Abstract
We investigated the effects of statins on tuberculosis (TB) and pneumonia risks in asthma–chronic pulmonary disease overlap syndrome (ACOS) patients. We extracted data of patients diagnosed as having ACOS during 2000–2010 from the Taiwan National Health Insurance Research Database and divided them into statin users and nonusers. All study participants were followed up from the index date until death, withdrawal from insurance, or TB and pneumonia occurred (31 December 2011). The cumulative TB and pneumonia incidence was analyzed using Cox proportional regression analysis with time-dependent variables. After adjustments for multiple confounding factors including age, sex, comorbidities, and use of medications [statins, inhaled corticosteroids (ICSs), or oral steroids (OSs)], statin use was associated with significantly lower TB [adjusted hazard ratio (aHR) 0.49, 95% confidence interval (CI) 0.34–0.70] and pneumonia (aHR 0.52, 95% CI 0.41–0.65) risks. Moreover, aHRs (95% CIs) for statins combined with ICSs and OSs were respectively 0.60 (0.31–1.16) and 0.58 (0.40–0.85) for TB and 0.61 (0.39–0.95) and 0.57 (0.45–0.74) for pneumonia. Thus, statin users had lower TB and pneumonia risks than did nonusers, regardless of age, sex, comorbidities, and ICS or OS use. Pneumonia risk was lower among users of statins combined with ICSs or Oss and TB risk was lower among the users of statins combined with OSs.
Keywords: tuberculosis, asthma–chronic pulmonary disease overlap syndrome, statins, pneumonia
1. Introduction
Pneumonia, a leading cause of mortality, places a heavy burden on both communities and healthcare systems [1,2]. Tuberculosis (TB) is a prevalent nosocomial infection, which often results in disability [3]. Chronic airway diseases, such as asthma and chronic obstructive pulmonary disease (COPD), are associated with pneumonia [4,5].
Hyperlipidemia was found to be a predisposing factor for pneumonia in one study [6] but was reported to be a factor potentially preventing TB in another study [7]. Cholesterol in the foamy macrophage plays a critical role in the reactivation of latent TB and the mentation of a persistent chronic TB infection. Phagosomal maturation and autophagy are responsible for the elimination of the bacteria and TB bacilli. A statin-mediated reduction in cholesterol levels within the phagosomal membranes potentially counteract the TB-induced inhibition of phagosomal maturation and promote host-induced autophagy, thereby augmenting protection against TB [8]. The role of cholesterol in pneumonia and TB development has been widely debated [9].
Statins exert anti-inflammatory effects on system inflammation and airway diseases such as asthma and COPD [10,11,12]. Pneumonia may increase an individual’s chances of contracting asthma and COPD later in life. Moreover, this condition is particularly responsible for the acute exacerbation of these two diseases. Fewer statin-using chronic respiratory disease patients [13] developed pneumonia than those not using statin [14]. Because bacteria attach to cell surfaces, cholesterol may be vital for the uptake and internal transport of certain bacteria [15]. Statins attenuate cholesterol levels, resulting in a lower chance of bacteria entering the cell to reproduce and destroy lung tissue. Moreover, cholesterol-lowering statins boost bacteria-killing cells [16]. By using human airway epithelial cells as an in vitro model, Statt et al. reported that prior exposure to physiological nanomolar serum concentrations of simvastatin (range 10–1000 nM) confers a significant cellular resistance to the cytotoxicity of pneumolysin, a pore-forming toxin that is the main virulence factor of Streptococcus pneumonia [17]. Therefore, hyperlipidemia with statin use may reduce the risk of pneumonia [18].
Asthma–chronic pulmonary disease overlap syndrome (ACOS) has components of both asthma and COPD. ACOS patients may have higher pneumonia [19] and TB [20] risks in the later course than do COPD patients [21]. Statins have anti-inflammatory and immunomodulatory properties, potentially resulting in reduced reactivation of latent TB; statins modulate T cell count and cytokine levels during pneumonia [22,23]. Few studies have reported on the relationship of statin, inhaled corticosteroid (ICS), or oral steroid (OS) use with TB or pneumonia in ACOS patients [24,25]. We explored this relationship in reference to the general population [26].
2. Methods
2.1. Data Source
This retrospective cohort study was conducted using data from the Longitudinal Health Insurance Database of one million enrollees extracted from the Taiwan National Health Insurance (NHI) Research Database (NHIRD) between 2000 and 2011 [27]. The NHI program, implemented since 1995, provides comprehensive medical care to nearly all of Taiwan’s population and covers the registry of beneficiaries, ambulatory care, inpatient care, prescriptions, and other medical services. This program has been described in numerous studies; for instance, by using 2005–2009 data from the NHIRD [28], Cheng et al. reported no association between cumulative statin use and intracranial hemorrhage risk in patients without a past history of stroke. This experience was incorporated into the 2017 Taiwan lipid guidelines for high risk patients [29]. Similarly, our study can provide baseline trends useful for further research on statins or other hyperlipidemia drug use [30]. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used to define the diseases. This research was approved by the Research Ethics Committee at China Medical University and Hospital, Taiwan (27 June 2018, CMUH104-REC2-115-CR3).
2.2. Patients
The patients aged ≥18 years diagnosed as having ACOS [i.e., COPD (ICD-9-CM codes 491, 492, and 496) and/or asthma (ICD-9-CM code 493)] between 2000 and 2010 were selected. The date of ACOS diagnosis was defined as the index date [26,31]. Patients aged <18 years or having baseline diagnoses of TB (ICD-9-CM codes 010–018) or pneumonia (ICD-9-CM codes 481–486) were excluded. Patients who did and did not receive statin treatment were defined as statin users and nonusers, respectively.
2.3. ACOS, Pneumonia, and TB Patient Validation
In Taiwan, COPD is diagnosed through chest X-ray (CXR; 84.7%), computed tomography (CT; 39.4%), pulmonary function test (PFT; 58.44%), and smoking status (82.9%) [32]. Su et al. report high sensitivity of these asthma (92.0%) and COPD (86.2%) [33] diagnosis methods. ACOS patients undergo these diagnostic procedures often because medical services for diseases, such as pneumonia and TB, are easily available [21,23]. Moreover, in the 12 months following the index date, ACOS patients undergo CXR, CT, and PFT more often than do COPD or asthma patients—which supports our speculations [26]. Thus, extracting data of ACOS patients from among COPD and asthma patients in the NHIRD is reasonable.
We validated ACOS patients based on the following information: (1) Clinical manifestation in form of clinical symptoms or signs, such as chronic productive cough for 3 months in 2 successive years in a patient, wheezing, and dyspnea, aid early ACOS detection [34]; (2) Imaging, particularly CXR, has a 90% sensitivity and 98% specificity for emphysema [35] (51.0% of ACOS patients undergo CXR [26]); (3) ICS or OS use is a useful tool for confirming ACOS patients [36]. We used the anatomical therapeutic chemical code R03 for confirming of ACOS (24.0% and 76.0% ICS and OS use, respectively). Similarly, Su et al. reported ICS use in 53.48% of ACOS patients during follow-up [33], and Shantakumar et al. reported ICS and OS use in 46.1% and 85.5% of ACOS patients during a 1-year follow-up [26]. In the current study, 11,129 (98.9%) of 11,256 ACOS patients undertook COPD-, asthma-, TB-, or pneumonia-related diagnostic tests, such as CXR, PFT, CT, or immunoglobulin E levels, validating our ACOS cohort from the NHIRD.
In Taiwan, TB is confirmed through sputum culture and smear, CXR with clinical symptoms and signs, and therapeutic response to anti-TB drugs [37]. Su et al. found that among 433 patients diagnosed as having TB, 326 received at least two anti-TB drugs for 4 weeks and were selected for validation of the TB definition. Of these, 314 were confirmed as having TB [38] at a sensitivity of 96.3%. Pneumonia was confirmed through sputum smear and culture, blood culture, elevation in serum blood titer or urinary antigen levels, CXR, and therapeutic response to antibiotics [39]. Overall, 284 of 300 inpatients and 277 of 300 outpatients were validated as having pneumonia, with a sensitivity was 94.7% and 92.3%, respectively.
Therefore, the TB and pneumonia codes in the NHIRD are typical reflect the real-world diagnosis of TB and pneumonia in Taiwan.
2.4. Outcome Measurement and Potential Comorbidities
The endpoint of this study was TB and pneumonia incidence. All study participants were followed up from the index date until death, withdrawal from insurance, or the study endpoint (31 December 2011). We also included potential TB- or pneumonia-related comorbidities, including sleep disorders, diabetes, hypertension, hyperlipidemia, mental disorders, alcohol-related illnesses, and chronic kidney disease. Other medications such as ICSs and OSs were included if they were potentially correlated with TB or pneumonia development.
2.5. Propensity Score Matching
Statin users and nonusers were matched at a 1:1 ratio based on their propensity scores. We used logistic regression to calculate the propensity score for each patient by estimating assignment probability according to the baseline variables of age, sex, comorbidities (sleep disorders, diabetes, hypertension, hyperlipidemia, mental disorders, alcohol-related illness, and chronic kidney disease), and use of other medications (ICSs and OSs). This ensured that a similar number of patients were included in both the cohorts with an equal probability to statin use.
2.6. Statistical Analysis
Differences in demographic distributions, baseline comorbidities, and medications between statin users and nonusers were compared using the chi-square test for categorical variables and Student’s t test for mean ages. The Kaplan–Meier method was used to measure the cumulative TB or pneumonia incidence among statin users and nonusers. The cumulative curves of the two cohorts were tested using the log-rank test. However, ACOS patients may have taken their prescription irregularly during the study period; this may have led to an overestimation of the drug effects. To reduce this bias, we used Cox proportional hazard models with time-dependent exposure covariates [40] to estimate the hazard ratios (HRs) and their 95% confidence intervals (CIs) for TB and pneumonia in statin users relative to nonusers. Next, aHRs were measured after adjustments for age, sex, comorbidities, and use of medications and stratification by age, sex, and ICS and OS use. Moreover, we performed sensitivity analysis by developing Cox models based on propensity score–matched cohorts to refine our results. All statistical analyses were performed using SAS (version 9.4 for Windows; SAS Institute, Inc., Cary, NC, USA). A two-tailed p of <0.05 was considered statistically significant.
3. Results
Table 1 demonstrates that compared with nonusers, statin users were slightly younger (62.7 ± 11.7 vs. 64.4 ± 14.8 years), with a higher female: male ratio, higher prevalence of comorbidities, and higher likelihood of ICS or OS use.
Table 1.
Demographic characteristics and clinical comorbidities.
| ACOS | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Statin | |||||||
| All (n = 11256) | No (n = 9206) | Yes (n = 2050) | |||||
| n | % | n | % | n | % | ||
| Age, years | <0.001 | ||||||
| <50 | 1960 | 17.4 | 1656 | 18.0 | 304 | 14.8 | |
| 50–64 | 3283 | 29.2 | 2502 | 27.2 | 781 | 38.1 | |
| 65+ | 6013 | 53.4 | 5048 | 54.8 | 965 | 47.1 | |
| Mean ± SD a | 64.1 | 14.3 | 64.4 | 14.8 | 62.7 | 11.7 | <0.001 |
| Gender | <0.001 | ||||||
| Women | 5029 | 44.7 | 3969 | 43.1 | 1060 | 51.7 | |
| Men | 6227 | 55.3 | 5237 | 56.9 | 990 | 48.3 | |
| Urbanization level § | 0.32 | ||||||
| 1 (highest) | 2888 | 25.7 | 2367 | 25.7 | 521 | 25.4 | |
| 2 | 3116 | 27.7 | 2522 | 27.4 | 594 | 29.0 | |
| 3 | 1822 | 16.2 | 1513 | 16.4 | 309 | 15.1 | |
| 4 (lowest) | 3430 | 30.5 | 2804 | 30.5 | 626 | 30.5 | |
| Monthly income (NTD) | 0.001 | ||||||
| <15,840 | 3427 | 30.5 | 2872 | 31.2 | 555 | 27.1 | |
| 15,840–25,200 | 5379 | 47.8 | 4357 | 47.3 | 1022 | 49.9 | |
| ≥25,200 | 2450 | 21.8 | 1977 | 21.5 | 473 | 23.1 | |
| Occupation | 0.005 | ||||||
| White collar | 3996 | 35.5 | 3287 | 35.7 | 709 | 34.6 | |
| Blue collar | 4924 | 43.8 | 3967 | 43.1 | 957 | 46.7 | |
| Others ‡ | 2336 | 20.8 | 1952 | 21.2 | 384 | 18.7 | |
| Comorbidity | |||||||
| Sleep disorder | 5262 | 46.8 | 4127 | 44.8 | 1135 | 55.4 | <0.001 |
| Diabetes | 2449 | 21.8 | 1740 | 18.9 | 709 | 34.6 | <0.001 |
| Hypertension | 7958 | 70.7 | 6219 | 67.6 | 1739 | 84.8 | <0.001 |
| Hyperlipidemia | 4662 | 41.4 | 3026 | 32.9 | 1636 | 79.8 | <0.001 |
| Mental disorders | 6858 | 60.9 | 5443 | 59.1 | 1415 | 69.0 | <0.001 |
| Alcohol-related illness | 1049 | 9.32 | 839 | 9.11 | 210 | 10.2 | 0.02 |
| Chronic kidney disease | 915 | 8.13 | 718 | 7.80 | 197 | 9.61 | <0.001 |
| Tobacco use | 526 | 4.67 | 426 | 4.63 | 100 | 4.88 | 0.63 |
| Coronary artery disease | 5492 | 48.8 | 4232 | 46.0 | 1260 | 61.5 | <0.001 |
| Stroke | 1986 | 17.6 | 1591 | 17.3 | 395 | 19.3 | 0.03 |
| Acute respiratory failure | 1405 | 12.5 | 1220 | 13.3 | 185 | 9.02 | <0.001 |
| Medication | |||||||
| Inhaled corticosteroids (ICSs) | 2706 | 24.0 | 2148 | 23.3 | 558 | 27.2 | <0.001 |
| Oral steroids (OSs) | 8550 | 76.0 | 6876 | 74.7 | 1674 | 81.7 | <0.001 |
Chi-square test; a Student’s t test; Monthly income in New Taiwan Dollar (NT$; NT$1 = US$0.03). § Urbanization level was categorized into four levels according to the population density of the residential area, with Level 1 and 4 being the most and least urbanized, respectively. ‡ Other occupational categories included primarily retired, unemployed, and low-income groups. ACOS: asthma–chronic pulmonary disease overlap syndrome.
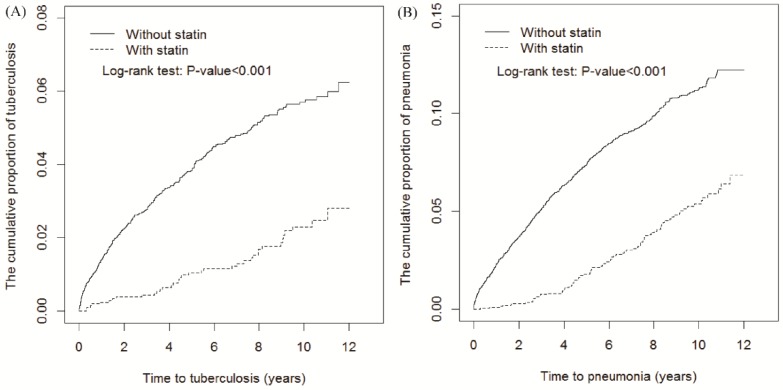
The follow-up period was longer in statin users (8.18 ± 2.76 years) than in nonusers (5.99 ± 3.88 years; Table 2). By the end of the 12-year follow-up, cumulative TB and pneumonia incidence was 3.39% and 5.3% lower in statin users than in nonusers, respectively (both p < 0.001, log-rank test; Figure 1A,B respectively). Moreover, the time-dependent regression analysis after adjustment for age, sex, urbanization level, monthly income, occupation, comorbidities, and other medication use revealed that statin users had aHRs (95% CIs) of 0.49 (0.34–0.70) and 0.52 (0.41–0.65) for TB and pneumonia, respectively. TB and pneumonia incidence was respectively 2.21 and 5.23 per 1000 person-years in statin users and 6.96 and 13.7 per 1000 person-years in statin nonusers.
Table 2.
Cumulative tuberculosis and pneumonia incidence and hazard ratios of statin users and nonusers.
| Statin | ||
|---|---|---|
| Variables | No (n = 9206) | Yes (n = 2050) |
| Tuberculosis | ||
| Person-years | 55,164 | 16,771 |
| Follow-up time (year), Mean ± SD | 5.99 ± 3.88 | 8.18 ± 2.76 |
| Event, n | 384 | 37 |
| Rate | 6.96 | 2.21 |
| cHR (95% CI) | 1 (Reference) | 0.34 (0.25, 0.48) *** |
| aHR (95% CI) a | 1 (Reference) | 0.49 (0.34, 0.70) *** |
| Pneumonia | ||
| Person-years | 54,267 | 16,649 |
| Follow-up time (year), Mean ± SD | 5.89 ± 3.41 | 8.12 ± 2.76 |
| Event, n | 742 | 87 |
| Rate | 13.7 | 5.23 |
| cHR (95% CI) | 1 (Reference) | 0.40 (0.32, 0.50) *** |
| aHR (95% CI) a | 1 (Reference) | 0.52 (0.41, 0.65) *** |
Incidence in per 1000 person-years. a Data adjusted for age, sex, urbanization level, monthly income, occupation, comorbidities (sleep disorders, diabetes, hypertension, hyperlipidemia, mental disorders, alcohol-related illness, chronic kidney disease, tobacco use, CAD, stroke, and acute respiratory failure), and use of medication (ICSs and OSs). Abbreviations: cHR, crude hazard ratio; aHR, adjusted hazard ratio; ICSs, inhaled corticosteroids; OSs, oral steroids. *** p < 0.001.
Figure 1.
Cumulative TB (A) and pneumonia (B) incidence curves for statin users and nonusers.
Table 3 lists TB and pneumonia incidence and HRs stratified by age and sex in both cohorts. Both male and female statin users had lower TB and pneumonia risks than did nonusers. Among patients aged ≥50 years, the TB and pneumonia risks were lower in statin users than in nonusers.
Table 3.
Age- and sex-stratified tuberculosis and pneumonia incidence and hazard ratios.
| Men | Women | |||
| Statin | Statin | |||
| No (n = 5237) | Yes (n = 990) | No (n = 3969) | Yes (n = 1060) | |
| Tuberculosis | ||||
| No. of event | 282 | 26 | 102 | 11 |
| Incidence rate | 9.30 | 3.29 | 4.11 | 1.24 |
| cHR (95% CI) | 1 (Reference) | 0.38 (0.26, 0.57) *** | 1 (Reference) | 0.33 (0.18, 0.61) *** |
| aHR (95% CI) a | 1 (Reference) | 0.53 (0.34, 0.81) ** | 1 (Reference) | 0.41 (0.21, 0.79) ** |
| Pneumonia | ||||
| No. of event | 525 | 57 | 217 | 30 |
| Incidence rate | 17.6 | 7.27 | 8.87 | 3.41 |
| cHR (95% CI) | 1 (Reference) | 0.43 (0.33, 0.57) *** | 1 (Reference) | 0.41 (0.28, 0.60) *** |
| aHR (95% CI) a | 1 (Reference) | 0.54 (0.41, 0.73) *** | 1 (Reference) | 0.47 (0.32, 0.70) *** |
| Age < 50 | Age ≥ 50 | |||
| Statin | Statin | |||
| No (n = 1656) | Yes (n = 304) | No (n = 7550) | Yes (n = 1746) | |
| Tuberculosis | ||||
| No. of event | 22 | 4 | 362 | 33 |
| Incidence rate | 1.84 | 1.50 | 8.38 | 2.34 |
| cHR (95% CI) | 1 (Reference) | 0.85 (0.29, 2.48) | 1 (Reference) | 0.31 (0.21, 0.44) *** |
| aHR (95% CI) a | 1 (Reference) | 0.74 (0.22, 2.49) | 1 (Reference) | 0.45 (0.31, 0.65) *** |
| Pneumonia | ||||
| No. of event | 33 | 2 | 709 | 85 |
| Incidence rate | 2.78 | 0.75 | 16.7 | 6.08 |
| cHR (95% CI) | 1 (Reference) | 0.29 (0.07, 1.21) | 1 (Reference) | 0.38 (0.30, 0.48) *** |
| aHR (95% CI) a | 1 (Reference) | 0.20 (0.05, 0.91) * | 1 (Reference) | 0.52 (0.41, 0.67) *** |
Incidence in per 1000 person-years. a Data adjusted for age, sex, urbanization level, monthly income, occupation, comorbidities (sleep disorders, diabetes, hypertension, hyperlipidemia, mental disorders, alcohol-related illness, chronic kidney disease, tobacco use, CAD, stroke, and acute respiratory failure), and use of medication (ICSs and OSs). Abbreviations: cHR, crude hazard ratio; aHR, adjusted hazard ratio; ICSs, inhaled corticosteroids; OSs, oral steroids. * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 4 presents TB and pneumonia incidence and HRs stratified by ICS and OS use in both cohorts. Compared with nonusers, statin use combined with ICS or OS use was significantly associated with lower pneumonia risk in statin users; however, statin use combined with OS use, but not ICS use, led to significantly lower TB risk.
Table 4.
ICS and OS use-stratified tuberculosis and pneumonia incidence and hazard ratios.
| With ICSs | Without ICSs | |||
| Statin | Statin | |||
| No (n = 2148) | Yes (n = 558) | No (n = 7058) | Yes (n = 1492) | |
| Tuberculosis | ||||
| No. of event | 76 | 12 | 308 | 25 |
| Incidence rate | 5.33 | 2.54 | 7.53 | 2.07 |
| cHR (95% CI) | 1 (Reference) | 0.49 (0.26, 0.89) * | 1 (Reference) | 0.31 (0.20, 0.46) *** |
| aHR (95% CI) a | 1 (Reference) | 0.60 (0.31, 1.16) | 1 (Reference) | 0.44 (0.29, 0.67) *** |
| Pneumonia | ||||
| No. of event | 151 | 26 | 591 | 61 |
| Incidence rate | 10.8 | 5.56 | 14.7 | 5.09 |
| cHR (95% CI) | 1 (Reference) | 0.52 (0.34, 0.78) ** | 1 (Reference) | 0.37 (0.29, 0.48) *** |
| aHR (95% CI) a | 1 (Reference) | 0.61 (0.39, 0.95) * | 1 (Reference) | 0.48 (0.36, 0.63) *** |
| With OSs | Without Oss | |||
| Statin | Statin | |||
| No (n = 6876) | Yes (n = 1674) | No (n = 2330) | Yes (n = 376) | |
| Tuberculosis | ||||
| No. of event | 237 | 34 | 147 | 3 |
| Incidence rate | 5.61 | 2.46 | 11.4 | 1.02 |
| cHR (95% CI) | 1 (Reference) | 0.46 (0.32, 0.66) *** | 1 (Reference) | 0.10 (0.03, 0.32) *** |
| aHR (95% CI) a | 1 (Reference) | 0.58 (0.40, 0.85) ** | 1 (Reference) | 0.16 (0.05, 0.53) ** |
| Pneumonia | ||||
| No. of event | 534 | 79 | 208 | 8 |
| Incidence rate | 12.9 | 5.76 | 16.1 | 2.72 |
| cHR (95% CI) | 1 (Reference) | 0.46 (0.36, 0.58) *** | 1 (Reference) | 0.19 (0.09, 0.38) *** |
| aHR (95% CI) a | 1 (Reference) | 0.57 (0.45, 0.74) *** | 1 (Reference) | 0.26 (0.12, 0.53) *** |
Incidence in per 1000 person-years. a Data adjusted for age, sex, urbanization level, monthly income, occupation, comorbidities (sleep disorders, diabetes, hypertension, hyperlipidemia, mental disorders, alcohol-related illness, chronic kidney disease, tobacco use, CAD, stroke, and acute respiratory failure), and use of medication (ICSs and OSs). Abbreviations: cHR, crude hazard ratio; aHR, adjusted hazard ratio; ICSs, inhaled corticosteroids; OSs, oral steroids. * p < 0.05, ** p < 0.01, *** p < 0.001.
The results of sensitivity analysis of propensity score–matched cohorts are listed in Table 5. TB incidence was 2.31 and 5.15 per 1000 person-years in statin users and nonusers, respectively. Statin users had a 0.37- and 0.47-fold lower TB and pneumonia risks, respectively (95% CI 0.25–0.56 and 0.36–0.61, respectively).
Table 5.
Overall tuberculosis and pneumonia incidence and hazard ratios in statin users vs. propensity score–matched statin nonusers.
| Statin | ||
|---|---|---|
| Variables | No (n = 1984) | Yes (n = 1984) |
| Tuberculosis | ||
| Person-years | 14,570 | 16,040 |
| Follow-up time (year), Mean ± SD | 7.34 ± 3.25 | 8.08 ± 2.75 |
| Event, n | 75 | 37 |
| Rate | 5.15 | 2.31 |
| cHR (95% CI) | 1 (Reference) | 0.38 (0.26, 0.57) *** |
| aHR (95% CI) a | 1 (Reference) | 0.37 (0.25, 0.56) *** |
| Pneumonia | ||
| Person-years | 14,308 | 15,924 |
| Follow-up time (year), Mean ± SD | 7.21 ± 3.32 | 8.03 ± 2.75 |
| Event, n | 150 | 86 |
| Rate | 10.5 | 5.40 |
| cHR (95% CI) | 1 (Reference) | 0.49 (0.38, 0.64) *** |
| aHR (95% CI) a | 1 (Reference) | 0.47 (0.36, 0.61) *** |
Incidence in per 1000 person-years. a Data adjusted for age, sex, urbanization level, monthly income, occupation, comorbidities (sleep disorders, diabetes, hypertension, hyperlipidemia, mental disorders, alcohol-related illness, chronic kidney disease, tobacco use, CAD, stroke, and acute respiratory failure), and use of medication (ICSs and OSs). Abbreviations: cHR, crude hazard ratio; aHR, adjusted hazard ratio; ICSs, inhaled corticosteroids; OSs, oral steroids. *** p < 0.001.
3.1. Sensitivity Analysis
Because most ACOS patients have higher readmission and mortality rates after discharge, we used a time-dependent model and long-term protective effects of statins to follow statin users (8.18 ± 2.76 years) and nonusers (5.99 ± 3.88 years). Long-term use was according to the higher cumulative dose recommended by the strict chronic prescription policy of Taiwan NHI. We further measured ICS and OS use among statin users to examine the effects of statins on TB and pneumonia risk further. Table 4 presents that the dichotomous effects of statins combined with ICSs or OSs noted in this study are similar to a forest plot.
3.2. Healthy User Bias
Considering “healthy user bias” is essential in the retrospective study of the pneumonia and TB outcomes of statin users. This bias is described as a higher awareness of health and a healthier lifestyle among statin users than that among nonusers. Therefore, statin users are potentially more likely to seek preventive health services, such as CXR, PFT, sputum culture, and vaccinations, particularly in the current ACOS cohort. However, measuring lifestyle factors, disease prevention behaviors, and drugs compliance in observational studies is difficult because lifestyle, income, and urbanization are confounded by alcohol-related diseases and sleep and mental disorders. To reduce the impact of the confounding healthy user bias, we measured alcohol-related diseases and sleep and mental disorders through individual insurance as a proxy to adjust for socioeconomic status. Propensity score matching, which included these as baseline variables, further reduced the healthy user bias. Furthermore, the factors associated with latent TB, such as nutrition (e.g., diabetes or hyperlipidemia), immune status, and steroid use, were included in the analysis. These statistical methods enable observational studies to simulate the results of randomized control trials.
3.3. Indication Bias of Statin Use with ICSs or OSs
The indication bias of statin may also be challenged in this study. In Taiwan, statins and ICSs are not available over the counter; the physicians’ decision to provide statin or ICS treatment should not only follow the treatment guidelines for the specific diseases but also the payment regulations by the NHI. If the prescriptions contravene Taiwan lipid guidelines and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, the NHI Administration may not only refuse to pay the medical fee but also punish the physicians with a maximum 100-fold penalty. This is because NHI is a single-payer compulsory insurance coverage policy and the NHI Administration has full authority to control all medical facilities and the concerned health care.
4. Discussion
The most crucial finding of this study was that statin-using ACOS patients had lower TB or pneumonia risk and that concurrent use of statin and ICSs or OSs was also associated a lower TB or pneumonia risk.
In asthma patients, statins can improve outcomes in combination with either ICSs or OSs [24,25]. Emergency rooms and hospitals are the sources of nosocomial infection; therefore, the cohort with superior outcomes and more infrequent visits may exhibit a lower incidence rate for nosocomial pneumonia and TB [19]. Moreover, the antibiotic effects of statins on TB during statin therapy are associated with a decreased active TB risk [41]—an observation supported by the effects of the duration of statin therapy in protecting against TB. A Taiwanese study using the NHIRD also reported that statin use is associated with a lower-than-usual TB risk [38].
According to an US study, ACOS patients can be classified as A (low risk, fewer symptoms, modified Medical Research Council (mMRC) score of 0–1, or COPD assessment test (CAT) score of <10), B (low risk, more symptoms, mMRC score of >2, or CAT score of >10), C (high risk, fewer symptoms, mMRC score of 0–1, or CAT score of <10), and D (high risk, more symptoms, mMRC score of >2, or CAT score of >10). The GOLD classifications characterizes A and B groups by a forced expiratory volume-1 second (FEV1) or forced vital capacity (FVC) of >50% with exacerbation of <1 per year and C and D groups by a FEV1 or FVC of <50% with exacerbation of >1 per year. Most ACOS patients are categorized in the B and D groups [34,42]. Poor lung function is associated with the development of TB or pneumonia classified in the D group (exacerbation of >1 per year) [31]. In a study, in COPD patients, statin use may have improved lung function, whereas it may have delayed decline in lung function in asthma patients [43]. These results are similar to those obtained by other studies, where after statin use in ACOS patients, some patients were reclassified from group D into group A (exacerbation of <1 per year), corresponding to the lowering of TB or pneumonia incidence [38,42]. These results are in accordance with those found in our study.
Improved lung function in COPD and asthma patients may be due to the anti-inflammatory effects of statin use [44,45,46]; moreover, concurrent use of ICSs or OSs with statin can lead to greater lung function improvement in an ACOS cohort [33]. These findings indicate the additive effects of statins and ICSs or OSs [47,48]; the combined use of statins and ICSs or OSs in ACOS patients may have therefore reduce the number of emergency hospital visits and exacerbation events [24] and thus the lower TB and pneumonia incidence, particularly in patients aged >50 years.
In another study, high long-term ICS or OS dosage was associated with an elevated TB risk in patients with asthma in the COPD cohort, with ICS use leading to the highest risk. Furthermore, exposure to ICSs was not associated with TB risk in the presence of OSs but with an increased TB risk in OS nonusers. The risk of TB among OS users has been well documented; moreover, the negative effects of ICSs have bed reported to be negligible, even at high doses. However, the previous studies have neither involved an ACOS cohort nor adjusted for statin and time dependency in their analyses. A population-based nested case–control study revealed that ICSs were not associated with TB development [49]; however, the benefits of ICSs and OSs may outweigh the risk of pneumonia in statin-using asthma patients [17,18]. ICSs and OSs demonstrated dichotomous effects on the incidence of TB and pneumonia in the ACOS cohort; hence, the effects of concurrent statin and ICS or OS treatment on TB development among patients in the ACOS cohort warrant further research.
The present work demonstrated that statins have beneficial anti-inflammatory and antioxidant effects beyond the reduction of cholesterol levels in TB or pneumonia patients. The accumulation of lipids in macrophages harboring TB is associated with persistent and phenotypic resistance to anti-TB drugs, leading to prolongation of treatment duration [8]. Statins play a role in the attenuation of both TB growth and infection-induced lipid accumulation in macrophages as well as the promotion of phagosomal maturation and autophagy. In line with the other studies, here, statins helped reduce TB development rate. Our study revealed that even with ICS or OS use, statin-using ACOS patients exhibited lower TB and pneumonia incidence. Determination of the correct statin administration dosage, timing, and duration among cohorts requires further confirmation.
By contrast, the reports mention of the statins without relationship or increase of the risk of pneumonia were found [50]. In a study, among 124,695 acute myocardial infarction (AMI) patients, 76,994 (61.9%) has statin prescription; of them, only 19,078 (15.3%) were diagnosed with pneumonia. Using an ordinary least squares method, the statin coefficient indicated that statin prescription was associated with a reduction in pneumonia incidence. However, the instrumental variables indicated that statin use was not associated with the reduction in pneumonia incidence. The study concluded that in AMI patients, the protective effect of statins against pneumonia is most likely the result of nonrandom treatment assignment (i.e., healthy user bias) [51].
In their meta-analysis, Chopra et al. revealed that two prospective studies (Majumdar and Yende et al.) found that statins did not reduce pneumonia-related mortality, but another prospective study (Chalmers et al.) found that they did [52]. Thus, Chopra et al. concluded that although statin use is associated with decreased pneumonia-related mortality, this effect weakens in important subgroups. According to Chalmers et al. statin use in animals reduced endothelial dysfunction and had antithrombotic effects, which improved pneumonia outcomes. Moreover, numerous clinical studies have suggested that statins may have a role in pneumonia prevention or prognosis improvement in hospitalized patients [53]. Only a randomized controlled study can completely explore the link among statins, pneumonia, and pneumonia-related mortality.
Our sensitivity analysis of propensity score–matched cohorts [54] revealed that statins were associated with a lower pneumonia and TB risks. This result may be explained by the different cohort and statistical methods used in the current research. We recommend using the optimal time, dose and duration of the statins may a conjunctive regimen [55] of the antibiotic, anti-TB or OSs/ICs in the ACOS cohort for attenuate the pneumonia sequelae or mortality [56]. These speculations warrant further large-scale research for confirmation.
4.1. Strengths
In traditional Kaplan–Meier or Cox regression analyses, a typical risk factor measured at baseline correlates with mortality thereafter. During follow-up, however, this may change: either the effect of a fixed baseline risk factor may vary over time, resulting in a weakening or strengthening of associations, or the risk factor itself may vary over time. A strength of the Cox model is its ability to encompass covariates this continually change. The reason for using time-dependent covariates is based on the underlying mechanisms of the Cox model; at the time of each event, the program compares current covariate values of the event subject to the current values of all others at risk at that given time. Time-dependent variables are those that can change in value over the course of the observation period [57]. Variables, such as medicine and the time at which patients receive ICSs or OSs and statins, may vary over time. Although researchers can hold the values of such variables fixed at a certain point in time (e.g., baseline), the changing values may have yielded different or even a more accurate analysis of data simply because we used as much data as possible. In this study, ACOS patients were identified based on COPD and medications used (e.g., ICSs) [26,36]. Moreover, pneumonia, TB, and ACOS diagnoses and follow-up were strongly validated by using other NHIRD-related study [26,31,37,58]. Furthermore, prescription and education policies regarding the use of ICSs or OSs and statins are well established in Taiwan [59,60]. Therefore, this NHIRD study (ACOS cohort and ICS, OS, or statin use) was representative of the real-world situation in Taiwan. We used propensity score matching to validate the sensitivity analysis and avoid selection bias.
4.2. Limitations
The following major points should be considered: (1) The modeling of a time-dependent covariate involves the selection of a function over time; the form this takes may not be obvious and requires considerable biological insight; (2) The selection of a complex functional form increases the possibility of excessive modeling and overfitting of a data set; (3) Numerous time-dependent covariates closely associated with the units under study are usually generated by that unit (e.g., ICS or OS and statin use) and alter the typical relationship between the hazard and survival functions; (4) time-dependent covariate models, except those under certain circumstances, do not allow the creation of individual predictive time-to-event curves; this is different from the Cox model, which only uses fixed covariate values; (5) Extreme caution must be exercised when modeling time-dependent exposure or treatments, particularly when changes in those variables are related to a participant’s health status, such as residential area (urban or rural) and insurance status (insured or uninsured). The opportunities inherent in time-dependent modeling (including the explicit relationship between longitudinal values and event occurrence) must be regarded considering potential biases, strong assumptions necessitated by the lack of other possible explanations, and need to select more complex functional forms for modeling [40]. Biochemical data of statin users and nonusers were unavailable in the NHIRD.
5. Conclusions
In ACOS patients, statin use was associated with lowest TB and pneumonia risks, regardless of age, sex, comorbidities, and ICS or OS use. Pneumonia risk was lower among users of statins combined with ICSs or Oss and TB risk was lower among the users of statins combined with OSs.
Acknowledgments
This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital (CMU106-ASIA-12, DMR-107-192); Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005-); Tseng-Lien Lin Foundation, Taichung, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Author Contributions
All authors contributed significantly and all agree with the manuscript content: Conception/Design: J.-J.Y. and C.-H.K.; Provision of study materials: C.-H.K.; Collection and/or assembly of data: All authors; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cao B., Huang Y., She D.Y., Cheng Q.J., Fan H., Tian X.L., Xu J.F., Zhang J., Chen Y., Shen N., et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin. Respir. J. 2018;12:1320–1360. doi: 10.1111/crj.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamauchi Y., Yasunaga H., Hasegawa W., Sakamoto Y., Takeshima H., Jo T., Matsui H., Fushimi K., Nagase T. Effect of outpatient therapy with inhaled corticosteroids on decreasing in-hospital mortality from pneumonia in patients with COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2016;11:1403–1411. doi: 10.2147/COPD.S107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambaw F., Mayston R., Hanlon C., Medhin G., Alem A. Untreated depression and tuberculosis treatment outcomes, quality of life and disability, Ethiopia. Bull. World Health Organ. 2018;96:243–255. doi: 10.2471/BLT.17.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restrepo M.I., Sibila O., Anzueto A. Pneumonia in Patients with Chronic Obstructive Pulmonary Disease. Tuberc. Respir. Dis. 2018;81:187–197. doi: 10.4046/trd.2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornheimer R., Shea K.M., Sato R., Pelton S.I., Weycker D. Pneumonia in Adults with Asthma: Impact on Subsequent Asthma Exacerbations. Open Forum Infect. Dis. 2016;3:1235. doi: 10.1093/ofid/ofw172.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen C.M., Varbo A., Nordestgaard B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017;38:2478–2486. doi: 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- 7.Gebremicael G., Amare Y., Challa F., Gebreegziabxier A., Medhin G., Wolde M., Kassa D. Lipid Profile in Tuberculosis Patients with and without Human Immunodeficiency Virus Infection. Int. J. Chron. Dis. 2017;2017 doi: 10.1155/2017/3843291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parihar S.P., Guler R., Khutlang R., Lang D.M., Hurdayal R., Mhlanga M.M., Suzuki H., Marais A.D., Brombacher F. Statin Therapy Reduces the Mycobacterium tuberculosis Burden in Human Macrophages and in Mice by Enhancing Autophagy and Phagosome Maturation. J. Infect. Dis. 2014;209:754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 9.Han R., Kornfeld H., Martens G. Is hypercholesterolemia a friend or a foe of tuberculosis? Infect. Immun. 2009;77:3514–3515. doi: 10.1128/IAI.00469-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee D., Chogtu B., Magazine R. Statins in Asthma: Potential Beneficial Effects and Limitations. Pulm. Med. 2015;2015:835204. doi: 10.1155/2015/835204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young R.P., Hopkins R.J. Statin use in pneumonia. Am. J. Med. 2013;126:e11–e12. doi: 10.1016/j.amjmed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Hothersall E., McSharry C., Thomson N.C. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61:729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinogradova Y., Coupland C., Hippisley-Cox J. Risk of pneumonia in patients taking statins: Population-based nested case-control study. Br. J. Gen. Pract. 2011;61:e742–e748. doi: 10.3399/bjgp11X606654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M.T., Lo Y.W., Tsai C.L., Chang L.C., Malone D.C., Chu C.L., Liou J.T. Statin use and risk of COPD exacerbation requiring hospitalization. Am. J. Med. 2013;126:598–606. doi: 10.1016/j.amjmed.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Goluszko P., Nowicki B. Membrane cholesterol: A crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect. Immun. 2005;73:7791–7796. doi: 10.1128/IAI.73.12.7791-7796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oczypok E.A., Oury T.D., Chu C.T. It’s a cell-eat-cell world: Autophagy and phagocytosis. Am. J. Pathol. 2013;182:612–622. doi: 10.1016/j.ajpath.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statt S., Ruan J.W., Hung L.Y., Chang C.Y., Huang C.T., Lim J.H., Li J.D., Wu R., Kao C.Y. Statin-conferred enhanced cellular resistance against bacterial pore-forming toxins in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2015;53:689–702. doi: 10.1165/rcmb.2014-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gowdy K.M., Fessler M.B. Emerging Roles for Cholesterol and Lipoproteins in Lung Disease. Pulm. Pharmacol. Ther. 2013;26:430–437. doi: 10.1016/j.pupt.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung W.-S., Lin C.-L., Kao C.-H. Comparison of Acute Respiratory Events Between Asthma–COPD Overlap Syndrome and COPD Patients: A Population-Based Cohort Study. Medicine. 2015;94:e755. doi: 10.1097/MD.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung J.W., Kong K.A., Lee J.H., Lee S.J., Ryu Y.J., Chang J.H. Characteristics and self-rated health of overlap syndrome. Int. J. Chron. Obstruct. Pulm. Dis. 2014;9:795–804. doi: 10.2147/COPD.S61093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh J.J., Wang Y.C., Kao C.H. Asthma-Chronic Obstructive Pulmonary Diseases Overlap Syndrome Increases the Risk of Incident Tuberculosis: A National Cohort Study. PLoS ONE. 2016;11:e0159012. doi: 10.1371/journal.pone.0159012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albanna A.S., Bachmann K., White D., Valiquette C., Menzies D. Serum lipids as biomarkers for therapeutic monitoring of latent tuberculosis infection. Eur. Respir. J. 2013;42:547–550. doi: 10.1183/09031936.00064713. [DOI] [PubMed] [Google Scholar]
- 23.Sasindran S., Torrelles J. Mycobacterium Tuberculosis Infection and Inflammation: What is Beneficial for the Host and for the Bacterium? Front. Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tse S.M., Li L., Butler M.G., Fung V., Kharbanda E.O., Larkin E.K., Vollmer W.M., Miroshnik I., Rusinak D., Weiss S.T., et al. Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. Am. J. Respir. Crit. Care Med. 2013;188:1076–1082. doi: 10.1164/rccm.201306-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokhandwala T., West-Strum D., Banahan B.F., Bentley J.P., Yang Y. Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective cohort analysis. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shantakumar S., Pwu R.F., D’Silva L., Wurst K., Kuo Y.W., Yang Y.Y., Juan Y.C., Chan K.A. Burden of asthma and COPD overlap (ACO) in Taiwan: A nationwide population-based study. BMC Pulm. Med. 2018;18:16. doi: 10.1186/s12890-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang I.K., Lin C.L., Yen T.H., Lin S.Y., Sung F.C. Comparison of survival between hemodialysis and peritoneal dialysis patients with end-stage renal disease in the era of icodextrin treatment. Eur. J. Intern. Med. 2018;50:69–74. doi: 10.1016/j.ejim.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Chang C.-H., Lin C.-H., Caffrey J.L., Lee Y.-C., Liu Y.-C., Lin J.-W., Lai M.-S. Risk of Intracranial Hemorrhage from Statin Use in Asians: A Nationwide Cohort Study. Circulation. 2015;131:2070–2078. doi: 10.1161/CIRCULATIONAHA.114.013046. [DOI] [PubMed] [Google Scholar]
- 29.Li Y.H., Ueng K.C., Jeng J.S., Charng M.J., Lin T.H., Chien K.L., Wang C.Y., Chao T.H., Liu P.Y., Su C.H., et al. 2017 Taiwan lipid guidelines for high risk patients. J. Formos. Med. Assoc. 2017;116:217–248. doi: 10.1016/j.jfma.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh H.-C., Hsu J.C., Lu C.Y. 10-year trends in statin utilization in Taiwan: A retrospective study using Taiwan’s National Health Insurance Research Database. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wurst K.E., St Laurent S., Hinds D., Davis K.J. Disease Burden of Patients with Asthma/COPD Overlap in a US Claims Database: Impact of ICD-9 Coding-based Definitions. J. Chron. Obstruct. Pulm. Dis. 2017;14:200–209. doi: 10.1080/15412555.2016.1257598. [DOI] [PubMed] [Google Scholar]
- 32.Cheng S.L., Chan M.C., Wang C.C., Lin C.H., Wang H.C., Hsu J.Y., Hang L.W., Chang C.J., Perng D.W., Yu C.J. COPD in Taiwan: A National Epidemiology Survey. Int. J. Chron. Obstruct. Pulm. Dis. 2015;10:2459–2467. doi: 10.2147/COPD.S89672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su V.Y.-F., Yang K.-Y., Yang Y.-H., Tsai Y.-H., Perng D.-W., Su W.-J., Chou K.-T., Su K.-C., Yen Y.-F., Chen P.-C. Use of ICS/LABA Combinations or LAMA Is Associated with a Lower Risk of Acute Exacerbation in Patients with Coexistent COPD and Asthma. J. Allergy Clin. Immunol. 2018 doi: 10.1016/j.jaip.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 34.Cosentino J., Zhao H., Hardin M., Hersh C.P., Crapo J., Kim V., Criner G.J. C45 ACROSS THE UNIVERSE OF COPD EPIDEMIOLOGY: Asthma-COPD Overlap Syndrome (ACOS) and the New GOLD Classification. Am. J. Respir. Crit. Care Med. 2015;191:1–2. [Google Scholar]
- 35.Miniati M., Monti S., Stolk J., Mirarchi G., Falaschi F., Rabinovich R., Canapini C., Roca J., Rabe K.F. Value of chest radiography in phenotyping chronic obstructive pulmonary disease. Eur. Respir. J. 2008;31:509–515. doi: 10.1183/09031936.00095607. [DOI] [PubMed] [Google Scholar]
- 36.Van Boven J.F.M., Román-Rodríguez M., Palmer J.F., Toledo-Pons N., Cosío B.G., Soriano J.B. Comorbidome, Pattern, and Impact of Asthma-COPD Overlap Syndrome in Real Life. Chest. 2016;149:1011–1020. doi: 10.1016/j.chest.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Yeh J. Validation of a model for predicting smear-positive active pulmonary tuberculosis in patients with initial acid-fast bacilli smear-negative sputum. Eur. Radiol. 2018;28:243–256. doi: 10.1007/s00330-017-4959-9. [DOI] [PubMed] [Google Scholar]
- 38.Su V.Y., Su W.J., Yen Y.F., Pan S.W., Chuang P.H., Feng J.Y., Chou K.T., Yang K.Y., Lee Y.C., Chen T.J. Statin use is associated with a lower risk of TB. Chest. 2017;152:598–606. doi: 10.1016/j.chest.2017.04.170. [DOI] [PubMed] [Google Scholar]
- 39.Yeh J.J., Chen S.C., Chen C.R., Yeh T.C., Lin H.K., Hong J.B., Wu B.T., Wu M.T. A high-resolution computed tomography-based scoring system to differentiate the most infectious active pulmonary tuberculosis from community-acquired pneumonia in elderly and non-elderly patients. Eur. Radiol. 2014;24:2372–2384. doi: 10.1007/s00330-014-3279-6. [DOI] [PubMed] [Google Scholar]
- 40.Wolkewitz M., Allignol A., Harbarth S., de Angelis G., Schumacher M., Beyersmann J. Time-dependent study entries and exposures in cohort studies can easily be sources of different and avoidable types of bias. J. Clin. Epidemiol. 2012;65:1171–1180. doi: 10.1016/j.jclinepi.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Su V.Y.-F. Statin use and the risk of tuberculosis: Data from 305,142 patients. Eur. Respir. J. 2016;48:OA4822. [Google Scholar]
- 42.Young R.P., Hopkins R.J. A new alphabet for COPD care: Where “E” stands for España. Eur. Respir. J. 2017;49:1601970. doi: 10.1183/13993003.01970-2016. [DOI] [PubMed] [Google Scholar]
- 43.Alexeeff S.E., Litonjua A.A., Sparrow D., Vokonas P.S., Schwartz J. Statin Use Reduces Decline in Lung Function: VA Normative Aging Study. Am. J. Respir. Crit. Care Med. 2007;176:742–747. doi: 10.1164/rccm.200705-656OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker D.Y., Edwards K.L. Statins in the treatment of asthma. Am. J. Health Syst. Pharm. 2013;70:1661–1669. doi: 10.2146/ajhp120680. [DOI] [PubMed] [Google Scholar]
- 45.Keddissi J.I., Younis W.G., Chbeir E.A., Daher N.N., Dernaika T.A., Kinasewitz G.T. The use of statins and lung function in current and former smokers. Chest. 2007;132:1764–1771. doi: 10.1378/chest.07-0298. [DOI] [PubMed] [Google Scholar]
- 46.Gan W., Man S., Senthilselvan A., Sin D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maneechotesuwan K., Ekjiratrakul W., Kasetsinsombat K., Wongkajornsilp A., Barnes P.J. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2,3-dioxygenase. J. Allergy Clin. Immunol. 2010;126:754–762. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Maneechotesuwan K., Kasetsinsombat K., Wamanuttajinda V., Wongkajornsilp A., Barnes P.J. Statins enhance the effects of corticosteroids on the balance between regulatory T cells and Th17 cells. Clin. Exp. Allergy. 2013;43:212–222. doi: 10.1111/cea.12067. [DOI] [PubMed] [Google Scholar]
- 49.Brode S.K., Campitelli M.A., Kwong J.C., Lu H., Marchand-Austin A., Gershon A.S., Jamieson F.B., Marras T.K. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur. Respir. J. 2017;50:1700037. doi: 10.1183/13993003.00037-2017. [DOI] [PubMed] [Google Scholar]
- 50.Yayan J. No significant detectable anti-infection effects of aspirin and statins in chronic obstructive pulmonary disease. Int. J. Med. Sci. 2015;12:280–287. doi: 10.7150/ijms.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polgreen L.A., Cook E.A., Brooks J.M., Tang Y., Polgreen P.M. Increased statin prescribing does not lower pneumonia risk. Clin. Infect. Dis. 2015;60:1760–1766. doi: 10.1093/cid/civ190. [DOI] [PubMed] [Google Scholar]
- 52.Chopra V., Rogers M.A., Buist M., Govindan S., Lindenauer P.K., Saint S., Flanders S.A. Is statin use associated with reduced mortality after pneumonia? A systematic review and meta-analysis. Am. J. Med. 2012;125:1111–1123. doi: 10.1016/j.amjmed.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Chalmers J.D., Short P.M., Mandal P., Akram A.R., Hill A.T. Statins in community acquired pneumonia: Evidence from experimental and clinical studies. Respir. Med. 2010;104:1081–1091. doi: 10.1016/j.rmed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Lee M.G., Lee C.C., Lai C.C., Hsu T.C., Porta L., Lee M., Chang S.S., Chien K.L., Chen Y.M. Preadmission statin use improves the outcome of less severe sepsis patients—A population-based propensity score matched cohort study. Br. J. Anaesth. 2017;119:645–654. doi: 10.1093/bja/aex294. [DOI] [PubMed] [Google Scholar]
- 55.Hennessy E., Adams C., Reen F.J., O’Gara F. Is there potential for repurposing statins as novel antimicrobials? Antimicrob. Agents Chemother. 2016;60:5111–5121. doi: 10.1128/AAC.00192-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia M., Huang W., Li L., Xu Z., Wu L. Statins reduce mortality after non-severe but not after severe pneumonia: A systematic review and meta-analysis. J. Pharm. Pharm. Sci. 2015;18:286–302. doi: 10.18433/J34307. [DOI] [PubMed] [Google Scholar]
- 57.Karim M.E., Gustafson P., Petkau J., Tremlett H. Long-Term B and Adverse Effects of Beta-Interferon for Multiple Sclerosis Study G. Comparison of Statistical Approaches for Dealing with Immortal Time Bias in Drug Effectiveness Studies. Am. J. Epidemiol. 2016;184:325–335. doi: 10.1093/aje/kwv445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh J.J., Wang Y.C., Hsu W.H., Kao C.H. Incident asthma and Mycoplasma pneumoniae: A nationwide cohort study. J. Allergy Clin. Immunol. 2016;137:1017–1023. doi: 10.1016/j.jaci.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 59.Yeh J.J., Wei Y.F., Lin C.L., Hsu W.H. Effect of the asthma-chronic obstructive pulmonary disease syndrome on the stroke, Parkinson’s disease, and dementia: A national cohort study. Oncotarget. 2018;9:12418–12431. doi: 10.18632/oncotarget.23811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jan C.-F., Chang H.-C., Tantoh D.M., Chen P.-H., Liu W.-H., Huang J.-Y., Wu M.-C., Liaw Y.-P. Duration-response association between exercise and HDL in both male and female Taiwanese adults aged 40 years and above. Oncotarget. 2018;9:2120–2127. doi: 10.18632/oncotarget.23251. [DOI] [PMC free article] [PubMed] [Google Scholar]