Abstract
Alzheimer’s disease (AD) is characterized by progressive memory dysfunction, oxidative stress, and presence of senile plaques formed by amyloid beta (Aβ) accumulation in the brain. AD is one of the most important causes of morbidity and mortality worldwide. AD has a variety of risk factors, including environmental factors, metabolic dysfunction, and genetic background. Recent research has highlighted the relationship between AD and systemic metabolic changes such as glucose and lipid imbalance and insulin resistance. Irisin, a myokine closely linked to exercise, has been associated with glucose metabolism, insulin sensitivity, and fat browning. Recent studies have suggested that irisin is involved in the process in central nervous system (CNS) such as neurogenesis and has reported the effects of irisin on AD as one of the neurodegenerative disease. Here, we review the roles of irisin with respect to AD and suggest that irisin highlight therapeutic important roles in AD. Thus, we propose that irisin could be a potential future target for ameliorating AD pathology and preventing AD onset.
Keywords: irisin, Alzheimer’s disease (AD), glucose metabolism, insulin resistance, neurogenesis
1. Introduction
Alzheimer’s disease (AD), which is characterized by the accumulation of amyloid beta (Aβ) plaques, neurofibrillary tangles (NFT), and oxidative stress in the brain, is the most common subtype of dementia [1]. The risk factors related with the onset and development of AD are diverse and include genetic factors, environmental factors, and impaired metabolic activity [2,3,4]. Recent studies have reported that many metabolic disorders such as obesity, type 2 diabetes (T2DM), atherosclerosis, and cardiovascular disease, have been observed in patients with AD [5,6]. Considering previous evidences, the risk factors for AD are not limited to the central nervous system (CNS) and include systemic metabolic abnormalities. Irisin, a myokine that is formed by fibronectin type III domain containing protein 5 (FNDC5, aka PeP, and FRCP2) [7,8,9] and produced by muscle tissue [10] increases energy metabolism [8], regulates glucose homeostasis [11], and is directly related to the browning process, converting white adipose tissue into brown adipose tissue [11]. Moreover, recent studies have highlighted that irisin enhances brain function by modulating neurotransmitter secretion [12,13], and could play a beneficial role in the AD brain [14,15]. Considering the significant evidences that suggest that AD onset is related to metabolic changes, we hypothesize that irisin which is involved in various metabolic pathways, may be linked to multiple aspects of AD pathology. Here, we broadly review the possible effect of irisin on AD pathology and propose that the roles of irisin in CNS should be further investigated as a means of alleviating AD pathology.
2. Alzheimer’s Disease (AD)
AD is the most common subtype of dementia and ultimately leads to death [16,17,18]. Its pathologies result from Aβ plaques, which are formed by the proteolysis of Aβ precursor protein (APP) by β- and γ-secretases, and the accumulation of intraneuronal amyloid plaques [1]. Aβ oligomer uptake are associated with the endocytosis of N-methyl-D aspartate receptor and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors [19,20], and is regulated by binding integrins [21] and are negatively related with the reduction of cellular cholesterol and sphingolipid levels [22]. Many studies suggested that decreased clearance process of Aβ in CNS leads to the development of AD [23]. The reduction of Aβ clearance process triggers the excessive accumulation of Aβ in the brain and cerebral amyloid angiopathy [24]. AD is characterized by cognitive impairment and memory loss [18], which are related to impaired synaptic plasticity and reduced long-term potentiation (LTP) [25]. Hippocampal neurogenesis plays a key role in synaptic plasticity and, ultimately influences learning and memory processes in the brain [26]. Patients with AD and AD animal models exhibit abnormal hippocampal neurogenesis [27,28,29]. The onset and development of AD are associated with genetic factors, mitochondrial dysfunction, oxidative damage, environmental factors, and energy metabolism impairment [2,3,4]. Clinically, the patients with AD typically have one or more comorbidity such as hypertension, obesity, diabetes, atherosclerosis, and metabolic deficiencies [5,6,30]. Most patients with the sporadic form AD are related to many pathogenic mechanisms, including brain atrophy, inflammation, oxidative stress, impaired glucose metabolism, insulin resistance, and T2DM [31,32,33]. As a result of findings from previous studies, recent research has highlighted the relationship between metabolic problems and AD [34,35]. One in vivo study has demonstrated that a high fat diet increases APP expression and APP processing enzyme levels [36]. Another in vivo study has reported that high fat diet fed rats exhibited increased levels of the APP-cleaving enzyme and Aβ in the hippocampus [37]. Furthermore, other studies have shown that a diet-induced obesity model exhibits memory dysfunction and neuroinflammation [35,38,39]. Moreover, the elevation of inflammatory responses in blood vessels triggers the reduction of synaptic plasticity and impairs neurogenesis [40]. Clinical studies have demonstrated that obesity results in decreased cognitive function, white matter atrophy, blood brain barrier disruption, and increases the risk of AD [41,42,43]. Even though the prevalence of AD is gradually increasing worldwide, effective therapeutic solutions and prevention methods are not fully found. As mentioned above, recent evidence has indicated a need for studying the relationship between AD onset and metabolic factors. Given the strong association between AD and metabolic changes, the study of metabolic problems in AD is necessary for finding therapeutic solutions for AD.
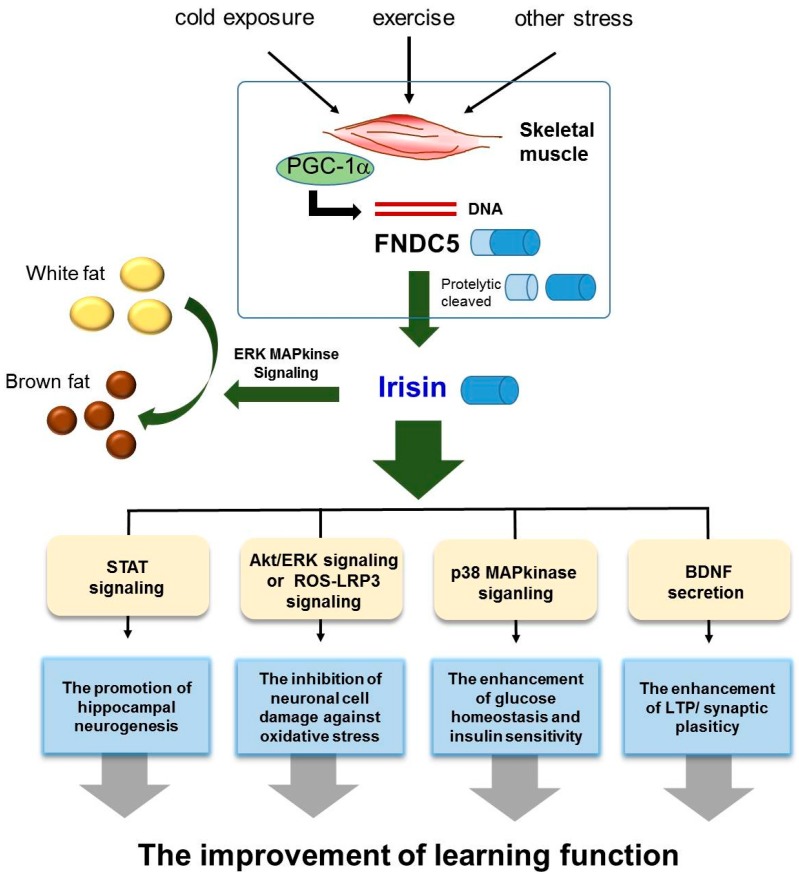
3. What Is Irisin?
Irisin as a 112-amino acid glycosylated protein-hormone is formed by the proteolytic cleavage of FNDC5 [7,8,9]. Briefly, the polypeptide is proteolytically cleaved from the C-terminal moiety after the N-terminal signal peptide is removed, then glycosylated, and released as a hormone of 112 amino acids [7,8,9]. The production of irisin is similar with that of other hormones and hormone-like polypeptides (i.e., transforming growth factor-α, and epidermal growth factor from transmembrane precursors) [7,8,9]. The formation of FNDC5 is promoted by exercise in muscle, and it could be changed into irisin by the transcriptional co-activator PPAR-γ co-activator-1 α (PGC1α) [8]. Irisin is secreted by muscle, circulates in the fat tissue and regulates energy metabolism [10,44,45]. It is known as the cold induced endocrine regulator of brown fat function [11,46] and its secretion is also promoted by the cold exposure [11,47]. In humans, mRNA expression of FNDC5 is approximately 100 times lower in the adipose tissue than in the skeletal muscle [48,49,50]. Secretion of irisin from adipocytes is also lower than that from skeletal muscle [49,50]. The irisin level in human white adipose tissue is only 5% of the level found in skeletal muscle [51]. Additionally, irisin, both from peripheral and neuronal origins, circulates throughout the body [52]. Mammals have two types of adipose tissue, white and brown [53]. Irisin promotes the browning process of white adipose tissue into brown adipose tissue [8,11,54,55]. The excess accumulation of white adipose tissue leads to obesity and results in an energy imbalance and chronic inflammation [56,57]. Brown adipose tissue is responsible for energy thermogenesis [58,59,60] due to the presence of many large mitochondria [61,62]. Irisin stimulates fat browning by activating mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling [63]. Furthermore, the secretion of irisin has been reported to increase after exercise [64,65] and influence the regulation of glucose homeostasis and lipid metabolism in skeletal muscle and adipose tissue [66,67]. Several studies have demonstrated that irisin, a contraction-regulated myokine, is secreted during exercise and exerts beneficial effects on glucose homeostasis [11,68]. In vivo studies have shown irisin injection to lead to an increase in energy expenditure and improvement in glucose metabolism [8,49,69,70]. Moreover, irisin is known to play crucial roles in the CNS [45]. Previous studies have reported that irisin is observed in the cerebrospinal fluid (CSF) and hypothalamus [71], and FNDC5 is known to be highly expressed in glia (e.g., astrocytes and microglia) and neurons in various brain regions [72,73,74,75]. Irisin is synthesized in the muscle tissue and is present in cerebellar Purkinje cells and intercellular nerve endings [73,76]. Zhang et al. demonstrated that administration of irisin by intracerebroventricular injection leads to an increase of the locomotor activity [77]. According to Brailoiu et al., irisin promotes neuronal depolarization of cardiac projecting neuron nucleus [78]. One study suggested that irisin contributes to neural differentiation by modulating metabolic responses in the CNS [77]. In rodents, FNDC5 is found in several brain regions, such as the midbrain and the hippocampus [79], and is known to be involved in reward-related models [80]. Irisin exerts antidepressant-like effects via modulation of energy metabolism in the prefrontal cortex [81]. A recent study demonstrated that skeletal muscle-derived irisin is linked to reward-related processes and motivation [82]. Several studies have highlighted that irisin secreted after exercise plays a beneficial role in brain function [12,13] and in neurodegenerative diseases such as AD [14,15]. Considering the multiple effects of irisin, we reviewed the various roles of irisin in the AD brain.
4. The Potential Therapeutic Roles of Irisin in Alzheimer’s Disease
4.1. Irisin Improves Learning and Memory Function by Regulating the Expression of Brain-Derived Neurotrophic Factor (BDNF)
Brain-derived neurotrophic factor (BDNF) as the widely distributed neurotrophin in the brain has been known to has a critical role in synaptic function and neuronal survival [83]. Also, BDNF suppresses the cytotoxic response of neuron and learning deficits against Aβ toxicity in AD [84,85]. Irisin is the upstream mediator of BDNF production [64] and triggers the expression of BDNF [64,86]. The exercise induced increase of FNDC5/irisin release from the periphery results in the increase of FNDC5/irisin in the neurons as well as the increase of BDNF production in the neuron [64]. They demonstrated that the manipulation of FNDC5 expression by using siRNA in cortical neuron results in the decrease of BDNF expression [64]. BDNF controls dopamine-3 signaling pathways, influencing dopamine’s effect on the brain [87,88] and modulating synaptic plasticity and neurogenesis in the amygdala, prefrontal cortex, and hippocampus [86,89]. BDNF induces LTP and increases the strength of synaptic connections [90]. Yan et al. reports the connection between BDNF and mesocortico-limbic system in brain and also proves the importance of BDNF in brain circuits [90]. Furthermore, BDNF is necessary for hippocampal neurogenesis and the hippocampal neural circuit [91,92]. BDNF is also known to play an important role in LTP as well as learning and memory function [93,94]. Decreased levels of neurotrophins such as BDNF have been shown in various regions of the AD brain [95,96,97]. Several studies have demonstrated that the administration of BDNF in patients with AD ameliorates AD pathology [98,99]. Recent studies have demonstrated that the irisin-BDNF axis could strengthen learning and memory function [82,100,101] and improve mood disorders and anxiety [102,103,104]. FNDC5/irisin contributes to the boosting of reward-related learning and motivation by enhancing the production of BDNF [82]. In addition, the increased expression of FNDC5 in primary cortical neurons promotes BDNF expression, while knockdown of FNDC5 leads to a decrease in BDNF expression [105]. Given that the expression of BDNF could potentially improve cognitive decline in AD [99], the stimulation of the irisin-BDNF axis in the brain may be a promising issue for AD treatment.
4.2. Irisin Promotes Neurogenesis and Protects against the Neuronal Damage Caused by Oxidative Stress
Neurogenesis which mainly occurs in the subventricular zone and the dentate gyrus of the hippocampus is important in in structural synaptic plasticity and neural network maintenance and contributes to the recovery of cognitive dysfunction [106,107]. Several studies reported that the neurogenesis as the new neuron’s replacement process in learning related hippocampus region is attenuated in AD brain [108,109]. Irisin has been shown to be essential for neural differentiation in mouse embryonic stem cells [110,111]. Knockdown of FNDC5 in neuronal precursor cells inhibits the differentiation of mouse embryonic stem cells into neurons, and the maturation of astrocytes [110]. In addition, FNDC5 boosts the differentiation of embryonic stem cells into neural cells [112]. One study found that irisin affects hippocampal neurogenesis and induces neuronal proliferation by modulating neurogenesis-related STAT3 signaling [111,113]. Huh et al. demonstrated that knockdown of the precursor of irisin suppresses the neural differentiation of embryonic stem cells in mice [48]. Irisin inhibits the neuronal damage caused by oxidative stress through activation of Akt/ERK1/2 signaling [114]. Irisin protects neurons by attenuating the secretion of pro-inflammatory cytokine such as tumor necrosis factor (TNF)-α through Akt/ERK1/2 signaling [114]. In addition, irisin protects neurons by suppressing ROS-NLRP3 inflammatory signaling in ischemic conditions [115]. This study demonstrated that PC12 cells as neuronal cells inhibited the expression of pro-inflammatory cytokine, interleukin (IL)-1β and the activation of caspase 1 signaling as apoptosis signaling under ischemic condition (i.e., oxygen and glucose deprivation conditions) [115]. Given that the enhancement of neurogenesis could potentially improve the impaired synaptic plasticity and memory dysfunction found in AD [26,28,29], irisin may be a potential therapeutic target for AD as it promotes both neurogenesis and neuronal cell survival.
4.3. Irisin Ameliorates Insulin Sensitivity and Improves Glucose Homeostasis
Impaired cerebral glucose metabolism and insulin signaling as a major pathological feature of AD is critically involved in the learning and memory loss [116]. The impairment of glucose uptake in the brain triggers brain atrophy and neuronal dysfunction in AD [117]. One study reported that glucose transporter (GLUT) 1 deficiency in brain endothelial cells aggravates AD neuropathology such as cognitive decline [118]. According to previous studies, irisin acts as a regulator of glucose utilization and browning of white adipose tissue in models of obesity and T2DM [63,119]. Serum irisin levels and single nucleotide polymorphisms in the FNDC5 are associated with glucose metabolism and insulin resistance [120]. A clinical study reported that circulating irisin levels are higher in patients with metabolic syndrome than in normal subjects [121]. A reduction in circulating irisin levels has been associated with insulin resistance [122,123]. Several studies have demonstrated that irisin has beneficial effects on insulin sensitivity and glucose metabolism in both skeletal muscle and adipose tissue [66,67]. Serum irisin levels were founded significantly lower in diabetic subjects than in normal subjects [124]. Irisin decreases the expression of G6Pase and PEPCK, both of which are critical for gluconeogenesis, in the liver [66]. Furthermore, irisin controls glucose utilization via p38 MAPK signaling [63]. Previous studies reported the strong association between FNDC5/irisin and insulin resistance estimated by homeostatic model assessment [125], and by testing glucose tolerance [50]. A study assessed the insulin action by irisin through Akt activation [124]. FNDC5/irisin overexpression improves insulin resistance and reduces blood glucose in the high fat diet fed mouse [126]. Plasma membrane GLUT-4 expression is activated in the skeletal muscles of irisin-treated rats [63,119]. Based on the previous reports, we also hypothesize that irisin may be a central modulator of glucose metabolism and insulin activity in the AD brain.
5. Conclusions
We reviewed the role of irisin in the AD brain, and demonstrated that irisin is involved in the modulation of several AD risk factors, including insulin resistance, impaired neurogenesis, oxidative stress, and imbalance of neurotrophic factors. In this review, we highlighted three points concerning the potential therapeutic role of irisin in AD (Figure 1). First, irisin boosts the production of BDNF, which could subsequently lead to cognitive improvement and a reduction in synaptic dysfunction in AD. Second, irisin could potentially enhance neurogenesis and protect against neuronal damage in AD. Finally, irisin might have a role in the regulation of insulin resistance and glucose homeostasis in AD. It is necessary to find a suitable treatment for alleviating AD pathology, and irisin holds promise as a therapeutic hormone. For example, several studies have demonstrated that irisin can be referred to as an “exercise-hormone” [8] since induction of FNDC5 by endurance exercise [8] has been observed in mice [71,127] and humans [128]. In addition, exercise induced irisin expression in the hippocampus could activate BDNF and other neuroprotective genes [64]. Based on previous studies, the treatment of irisin could suppress the expression of pro-inflammatory cytokines such as TNF-alpha and IL-6, reduce the expression of monocyte chemoattractant protein-1 in adipocytes, subsequently attenuate migration of macrophages and induce the phenotypic switching of macrophages from M1 (pro-inflammatory) to M2 (anti-inflammatory) state [129]. Also, in brain ischemia state, the researchers observed that marked increased levels of ROS and malondialdehyde were reduced by irisin treatment in peri-infarct brain tissues [130]. Furthermore, uncoupling proteins, as a regulator in mitochondrial biogenesis and neuroprotection and synaptic function in the CNS, was increased by high dose administration of irisin in all brain areas [131,132,133]. Given these clinical application of irisin, we speculate that irisin may contribute to the improvement of neuropathy by reducing neuroinflammation, and enhancing synaptic functions in AD brain. In light of the presented evidence, we propose that AD patients require the induction of FNDC5 through an appropriate amount of exercise to promote neurogenesis, neuronal survival, and synaptic plasticity. Therefore, we propose that irisin could be a potential future target for ameliorating AD pathology and preventing AD onset.
Figure 1.
Scheme for the therapeutic role of irisin in Alzheimer’s disease.
Author Contributions
O.Y.K. wrote the draft of the manuscript and J.S. revised the manuscript.
Funding
This study was supported by funded by grants (2016R1D1A1B03930394 to Juhyun Song, and 2016R1A2B4013627 to Oh Yoen Kim) of the Basic Science Research Program through the National Research Foundation of Korea (NRF). This study was also financially supported by Chonnam National University (2017-2859 to Juhyun Song).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Reitz C., Honig L., Vonsattel J.P., Tang M.X., Mayeux R. Memory performance is related to amyloid and tau pathology in the hippocampus. J. Neurol. Neurosurg. Psychiatry. 2009;80:715–721. doi: 10.1136/jnnp.2008.154146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrer I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog. Neurobiol. 2012;97:38–51. doi: 10.1016/j.pneurobio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Cai Z., Zhao B., Ratka A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromol. Med. 2011;13:223–250. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 4.Cacabelos R. Molecular pathology and pharmacogenomics in Alzheimer’s disease: Polygenic-related effects of multifactorial treatments on cognition, anxiety and depression. Methods Find. Exp. Clin. Pharmacol. 2007;29(Suppl. A):1–91. [PubMed] [Google Scholar]
- 5.Lutz M.W., Crenshaw D., Welsh-Bohmer K.A., Burns D.K., Roses A.D. New Genetic Approaches to AD: Lessons from APOE-TOMM40 Phylogenetics. Curr. Neurol. Neurosci. Rep. 2016;16:48. doi: 10.1007/s11910-016-0643-8. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman R.F., Schneider A.L., Zhou Y., Coresh J., Green E., Gupta N., Knopman D.S., Mintz A., Rahmim A., Sharrett A.R., et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teufel A., Malik N., Mukhopadhyay M., Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297:79–83. doi: 10.1016/S0378-1119(02)00828-4. [DOI] [PubMed] [Google Scholar]
- 8.Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Bostrom E.A., Choi J.H., Long J.Z., et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher M.A., Chinnam N., Ohashi T., Shah R.S., Erickson H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013;288:33738–33744. doi: 10.1074/jbc.M113.516641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson H.P. Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte. 2013;2:289–293. doi: 10.4161/adip.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P., Linderman J.D., Smith S., Brychta R.J., Wang J., Idelson C., Perron R.M., Werner C.D., Phan G.Q., Kammula U.S., et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattson M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson K.I., Weinstein A.M., Lopez O.L. Physical activity, brain plasticity, and Alzheimer’s disease. Arch. Med. Res. 2012;43:615–621. doi: 10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okonkwo O.C., Schultz S.A., Oh J.M., Larson J., Edwards D., Cook D., Koscik R., Gallagher C.L., Dowling N.M., Carlsson C.M., et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83:1753–1760. doi: 10.1212/WNL.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker J.M., Klakotskaia D., Ajit D., Weisman G.A., Wood W.G., Sun G.Y., Serfozo P., Simonyi A., Schachtman T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015;44:561–572. doi: 10.3233/JAD-140981. [DOI] [PubMed] [Google Scholar]
- 16.Xu J., Murphy S.L., Kochanek K.D., Bastian B.A. Deaths: Final Data for 2013. Natl. Vital Stat. Rep. 2016;64:1–119. [PubMed] [Google Scholar]
- 17.Brookmeyer R., Evans D.A., Hebert L., Langa K.M., Heeringa S.G., Plassman B.L., Kukull W.A. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011;7:61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitz C., Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang B.L. Neuronal protein trafficking associated with Alzheimer disease: From APP and BACE1 to glutamate receptors. Cell Adhes. Migr. 2009;3:118–128. doi: 10.4161/cam.3.1.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rui Y., Gu J., Yu K., Hartzell H.C., Zheng J.Q. Inhibition of AMPA receptor trafficking at hippocampal synapses by β-amyloid oligomers: The mitochondrial contribution. Mol. Brain. 2010;3:10. doi: 10.1186/1756-6606-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi X., Gall C.M., Zhou J., Lynch G. Uptake and pathogenic effects of amyloid β peptide 1-42 are enhanced by integrin antagonists and blocked by NMDA receptor antagonists. Neuroscience. 2002;112:827–840. doi: 10.1016/S0306-4522(02)00132-X. [DOI] [PubMed] [Google Scholar]
- 22.Saavedra L., Mohamed A., Ma V., Kar S., de Chaves E.P. Internalization of β-amyloid peptide by primary neurons in the absence of apolipoprotein E. J. Biol. Chem. 2007;282:35722–35732. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- 23.Weller R.O., Massey A., Kuo Y.M., Roher A.E. Cerebral amyloid angiopathy: Accumulation of A β in interstitial fluid drainage pathways in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000;903:110–117. doi: 10.1111/j.1749-6632.2000.tb06356.x. [DOI] [PubMed] [Google Scholar]
- 24.Love S. Contribution of cerebral amyloid angiopathy to Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:1–4. [PMC free article] [PubMed] [Google Scholar]
- 25.Neves G., Cooke S.F., Bliss T.V. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 26.van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin K., Galvan V., Xie L., Mao X.O., Gorostiza O.F., Bredesen D.E., Greenberg D.A. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw,Ind) mice. Proc. Natl. Acad. Sci. USA. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J.M., Singh C., Liu L., Irwin R.W., Chen S., Chung E.J., Thompson R.F., Brinton R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demars M., Hu Y.S., Gadadhar A., Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J. Neurosci. Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bis J.C., Sitlani C., Irvin R., Avery C.L., Smith A.V., Sun F., Evans D.S., Musani S.K., Li X., Trompet S., et al. Drug-Gene Interactions of Antihypertensive Medications and Risk of Incident Cardiovascular Disease: A Pharmacogenomics Study from the CHARGE Consortium. PLoS ONE. 2015;10:e0140496. doi: 10.1371/journal.pone.0140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iqbal K., Grundke-Iqbal I. Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2010;6:420–424. doi: 10.1016/j.jalz.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira S.T., Clarke J.R., Bomfim T.R., De Felice F.G. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014;10:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Liang Z., Tian Z., Blanchard J., Dai C.L., Chalbot S., Iqbal K., Liu F., Gong C.X. Intracerebroventricular streptozotocin exacerbates Alzheimer-like changes of 3xTg-AD mice. Mol. Neurobiol. 2014;49:547–562. doi: 10.1007/s12035-013-8539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottesman R.F., Albert M.S., Alonso A., Coker L.H., Coresh J., Davis S.M., Deal J.A., McKhann G.M., Mosley T.H., Sharrett A.R., et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74:1246–1254. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang Y.F., An Y., Bilgel M., Wong D.F., Troncoso J.C., O’Brien R.J., Breitner J.C., Ferruci L., Resnick S.M., Thambisetty M. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol. Psychiatry. 2016;21:910–915. doi: 10.1038/mp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puig K.L., Floden A.M., Adhikari R., Golovko M.Y., Combs C.K. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS ONE. 2012;7:e30378. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T., Pan B.S., Zhao B., Zhang L.M., Huang Y.L., Sun F.Y. Exacerbation of poststroke dementia by type 2 diabetes is associated with synergistic increases of β-secretase activation and β-amyloid generation in rat brains. Neuroscience. 2009;161:1045–1056. doi: 10.1016/j.neuroscience.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 38.Jurdak N., Lichtenstein A.H., Kanarek R.B. Diet-induced obesity and spatial cognition in young male rats. Nutr. Neurosci. 2008;11:48–54. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- 39.Kanoski S.E., Davidson T.L. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J. Exp. Psychol. Anim. Behav. Process. 2010;36:313–319. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 40.Kiliaan A.J., Arnoldussen I.A., Gustafson D.R. Adipokines: A link between obesity and dementia? Lancet Neurol. 2014;13:913–923. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Droogsma E., van Asselt D., Bieze H., Veeger N., De Deyn P.P. The relationship of weight change trajectory with medial temporal lobe atrophy in patients with mild Alzheimer’s disease: Results from a cohort study. Alzheimer’s Res. Ther. 2015;7:18. doi: 10.1186/s13195-015-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustafson D.R., Luchsinger J.A. High adiposity: Risk factor for dementia and Alzheimer’s disease? Alzheimer’s Res. Ther. 2013;5:57. doi: 10.1186/alzrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanz C.M., Ruidavets J.B., Bongard V., Marquie J.C., Hanaire H., Ferrieres J., Andrieu S. Relationship between markers of insulin resistance, markers of adiposity, HbA1c, and cognitive functions in a middle-aged population-based sample: The MONA LISA study. Diabetes Care. 2013;36:1512–1521. doi: 10.2337/dc12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grygiel-Gorniak B., Puszczewicz M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4687–4693. [PubMed] [Google Scholar]
- 45.Novelle M.G., Contreras C., Romero-Pico A., Lopez M., Dieguez C. Irisin, two years later. Int. J. Endocrinol. 2013;2013:746281. doi: 10.1155/2013/746281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung N., Park J., Lim K. The effects of exercise and cold exposure on mitochondrial biogenesis in skeletal muscle and white adipose tissue. J. Exerc. Nutr. Biochem. 2017;21:39–47. doi: 10.20463/jenb.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celi F.S., Brychta R.J., Linderman J.D., Butler P.W., Alberobello A.T., Smith S., Courville A.B., Lai E.W., Costello R., Skarulis M.C., et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur. J. Endocrinol. 2010;163:863–872. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huh J.Y., Panagiotou G., Mougios V., Brinkoetter M., Vamvini M.T., Schneider B.E., Mantzoros C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metab. Clin. Exp. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurdiova T., Balaz M., Vician M., Maderova D., Vlcek M., Valkovic L., Srbecky M., Imrich R., Kyselovicova O., Belan V., et al. Effects of obesity, diabetes and exercise on FNDC5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014;592:1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreno-Navarrete J.M., Ortega F., Serrano M., Guerra E., Pardo G., Tinahones F., Ricart W., Fernandez-Real J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 51.Norheim F., Langleite T.M., Hjorth M., Holen T., Kielland A., Stadheim H.K., Gulseth H.L., Birkeland K.I., Jensen J., Drevon C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 52.Jedrychowski M.P., Wrann C.D., Paulo J.A., Gerber K.K., Szpyt J., Robinson M.M., Nair K.S., Gygi S.P., Spiegelman B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015;22:734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gil A., Olza J., Gil-Campos M., Gomez-Llorente C., Aguilera C.M. Is adipose tissue metabolically different at different sites? Int. J. Pediatr. Obes. 2011;6(Suppl. 1):13–20. doi: 10.3109/17477166.2011.604326. [DOI] [PubMed] [Google Scholar]
- 54.Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J.P., De Matteis R., Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 55.Lecker S.H., Zavin A., Cao P., Arena R., Allsup K., Daniels K.M., Joseph J., Schulze P.C., Forman D.E. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ. Heart Fail. 2012;5:812–818. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill J.O., Wyatt H.R., Peters J.C. Energy balance and obesity. Circulation. 2012;126:126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vazquez-Vela M.E., Torres N., Tovar A.R. White adipose tissue as endocrine organ and its role in obesity. Arch. Med. Res. 2008;39:715–728. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Cannon B., Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 59.Whittle A.J., Lopez M., Vidal-Puig A. Using brown adipose tissue to treat obesity—The central issue. Trends Mol. Med. 2011;17:405–411. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zingaretti M.C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 62.Villarroya F., Vidal-Puig A. Beyond the sympathetic tone: The new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y., Li R., Meng Y., Li S., Donelan W., Zhao Y., Qi L., Zhang M., Wang X., Cui T., et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–525. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 64.Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J.D., Greenberg M.E., Spiegelman B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huh J.Y., Mougios V., Kabasakalis A., Fatouros I., Siopi A., Douroudos I.I., Filippaios A., Panagiotou G., Park K.H., Mantzoros C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014;99:E2154–E2161. doi: 10.1210/jc.2014-1437. [DOI] [PubMed] [Google Scholar]
- 66.Xin C., Liu J., Zhang J., Zhu D., Wang H., Xiong L., Lee Y., Ye J., Lian K., Xu C., et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016;40:443–451. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- 67.Lee H.J., Lee J.O., Kim N., Kim J.K., Kim H.I., Lee Y.W., Kim S.J., Choi J.I., Oh Y., Kim J.H., et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015;29:873–881. doi: 10.1210/me.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J.Q., Huang Y.Y., Gusdon A.M., Qu S. Irisin: A new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015;14:2. doi: 10.1186/1476-511X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zafrir B. Brown adipose tissue: Research milestones of a potential player in human energy balance and obesity. Horm. Metab. Res. 2013;45:774–785. doi: 10.1055/s-0033-1348264. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez A., Ezquerro S., Mendez-Gimenez L., Becerril S., Fruhbeck G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015;309:E691–E714. doi: 10.1152/ajpendo.00297.2015. [DOI] [PubMed] [Google Scholar]
- 71.Quinn L.S., Anderson B.G., Conner J.D., Wolden-Hanson T. Circulating irisin levels and muscle FNDC5 mRNA expression are independent of IL-15 levels in mice. Endocrine. 2015;50:368–377. doi: 10.1007/s12020-015-0607-9. [DOI] [PubMed] [Google Scholar]
- 72.Albayrak S., Atci I.B., Kalayci M., Yilmaz M., Kuloglu T., Aydin S., Kom M., Ayden O., Aydin S. Effect of carnosine, methylprednisolone and their combined application on irisin levels in the plasma and brain of rats with acute spinal cord injury. Neuropeptides. 2015;52:47–54. doi: 10.1016/j.npep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Piya M.K., Harte A.L., Sivakumar K., Tripathi G., Voyias P.D., James S., Sabico S., Al-Daghri N.M., Saravanan P., Barber T.M., et al. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 2014;306:E512–E518. doi: 10.1152/ajpendo.00308.2013. [DOI] [PubMed] [Google Scholar]
- 74.Dun S.L., Lyu R.M., Chen Y.H., Chang J.K., Luo J.J., Dun N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrer-Martinez A., Ruiz-Lozano P., Chien K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002;224:154–167. doi: 10.1002/dvdy.10099. [DOI] [PubMed] [Google Scholar]
- 76.Aydin S., Kuloglu T., Aydin S., Kalayci M., Yilmaz M., Cakmak T., Albayrak S., Gungor S., Colakoglu N., Ozercan I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–136. doi: 10.1016/j.peptides.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 77.Zhang W., Chang L., Zhang C., Zhang R., Li Z., Chai B., Li J., Chen E., Mulholland M. Irisin: A myokine with locomotor activity. Neurosci. Lett. 2015;595:7–11. doi: 10.1016/j.neulet.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brailoiu E., Deliu E., Sporici R.A., Brailoiu G.C. Irisin evokes bradycardia by activating cardiac-projecting neurons of nucleus ambiguus. Physiol. Rep. 2015;3:e12419. doi: 10.14814/phy2.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips C., Baktir M.A., Srivatsan M., Salehi A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front. Cell. Neurosci. 2014;8:170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zsuga J., Biro K., Papp C., Tajti G., Gesztelyi R. The “proactive” model of learning: Integrative framework for model-free and model-based reinforcement learning utilizing the associative learning-based proactive brain concept. Behav. Neurosci. 2016;130:6–18. doi: 10.1037/bne0000116. [DOI] [PubMed] [Google Scholar]
- 81.Wang S., Pan J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 2016;474:22–28. doi: 10.1016/j.bbrc.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 82.Zsuga J., Tajti G., Papp C., Juhasz B., Gesztelyi R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med. Hypotheses. 2016;90:23–28. doi: 10.1016/j.mehy.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 83.Diniz B.S., Teixeira A.L. Brain-derived neurotrophic factor and Alzheimer’s disease: Physiopathology and beyond. Neuromol. Med. 2011;13:217–222. doi: 10.1007/s12017-011-8154-x. [DOI] [PubMed] [Google Scholar]
- 84.Arancibia S., Silhol M., Mouliere F., Meffre J., Hollinger I., Maurice T., Tapia-Arancibia L. Protective effect of BDNF against β-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L., Fang Y., Lian Y., Chen Y., Wu T., Zheng Y., Zong H., Sun L., Zhang R., Wang Z., et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by aβ1-42. PLoS ONE. 2015;10:e0122415. doi: 10.1371/journal.pone.0122415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Camandola S., Mattson M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017;36:1474–1492. doi: 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guillin O., Griffon N., Bezard E., Leriche L., Diaz J., Gross C., Sokoloff P. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: Therapeutic implications in Parkinson’s disease. Eur. J. Pharmacol. 2003;480:89–95. doi: 10.1016/j.ejphar.2003.08.096. [DOI] [PubMed] [Google Scholar]
- 88.Collo G., Cavalleri L., Spano P. Structural plasticity in mesencephalic dopaminergic neurons produced by drugs of abuse: Critical role of BDNF and dopamine. Front. Pharmacol. 2014;5:259. doi: 10.3389/fphar.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen N., Li Q., Liu J., Jia S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes/Metab. Res. Rev. 2016;32:51–59. doi: 10.1002/dmrr.2660. [DOI] [PubMed] [Google Scholar]
- 90.Yan Q.S., Feng M.J., Yan S.E. Different expression of brain-derived neurotrophic factor in the nucleus accumbens of alcohol-preferring (P) and -nonpreferring (NP) rats. Brain Res. 2005;1035:215–218. doi: 10.1016/j.brainres.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 91.Lee J., Duan W., Mattson M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 92.Vilar M., Mira H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji Y., Lu Y., Yang F., Shen W., Tang T.T., Feng L., Duan S., Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu Y., Christian K., Lu B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Budni J., Bellettini-Santos T., Mina F., Garcez M.L., Zugno A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015;6:331–341. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J., Fukumoto H., Orne J., Klucken J., Raju S., Vanderburg C.R., Irizarry M.C., Hyman B.T., Ingelsson M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 97.Michalski B., Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Brain Res. Mol. Brain Res. 2003;111:148–154. doi: 10.1016/S0169-328X(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 98.Chen Z.Y., Cao L., Wang L.M., Guo C., Ye J.L., Chai Y.F., Yan Z.Y. Development of neurotrophic molecules for treatment of neurodegeneration. Curr. Protein Pept. Sci. 2001;2:261–276. doi: 10.2174/1389203013381125. [DOI] [PubMed] [Google Scholar]
- 99.Longo F.M., Yang T., Knowles J.K., Xie Y., Moore L.A., Massa S.M. Small molecule neurotrophin receptor ligands: Novel strategies for targeting Alzheimer’s disease mechanisms. Curr. Alzheimer Res. 2007;4:503–506. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- 100.Zsuga J., Biro K., Tajti G., Szilasi M.E., Papp C., Juhasz B., Gesztelyi R. ‘Proactive’ use of cue-context congruence for building reinforcement learning’s reward function. BMC Neurosci. 2016;17:70. doi: 10.1186/s12868-016-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nascimento C.M., Pereira J.R., Pires de Andrade L., Garuffi M., Ayan C., Kerr D.S., Talib L.L., Cominetti M.R., Stella F. Physical exercise improves peripheral BDNF levels and cognitive functions in mild cognitive impairment elderly with different bdnf Val66Met genotypes. J. Alzheimer’s Dis. 2015;43:81–91. doi: 10.3233/JAD-140576. [DOI] [PubMed] [Google Scholar]
- 102.Latsko M.S., Gilman T.L., Matt L.M., Nylocks K.M., Coifman K.G., Jasnow A.M. A Novel Interaction between Tryptophan Hydroxylase 2 (TPH2) Gene Polymorphism (rs4570625) and BDNF Val66Met Predicts a High-Risk Emotional Phenotype in Healthy Subjects. PLoS ONE. 2016;11:e0162585. doi: 10.1371/journal.pone.0162585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cagni F.C., Campelo C., Coimbra D.G., Barbosa M.R., Junior L.G.O., Neto A.B.S., Ribeiro A.M., Junior C.O.G., Gomes de Andrade T., Silva R.H. Association of BDNF Val66MET Polymorphism With Parkinson’s Disease and Depression and Anxiety Symptoms. J. Neuropsychiatry Clin. Neurosci. 2017;29:142–147. doi: 10.1176/appi.neuropsych.16040062. [DOI] [PubMed] [Google Scholar]
- 104.Satoh A., Imai S.I., Guarente L. The brain, sirtuins, and ageing. Nat. Rev. Neurosci. 2017;18:362–374. doi: 10.1038/nrn.2017.42. [DOI] [PubMed] [Google Scholar]
- 105.Liu P., Zou D., Yi L., Chen M., Gao Y., Zhou R., Zhang Q., Zhou Y., Zhu J., Chen K., et al. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restor. Neurol. Neurosci. 2015;33:143–157. doi: 10.3233/RNN-140446. [DOI] [PubMed] [Google Scholar]
- 106.Hollands C., Bartolotti N., Lazarov O. Alzheimer’s Disease and Hippocampal Adult Neurogenesis; Exploring Shared Mechanisms. Front. Neurosci. 2016;10:178. doi: 10.3389/fnins.2016.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ninkovic J., Gotz M. Signaling in adult neurogenesis: From stem cell niche to neuronal networks. Curr. Opin. Neurobiol. 2007;17:338–344. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Sotthibundhu A., Li Q.X., Thangnipon W., Coulson E.J. Aβ(1-42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol. Aging. 2009;30:1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Demars M.P., Hollands C., Zhao Kda T., Lazarov O. Soluble amyloid precursor protein-α rescues age-linked decline in neural progenitor cell proliferation. Neurobiol. Aging. 2013;34:2431–2440. doi: 10.1016/j.neurobiolaging.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hashemi M.S., Ghaedi K., Salamian A., Karbalaie K., Emadi-Baygi M., Tanhaei S., Nasr-Esfahani M.H., Baharvand H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 111.Moon H.S., Dincer F., Mantzoros C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metab. Clin. Exp. 2013;62:1131–1136. doi: 10.1016/j.metabol.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ostadsharif M., Ghaedi K., Hossein Nasr-Esfahani M., Mojbafan M., Tanhaie S., Karbalaie K., Baharvand H. The expression of peroxisomal protein transcripts increased by retinoic acid during neural differentiation. Differ. Res. Biol. Divers. 2011;81:127–132. doi: 10.1016/j.diff.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Jung K.H., Chu K., Lee S.T., Kim S.J., Sinn D.I., Kim S.U., Kim M., Roh J.K. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res. 2006;1073–1074:190–201. doi: 10.1016/j.brainres.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 114.Li D.J., Li Y.H., Yuan H.B., Qu L.F., Wang P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metab. Clin. Exp. 2017;68:31–42. doi: 10.1016/j.metabol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 115.Peng J., Deng X., Huang W., Yu J.H., Wang J.X., Wang J.P., Yang S.B., Liu X., Wang L., Zhang Y., et al. Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol. Immunol. 2017;91:185–194. doi: 10.1016/j.molimm.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 116.Chen Z., Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Willette A.A., Bendlin B.B., Starks E.J., Birdsill A.C., Johnson S.C., Christian B.T., Okonkwo O.C., La Rue A., Hermann B.P., Koscik R.L., et al. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D., Sengillo J.D., Hillman S., Kong P., Nelson A.R., et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Al-Daghri N.M., Mohammed A.K., Al-Attas O.S., Amer O.E., Clerici M., Alenad A., Alokail M.S. SNPs in FNDC5 (irisin) are associated with obesity and modulation of glucose and lipid metabolism in Saudi subjects. Lipids Health Dis. 2016;15:54. doi: 10.1186/s12944-016-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanisawa K., Taniguchi H., Sun X., Ito T., Cao Z.B., Sakamoto S., Higuchi M. Common single nucleotide polymorphisms in the FNDC5 gene are associated with glucose metabolism but do not affect serum irisin levels in Japanese men with low fitness levels. Metab. Clin. Exp. 2014;63:574–583. doi: 10.1016/j.metabol.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 121.Park K.H., Zaichenko L., Peter P., Davis C.R., Crowell J.A., Mantzoros C.S. Diet quality is associated with circulating C-reactive protein but not irisin levels in humans. Metab. Clin. Exp. 2014;63:233–241. doi: 10.1016/j.metabol.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi Y.K., Kim M.K., Bae K.H., Seo H.A., Jeong J.Y., Lee W.K., Kim J.G., Lee I.K., Park K.G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Shoukry A., Shalaby S.M., El-Arabi Bdeer S., Mahmoud A.A., Mousa M.M., Khalifa A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life. 2016;68:544–556. doi: 10.1002/iub.1511. [DOI] [PubMed] [Google Scholar]
- 124.Yang Z., Chen X., Chen Y., Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 2015;8:6490–6497. [PMC free article] [PubMed] [Google Scholar]
- 125.Hu W., Wang R., Li J., Zhang J., Wang W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann. Clin. Biochem. 2016;53:67–74. doi: 10.1177/0004563215582072. [DOI] [PubMed] [Google Scholar]
- 126.Xiong X.Q., Chen D., Sun H.J., Ding L., Wang J.J., Chen Q., Li Y.H., Zhou Y.B., Han Y., Zhang F., et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta. 2015;1852:1867–1875. doi: 10.1016/j.bbadis.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 127.Tiano J.P., Springer D.A., Rane S.G. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) during exercise. J. Biol. Chem. 2015;290:11431. doi: 10.1074/jbc.A114.617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Albrecht E., Norheim F., Thiede B., Holen T., Ohashi T., Schering L., Lee S., Brenmoehl J., Thomas S., Drevon C.A., et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015;5:8889. doi: 10.1038/srep08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park S.Y., Kim H.J., Wang S., Higashimori T., Dong J., Kim Y.J., Cline G., Li H., Prentki M., Shulman G.I., et al. Hormone-sensitive lipase knockout mice have increased hepatic insulin sensitivity and are protected from short-term diet-induced insulin resistance in skeletal muscle and heart. Am. J. Physiol. Endocrinol. Metab. 2005;289:E30–E39. doi: 10.1152/ajpendo.00251.2004. [DOI] [PubMed] [Google Scholar]
- 130.Gaggini M., Cabiati M., Del Turco S., Navarra T., De Simone P., Filipponi F., Del Ry S., Gastaldelli A., Basta G. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides. 2017;88:62–66. doi: 10.1016/j.peptides.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 131.Erden Y., Tekin S., Sandal S., Onalan E.E., Tektemur A., Kirbag S. Effects of central irisin administration on the uncoupling proteins in rat brain. Neurosci. Lett. 2016;618:6–13. doi: 10.1016/j.neulet.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 132.Sullivan P.G., Dube C., Dorenbos K., Steward O., Baram T.Z. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann. Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horvath T.L., Diano S., Leranth C., Garcia-Segura L.M., Cowley M.A., Shanabrough M., Elsworth J.D., Sotonyi P., Roth R.H., Dietrich E.H., et al. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology. 2003;144:2757–2760. doi: 10.1210/en.2003-0163. [DOI] [PubMed] [Google Scholar]