (100 plants/type) at their flowering stages. Plants were grown at Mae-Hia Agricultural Training and Research Centre, Division of Horticulture, Faculty of Agriculture, Chiang Mai University in an open-area (100 × 2500 cm plot with a plant spacing of 50 cm2) and maintained by watering for 2 h, 3 times per week using drip irrigation, and fertilising (Urea (46-0-0) and NPK fertiliser (15-15-15) in the ratio 1:3) once a month until flowering. After collection, plant specimens (leaf and floral specimens) were separated and their morphological appearances were recorded prior to sending them to the Department of Biology, Faculty of Science, Chiang Mai University for taxonomic confirmation.
2.2. Essential Oil Extraction
At the laboratory, leaf tissues were parted from the stems and inflorescence. Fresh leaves (~300 g) were used for essential oil extraction in a 5 L Clevenger hydro-distillation apparatus containing 2.5 L of distilled water and the extraction was for 2 h at 150 °C (MTopo®, heating mantle, Korea). After cooling to room temperature, the essential oil was collected and treated with anhydrous sodium sulphate to remove the remaining water. The yields were averaged from three separate extractions and calculated according to fresh weight of plant material [15].
2.3. Physical Characteristics of Essential Oil
Colour of the essential oil was determined by physical observation in day light and under ultraviolet radiation using an ultra-violet chamber [16]. The essential oil from water distillation was dissolved in methanol (10% v/v) and scanned for the absorbance with the wavelengths ranging from 220 to 500 nm by an UV-visible spectrophotometer (SPECTROstar Nano; BMG LABTECH, Offenburg, Germany) [17].
2.4. Gas Chromatography–Mass Spectrometry (GC-MS)
The Gas Chromatography–Mass Spectrometry (GC-MS) analyses were accomplished with a Bruker-scion 436 GC-MS equipped with 30 m × 0.25 mm, Rxi-5Sil MS column (Restek, Bellefonte, PA, USA). Essential oil samples (2 µL at the dilution of 1%, v/v, in dichloromethane with a presence of 0.003% w/v toluene as an internal standard) were injected in a split mode (1:20). The oven temperature was set at 60 °C for 3 min, and increased by 2.5 °C/min until 240 °C where it was held at this temperature for 10 min. The carrier gas was helium with a flow rate of 1.1 mL /min. The interface with MS was at 200 °C and mass spectra were taken at 70 eV in electron impact ionisation mode, with a scanning speed of 0.5 scans/s from m/z 20–350 [18]. The standard solution of C8–C20 n-alkane (Fluka® Analytical, Munich, Germany) in hexane was also used for the calculation of retention indices (RI) [19]. The identification of the volatile compositions was by comparison with mass spectra in NIST 05.L and NIST 98.L libraries with >70% similarity. The compounds were confirmed by their RI as well as those from the literature [11]. The amount in µg/mL of essential oil was calculated as relative to that of internal standard.
2.5. Descriptive Analysis
Prior to sensory testing, five semi-trained panellists with extensive experience in Thai food (two males, three females, and age ranged from 25 to 37 years old) were chosen based on their abilities to discriminate differences and ranking/rating the intensity scents. They also completed a 3-day orientation and a 12 h descriptive training course. Thereafter, they then developed attribute terms describing the odour of five essential oils on cotton balls (200 µL). Panellists tasted all samples and discussed the attribute definitions, attribute references, reference intensities, and evaluation procedures. References were consistent with those of the previous studies as described in Table 1, although some additional terms (anise, herb, and woody) were added.
Table 1.
Attributes and references used in evaluating five essential oil odour.
| Odour Attributes | Reference Standard | n/15 | Reference |
|---|---|---|---|
| Anise | anise powder, 2 g | 10/15 | * |
| Citrus | lemon extract (McCormick), 200 µL | 8/15 | [21] |
| Herb | thyme (McCormick), 0.5 g | 10/15 | * |
| Spice | ground allspice (McCormick), 0.5 g | 8/15 | [22] |
| Sweet | vanilla flavour (McCormick), 200 µL | 10/15 | [23] |
| Woody | peanut peel 2 g with 100 µL DI water | 7/15 | * |
* Added terms from the panellists.
At the day of testing, the essential oil (200 µL oil pipetted on clean and deodourised cotton ball) and reference attributes were presented in front of the same group of the panellists. They then evaluated the intensity of the given attributes in triplicates on the 15-point interval scale (0 = none, 15 = extra strong) [20]. Clean air was obtained between each assessment. A gap of 20 s was sufficient to the individual odour assessments.
2.6. Statistical Analysis
Differences of the descriptive analysis data was determined using Analysis of Variance (ANOVA) by SPSS (IBM, Armonk, NY, USA) with a significance level of 0.05. Principal Component Analysis (PCA) was used to summarise graphical differences of volatile components of the essential oil and the descriptive data among the Ocimum spp. using XLSTAT ver. 2018.5 (New York, NY, USA).
3. Results and Discussion
3.1. Plant Identification and Physiochemical Characteristics of the Essential Oils
Basils are widely distributed in tropical areas and are likely to have originated in South Asia (India). These herbaceous plants are of annual type, usually propagated through seeds [4]. The genus of Ocimum comprises of more than 65 species and is the biggest genera in Lamiaceae family worldwide [24]. Different basil species can be identified by morphological characterisations such as leaf shape and its colour, flower structures and its colour, seed structures and its characteristics (Table 2). However, due to extensive cultivation, inter and intra-specific cross hybridisation has occurred leading to polyploidy and different numbers of species, subspecies, and varieties that are not significantly different in their appearances [24]. In our study, five Ocimum spp. types had distinct morphological characteristics. The O. sanctum of white and red varieties (viz., Rama and Shyama) possessed different leaf colours. O. citriodorum and O. basilicum var. thyrsiflorum illustrated unique seed characteristic which was mucilaginous after soaking in water. O. gratissimum possessed a large leaf size about 45 cm2 while O. citriodorum conferred leaf size around 3.5 cm2.
Table 2.
Morphology of five Ocimum spp. and physiochemical characteristics of their essential oils.
| Characteristics | LB | RB | ThB | TrB | WB |
|---|---|---|---|---|---|
| Plant morphology | |||||
| General | 50–105 cm tall
|
70–150 cm tall
|
45–100 cm tall
|
140–200 cm tall
|
70–160 cm tall
|
| Leaf structure | leaf size ~3.5 × 1 cm, leaf elliptic-broadly obovate, glabrous except hairy midrib, veinlets and margin | leaf size ~4 × 1.5 cm, ovate-obovate, elliptic-oblong, surface patently hairy to clothed with soft spreading hair, Purple leaf | leaf size ~5.5 × 2 cm, leaf ovate-lanceolate to oblong-lanceolate, glabrous except hairy midrib, veinlets and margin | leaf size ~9 × 5 cm, leaf lanceolate, ovate or ovate-lanceolate, glabrous except hairy midrib | leaf size ~4 × 1.5 cm, leaf ovate-obovate, elliptic-oblong, surface patently hairy to clothed with soft spreading hair, green leaf |
| Inflorescence and floral structure | inflorescence greenish, flowers white, calyx green, long hairy | inflorescence purple, flowers purplish, calyx purple, patently hairy to densely pubescent | inflorescence greenish, flowers whitish pink, calyx green, long hairy | inflorescence greenish purple, flowers yellowish white, calyx greenish purple, hairy | inflorescence green-greenish purple, flowers purplish, calyx green, patently hairy to densely pubescent |
| Seed characteristics | seed brownish black, ellipsoid, mucilaginous | seed brown, globose, non-mucilaginous | seed brownish black, ellipsoid, mucilaginous | seed brown, subglobose, non-mucilaginous | seed brown, globose, non-mucilaginous |
| Physiochemical Characteristics of essential oils | |||||
| Yield *,** | 0.37% ± 0.12 b | 0.33% ± 0.06 b | 0.43% ± 0.09 c | 0.19% ± 0.05 a | 0.47% ± 0.09 c |
| Colour under day light | yellow | clear | yellow | orange | clear |
| Colour under UV light *** | + | +++ | ++ | ++ | +++ |
LB = Lemon basil (O. citriodorum); RB = red Holy basil (O. sanctum var. Shyama); ThB = Thai basil (O. basilicum var. thyrsiflorum); TrB = Tree basil (O. gratissimum); WB = white Holy basil (O. sanctum var. Rama); * Values are mean ± SE (standard error) of 3 replications. ** Values followed by the different superscript letters (a–c) are significantly different at p = 0.05. *** + = degree of UV light reflection intensity.
To obtain the essential oil, fresh basil leaves were extracted by hydro-distillation and the physiochemical characteristics were shown in Table 2. White Holy basil (O. sanctum var. Rama) and Thai basil (O. basilicum var. thyrsiflorum) illustrated maximum yield ~0.4%, Lemon basil (O. citriodorum) and red Holy basil (O. sanctum var. Shyama) yielded the essential oil of ~0.33%. However, Tree basil (O. gratissimum) gave the least essential oil content (<~0.2%). The colour (orange, yellow, and colourless) of essential oils under day light was different from species to species (O. gratissimum, O. citriodorum, O. sanctum, and O. basilicum var. thyrsiflorum) but not from variety to variety (O. sanctum var. Rama and Shyama). Variations in essential oil colours not only depends upon taxonomical characteristics but also relies on age of the plants as well as time of harvesting and different extraction techniques [25,26,27]. Siddique et al. [27] suggested that the chemical compositions of essential oil depend largely on its colour. Moreover, the alteration of the essential oil colour as a result of their compositions is suggested to be due to thermal degradation, oxidation, isomerisation, dehydrogenation and polymerisation [17,27,28,29].
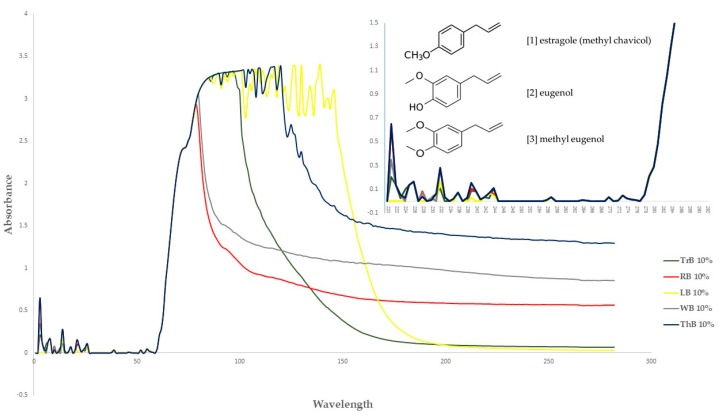
Essential oils with different chemical compositions can absorb UV light at different wavelength therefore, illustrating variation of light reflection intensity [30,31]. In our experiment, white and red Holy basils essential oils reflected high UV light intensity, followed by moderate reflection intensity (Tree and Thai basils) and low reflection intensity (Lemon basil) (Table 2). These were confirmed by absorbance spectrum patterns under various UV-Visible wavelengths (220–500 nm) (Figure 1). The active chemical components of essential oil from Ocimum spp. plants are estragole (methyl chavicol), eugenol, and methyl eugenol [11,32,33]. Dighe et al. [34] advised that UV spectrum of eugenol gave the maximum absorption at 220–230 nm and a smaller peak at 278 nm which agree with our results. However, we found the tiny peaks at 269–275 instead of 278 nm (Figure 1).
Figure 1.
UV-Visible spectra of the essential oils from five Ocimum spp. The insertion is the inset evidences of the peaks between 220–280 nm and chemical structures of (1) estragole, (2) eugenol, (3) methyl eugenol. The essential oil was diluted in methanol. LB = Lemon basil (O. citriodorum); RB = red Holy basil (O. sanctum var. Shyama); ThB = Thai basil (O. basilicum var. thyrsiflorum); TrB = Tree basil (O. gratissimum); WB = white Holy basil (O. sanctum var. Rama).
3.2. Chemical Profile of the Essential Oil
The concentrations and the calculated retention indices of volatile compounds from the five Ocimum spp. essential oils are given in Table 3. Sixty-seven compounds were identified. The major components of essential oil from Lemon basil (O. citriodorum) volatile oil were estragole (methylchavicol) (98.22 µg/mL), citral (9.55 µg/mL), and neral (6.32 µg/mL). The principle components of red Holy basil (O. sanctum var. Shyama) were methyl eugenol (683.90 µg/mL), β-caryophyllene (145.81 µg/mL), and α-cubebene (104.70 µg/mL), and for Thai basil (O. basilicum var. thyrsiflorum) were estragole (methyl chavicol) (452.80 µg/mL), geranial (180.57 µg/mL), and neral (151.16 µg/mL). The main compositions of Tree basil (O. gratissimum) were eugenol (408.00 µg/mL), α-ocimene (256.45 µg/mL), and γ-muurolene (91.57 µg/mL) and in oil of white Holy basil (O. sanctum var. Rama) the major components were methyl eugenol (98.44 µg/mL), α-cubebene (9.94 µg/mL), and α-copaene (4.74 µg/mL).
Table 3.
Chemical compositions of the essential oils from five Ocimum spp. plants.
| No. | Compounds | Retention Index | Retention Index * | Amount of Chemicals | ||||
|---|---|---|---|---|---|---|---|---|
| (µg/mL Essential Oils) ** | ||||||||
| LB | RB | ThB | TrB | WB | ||||
| 1 | methyl 2-methylbutanoate | - | - | nd | nd | nd | 0.89 | nd |
| 2 | 3-hexen-1-ol | 850 | - | nd | nd | nd | 0.79 | nd |
| 3 | α-pinene | 930 | 938 | nd | nd | nd | nd | 0.32 |
| 4 | camphene | 944 | - | nd | nd | nd | nd | 0.42 |
| 5 | β-pinene | 973 | 981 | 1.31 | nd | 2.58 | nd | 0.52 |
| 6 | 1-octen-3-ol | 979 | - | nd | nd | nd | 2.77 | nd |
| 7 | myrcene | 989 | 995 | nd | nd | nd | 5.13 | nd |
| 8 | 1,8-cineole | 1028 | 1034 | 2.82 | nd | 5.64 | nd | nd |
| 9 | α-ocimene | 1049 | 1046 | nd | nd | 13.0 | 257 | nd |
| 10 | z-ocimene | 1051 | - | 5.83 | nd | nd | 17.7 | nd |
| 11 | γ-terpinene | 1058 | - | 0.36 | nd | 2.39 | nd | nd |
| 12 | 3-carene | 1101 | - | nd | 1.76 | 14.61 | 22.3 | nd |
| 13 | linalool | 1104 | 1097 | 1.12 | nd | nd | nd | 1.09 |
| 14 | (4e,6z)-allo-ocimene | 1132 | - | nd | nd | nd | 14.9 | nd |
| 15 | d-camphor | 1145 | 1144 | 1.34 | nd | 3.54 | nd | nd |
| 16 | trans-chrysanthemal | 1152 | - | nd | nd | 2.20 | nd | nd |
| 17 | borneol | 1168 | - | nd | 7.79 | nd | nd | 2.80 |
| 18 | 1,4-heptadiene, 3-methyl- | 1169 | - | nd | nd | 5.54 | nd | nd |
| 19 | terpinen-4-ol | 1180 | - | 1.78 | nd | 15.4 | nd | nd |
| 20 | cyclohexane, ethenyl- | 1189 | - | nd | nd | 6.78 | nd | nd |
| 21 | estragole | 1211 | 1196 | 98.2 | nd | 453 | nd | nd |
| 22 | (r)-α-pinene | 1236 | - | nd | nd | 8.60 | nd | nd |
| 23 | neral | 1251 | 1238 | 6.32 | nd | 151 | nd | nd |
| 24 | (+) -(−)-3-carene | 1264 | - | nd | nd | 6.30 | nd | nd |
| 25 | geranial | 1282 | 1268 | nd | nd | 181 | nd | nd |
| 26 | citral | 1283 | - | 9.55 | nd | nd | nd | nd |
| 27 | eugenol | 1371 | 1361 | nd | nd | nd | 408 | 1.50 |
| 28 | α-copaene | 1379 | - | nd | 20.7 | nd | 16.1 | 4.74 |
| 29 | β-bourbonene | 1388 | 1383 | nd | 7.68 | nd | 1.88 | nd |
| 30 | 4-methylpyrazole | 1389 | - | nd | nd | 1.82 | nd | nd |
| 31 | n-butylpyrrole | 1391 | - | nd | nd | nd | nd | 2.25 |
| 32 | β-cubebene | 1396 | 1389 | nd | 1.98 | nd | 7.11 | nd |
| 33 | β-elemene | 1396 | 1391 | 0.77 | 65.7 | 4.30 | 3.65 | 2.79 |
| 34 | methyl eugenol | 1409 | 1411 | 0.77 | 684 | 2.87 | nd | 98.4 |
| 35 | β-caryophyllene | 1424 | 1420 | 1.34 | 146 | 10.6 | 17.3 | nd |
| 36 | α-bergamotene | 1439 | 1437 | 1.00 | nd | 4.49 | 0.69 | nd |
| 37 | (z,e)-α-farnesene | 1442 | - | nd | nd | nd | 51.6 | nd |
| 38 | α-guaiene | 1443 | 1439 | 0.19 | nd | nd | nd | nd |
| 39 | β-sesquiphellandrene | 1447 | - | nd | nd | nd | 0.59 | nd |
| 40 | α-humulene | 1458 | 1454 | 3.42 | 13.4 | 6.88 | 3.36 | 1.42 |
| 41 | bicyclo sesquiphellandrene | 1468 | - | 2.43 | nd | 10.8 | 0.59 | nd |
| 42 | germacrene d | 1469 | 1482 | 2.26 | 0.55 | 14.3 | 0.89 | 1.23 |
| 43 | γ-muurolene | 1490 | - | nd | nd | nd | 91.6 | nd |
| 44 | α-cubebene | 1490 | - | nd | 105 | nd | nd | 9.94 |
| 45 | bicyclo [3.1.1] hept-3-ene-spiro-2,4′-(1′,3′-dioxane), 7,7-dimethyl- | 1493 | - | nd | 1.87 | nd | nd | nd |
| 46 | bicyclogermacrene | 1500 | 1497 | 0.81 | nd | 3.63 | 3.55 | nd |
| 47 | β-gurjunene | 1501 | - | nd | 6.36 | nd | nd | nd |
| 48 | α-selinene | 1505 | - | nd | nd | nd | nd | 0.40 |
| 49 | α-bulnesene | 1509 | 1506 | 1.06 | nd | 3.92 | nd | nd |
| 50 | α-farnesene | 1511 | - | nd | nd | nd | 42.3 | nd |
| 51 | α-amorphene | 1518 | - | nd | nd | 2.58 | nd | nd |
| 52 | phenylethanolamine | 1519 | - | nd | nd | nd | 0.69 | nd |
| 53 | δ-cadinene | 1525 | 1524 | nd | 8.12 | nd | 11.1 | 0.56 |
| 54 | 1-bromo-8-heptadecyne | 1539 | - | 0.44 | nd | nd | nd | nd |
| 55 | (z)-4-decen-1-ol | 1539 | - | nd | 3.84 | nd | nd | nd |
| 56 | (z)-α-bisabolene | 1546 | 1544 | nd | nd | 11.1 | nd | nd |
| 57 | eremophilene | 1556 | - | nd | 2.52 | nd | nd | nd |
| 58 | elemol | 1557 | - | nd | nd | nd | nd | 0.23 |
| 59 | 4-ethylphenethylamine | 1582 | - | nd | nd | nd | 0.59 | nd |
| 60 | ethyl trichloroacetate | 1582 | - | nd | 1.75 | nd | nd | nd |
| 61 | benzofuran, 7-(2,4-dinitrophenoxy)-3-ethoxy-2,3-dihydro-2,2-dimethyl- | 1590 | - | nd | 0.99 | nd | nd | nd |
| 62 | 1,3-diisopropyl-1,3-cyclopentadiene | 1602 | - | nd | nd | nd | 0.99 | nd |
| 63 | cadina-1,4-diene | 1622 | - | nd | nd | 1.24 | nd | nd |
| 64 | naphthalene, 1,2,3,4,4a,7-hexahydro-1,6-dimethyl-4-(1-methylethyl)- | 1622 | - | 0.31 | nd | nd | nd | nd |
| 65 | bromoacetonitrile | 1650 | - | nd | nd | nd | 0.50 | nd |
| 66 | α-muurolene | 1663 | - | nd | 2.41 | nd | nd | nd |
| 67 | β-bisabolene | 1690 | - | 0.27 | nd | nd | nd | nd |
* Retention index [11]. ** Values are calculated as reference to the internal standard toluene (0.003% w/v); nd = not detected. LB = Lemon basil (O. citriodorum); RB = red Holy basil (O. sanctum var. Shyama); ThB = Thai basil (O. basilicum var. thyrsiflorum); TrB = Tree basil (O. gratissimum); WB = white Holy basil (O. sanctum var. Rama). Values are proportionate to the internal toluene standard (0.003%). The Limit of Detection (LOD) and Limit of Quantitation (LOQ) are calculated from ranges of toluene concentrations (0.0015–0.03%). LOD = 0.083 µg/mL and LOQ = 0.835 µg/mL.
From the literature, three major compounds were generally identified from the essential oil of Ocimum spp., viz. estragole (1), eugenol (2), and methyl eugenol (3) (Figure 1). Estragole (methyl chavicol) (1) is found in O. basilicum [11,13,35,36,37,38] and O. citriodorum [39]. Eugenol (2) is detected in O. basilicum [11,13], O. utricifolium [39], O. sanctum var. green and var. purple [14,40], O. gratissimum [38,41,42] essential oils. Methyl eugenol (3) is the principal composition in O. sanctrm var. Shyama [43], O. uttricifolium [44], O. campechianum Mill. [45] and some varieties of O. basilicum (i.e., var. dark green and var. purple opal) [12]. These results are in line with our work, however, the quantitative compositions of such chemicals may vary. Joshi and Si [46] and Murarikova et al. [12] described that variation in the qualitative and quantitative chemical profiles of the basil essential oil may be due to the variety of plant and their growing conditions such as season, climate or soil conditions. There was also some chemical variability of the essential oil of plants belonging to the Lamiacea family but within the same species, the major constituents can be similar [47,48]. In other plants, the factors being geographical differences and phenological stages of raw materials, such as in Thymus algeriensis [49] and Hypericum spp. [50].
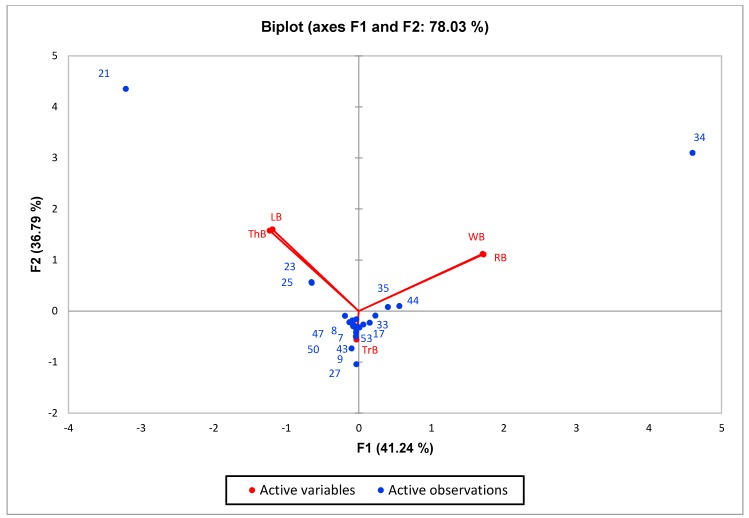
The PCA between the volatile compositions and the essential oil types revealed three major clustering groups (Figure 2). The first group included Thai basil and Lemon basil with the evident aromatic compounds (viz., estragole, geranial, and neral) which represent anise, lime-like, and fresh aroma [51]. The second cluster was of the white Holy basil and red Holy basil group with methyl eugenol, β-caryophyllene, and α-cubebene (herb and spice) as distinctive compounds [52]. White and red Holy basils are also variety related species (O. sanctum). The last group is the Tree basil that did not correlate with any groups described previously.
Figure 2.
Principal Component Analysis (PCA) biplot illustrating the relationships among the chemical components and five Ocimum spp. Odour active compounds of 1–64 correspond to the code compounds in Table 3. LB = Lemon basil (O. citriodorum); RB = red Holy basil (O. sanctum var. Shyama); ThB = Thai basil (O. basilicum var. thyrsiflorum); TrB = Tree basil (O. gratissimum); WB = white Holy basil (O. sanctum var. Rama).
3.3. Sensory Profile of the Essential Oil
Overall sensory scores obtained by the different five Ocimum spp. essential oils are illustrated in Table 4. The intensity (0–15) represent the maximum possible aroma quantity of each identified attributes judged by the panelists. It was observed that the sweet attribute (scores ~2.4–3.4) was not significantly different among all types of the essential oils. The essential oils of Tree basil and Thai basil gave the highest intensity of herb odour (score ~9.0–11.0). Essential oils of white and red Holy basils provided the maximum woody scent (score ~2.2–3.4), while the Tree basil and white Holy basil dominated anisic attribute (score ~5.9–7.4). Tree basil and Thai basil oils illustrated the highest spice aroma intensity (score ~6.4–7.3). The citrus scent was the highest in the oil of Lemon basil with the score of 12.8.
Table 4.
The mean intensity values of the six attributes for the five Ocimum spp. essential oils in descriptive sensory evaluation.
| Basils | Sweet | Herb | Woody | Anise | Citrus | Spice |
|---|---|---|---|---|---|---|
| LB | 3.4 ± 0.24 a | 7.2 ± 0.49 bc | 0.98 ± 0.02 a | 4.72 ± 0.70 bc | 12.8 ± 0.58 d | 5.2 ± 0.66 b |
| RB | 2.6 ± 0.51 a | 5.68 ± 1.14 b | 2.26 ± 0.55 bc | 3.6 ± 0.51 ab | 2.7 ± 0.37 a | 3.5 ± 0.67 a |
| ThB | 2.4 ± 0.24 a | 11.2 ± 0.86 d | 1.6 ± 0.33 ab | 2.34 ± 0.52 a | 10.62 ± 0.69 c | 6.4 ± 0.24 bc |
| TrB | 2.46 ± 0.63 a | 9.2 ± 0.80 cd | 1.8 ± 0.20 ab | 7.4 ± 0.40 d | 8.9 ± 0.56 b | 7.28 ± 0.42 c |
| WB | 2.42 ± 0.50 a | 1.1 ± 0.10 a | 3.34 ± 0.50 c | 5.96 ± 0.87 cd | 4.2 ± 0.37 a | 2.7 ± 0.44 a |
Mean scores (n = 5) for each attribute within a column with different superscript letters (a–d) are significantly different at p = 0.05 using Duncan’s multiple comparison test. LB = Lemon basil (O. citriodorum); RB = red Holy basil (O. sanctum var. Shyama); ThB = Thai basil (O. basilicum var. thyrsiflorum); TrB = Tree basil (O. gratissimum); WB = white Holy basil (O. sanctum var. Rama).
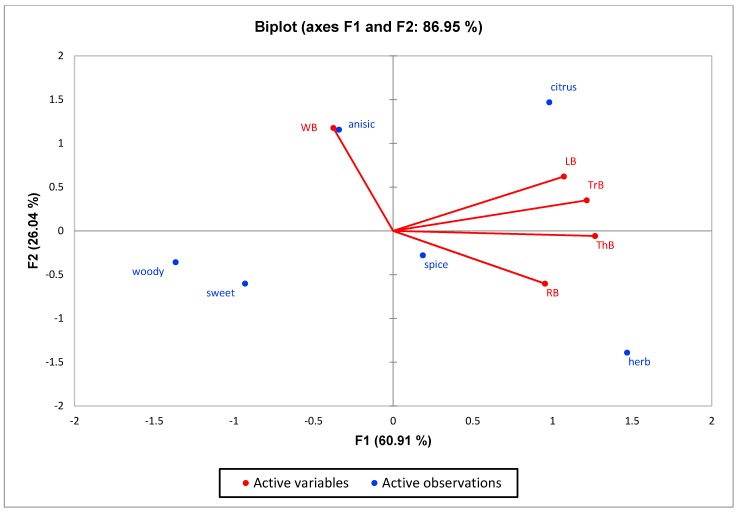
The PCA was able to split aromatic profile of white Holy basil relating with the higher intensity of anisic attribute (Figure 3). However, there was no correlation of woody and sweet attributes across the five essential oil types.
Figure 3.
PCA biplot illustrating the relationships among the odour attributes and five Ocimum spp. LB = Lemon basil (O. citriodorum); RB = red Holy basil (O. sanctum var. Shyama); ThB = Thai basil (O. basilicum var. thyrsiflorum); TrB = Tree basil (O. gratissimum); WB = white Holy basil (O. sanctum var. Rama).
A study on the attribute of essential oil from Ocimum spp. (O. basilicum L.) by Calín-Sánchez et al. [53] evaluated the same attributes that are herbaceous (herb), spice, woody, and sweet. These attributes were also identified in our study. This group of researchers also found that the Sweet basil (O. basilicum) essential oil extracted from the dried leaves, gave stronger sweet and woody attributes than the essential oil extracted from fresh leaves. In our study, the citrus attribute is a principle characteristic of essential oils from most Thai basil types analyses especially the Lemon basil as confirmed by both chemical and sensory evaluations. This is consistent with the previous research by Al-Kateb and Mottram [54] who studied the relationship between growth stages and volatile compositions of Lemon basil (O. citriodorum Vis.). They found that citral, linalool, and estragole are major compounds of the essential oil and they contributed to the citrusy aroma.
4. Conclusions
From this research, we conclude that the major components of essential oils from the five types of basils used as food ingredients in Thailand are estragole, eugenol, and methyl eugenol. From this chemical analysis we can then distinguish Ocimum spp. plants from the odour characteristics into two groups: citrus and spice-like. Sensory analysis also confirmed that citrus is the main feature in these Ocimum spp. essential oils. It is also possible to sensorially separate the aromatic scent of the two Holy basil (var. red and white) essential oils by using anisic attributes. Future research could be extended to the use of the natural products from these plant species in food as additives active against food microbials and in agriculture (i.e., methyl eugenol) as bio-control agent.
Acknowledgments
We would like to especially thanks Ersatz Professor Michael D Burgett for his kind supports and generosity to BAC group and Paul Page for assisting in the volatile identification.
Author Contributions
Conceptualisation, S.R.S.; Methodology, T.T. and S.R.S.; Formal Analysis, T.T.; Investigation, T.T. and S.R.S.; Writing-Original Draft Preparation, T.T.; Writing-Review & Editing, S.R.S. and H.C.; Supervision, S.R.S. and H.C.; Project Administration, T.B.; Funding Acquisition, S.S.
Funding
This research was funded by Thai Research Fund (TRF) under the Industrial-Academic Research Fellowship Program (IRF) and Handharvest, co. Ltd., Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pandey A.K., Singh P., Tripathi N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014;4:682–694. doi: 10.12980/APJTB.4.2014C77. [DOI] [Google Scholar]
- 2.Patel R.P., Singh R., Rao B.R.R., Singh R.R., Srivastava A., Lal R.K. Differential response of genotype×environment on phenology, essential oil yield and quality of natural aroma chemicals of five Ocimum species. Ind. Crops Prod. 2016;87:210–217. doi: 10.1016/j.indcrop.2016.04.001. [DOI] [Google Scholar]
- 3.Juntachote T., Berghofer E., Siebenhandl S., Bauer F. The antioxidative properties of holy basil and galangal in cooked ground pork. Meat Sci. 2006;72:446–456. doi: 10.1016/j.meatsci.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan N., Rawal S., Verma M., Poddar M., Alok S. A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum. Biomed. Prev. Nutr. 2013;3:185–192. doi: 10.1016/j.bionut.2012.08.002. [DOI] [Google Scholar]
- 5.Siripongvutikorn S., Thummaratwasik P., Huang Y.-W. Antimicrobial and antioxidation effects of thai seasoning, tom-yum. LWT—Food Sci. Technol. 2005;38:347–352. doi: 10.1016/j.lwt.2004.06.006. [DOI] [Google Scholar]
- 6.Wandersleben T., Morales E., Burgos-Díaz C., Barahona T., Labra E., Rubilar M., Salvo-Garrido H. Enhancement of functional and nutritional properties of bread using a mix of natural ingredients from novel varieties of flaxseed and lupine. LWT—Food Sci. Technol. 2018;91:48–54. doi: 10.1016/j.lwt.2018.01.029. [DOI] [Google Scholar]
- 7.Burdock G.A., Wang W. Our unrequited love for natural ingredients. Food Chem. Toxicol. 2017;107:37–46. doi: 10.1016/j.fct.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Imen T., Olfa B., Jamel H., Luigi C.P., Mokhtar L., Guido F., Zeineb O. Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to nacl or Na2SO4 salinity. J. Plant Nutr. Soil Sci. 2013;176:748–755. [Google Scholar]
- 9.Kwee E.M., Niemeyer E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011;128:1044–1050. doi: 10.1016/j.foodchem.2011.04.011. [DOI] [Google Scholar]
- 10.Jordán M.J., Quílez M., Luna M.C., Bekhradi F., Sotomayor J.A., Sánchez-Gómez P., Gil M.I. Influence of water stress and storage time on preservation of the fresh volatile profile of three basil genotypes. Food Chem. 2017;221:169–177. doi: 10.1016/j.foodchem.2016.10.059. [DOI] [PubMed] [Google Scholar]
- 11.Bernhardt B., Sipos L., Kókai Z., Gere A., Szabó K., Bernáth J., Sárosi S. Comparison of different Ocimum basilicum L. Gene bank accessions analyzed by GC-MS and sensory profile. Ind. Crops Prod. 2015;67:498–508. doi: 10.1016/j.indcrop.2015.01.013. [DOI] [Google Scholar]
- 12.Muráriková A., Ťažký A., Neugebauerová J., Planková A., Jampílek J., Mučaji P., Mikuš P. Characterization of essential oil composition in different basil species and pot cultures by a GC-MS method. Molecules. 2017;22:1221. doi: 10.3390/molecules22071221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurzyñska-Wierdak R. Morphological variability and essential oil composition of four Ocimum basilicum L. Cultivars. J. Essent. Oil Bear. Pl. 2014;17:112–119. doi: 10.1080/0972060X.2013.855362. [DOI] [Google Scholar]
- 14.Zheljazkov V.D., Cantrell C.L., Evans W.B., Ebelhar M.W., Coker C. Yield and composition of Ocimum basilicum L. and Ocimum sanctum L. grown at four locations. HortScience. 2008;43:737–741. [Google Scholar]
- 15.Jofré Barud F., López S., Tapia A., Feresin G.E., López M.L. Attractant, sexual competitiveness enhancing and toxic activities of the essential oils from Baccharis spartioides and Schinus polygama on Ceratitis capitata wiedemann. Ind. Crops Prod. 2014;62:299–304. doi: 10.1016/j.indcrop.2014.08.045. [DOI] [Google Scholar]
- 16.Barkatullah;I.M.;Rauf A., Inyat-Ur-Rahman K. Physicochemical characterization of essential and fixed oils of Skimmia laureola and Zanthoxylum armatum. J. Med. Plant Res. 2012;1:51–58. [Google Scholar]
- 17.Taraj K., Delibashi A., Andoni A., Lazo P., Kokalari E., Lame A., Xhaxhiu K., Çomo A. Extraction of chamomile essential oil by subcritical CO2 and its analysis by UV-Vis spectrophotometer. Asian J. Chem. 2013;25:7361–7364. [Google Scholar]
- 18.De Lira C.S., Pontual E.V., de Albuquerque L.P., Paiva L.M., Paiva P.M.G., de Oliveira J.V., Napoleão T.H., Navarro D.M.d.A.F. Evaluation of the toxicity of essential oil from Alpinia purpurata inflorescences to Sitophilus zeamais (maize weevil) Crop Prot. 2015;71:95–100. doi: 10.1016/j.cropro.2015.02.004. [DOI] [Google Scholar]
- 19.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Pub. Corporation; Carol Stream, IL, USA: 2001. [Google Scholar]
- 20.Pimentel T.C., Gomes da Cruz A., Deliza R. Sensory evaluation: Sensory rating and scoring methods. In: Caballero B., Finglas P.M., Toldrá F., editors. Encyclopedia of Food and Health. Academic Press; Oxford, UK: 2016. pp. 744–749. [Google Scholar]
- 21.Lee J., Chambers D. A lexicon for flavor descriptive analysis of green tea. J. Sens. Stud. 2007;22:256–272. doi: 10.1111/j.1745-459X.2007.00105.x. [DOI] [Google Scholar]
- 22.Ledeker C.N., Suwonsichon S., Chambers D.H., Adhikari K. Comparison of sensory attributes in fresh mangoes and heat-treated mango purées prepared from thai cultivars. LWT—Food Sci. Technol. 2014;56:138–144. doi: 10.1016/j.lwt.2013.11.011. [DOI] [Google Scholar]
- 23.Chambers E., Sanchez K., Phan U.X.T., Miller R., Civille G.V., Di Donfrancesco B. Development of a “living” lexicon for descriptive sensory analysis of brewed coffee. J. Sens. Stud. 2016;31:465–480. doi: 10.1111/joss.12237. [DOI] [Google Scholar]
- 24.Chowdhury T., Mandal A., Roy S.C., De Sarker D. Diversity of the genus Ocimum (lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J. Genet. Eng. Biotechnol. 2017;15:275–286. doi: 10.1016/j.jgeb.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Özcan M., Chalchat J.-C. Essential oil composition of Ocimum basilicum L. and Ocimum minimum L. in turkey. Czech J. Food Sci. 2002;20:223–228. doi: 10.17221/3536-CJFS. [DOI] [Google Scholar]
- 26.Sims C.A., Juliani H.R., Mentreddy S.R., Simon J.E. Essential oils in holy basil (Ocimum tenuiflorum L.) as influenced by planting dates and harvest times in north alabama. J. Med. Active Pl. 2014;2:33–41. [Google Scholar]
- 27.Siddique A.B., Mizanur Rahman S.M., Hossain M.A. Chemical composition of essential oil by different extraction methods and fatty acid analysis of the leaves of Stevia rebaudiana bertoni. Arab. J. Chem. 2016;9:S1185–S1189. doi: 10.1016/j.arabjc.2012.01.004. [DOI] [Google Scholar]
- 28.Turek C., Stintzing F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013;12:40–53. doi: 10.1111/1541-4337.12006. [DOI] [Google Scholar]
- 29.Hădărugă D.I., Hădărugă N.G., Costescu C.I., David I., Gruia A.T. Thermal and oxidative stability of the Ocimum basilicum L. Essential oil/β-cyclodextrin supramolecular system. Beilstein J. Org. Chem. 2014;10:2809–2820. doi: 10.3762/bjoc.10.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beltramea J.M., Angnesa R.A., Chiavellib L.U.R., Costab W.F.d., Rosac M.F.d., Loboa V.d.S., Pominib A.M. Photodegradation of essential oil from marjoram (Origanum majorana L.) studied by GC-MS and UV-Vis spectroscopy. Rev. Latinoam. Quím. 2013;41:81–88. [Google Scholar]
- 31.Le Borgne E., Cicchetti E., Bertrand T. HPTLC methods for qualitative and quantitative analysis of selected furocoumarins in essential oils. Flavour Fragr. J. 2017;32:330–339. doi: 10.1002/ffj.3394. [DOI] [Google Scholar]
- 32.Simon J.E., Morales M.R., Phippen W.B., Vieira R.F., Hao Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. In: Janick J., editor. Perspectives on New Crops and New Uses. ASHS Press; Alexandria, VA, USA: 1999. pp. 499–505. [Google Scholar]
- 33.Singh D., Chaudhuri P.K. A review on phytochemical and pharmacological properties of holy basil (Ocimum sanctum L.) Ind. Crops Prod. 2018;118:367–382. doi: 10.1016/j.indcrop.2018.03.048. [DOI] [Google Scholar]
- 34.Dighe V.V., Gursale A.A., Sane R.T., Menon S., Patel P.H. Quantitative determination of eugenol from Cinnamomum tamala nees and eberm. leaf powder and polyherbal formulation using reverse phase liquid chromatography. Chromatographia. 2005;61:443–446. doi: 10.1365/s10337-005-0527-6. [DOI] [Google Scholar]
- 35.Sajjadi S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from iran. DARU J. Pharm. Sci. 2006;14:128–130. [Google Scholar]
- 36.Mondawi B., Duprey R., Magboul A., Satti A. Constituents of essential oil of Ocimum basilicum var. Thyrsiflorum. Fitoterapia. 1984;55:60–61. [Google Scholar]
- 37.Khatri L., Nasir M., Saleem R., Noor F. Evaluation of pakistani sweet basil oil for commercial exploitation. Pak. J. Sci. Ind. Res. 1995;38:281–282. [Google Scholar]
- 38.Verma R.S., Bisht P.S., Padalia R.C., Saikia D., Chauhan A. Chemical composition and antibacterial activity of essential oil from two Ocimum spp. grown in sub-tropical india during spring-summer cropping season. Asian J. Tradit. Med. 2011;6:211–217. [Google Scholar]
- 39.Avetisyan A., Markosian A., Petrosyan M., Sahakyan N., Babayan A., Aloyan S., Trchounian A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement Altern. Med. 2017;17:60. doi: 10.1186/s12906-017-1587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondello L., Zappia G., Cotroneo A., Bonaccorsi I., Chowdhury J.U., Yusuf M., Dugo G. Studies on the essential oil-bearing plants of bangladesh. Part viii. Composition of some Ocimum oils O. basilicum L. Var. Purpurascens; O. sanctum L. Green; O. sanctum L. Purple; O. americanum L., citral type; O. americanum L., camphor type. Flavour Fragr. J. 2002;17:335–340. doi: 10.1002/ffj.1108. [DOI] [Google Scholar]
- 41.Silva M.G.V., Matos F.J.A., Lopes P.R.O., Silva F.O., Holanda M.T. Composition of essential oils from three Ocimum species obtained by steam and microwave distillation and supercritical CO2 extraction. Arkivoc. 2004;6:66–71. [Google Scholar]
- 42.Sastry K.P., Kumar R.R., Arigar Kumar N., Sneha G., Elizabeth M. Morpho-chemical description and antimicrobial activity of different Ocimum species. J. Plant Dev. Sci. 2012;19:53–64. [Google Scholar]
- 43.Awasthi P.K., Dixit S.C. Chemical compositions of Ocimum sanctum shyama and Ocimum sanctum rama oils from the plains of northern india. J. Essent. Oil Bear. Pl. 2007;10:292–296. doi: 10.1080/0972060X.2007.10643557. [DOI] [Google Scholar]
- 44.Janssen A.M., Scheffer J.J.C., Ntezurubanza L., Svendsen A.B. Antimicrobial activities of some Ocimum species grown in rwanda. J. Ethnopharmacol. 1989;26:57–63. doi: 10.1016/0378-8741(89)90113-X. [DOI] [PubMed] [Google Scholar]
- 45.Zoghbi M.D.G.B., Oliveira J., Andrade E.H.A., Trigo J.R., Fonseca R.C.M., Rocha A.E.S. Variation in volatiles of Ocimum campechianum mill. And Ocimum gratissimum L. cultivated in the north of brazil. J. Essent. Oil Bear. Pl. 2007;10:229–240. doi: 10.1080/0972060X.2007.10643547. [DOI] [Google Scholar]
- 46.Joshi R.K., Si H. Chemical composition of the essential oil of Ocimum tenuiflorum L. (krishna tulsi) from north west karnataka, india. Plant Science Today. 2014;1:99–102. doi: 10.14719/pst.2014.1.3.52. [DOI] [Google Scholar]
- 47.Rajabi Z., Ebrahimi M., Farajpour M., Mirza M., Ramshini H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind. Crops Prod. 2014;61:233–239. doi: 10.1016/j.indcrop.2014.06.038. [DOI] [Google Scholar]
- 48.Agostini F., Santos A.C.A.d., Rossato M., Pansera M.R., Santos P.L.d., Serafini L.A., Molon R., Moyna P. Essential oil yield and composition of Lamiaceae species growing in southern Brazil. Braz. Arch. Biol. Technol. 2009;52:473–478. doi: 10.1590/S1516-89132009000200026. [DOI] [Google Scholar]
- 49.Zouari N., Ayadi I., Fakhfakh N., Rebai A., Zouari S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis boiss. Et reut., a North African endemic species. Lipids Health Dis. 2012;11:28. doi: 10.1186/1476-511X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagci E., Bekci F. Variation in essential oil composition of Hypericum scabrum L. and H. scabroides N. Robson & Poulter (Hypericaceae) aerial parts during its phenological cycle. Acta Bot. Gall. 2010;157:247–254. [Google Scholar]
- 51.Lan Phi N.T., Minh Tu N.T., Nishiyama C., Sawamura M. Characterisation of the odour volatiles in Citrus aurantifolia persa lime oil from Vietnam. In: Bredie W.L.P., Petersen M.A., editors. Developments in Food Science. Volume 43. Elsevier; Amsterdam, The Netherlands: 2006. pp. 193–196. [Google Scholar]
- 52.Xiao Z., Chen J., Niu Y., Chen F. Characterization of the key odorants of fennel essential oils of different regions using GC–MS and GC–O combined with partial least squares regression. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1063:226–234. doi: 10.1016/j.jchromb.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 53.Calín-Sánchez Á., Lech K., Szumny A., Figiel A., Carbonell-Barrachina Á.A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012;48:217–225. doi: 10.1016/j.foodres.2012.03.015. [DOI] [Google Scholar]
- 54.Al-Kateb H., Mottram D.S. The relationship between growth stages and aroma composition of lemon basil Ocimum citriodorum vis. Food Chem. 2014;152:440–446. doi: 10.1016/j.foodchem.2013.12.001. [DOI] [PubMed] [Google Scholar]