Abstract
Spinach is one of the most widely consumed vegetables, and is known as for both physical and mental health maintenance. However, there is little information about how spinach protects one from stress. In the present study, we created three extracts from Spinach oleracea L., (frozen powder (FP), water extract (WE), and ethanol extract (EE)), and examined their anti-stress and anti-depressive effects on mouse using a chronic immobilization stress (CIS) regimen. FP, WE, and EE showed different free amino acid constituents. Calorie-balanced diets derived from each extract were tested for their ability to reduce blood corticosterone (CORT) levels in naïve mice. Diets supplemented with FP or EE induced lower blood CORT levels than a normal diet, but the WE diet did not. Mobility duration and sucrose preference were increased by FP and EE supplementation in the CIS-induced depression animal models. Moreover, FP and EE increased glutamate and glutamine levels in the medial prefrontal cortex (mPFC) compared with CIS-induced depressed group. These results suggest that spinach has anti-stress and anti-depressive properties by lowering CORT and increasing glutamate and glutamine levels in the mPFC.
Keywords: spinach, chronic stress, corticosterone, depressive behavior
1. Introduction
Stress challenges the homeostasis of animals and requires adaptive responses. Animals adapt to various stressors by the endogenous stress response system initiated through the hypothalamus pituitary adrenal (HPA) axis, leading to increased serum corticosteroid levels. However, the prolonged and excessive elevation of stress or stress hormone levels is deleterious to the animal [1]. Many modern lifestyles expose humans to diverse and consistent stressors that cause various diseases, including major depressive disorder (MDD), a devastating psychiatric illness that induces disability and sometimes leads to suicide. The World Health Organization predicted that depression would be the second leading cause of disability by 2020 in 2001, but in fact, by 2013 MDD was already the second leading cause of disability [2]. Thus, finding a safe and effective anti-depressive alternative medicine for regulating daily stress hormone levels is crucial. Currently available antidepressants are monoaminergic and have been prescribed to MDD patients following their serendipitous discovery in the 1950s. Over the next three decades, persistent research efforts have improved monoaminergic antidepressants as selective agents (e.g., selective serotonin reuptake inhibitors (SSRIs)) and patient quality of life. Nevertheless, the clinical limitations of these compounds still exist [3]. For example, MDD patients must wait weeks or months to benefit from the antidepressant’s efficacy, and one-third of patients do not respond to the medication. Up to 70% of recovered MDD patients experience minor residual symptoms and some major recurring symptoms. Additionally, patients taking antidepressants often suffer side effects such as nausea, insomnia, weight gain or loss, decreased sexual drive, drowsiness, fatigue, and headaches [4]. These limitations demonstrate the need to find new anti-depressant targets and drugs for treating MDD.
A high concentration of glucocorticoids is one cause of MDD [5], according to preclinical studies. Chronic glucocorticoid treatment changes cellular functions and structures in the brain and induces depressive behaviors [6,7]. Cellular atrophy induced by glucocorticoids is similar to neuronal atrophy found in depressed patients [8]. Moreover, many studies have shown that successful antidepressant therapies are associated with normalization of impairments in negative feedback of the HPA axis [9]. Therefore, elevated glucocorticoids are a key neurobiological factor in eliciting depressive behaviors, suggesting that maintaining homeostatic levels of glucocorticoids may be an effective therapeutic strategy against MDD.
In a previous study, we reported that exogenous glutamine (Gln) directly infused into the medial prefrontal cortex (mPFC) attenuates depressive-like behaviors of mice [10]. We recently reported that Gln-supplemented diet reduced blood corticosterone (CORT) levels and maintained normal growth performance of cage-reared chicks [11]. Moreover, the Gln-supplemented diet ameliorated the deleterious effects of the chronic immobilization stress (CIS)-induced depression mouse model [12]. In that study, we found that the Gln-supplemented diet reversed their depressive behaviors and restored brain glutamate (Glu) and Gln levels. It is well-known that spinach contains many amino acids, including Gln, and other beneficial compounds, such as minerals, essential vitamins, folic acid, lecithin, secretin, saponins, and flavonoids [13]. Spinach’s possible beneficial effects on stress and mood are also of interest, but no scientific research regarding this issue has been published. Therefore, we investigated if spinach extracts attenuate blood CORT levels and depressive-like behaviors induced by CIS.
2. Materials and Methods
2.1. Animals
Male C57BL/6 mice (Koatech, Co. Ltd., Pyeongtaek, Korea) weighing 22–24 g at the start of the experiment were used for all studies. All animals were single-caged in a temperature- and humidity- controlled room (lights on 06:00–18:00) with food and water available ad libitum. Mice were randomly grouped according to body weight using a computer-generated list. Animal use procedures were performed in accordance with National Institutes of Health guidelines and an approved protocol (GLA-100917-M0093) by the Gyeongsang National University Institutional Animal Care & Use Committee.
2.2. Spinach Extracts
Spinach (Spinach oleracea L.) was purchased from Namhae County, Republic of Korea. After cutting roots, spinach was washed, lyophilized, and powdered to generate a frozen powder (FP). Separately, 10 L water or ethanol were added to 500 g powdered spinach, and the mixture was normalized to room temperature for 24 h and filtered (No. 2 filter paper, Advantec, Tokyo, Japan). The water extract (WE) was generated through lyophilizing, and the ethanol extract (EE) was concentrated using a rotary evaporator (N-1200 AVW, EYELA, Tokyo, Japan).
2.3. Free Amino Acids Analysis of Spinach Extracts
To determine free amino acid content, 150 mL ethanol were added to 3 g spinach extract, and the mixture was homogenized and centrifuged (5000 rpm for 10 min). The residue was extracted twice with 75 mL 80% ethanol, and the supernatant was collected, concentrated, and defatted with ether to a final volume of 50 mL. The mixture was concentrated using a rotary vacuum evaporator and adjusted to 10 mL with lithium citrate buffer (pH 2.2). The sample was filtered through a 0.25 µm-membrane. We identified the amino acid peaks in the samples by their retention times compared with external standards. Amino acid concentrations were quantified according to the relative peak height measured (Biochrom 30+, Biochrom Ltd., Cambridge, UK) [14,15].
2.4. Free Glu and Gln Analysis in the PFC
L-Glu and L-Gln contents were determined by ultra-performance liquid chromatography (UPLC) with an AccQ-Tag Ultra system (Waters, Milford, MA, USA) as previously reported [16]. The brain tissues were homogenized in T-PER (tissue protein extraction reagent) (Pierce, Rockford, IL, USA) and the supernatant was collected after centrifugation at 12,000 rpm for 30 min. Protein concentration in the supernatant was determined using the Bicinchoninic Acid (BCA) reagent (Pierce, Rockford, IL, USA). The sample was ultra-filtered using a SmarPor Syringe Filter (25 mm, 0.2 µm, Woongki Ltd., Seoul, Korea). Once the filtrate was diluted to a suitable concentration, fluorescence derivatization was performed according to the AccQ-Tag manufacturer’s instruction. A 20-µL sample solution, 60 µL of AccQ-fluor borate buffer, and 20 µL of AccQ-fluor reagent were mixed together, and the mixture was incubated for 5 min at 55 °C. Derivatized amino acids were separated on an AccQ-Tag Ultra column (2.1 × 100 mm, Waters) by gradient elution (AccQ-Tag Ultra eluent A and B) at 30 °C. The derivatized amino acids were detected by a PDA eλ detector (Waters) [17,18]. The chromatography data were analyzed using Empower software (Waters). To determine amino acid concentrations, a standard solution containing known concentrations of amino acids were analyzed with samples in every series.
2.5. CIS Regimen
The regimen was carried out as previously described [19,20]. Briefly, mice were repeatedly placed in a restrainer 2 h/day for 15 days under 100 lx light. Body weight and food intake were evaluated every other day throughout the experiment. Mice were fed a diet containing one of the spinach extracts (FP (80 g), WE (40 g), and EE (34 g)) per one kilogram of feed or a normal diet for two weeks, and total calories fed were adjusted to 4000 kcal/kg (AIN 93G, UniFaith, Seoul, Korea).
2.6. Behavioral Tests
The tail suspension test (TST) was conducted as previously described [12,21] with some modification. Mice were individually suspended by the tail via a horizontal bar approximately 30 cm from the floor using tape approximately 1 cm from the tip of the tail. The 6 min motion was recorded and analyzed with an animal behavior video program (EthoVision, Noldus Information Technology, Wageningen, The Netherlands).
The sucrose preference test (SPT) was performed to determine symptoms of anhedonia as previously described [20] with some modification. Briefly, mice were habituated for 48 h with a palatable sucrose solution (0.1 M), followed by a 24 h water deprivation period and 6 h access to two identical bottles, one filled with sucrose solution and the other with water. The consumption of sucrose solution and water was measured throughout the 6 h period, and sucrose preference was represented as the ratio of sucrose-to-water consumption.
2.7. Measurement of Plasma CORT
Plasma CORT was measured as previously described [22]. Mouse blood was collected at 09:00 into vacutainers containing K3 EDTA. Plasma was isolated via centrifugation at 1000× g for 15 min at 4 °C. The samples were stored at −80 °C until the assay was performed. Quantitation of plasma CORT levels was carried out using the CORT EIA kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s protocol.
2.8. Statistical Analyses
All data evaluated by ANOVA and a Newman Keuls multiple comparison post-hoc test using Prism (GraphPad Software, La Jolla, CA, USA). Data are represented as means ± SEM. The cut-off for statistical significance was p < 0.05.
3. Results
3.1. Free Amino Acid Contents of Spinach Extracts Differ by the Extraction Method
Total free amino acids in the WE were 5.56 times higher than the FP extract (Table 1). Interestingly, Gln was detected in the EE (29.13 mg/100 g) and FP (62.88 mg/100 g), but not in the WE. Instead of Gln, WE contained a high level of Glu (1171.38 mg/100 g) compared with FP (146.69 mg/100 g) and EE (23.65 mg/100 g). Conversely, L-tyrosine was not detected from WE, but was found in EE and FP at 162.88 and 38.75 mg/100 g, respectively.
Table 1.
Contents of free amino acids in spinach extracts (mg/100 g).
| Amino Acids | Ethanol Extract | Water Extract | Frozen Powder |
|---|---|---|---|
| L-Phenylalanine | ND | 44.13 ± 0.21 | 1.48 ± 0.15 |
| L-Aspartic Acid | 26.11 ± 1.31 | ND | ND |
| L-Threonine | 32.28 ± 1.54 | 68.44 ± 1.36 | 11.18 ± 0.45 |
| L-Serine | 32.11 ± 1.36 | 98.80 ± 6.32 | 23.78 ± 0.36 |
| L-Asparagine | 23.68 ± 0.25 | 3.43 ± 0.24 | 30.55 ± 0.33 |
| L-Glutamate | 23.65 ± 0.84 | 1171.38 ± 15.27 | 146.69 ± 2.58 |
| L-Glutamine | 29.13 ± 0.25 | ND | 62.88 ± 3.36 |
| L-Proline | 549.98 ± 5.64 | 564.45 ± 4.25 | 145.72 ± 4.68 |
| Glycine | 9.24 ± 0.24 | 66.79 ± 0.65 | 5.45 ± 0.65 |
| L-Alanine | 62.88 ± 2.65 | 233.74 ± 3.54 | 20.23 ± 0.36 |
| L-Citrulline | 13.98 ± 0.25 | 44.80 ± 3.54 | 2.54 ± 0.04 |
| α-Aminobutyric acid | 8.74 ± 0.21 | 17.03 ± 0.25 | 0.65 ± 0.15 |
| L-Valine | 80.82 ± 0.14 | 105.14 ± 4.62 | 24.23 ± 1.23 |
| L-Cystine | 2.36 ± 0.73 | 0.35 ± 0.03 | 2.46 ± 0.54 |
| L-Methionine | 11.19 ± 0.21 | 58.20 ± 1.75 | 16.39 ± 0.36 |
| L-Isoleucine | 104.12 ± 0.41 | 168.15 ± 4.56 | 40.07 ± 0.45 |
| L-Leucine | 43.77 ± 0.36 | 94.14 ± 2.36 | 17.09 ± 0.50 |
| L-Tyrosine | 162.88 ± 10.94 | ND | 38.75 ± 0.61 |
| β-Alanine | 29.41 ± 0.21 | 79.61 ± 1.25 | 14.30 ± 0.45 |
| L-Homocystine | 189.05 ± 8.50 | 668.55 ± 4.66 | 83.38 ± 0.86 |
| γ-Amino-n-butyric acid | 158.99 ± 7.64 | 231.14 ± 1.35 | 42.32 ± 0.64 |
| Ethanolamine | 108.29 ± 3.50 | 448.94 ± 4.25 | 20.58 ± 0.03 |
| δ-Hydroxylysine | 0.10 ± 0.02 | 89.18 ± 0.58 | 2.43 ± 0.05 |
| Ornithine | 5.48 ± 0.14 | 75.49 ± 0.25 | 1.73 ± 0.04 |
| 1-Methylhistidine | 8.96 ± 0.65 | 43.87 ± 0.36 | 13.21 ± 0.10 |
| L-Histidine | 9.47 ± 1.58 | 26.47 ± 1.27 | 2.77 ± 0.05 |
| L-Arginine | 16.84 ± 0.24 | 0.28 ± 0.04 | 19.97 ± 1.25 |
| Total | 1743.51 ± 49.81 | 4402.50 ± 62.96 | 790.83 ± 20.27 |
ND: not detected.
3.2. FP and EE-Supplemented Diets Decrease Blood CORT Levels in Mice
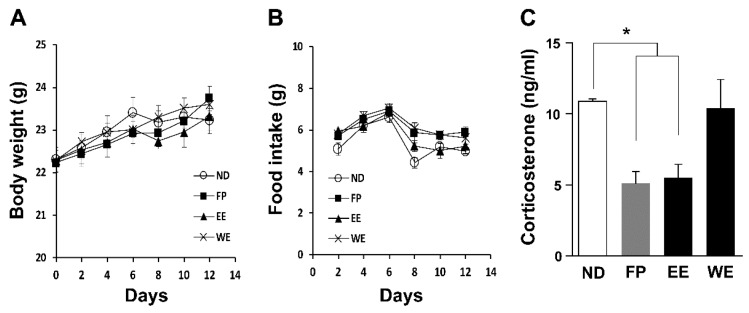
We observed no changes in body weight and food intake between mice fed diets containing different spinach extracts (Figure 1A,B), it would likely to be due to the calorie-balanced diet prepared for this experiment. Because we recently found that Gln-supplemented diet decreased blood CORT level in growing chicks [11] and CIS-induced depressive mice [12], we expected that Gln-containing extract would be effective on reduction of blood CORT level. Expectedly, blood CORT levels were remarkably decreased by EE- and FP-supplemented diets (Figure 1C) although all groups suffered from same daily stressors. This result suggested that FP and EE could have anti-stress and anti-depressive properties; thus we used EE and FP for further analyses but did not use WE.
Figure 1.
A spinach extract diet reduced blood CORT level. (A) Body weight and (B) food intake (n = 8 mice per group). (C) Blood CORT decreased in mice fed an FP and EE diet but not a WE diet (n = 5 mice per group). All values are means ± SEM. * p < 0.05. EE, ethanol extract; FP, frozen powder; ND, normal diet; WE, water extract; CORT, corticosterone.
3.3. FP and EE-Supplemented Diet Attenuates Depressive-Like Behaviors Induced by CIS
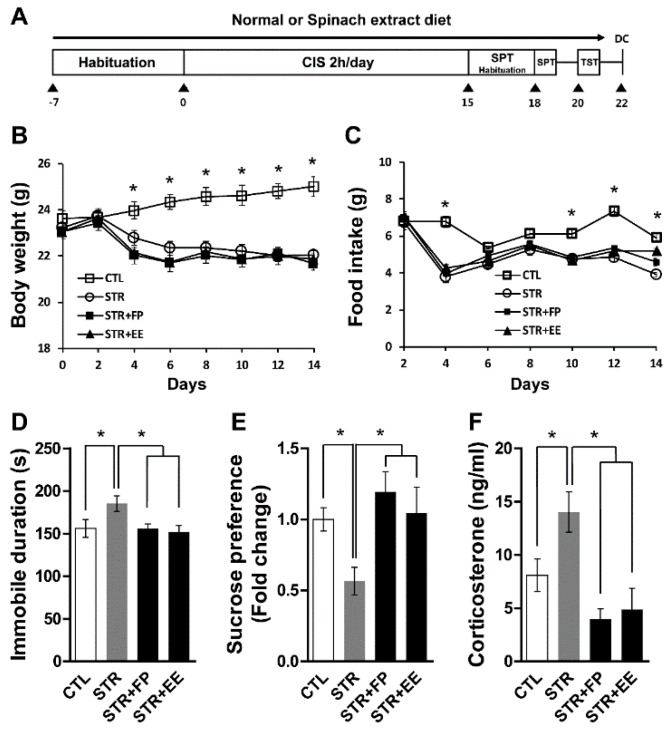
We determined if spinach extracts have anti-depressive effects on mice exposed to CIS. Mice were fed with normal or FP- or EE-supplemented diets during the experiments depicted in Figure 2A. The body weight and food intake significantly decreased in the stress groups (STR, STR + FP, STR + EE) compared with that of the control group (CTL) (Figure 2B,C) were found. Significantly decreased sucrose preference and increased immobility were seen in mice given CIS compared with the CTL. These depressive-like behaviors were reversed by FP and EE supplementation compared with the STR (Figure 2D,E), highlighting the antidepressant properties of these particular spinach extracts. We also found that FP and EE significantly attenuated increased blood CORT levels by CIS (Figure 2F). These results demonstrate that spinach extract-supplemented diets decrease stress-associated blood CORT levels and ameliorate depressive-like behaviors induced by chronic stress.
Figure 2.
Spinach extract diet attenuated depressive behaviors and increased CORT in a CIS-induced depression model. (A) Timeline of CIS experiments. (B) Body weight and (C) food intake (n = 7–8 mice per group). (D) Immobile duration and (E) sucrose preference, assessed by the TST and SPT, respectively (n = 7–8 mice per group). (F) Blood CORT level (n = 4–5 mice per group). All values are means ± standard error of mean (SEM). * p < 0.05. CTL, control; DC, decapitation; EE, ethanol extract; FP, frozen powder; ND, normal diet; STR, stress; CIS, chronic immobilization stress; SPT, sucrose preference test; TST, tail suspension test.
3.4. FP and EE-Supplemented Diet Increases Brain Glu and Gln Levels
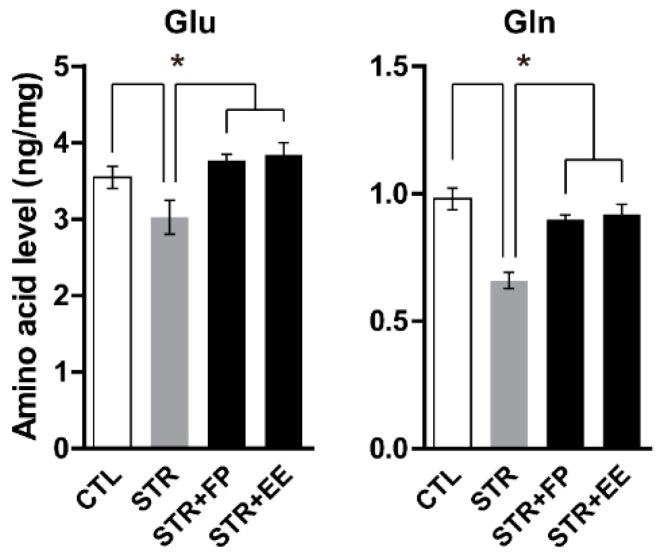
Previously, we demonstrated that increased level of Gln by direct infusion into the mPFC has an anti-depressant effect on chemically induced depressive mice [10] and recently found the causal relationship between low levels of cortical Glu and Gln and depressive behaviors of stressed mice [12]. Moreover, we showed that Gln-supplemented diet has antidepressant effects through increments of brain Glu and Gln levels [12]. Therefore, we examined the possibility the FP- and EE-supplemented diets could change brain Glu and Gln levels and found that there were remarkable increments of Glu and Gln levels in the PFC in the FP- and EE-supplemented groups compared with the normal diet STR group (Figure 3).
Figure 3.
Glu and Gln changed by CIS and spinach extract-supplemented diet during chronic immobilization stress. Glu and Gln levels in the prefrontal cortex (PFC; n = 5–6 mice per group). All values are means ± standard error of mean (SEM). * p < 0.05. CTL, control; EE, ethanol extract; FP, frozen powder; STR, stress.
4. Discussion
In the present study, we found that FP and EE from spinach decreased blood CORT levels in human-handled mice. We also showed that FP and EE reversed blood CORT levels and depressive behaviors induced by CIS. Moreover, FP and EE increased Glu and Gln levels compared with the STR group. Thus, it is suggested that the FP and EE spinach extracts have anti-stress and anti-depressant properties against chronic stress.
Natural products from foods can be good candidates for alternative medical therapies and have attracted the attention of many researchers and pharmaceutical companies because their bioactivities are comparably effective and safe compared with chemical drugs. Numerous natural product-derived medicines have been developed for various diseases [23]. Only St John’s wort, an herbaceous perennial plant commonly found in Asia and Europe, has been licensed and widely prescribed to MDD patients in many European countries. Recent meta-analyses suggest that St John’s wort is safer than and comparably efficacious with SSRIs in patients with MDD [24]. As a result, many studies have examined natural extracts’ effects on depressive-like behaviors using animal models [25,26,27].
Spinach is called a “superfood” because of its constituents and is traditionally used as a functional food for various purposes. The functionality of spinach has been confirmed with respect to its anti-anemia, anti-microbial, anti-convulsant, anti-diabetic, anti-hyperlipidemic, anti-inflammatory, anti-oxidant, and anti-ulcer activities [28], suggesting the potential anti-stress property of spinach extracts in a supplemented diet. Moreover, the vitamins, folic acid, and flavonoids present in spinach exert anti-depressive properties [29,30,31,32]. Our results support the anti-stress and anti-depressive activities of spinach being alluded by previous studies.
Depending on the extraction method, the constituents of amino acids from different organisms vary [33,34,35]. We used three extraction methods in our current study, and although only free amino acids were analyzed, the extracts’ contents were significantly different depending on the extraction method. Only FP and EE of spinach reduced CORT levels, further demonstrating that beneficial properties of a natural product may depend on the extraction method. From this result, we hypothesize that the absence of L-Gln and L-tyrosine in the WE spinach may explain its lack of effect on blood CORT levels in the mice. People take L-tyrosine supplements for improving depression, attention-deficit/hyperactivity disorder, cognitive performance, narcolepsy, and alertness following sleep deprivation [36]. Tyrosine positively affects the activity of catecholamines, including epinephrine and norepinephrine, which regulate stress responses in the adrenal glands and brain [37]. Thus, tyrosine can confer benefits under conditions such as stress, cold, fatigue, and prolonged sleep deprivation [38].
In addition, Gln is an important nitrogen and carbon source for many cell types [39] and may promote gut function, immune responses, and other essential physiological processes during times of stress, such as physical exhaustion and post-operative periods [40]. Gln is also essential for maintaining the homeostasis of Glu, which is involved in brain neurotransmission [10]. Previously, we demonstrated Gln deficiency in prefrontal cortical neurons could evoke depressive-like behaviors in rodents [10] and low levels of Glu and Gln by CIS would be a cause for depressive behaviors [12]. There were several lines of evidence showing low levels of Glu and Gln in certain brain regions of MDD patients [41,42,43]. In this study, we also found the lower levels of Glu and Gln in the PFC of the STR group (Figure 3) which is consistent with our previous reports [10,12]. Interestingly, FP and EE increased the levels of Glu and Gln in the PFC (Figure 3), which may activate glutamatergic neurons being suppressed by CIS [12]. These findings suggest that the beneficial effects of spinach FP and EE on stress hormones and depressive-like behaviors may be due to decreasing blood CORT and increasing Glu and Gln levels in the PFC.
Although we confirmed FP and EE of spinach relieve stress-related and depressive-like symptoms induced by CIS, we only analyzed their free amino acid contents. Spinach contains other beneficial compounds, including minerals, vitamins A, B, C, D, E, and K, folic acid, lecithin, secretin; saponins and flavonoids [13]. Thus, further analyses of FP, EE, and WE content are necessary to identify other candidates that may reduce stress and depressive-like behaviors.
Acknowledgments
This research was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF-2014R1A1A4A01006451 and NRF-2015R1A5A2008833).
Author Contributions
Investigation, H.S., S.J., J.H.S., and M.J.K.; Supervision, H.J.K.; Writing—Original Draft Preparation, H.S.; Writing—Review and Editing, H.J.K.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J.L., Vos T., Whiteford H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Gartlehner G., Hansen R.A., Morgan L.C., Thaler K., Lux L.J., Van Noord M., Mager U., Gaynes B.N., Thieda P., Strobelberger M., et al. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. [(accessed on 30 October 2018)]; Available online: http://www.diva-portal.org/smash/record.jsf?pid=diva2%3A810275&dswid=-7243. [PubMed]
- 5.Checkley S. The neuroendocrinology of depression and chronic stress. Br. Med. Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 6.Gregus A., Wintink A.J., Davis A.C., Kalynchuk L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005;156:105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Woolley C.S., Gould E., McEwen B.S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-A. [DOI] [PubMed] [Google Scholar]
- 8.Sapolsky R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 9.Parker K.J., Schatzberg A.F., Lyons D.M. Neuroendocrine aspects of hypercortisolism in major depression. Horm. Behav. 2003;43:60–66. doi: 10.1016/S0018-506X(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y., Son H., Kim G., Kim S., Lee D.H., Roh G.S., Kang S.S., Cho G.J., Choi W.S., Kim H.J. Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. J. Psychiatry Neurosci. 2013;38:183–191. doi: 10.1503/jpn.120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M., Jung S., Son H., Joon H. Glutamine-supplement diet maintains growth performance and reduces blood corticosterone level in cage-reared growing chicks. J. Agric. Life Sci. 2018;52:91–102. doi: 10.14397/jals.2018.52.3.91. [DOI] [Google Scholar]
- 12.Son H., Baek J.H., Go B.S., Jung D., Sontakke S.B., Chung H.J., Lee D.H., Roh G.S., Kang S.S., Cho G.J., et al. Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology. 2018 doi: 10.1016/j.neuropharm.2018.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Bergman M., Varshavsky L., Gottlieb H.E., Grossman S. The antioxidant activity of aqueous spinach extract: chemical identification of active fractions. Phytochemistry. 2001;58:143–152. doi: 10.1016/S0031-9422(01)00137-6. [DOI] [PubMed] [Google Scholar]
- 14.Le A., Ng A., Kwan T., Cusmano-Ozog K., Cowan T.M. A rapid, sensitive method for quantitative analysis of underivatized amino acids by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;944:166–174. doi: 10.1016/j.jchromb.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 15.De Angelis M., Montemurno E., Piccolo M., Vannini L., Lauriero G., Maranzano V., Gozzi G., Serrazanetti D., Dalfino G., Gobbetti M., et al. Microbiota and metabolome associated with Immunoglobulin A Nephropathy (IgAN) PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0099006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son H., Jung S., Kim J.Y., Goo Y.M., Cho K.M., Lee D.H., Roh G.S., Kang S.S., Cho G.J., Choi W.S., et al. Type 1 diabetes alters astrocytic properties related with neurotransmitter supply, causing abnormal neuronal activities. Brain Res. 2015;1602:32–43. doi: 10.1016/j.brainres.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 17.Fiechter G., Mayer H.K. UPLC analysis of free amino acids in wines: Profiling of on-lees aged wines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879:1361–1366. doi: 10.1016/j.jchromb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Qu J., Chen W., Luo G., Wang Y., Xiao S., Ling Z., Chen G. Rapid determination of underivatized pyroglutamic acid, glutamic acid, glutamine and other relevant amino acids in fermentation media by LC-MS-MS. Analyst. 2002;127:66–69. doi: 10.1039/b108422b. [DOI] [PubMed] [Google Scholar]
- 19.Joo Y., Choi K.M., Lee Y.H., Kim G., Lee D.H., Roh G.S., Kang S.S., Cho G.J., Choi W.S., Kim H.J. Chronic immobilization stress induces anxiety- and depression-like behaviors and decreases transthyretin in the mouse cortex. Neurosci. Lett. 2009;461:121–125. doi: 10.1016/j.neulet.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Jung S., Lee Y., Kim G., Son H., Lee D.H., Roh G.S., Kang S.S., Cho G.J., Choi W.S., Kim H.J. Decreased expression of extracellular matrix proteins and trophic factors in the amygdala complex of depressed mice after chronic immobilization stress. BMC Neurosci. 2012;13:58. doi: 10.1186/1471-2202-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim G., Jung S., Son H., Kim S., Choi J., Lee D.H., Roh G.S., Kang S.S., Cho G.J., Choi W.S., et al. The GABAB receptor associates with regulators of G-protein signaling 4 protein in the mouse prefrontal cortex and hypothalamus. BMB Rep. 2014;47:324–329. doi: 10.5483/BMBRep.2014.47.6.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 24.Ng Q.X., Venkatanarayanan N., Ho C.Y. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017;210:211–221. doi: 10.1016/j.jad.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 25.Ge J.F., Gao W.C., Cheng W.M., Lu W.L., Tang J., Peng L., Li N., Chen F.H. Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur. Neuropsychopharmacol. 2014;24:172–180. doi: 10.1016/j.euroneuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Li L.-F., Yang J., Ma S.-P., Qu R. Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model of depression. Eur. J. Pharmacol. 2013;711:42–49. doi: 10.1016/j.ejphar.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Xing H., Zhang K., Zhang R., Shi H., Bi K., Chen X. Antidepressant-like effect of the water extract of the fixed combination of Gardenia jasminoides, Citrus aurantium and Magnolia officinalis in a rat model of chronic unpredictable mild stress. Phytomedicine. 2015;22:1178–1185. doi: 10.1016/j.phymed.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Jiraungkoorskul W. Review of Neuro-nutrition Used as Anti-Alzheimer Plant, Spinach, Spinacia oleracea. Pharmacogn. Rev. 2016;10:105–108. doi: 10.4103/0973-7847.194040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brocardo P.S., Budni J., Kaster M.P., Santos A.R., Rodrigues A.L. Folic acid administration produces an antidepressant-like effect in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology. 2008;54:464–473. doi: 10.1016/j.neuropharm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Gowda U., Mutowo M.P., Smith B.J., Wluka A.E., Renzaho A.M. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition. 2015;31:421–429. doi: 10.1016/j.nut.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Guan L.P., Liu B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016;121:47–57. doi: 10.1016/j.ejmech.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Mikkelsen K., Stojanovska L., Apostolopoulos V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016;23:4317–4337. doi: 10.2174/0929867323666160920110810. [DOI] [PubMed] [Google Scholar]
- 33.Park S.H., Kim J.H., Min S.G., Jo Y.J., Chun J.Y. Effects of ethanol addition on the efficiency of subcritical water extraction of proteins and amino acids from porcine placenta. Korean J. Food Sci. Anim. Resour. 2015;35:265–271. doi: 10.5851/kosfa.2015.35.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dashdorj D., Hwang I. Effect of extraction methods on the types and levels of free amino acid of beef longissimus muscle. Korean J. Food Sci. Anim. Resour. 2012;32:369–375. doi: 10.5851/kosfa.2012.32.3.369. [DOI] [Google Scholar]
- 35.Houpert Y., Tarallo P., Siest G. Comparison of procedures for extracting free amino acids from polymorphonuclear leukocytes. Clin. Chem. 1976;22:1618–1622. [PubMed] [Google Scholar]
- 36.Jongkees B.J., Hommel B., Kühn S., Colzato L.S. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—A review. J. Psychiatr. Res. 2015;70:50–57. doi: 10.1016/j.jpsychires.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Kabuki Y., Mizobe Y., Yamada S., Furuse M. Dietary l-tyrosine alleviates the behavioral alterations induced by social isolation stress in mice. Brain Res. Bull. 2009;80:389–396. doi: 10.1016/j.brainresbull.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Young S.N. Behavioral effects of dietary neurotransmitter precursors: basic and clinical aspects. Neurosci. Biobehav. Rev. 1996;20:313–323. doi: 10.1016/0149-7634(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 39.Ajami V.R.Y., Ajami A.M. Glutamine: The emperor or his clothes? J. Nutr. 2001;131:2505–2508. doi: 10.1093/jn/131.9.2505S. [DOI] [PubMed] [Google Scholar]
- 40.Wilmore D.W. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J. Nutr. 2001:2543–2549. doi: 10.1093/jn/131.9.2543S. [DOI] [PubMed] [Google Scholar]
- 41.Arnone D., Mumuni A.N., Jauhar S., Condon B., Cavanagh J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: Meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur. Neuropsychopharmacol. 2015:1–9. doi: 10.1016/j.euroneuro.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Luykx J.J., Laban K.G., van den Heuvel M.P., Boks M.P.M., Mandl R.C.W., Kahn R.S., Bakker S.C. Region and state specific glutamate downregulation in major depressive disorder: A meta-analysis of (1)H-MRS findings. Neurosci. Biobehav. Rev. 2011:1–8. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Taylor M.J. Could glutamate spectroscopy differentiate bipolar depression from unipolar? J. Affect. Disord. 2014;167:80–84. doi: 10.1016/j.jad.2014.05.019. [DOI] [PubMed] [Google Scholar]