Abstract
CD117 is a putative marker of luminal progenitor cells in the human breast. However, so far mapping the expression pattern of CD117 within the normal gland has not been reported. Here, we examined the anatomical distribution of CD117-expressing cells in lobular and ductal structures by immunohistochemistry. The presence of CD117-positive luminal cells could be divided into three distinct patterns: (1) contiguous, with coherent positive cells and rare negative cells interspaced; (2) patched, with a roughly equal frequency of positive and negative cells distributed focally; or (3) scattered, with few or no positive cells in the structure. Generally, a patched or scattered expression pattern was more frequent in lobules compared with ducts. Furthermore, an age-correlated increase in heterogeneity was observed. When comparing women below and above 21 years of age this heterogeneity was evident for both lobules and ducts. Although CD117-expression was generally segregated from luminal-lineage transcription factor GATA3-positive cells, some did co-express both markers. Finally, co-staining with Ki-67 revealed that a prominent part of cycling cells belonged to the CD117-positive population. Together these data demonstrate the presence of a CD117-expressing progenitor compartment with the capacity to replenish the luminal lineage of the breast gland.
Keywords: CD117, c-Kit, GATA3, immunohistochemistry, mammary gland
Introduction
Identifying the normal cell(s) of origin for breast carcinomas has implications both for prognosis and treatment intervention. Recent data suggest that at least a subset of breast carcinomas are initiated in progenitors within the luminal epithelial compartment. Molyneux et al. provided a new insight in to the cell of origin concept by deletion of the BRCA1 gene in murine models.1 Luminal cells, rather than basal cells of BRCA1 mutation carriers were able to give rise to estrogen receptor (ER)-negative basal-like tumors. In humans, Lim et al. found that the breast gland epithelium of BRCA1 mutation carriers was enriched for luminal progenitors.2 Furthermore, they reported that the gene expression profile of basal-like breast cancer had most similarity to that of luminal progenitors among the different lineages in the normal breast. Thus, a thorough characterizing of the luminal progenitor compartment of the normal human breast encourages for new strategies that eventually allow for early diagnosis and a more effective treatment.
CD117, also named c-Kit or stem cell growth factor receptor, is a transmembrane receptor tyrosine kinase which is activated when associating with its ligand, stem cell factor (SCF).3 CD117 is presumably expressed in progenitor cells of different tissues that include the hematopoietical system, testis, myocardium, lung, and breast.4–9 In the adult human breast, a substantial number of luminal epithelial cells express CD117.10 Transcriptome analysis of populations isolated by flow cytometry suggest that expression of CD117 correlates to the luminal progenitor compartment.9 In support of this finding, the CD117-positive compartment can be segregated from a CD166-expressing population enriched for mature luminal markers including steroid receptors.11 Thus, currently the proposed hypothesis dictates that early and late luminal progenitors express significant levels of CD117, which is reduced as the cells differentiate along the luminal hierarchy. Regan and colleagues demonstrated that knocking down CD117 reduced proliferation and increased the level of apoptosis of mouse mammary epithelial cells.12 However, it is still not known which functional role CD117 plays with regard to sustaining cells in a progenitor-state, how the expression is modulated during differentiation, and if any changes in the balance between differentiation states can affect the susceptibility to breast cancer initiation and progression.
To get more insight into the distribution and dynamics of the CD117-positive luminal subpopulation in the normal breast gland, here we have mapped the expression pattern including the lobular and ductal distribution of these cells in normal breast tissue from a large selection of female individuals of different ages.
Materials and Methods
Acquisition of Human Tissue Samples
Normal human breast specimens were obtained from consenting patients that have undergone reduction mammoplasty for cosmetic reasons. The operations were performed at CFR Hospitaler, Lyngby, Denmark. The only information that was passed on was the current age. Material from some of the biopsies have been included in previous studies. However, picking the sample material was done randomly, and all relevant tissue stainings were done specifically for this study. Normal-derived tissue specimens from lactating patients were obtained from mastectomies performed at the State University Hospital, Copenhagen, Denmark. The use of human material complies with the ethical committee approval (Regional Scientific Ethical Committees for Copenhagen and Frederiksberg (KF) (11)263995 and for Region Hovedstaden H-2-2011-052). Fresh tissue specimens were snap frozen and conserved at −80C until sectioning. Material for flow cytometric analysis was digested by collagenase treatment and harvesting of epithelial organoids as described previously13,14 followed by preservation in liquid nitrogen until use.
Immunohistochemistry
Cryopreserved tissue specimens were cut at 6 µm on a cryostat (Microm HM560, Axlab, Vedbæk, Denmark) and were subsequently fixed in 3.7% formaldehyde (Merck, Darmstadt, Germany) followed by permeabilization with 0.1% Tritin X-100 detergent (Sigma-Aldrich, Søborg, Denmark). For peroxidase stainings, sections were incubated with primary antibody for 1 hr followed by utilization of Ultravision ONE Detection System according to the manufacturer’s instructions (Lab Vision, ThermoFisher Scientific, Roskilde, Denmark). Nuclei were stained with hematoxylin. For immunofluorescence specimens were incubated for 2 hr with primary antibody followed by incubation with isotype-specific Alexa Fluor secondary antibodies (Life Technologies, Roskilde, Denmark). Nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole). Antibody specificity was routinely controlled by incubating with an irrelevant primary antibody or by omitting primary antibody from the staining procedure. Antibodies and dilutions are listed in Table 1.
Table 1.
Antibodies Used for Tissue Stainings and Flow Cytometry.
| Antibody | Clone | Isotype | Company (Cat. No.) | Dilution, Peroxidase | Dilution, Fluorescence | Dilution, Flow Cytometry |
|---|---|---|---|---|---|---|
| CAM5.2 | CAM5.2 | IgG2a | BD Biosciences (345779) | 1:25 | 1:10 | — |
| CD117 | K45 | IgG2a | Thermo Scientific (MS-289-P) | 1:100 | 1:50 | — |
| CD117 | 104D2 | IgG1 | Aviva Systems (OAEE00056) | 1:100 | 1:50 | — |
| CD326 | 9C4 | IgG2b | BioLegend (324202) | — | 1:25 | — |
| ERα | 1D5 | IgG1 | Dako (M7047) | 1:50 | — | — |
| ERα | Sp1 | Rabbit | Thermo Scientific (RM-9101-S) | 1:50 | — | |
| GATA3 | HG3-31 | IgG1 | Santa Cruz (sc-268) | 1:200 | 1:50 | — |
| Ki-67 | MIB-1 | IgG1 | Dako (M7240) | 1:100 | 1:50 | — |
| Ki-67 | Sp6 | Rabbit | Thermo Scientific (RM-9106-S) | 1:100 | 1:25 | — |
| CD117-PE | 104D2 | IgG1 | BD Biosciences (332785) | — | — | 1:20 |
| CD166-Alexa Fluor 488 | 3A6 | IgG1 | AbD Serotec (MCA1926A488) | — | — | 1:20 |
| CD271-APC | ME20.4 | IgG1 | Cedarlane (CL10013APC) | — | — | 1:50 |
| CD326-PerCP/Cy5.5 | 9C4 | IgG2b | BioLegend (324214) | — | — | 1:20 |
Microscopy
Routine analysis by brightfield and fluorescence were performed on a Leica DM550B microscope. Peroxidase images were acquired on this microscope equipped with a DFC550 camera (Leica Microsystems, Copenhagen, Denmark). Fluorescent images were captured using a confocal microscope (Zeiss LSM 700) at the Core Facility for Integrated Microscopy, Faculty of Health Sciences, University of Copenhagen.
Flow Cytometry
Preparation of single cells from primary organoids and staining with fluorescent-conjugated primary antibodies were done as described previously.11 Analysis was performed on a flow cytometer (FACSAria II, Becton Dickinson, Lyngby, Denmark).
Statistical Analysis
To evaluate a significant difference between two groups of which variables were not fit into a normal distribution, Mann–Whitney was performed. Spearman rank correlation test was performed to measure the degree of association between age and CD117-expression. All the statistical analyses were tested using a statistical computing and graphic program R (version 3.3.2).
Results
CD117-Positive Cells Are Distributed in Different Patterns in Normal Human Breast Gland Structures
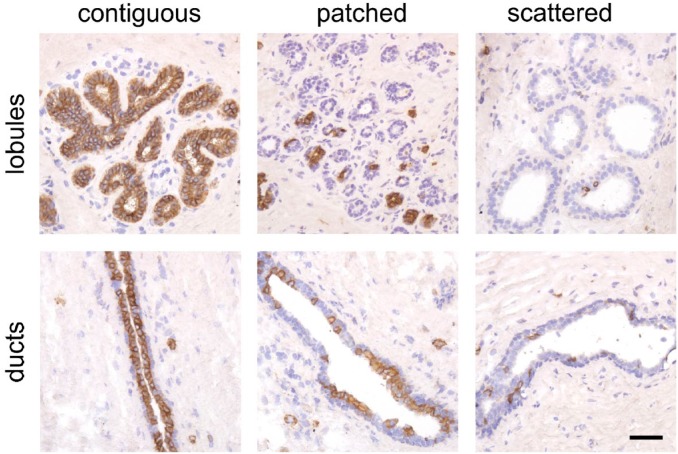
Although expression of CD117 has been correlated to a luminal progenitor compartment in the human breast, a more detailed evaluation of the distribution of CD117-positive cells in the normal human breast gland has yet to be reported. Here we analyzed the expression of CD117 by immunohistochemistry in tissue sections from 57 female donors (age range 13–75 years) who underwent breast gland reduction mammoplasty. A total of 1209 individual lobules or ducts were evaluated and we found three distinct patterns of CD117-expression in the luminal compartment of these structures: (1) contiguous segments of positive cells interspaced by infrequent negative cells; (2) patches of CD117-positive mixed with patches of negative cells at somewhat varying frequencies; and (3) infrequent CD117-positive cells scattered as single cells or in some cases completely missing (Fig. 1). We utilized two different antibody clones for CD117 stainings, K45 and 104D2, and essentially they reacted identically (Fig. S1). Overall, the majority of structures (77%) were characterized by a contiguous expression pattern (Table S1). However, a contiguous pattern was more frequently observed in ducts (306 out of 361, 85%) compared with lobules (621 out of 848, 68%) (p<0.001, Mann–Whitney test).
Figure 1.
CD117 is expressed in different patterns in the breast gland. Representative images of immunohistochemically stained normal breast tissue sections with luminal cells expressing CD117 in different patterns from lobules and ducts: contiguous, patched, and scattered. Bar, 50 µm.
Thus, while CD117-expressing cells are widely present throughout the breast gland, foci of CD117-negative luminal cells are more frequently found in lobules compared with ducts.
CD117-Heterogeneity in Breast Gland Structures Correlates With Age
Next, we analyzed flow cytometric data based on uncultured breast epithelium derived from 11 female donors (aged 18–45 years). Quantifying the subpopulations within the total EpCAM-expressing luminal fraction by FACS analysis revealed that CD117-positive cells constituted a frequency ranging from 20% to 60% of the luminal lineage, and did not show any significant age-related correlation (Figs. 2A and S2A). However, when correlating the percentage of immunostained structures expressing CD117 in a contiguous pattern, a significant age-related decrease was evident (Spearman rank correlation ρ = −0.36, p<0.01, Fig. 2B). In particular, when we compared contiguous CD117-expression in structures based on the anatomical location, an age-related decrease was found to be statistically significant in lobular regions (Spearman rank correlation ρ = −0.31, p<0.05), but not in ducts (Fig. S2B). Apart from age, we do not have access to any personal information on the women that donate breast tissue, including parity. However, based on data from Statistics Denmark, in a previous study, we utilized the fact that less than 2% of Danish women younger than 21 years of age have given birth.15 When comparing the expression pattern in patients aged <21 years with those >21 years of age we found significant differences in distribution for both lobules and ducts (p<0.0005 and p<0.05, respectively, by Mann–Whitney test, Fig. 2C). For lobules an average of 90% (median 100%) of structures were contiguous for women <21 years, while the corresponding average was 61% (median 67%) for women >21 years. Specifically, in 11 of 13 women (85%) <21 years of age ≥80% of lobular structures were contiguous, while it was only the case in 14 of 44 (32%) women aged >21 years. For ducts an average of 96% (median 100%) were contiguous for women <21 years and 81% (median 89%) for women >21 years of age.
Figure 2.
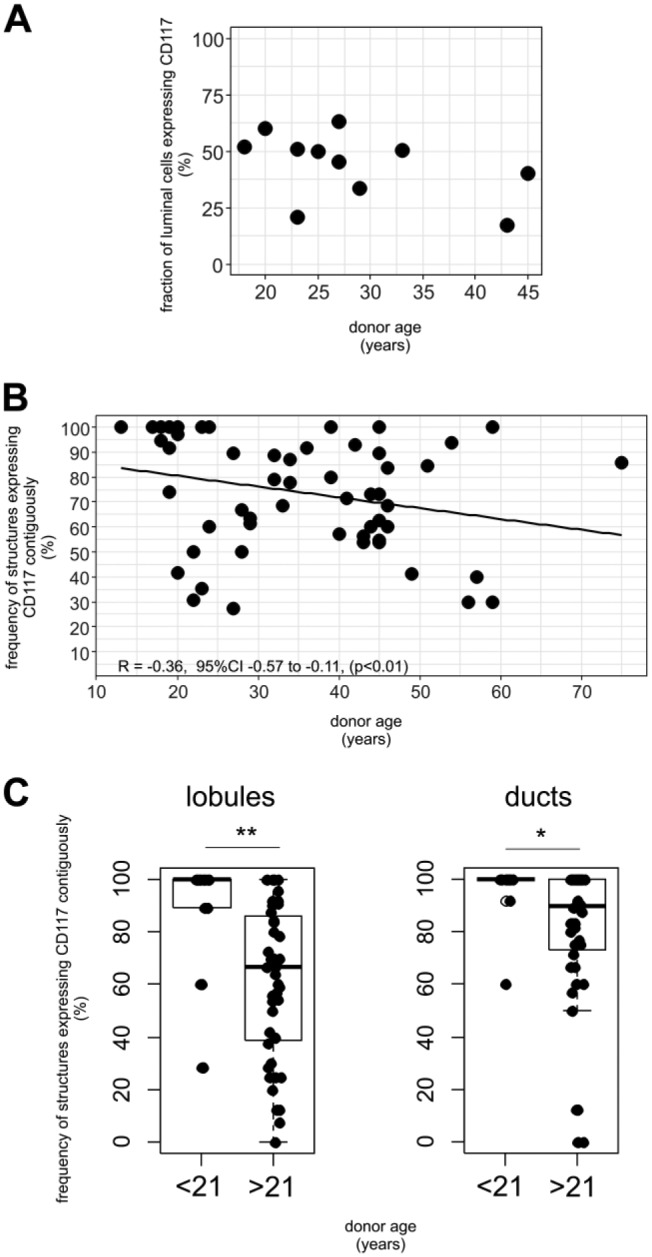
The pattern of CD117-expression varies with age. (A) Scatter plot showing the frequency of CD117-positive cells in 11 individuals by flow cytometry. (B) Scatter plot showing the percentage of contiguous structures of all evaluated structures according to age. Spearman correlation test revealing a significant reduction of contiguous structures with increased age (ρ = −0.36, p<0.01). (C) Box plots showing the distribution of glandular structures with a contiguous pattern of CD117-expression in lobules and ducts, respectively, divided into two age groups: <21 and >21 years of age.*p<0.05, **p<0.0005.
Thus, generally expression of CD117 becomes more heterogeneous in the breast gland with increasing age which is especially evident in lobular structures.
GATA3-Expression Defines a Luminal Differentiation Stage That Is Mostly Segregated From the CD117-Positive Compartment
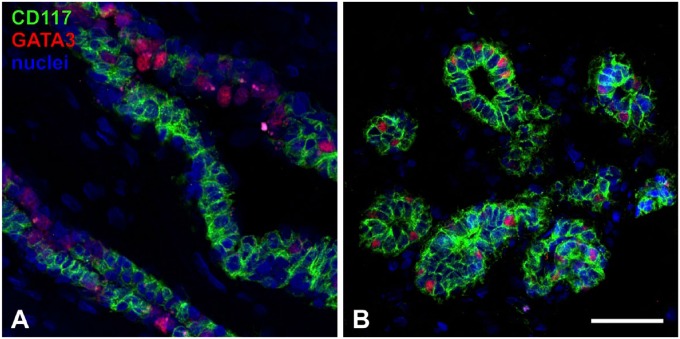
It has been previously shown that cells expressing CD117 and putative mature luminal ERα-positive cells generally are mutually exclusive.11,16 However, ERα-positive cells typically contribute with less than 15% of luminal cells17,18 which means a segment of cells resides in between these two subcompartments. GATA3 is a transcription factor that has been reported as a crucial regulator of luminal epithelial differentiation.19 Analyzing the distribution of CD117- and GATA3-positive cells in immunofluorescent doublestainings in a selection of normal biopsies revealed that while generally the two markers are segregated (Fig. 3A), in a distinct subset of cells CD117 and GATA3 are co-expressed (Fig. 3B). The presence of luminal cells co-expressing both markers was most prominent in ductal structures in 5 of 8 normal biopsies examined. Although ERα-expressing cells are also GATA3-positive, the latter is more widely distributed than this steroid receptor (Fig. S3A) indicating that GATA3 may mark a continuum of luminal cells that have the capacity to mature into terminally differentiated steroid receptor expressing cells.
Figure 3.
CD117 and GATA3 are segregated in to different luminal subcompartments in the majority of cells. Tissue sections stained with antibodies against CD117 (green) and GATA3 (red). Nuclei were stained with DAPI. (A) Segregation of the two markers in different luminal cells. (B) Example of cells expressing both markers. Bar, 50 µm. Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
Furthermore, to evaluate expression of CD117 in a highly differentiated state of the breast gland, we analyzed three biopsies with lactating tissue. We found that CD117-expression was highly diminished in all three lactating biopsies (Fig. S3B).
Together this suggest that CD117-expression is inversely associated with mature differentiation within the luminal lineage.
The CD117-Positive Luminal Compartment Is a Proliferating Population
To demonstrate whether the putative CD117-positive progenitor compartment has a capacity to be responsible for tissue homeostasis processes in the resting gland, sections from eight biopsies were analyzed by immunofluorescent triple-stainings utilizing antibodies against CD117 and proliferation marker Ki-67 together with either CAM5.2 or EpCAM that targets the luminal cells in general (Fig. 4A). Specifically, it was determined at which frequency Ki-67-positive luminal cells were contained within the CD117-expressing compartment. In all biopsies analyzed, the majority of luminal cycling cells were CD117-positive, demonstrating that this compartment can actively contribute to renewal of the luminal epithelium in the resting breast gland (Fig. 4B).
Figure 4.
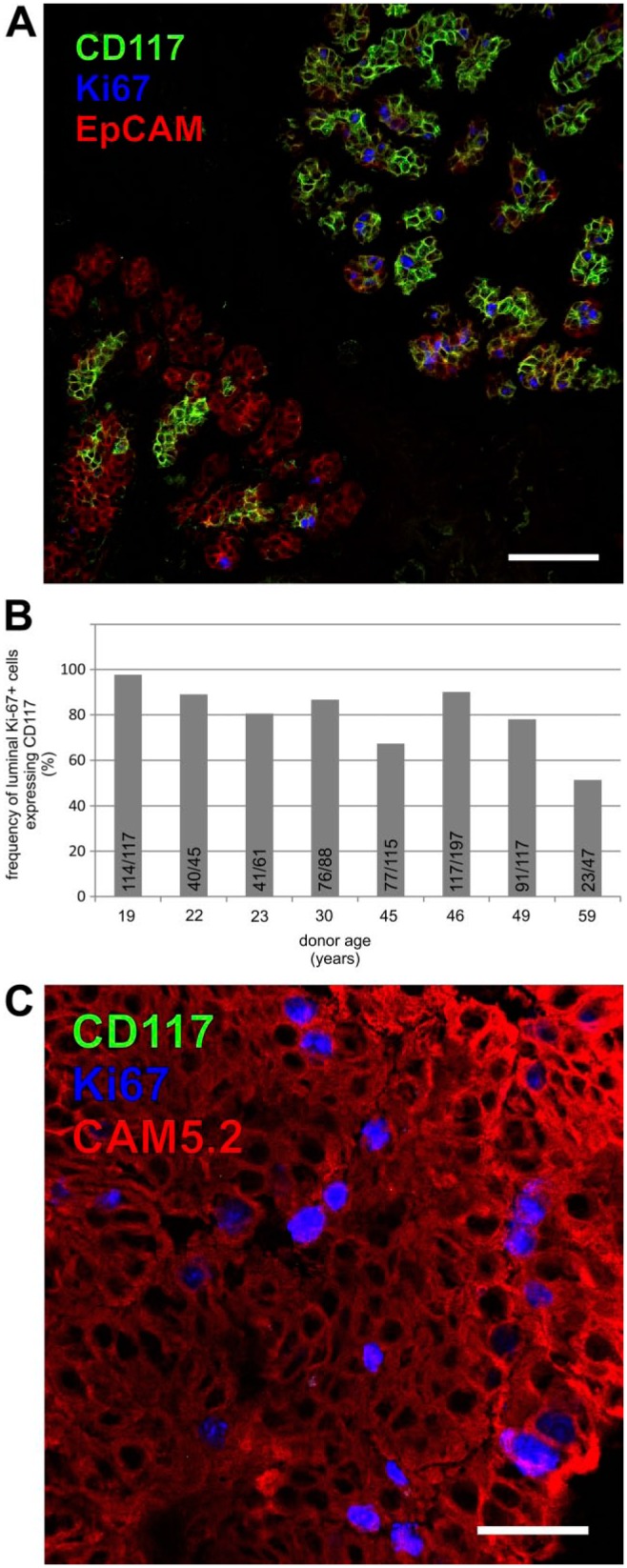
CD117-positive cells constitute a proliferative compartment in the normal breast gland. (A) Immunofluorescent staining of normal breast tissue with CD117 (green), cell cycle marker Ki-67 (blue) and EpCAM (red). Bar, 100 µm. (B) Bar graph showing the frequency of Ki-67-positive luminal cells also expressing CD117 in eight individuals counted on immunostained sections. The individual counts for each biopsy are listed within the bars. (C) Immunofluorescent staining of aberrant breast gland structure from normal tissue with CD117 (green), cell cycle marker Ki-67 (blue) and CAM5.2 (red). Bar, 50 µm.
To clarify whether CD117 can segregate from a proliferative compartment, we selected three normal-derived biopsies with aberrant proliferative structures to test for expression of CD117 and Ki-67. In these proliferative structures, Ki-67-expression mostly correlated to CD117-negative epithelial cells implicating a role for a CD117-negative compartment in the development of at least some preneoplastic structures (Figs. 4C and S3).
Taken altogether, CD117-positive cells constitute a proliferating luminal progenitor compartment with an age-related change in cellular distribution in which a contiguous pattern preferably is retained in ductal structures.
Discussion
A major part of this study was to detail the anatomical distribution of CD117-positive cells in the normal human breast gland. This distribution of different subpopulations in the luminal compartment may be of importance both with regard to identifying the cells that are actively contributing to tissue renewal, and to characterize the cellular origin of breast carcinoma.
As of late breast gland lobules have been studied with a renewed interest as the majority of breast carcinomas arise in these structures.15,20–22 Generally, epithelial cells in lobules are more frequently expressing CD117 in a patched or scattered pattern compared with ducts, suggesting that the highest frequency of CD117-positive luminal cells are located in the latter structures. As CD117 is considered a marker of progenitors this observation is in line with our previous characterization of increased levels of progenitor cell activity in ducts compared with lobules.23 Comparably, ERα-positive cells are typically more frequent in lobules,17 which correlates with the observation that CD117-positive cells are generally segregated from ERα-positive cells.11,16 In support of this we have previously suggested that a scattered distribution with rare CD117-positive cells can coincide with the presence of a contiguous pattern of ERα-positive cells.14 Interestingly, a higher frequency of ERα-expressing cells have been observed in normal-derived tissue of breast cancer patients, which have led to the speculation that the presence of larger numbers of steroid receptor-positive cells contribute to an increased risk of developing breast carcinoma.24–27 Accordingly, a decreased frequency of CD117-positive cells within the luminal compartment may be an indicator of this risk. When analyzing the frequency of CD117-positive cells in the breast gland epithelium of 11 women, two stood out with frequencies well below 25% of the luminal population. However, the material from breast reduction mammoplasties was donated anonymously, so at present we are not able to extrapolate on this. Conversely, an increase in the frequency of CD117-progenitors could potentially pose a higher risk for developing other breast cancer subtypes. Lim et al. demonstrated that CD117 mRNA expression was significantly higher in normal breast tissue from BRCA1-mutant carriers compared with tissue from nonmutated women, indicating that an accumulation of luminal progenitors could play a role as a target for oncogenic events leading to BRCA1-associated breast carcinoma.2 Thus there is still a viable debate with regard to the identity of the cell of origin for breast cancer and it may vary with the type of tumor that develops.28,29
The presence of CD117-positive cells in the luminal fraction did not correlate to age based on the flow cytometry analysis on 11 women, of which only two were <21 years of age and the oldest was 45 years of age. Previously it has been reported that CD117-expression in breast tissue does increase with age of the women.10 Although the previous study also includes the myoepithelial compartment, here we only account for distribution within the luminal compartment which may explain the apparent discrepancy. Also, it should be taken into account that we have no information on incidents that can affect the dynamics of the breast gland, like phase in menstrual cycle, history of ingestion of contraceptives or parity of the tested women. However, when analyzing the expression pattern of CD117 in a sample based on 57 women, we did observe that young women, <21 years of age, in general had a much higher frequency of contiguous structures both in lobules and ducts than women >21 years of age. In women in their twenties and above the expression pattern varied quite significantly. This could mean that certain events, for example, repeated cycles of menstruation or parity can affect the distribution of CD117-positive and CD117-negative cells. Interestingly, in Denmark less than 2% of women give birth before the age of 21 years.11
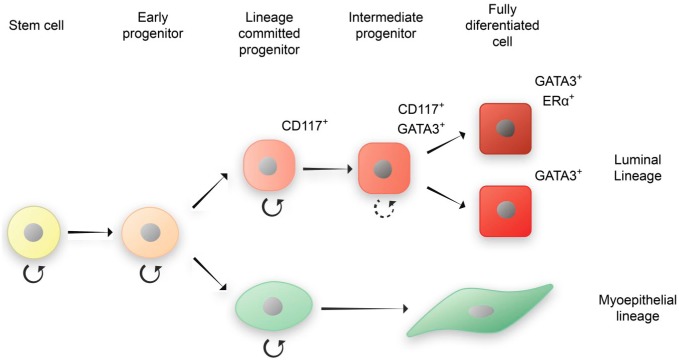
Although CD117 and GATA3 generally segregate luminal cells into different subcompartments some CD117-positive cells also co-express GATA3. Conditional deletion of GATA3 in mouse strains have revealed that mammary gland development, in particular luminal differentiation, was severely compromised, indicating that luminal cells were blocked in a progenitor state.19 As GATA3 plays a crucial role for maturation of cells in the luminal compartment it is feasible to suggest that the two subcompartments are hierarchically connected, with intermediate CD117/GATA3-expressing cells. This thesis is supported by recent data, showing that CD117-positive luminal cells isolated by FACS can be induced to differentiate into an ERα-expressing state in culture.11 Thus, CD117-expressing cells may designate early luminal progenitors, CD117/GATA3 intermediate cells, GATA3 late progenitors and GATA3/ERα mature luminal cells (Fig. 5). As CD117-positive cells account for a distinct number of cells in the gland it is quite possible that this population can be subdivided even further, containing a subset of luminal progenitors that are even closer to the apex of the hierarchy. For example, putative luminal progenitor marker cytokeratin 14 is expressed in a minor subset of luminal cells that may very well overlap with CD117-positive cells.23,30,31 It should also be noted that a subset of luminal cells can be observed expressing neither CD117 nor GATA3, and these may require further characterization.
Figure 5.
Proposed model of luminal lineage differentiation in the human breast gland implicating CD117, GATA3, and ERα.
How tissue homeostasis is maintained in the adult human breast gland is still a matter of debate. Based on lineage tracing experiments in mice Van Keymeulen and colleagues have demonstrated the presence of long lived unipotent lineage-restricted stem cells or progenitors responsible for continuous renewal of the luminal and myoepithelial lineages.32 By utilizing cell cycle marker Ki-67, we determined that typically the majority of cycling cells within the luminal compartment belonged to the CD117-positive population, arguing that these cells contribute actively to tissue homeostasis in the resting gland. However, staining of a small sample of aberrant proliferative structures derived from normal breast glands showed that largely the proliferative cells are found outside the CD117-expressing compartment. This indicate that either these presumed preneoplastic aberrations arise in luminal epithelial cells that have differentiated further along the luminal lineage, or they get primed to differentiate as they enter a hyperproliferative state. A deeper understanding of these dynamics can have important implications for unraveling the basis for breast cancer development.
Supplemental Material
Supplemental material, DS_10.1369_0022155418788845 for Expression of Luminal Progenitor Marker CD117 in the Human Breast Gland by Jiyoung Kim and René Villadsen in Journal of Histochemistry & Cytochemistry
Acknowledgments
We thank Tove Marianne Lund and Lena Kristensen for expert technical assistance. Benedikte Thuesen, CFR Hospitaler and Vera Timmermanns Wielenga, Rigshospitalet are acknowledged for providing breast biopsy material. Furthermore, we thank Agla Fridriksdottir for assistance with FACS data and Benjamin Villadsen for assistance with schematic drawings. The Core Facility for Integrated Microscopy (University of Copenhagen) is acknowledged for confocal microscope accessibility.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RV conceived the project, designed the study, performed the experiments, analyzed the data, and wrote the manuscript. JK designed the study, analyzed the data, performed the statistical calculations, and wrote the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Novo Nordisk Fonden and Danish Research Council grant 10-092798 (to DanStem), Toyota-Fonden Denmark, and Anita og Tage Therkelsens Fond (to RV), Familien Erichsens Mindefond and Vera og Carl Johan Michaelsens Legat (to JK).
ORCID iD: R Villadsen  https://orcid.org/0000-0002-5226-381X
https://orcid.org/0000-0002-5226-381X
Contributor Information
Jiyoung Kim, Department of Cellular and Molecular Medicine, and Novo Nordisk Center for Stem Cell Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
René Villadsen, Department of Cellular and Molecular Medicine, and Novo Nordisk Center for Stem Cell Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Literature Cited
- 1. Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, MacKay A, Grigoriadis A, Tutt A, Ashworth A, Reis-Filho JS, Smalley MJ. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–17. [DOI] [PubMed] [Google Scholar]
- 2. Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat M-L, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13. [DOI] [PubMed] [Google Scholar]
- 3. Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92:1619–49. [DOI] [PubMed] [Google Scholar]
- 4. Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shin JY, Hu W, Naramura M, Park CY. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med. 2014;211:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang M, Zhou H, Zheng C, Xiao J, Zuo E, Liu W, Xie D, Shi Y, Wu C, Wang H, Li D, Li J. The roles of testicular c-kit positive cells in de novo morphogenesis of testis. Sci Rep. 2015;4:5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. [DOI] [PubMed] [Google Scholar]
- 8. Kajstura J, Rota M, Hall SR, Hosoda T, D’Amario D, Sanada F, Zheng H, Ogórek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Lim E, Wu D, Pal B, Bouras T, Asselin-Labat M-L, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garbe JC, Pepin F, Pelissier FA, Sputova K, Fridriksdottir AJ, Guo DE, Villadsen R, Park M, Petersen OW, Borowsky AD, Stampfer MR, LaBarge MA. Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia. Cancer Res. 2012;72:3687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fridriksdottir AJ, Kim J, Villadsen R, Klitgaard MC, Hopkinson BM, Petersen OW, Rønnov-Jessen L. Propagation of oestrogen receptor-positive and oestrogen-responsive normal human breast cells in culture. Nat Commun. 2015;6:8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Regan JL, Kendrick H, Magnay F-A, Vafaizadeh V, Groner B, Smalley MJ. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene. 2012;31:869–83. [DOI] [PubMed] [Google Scholar]
- 13. Rønnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 14. Balk-Møller E, Kim J, Hopkinson B, Timmermans-Wielenga V, Petersen OW, Villadsen R. A marker of endocrine receptor-positive cells, CEACAM6, is shared by two major classes of breast cancer: luminal and HER2-enriched. Am J Pathol. 2014;184:1198–208. [DOI] [PubMed] [Google Scholar]
- 15. Fridriksdottir AJ, Villadsen R, Morsing M, Klitgaard MC, Kim J, Petersen OW, Rønnov-Jessen L. Proof of region-specific multipotent progenitors in human breast epithelia. Proc Natl Acad Sci U S A. 2017;114:E10102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westbury CB, Reis-Filho JS, Dexter T, Mahler-Araujo B, Fenwick K, Iravani M, Grigoriadis A, Parry S, Robertson D, Mackay A, Ashworth A, Yarnold JR, Isacke CM. Genome-wide transcriptomic profiling of microdissected human breast tissue reveals differential expression of KIT (c-Kit, CD117) and oestrogen receptor-α (ERα) in response to therapeutic radiation. J Pathol. 2009;219:131–40. [DOI] [PubMed] [Google Scholar]
- 17. Petersen OW, Høyer PE, van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Res. 1987;47:5748–51. [PubMed] [Google Scholar]
- 18. Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–91. [PubMed] [Google Scholar]
- 19. Asselin-Labat M-L, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9. [DOI] [PubMed] [Google Scholar]
- 20. Tabár L, Dean PB, Yen AM, Tarján M, Chiu SY, Chen SL, Fann JC, Chen TH. A proposal to unify the classification of breast and prostate cancers based on the anatomic site of cancer origin and on long-term patient outcome. Breast Cancer. 2014;8:15–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santagata S, Thakkar A, Ergonul A, Wang B, Woo T, Hu R, Harrell JC, Mcnamara G, Schwede M, Culhane AC, Kindelberger D, Rodig S, Richardson A, Schnitt SJ, Tamimi RM, Ince TA. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J Clin Invest. 2014;124:858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honeth G, Schiavinotto T, Vaggi F, Marlow R, Kanno T, Shinomiya I, Lombardi S, Buchupalli B, Graham R, Gazinska P, Ramalingam V, Burchell J, Purushotham AD, Pinder SE, Csikasz-Nagy A, Dontu G. Models of breast morphogenesis based on localization of stem cells in the developing mammary lobule. Stem Cell Reports. 2015;4:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ, Petersen OW. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Umekita Y, Souda M, Ohi Y, Rai Y, Sagara Y, Sagara Y, Yoshida H. Expression of estrogen receptor alpha and progesterone receptor in normal human breast epithelium. In Vivo. 2007;21:535–9. [PubMed] [Google Scholar]
- 25. Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst. 1998;90:37–42. [DOI] [PubMed] [Google Scholar]
- 26. Khan SA, Yee KA, Kaplan C, Siddiqui JF. Estrogen receptor α expression in normal human breast epithelium is consistent over time. Int J Cancer. 2002;102:334–7. [DOI] [PubMed] [Google Scholar]
- 27. Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol. 1999;188:237–44. [DOI] [PubMed] [Google Scholar]
- 28. Villadsen R. In search of a stem cell hierarchy in the human breast and its relevance to breast cancer evolution. APMIS. 2005;113:903–21. [DOI] [PubMed] [Google Scholar]
- 29. Skibinski A, Kuperwasser C. The origin of breast tumor heterogeneity. Oncogene. 2015;34:5309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor-Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989;94(Pt 3):403–13. [DOI] [PubMed] [Google Scholar]
- 31. Wetzels RH, Kuijpers HJ, Lane EB, Leigh IM, Troyanovsky SM, Holland R, van Haelst UJ, Ramaekers FC. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991;138:751–63. [PMC free article] [PubMed] [Google Scholar]
- 32. Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1369_0022155418788845 for Expression of Luminal Progenitor Marker CD117 in the Human Breast Gland by Jiyoung Kim and René Villadsen in Journal of Histochemistry & Cytochemistry