Abstract
Objective:
As part of the Care and Prevention in the United States Demonstration Project (2012-2016), which aimed to reduce HIV-related morbidity and mortality among racial/ethnic minority groups in 8 states, the Virginia Department of Health (VDH) funded Walgreens to provide HIV testing in retail pharmacies in areas with large racial/ethnic minority communities and high rates of poverty. We describe this program and summarize its outcomes. We hypothesized that (1) offering walk-in HIV testing outside of traditional business hours and alongside other point-of-care tests in retail pharmacies would increase rates of first-time testers and (2) using data on social determinants of health associated with higher rates of HIV infection to locate test sites would increase the identification of people who were previously undiagnosed.
Methods:
Using 2010 US Census data and 2007-2011 five-year population estimates from the American Community Survey, VDH selected 32 Walgreens stores located in census tracts where at least 30% of the population was black and/or Hispanic/Latino and/or where at least 20% of the population was living at or below the federal poverty level. Pharmacists administered the INSTI HIV-1/HIV-2 Rapid Antibody Test. Clients with a reactive test result were linked to confirmatory testing and medical care.
Results:
Between June 1, 2014, and September 29, 2016, Walgreens pharmacists performed HIV tests on 3630 clients, of whom 1668 (46.0%) had either never been tested or were unsure if they had been tested. Of all clients tested, 30 (0.8%) had a reactive test result. Of 26 clients who also had positive confirmatory testing, 22 (84.6%) were linked to care. The mean cost per person tested was $41.79, and the mean cost per reactive result was $5057.
Conclusions:
Retail pharmacies may be an effective venue for those who have never been tested for HIV to access HIV testing, particularly if the pharmacies are located in priority areas or where community-based organizations are unable to operate.
Keywords: Care and Prevention in the United States, HIV, HIV prevention, HIV testing, Secretary’s Minority AIDS Initiative Fund
The National HIV/AIDS Strategy1 and guidelines from the Centers for Disease Control and Prevention (CDC)2 and the National Institutes of Health3 emphasize the need for improved access to HIV testing. Research has shown that people who are aware that they are infected with HIV are more likely than those who are unaware of their infection to reduce risk behaviors that can lead to HIV transmission.4 In addition, early HIV detection and linkage to medical care can result in considerable health benefits and a reduction in the overall risk for HIV-related illness and death.2
The Virginia Department of Health (VDH) provides access to publicly funded HIV testing through local health departments and nonclinical community-based organizations (CBOs), as well as to clinic-based HIV testing at hospitals, community health centers, and behavioral health organizations.5 However, geographic and scheduling challenges can impede access to free HIV testing at these sites, particularly in rural regions; in addition, HIV-related stigma is a deterrent to HIV testing.6,7 A 2010 CDC report pertaining to HIV and other communicable diseases, and focusing on social determinants of health, stressed the need for innovative approaches to “reduce the impact of poverty, unequal access to health care, incarceration, lack of education, stigma, homophobia, sexism, racism, and other factors that result in disproportionate health impact.”8 Along these lines, research has shown that comprehensive prevention programs that bring health services to the places where people “live, work, learn, and play” have positive effects on health outcomes.7,8
Retail pharmacies can play a role in this process because they are typically located in neighborhoods and shopping centers, making them accessible to a large and diverse population of potential testers.9 Retail pharmacies have been cited as promising venues for expanding access to HIV testing.9,10 Furthermore, published studies detailed pharmacist-provided HIV testing programs in Michigan11 and New York City,12 counselor-provided HIV testing in New York City,13 and HIV testing in pharmacies by various providers in Georgia, Illinois, Montana, New York, and Washington, DC.14
VDH used funds from the US Department of Health and Human Services Secretary’s Minority AIDS Initiative Fund for the Care and Prevention in the United States Demonstration Project (hereinafter, CAPUS), which aims to create more efficient and effective systems to improve HIV testing, linkage to and retention in care, and adherence to antiretroviral therapy, targeting highest-risk racial/ethnic minority populations.15 VDH developed a statewide retail pharmacy–based HIV testing program, focusing on areas with large racial/ethnic minority communities and high rates of poverty, with the goal of improving access to HIV testing. The objective of this analysis was to describe this program and summarize its outcomes. We hypothesized that (1) offering walk-in HIV testing outside of traditional business hours and alongside other point-of-care tests in retail pharmacies would increase rates of first-time testers and (2) locating stores in communities disproportionately affected by HIV would lead to an increase in the identification of people who had previously undiagnosed HIV infection.
Methods
VDH selected a vendor and HIV test; chose store sites; trained pharmacists; established protocols for the testing session, referrals, and linkage to care; and determined costs of the intervention.
Vendor and HIV Test Selection
VDH issued a request for proposals for retail pharmacy–based HIV testing and selected Walgreens as the vendor for the pilot program. Walgreens has demonstrated enthusiasm for HIV-related issues by certifying several locations as HIV Centers of Excellence15 (ie, employees at that location have completed additional training on HIV care and HIV-related stigma)16 and by participating in a CDC pharmacy HIV testing pilot project from 2011 to 2013.14 Walgreens proposed that the INSTI HIV-1/HIV-2 Rapid Antibody Test (bioLytical Laboratories Inc, Richmond, BC, Canada) (hereinafter, INSTI test) be used for this program. The INSTI test is a manual, visually read immunoassay for the qualitative detection of HIV antibodies in human blood. It has been granted Clinical Laboratory Improvement Amendments–waived status for fingerstick blood (signifying that it is a minimally complex test to administer and interpret), has a 1-minute incubation time, and is 100% sensitive and 99.8% specific for detecting HIV in blood samples.17
Store Site Selection
VDH analyzed 2010 US Census data and 2007-2011 five-year population estimates from the American Community Survey to identify priority census tracts in which at least 30% of the population was black and/or Hispanic/Latino and/or in which at least 20% of the population was living at or below the federal poverty level.18,19 These 2 metrics are associated with increased HIV infection risk and are consistent with populations targeted by CAPUS.15,20 VDH selected Walgreens stores in or near these priority census tracts rather than using epidemiological indicators (eg, incidence or prevalence, indicators of high primary risk of transmission) to select stores because we noted that HIV testing in many of these priority areas was limited to only local health department clinics, where stigma, inconvenience, and inaccessibility may have created barriers to testing for clients in our target population.21
VDH and Walgreens agreed on 13 stores with private rooms available for testing that would serve as pilot sites. After 12 months, the program expanded to include 19 additional stores, also with private rooms, in priority areas across the state. Additional stores were selected from those Walgreens locations that already had a private consultation room and were not located near another pharmacy testing location. We classified pharmacies located in cities and counties with <15 000 residents as rural stores (n = 6), with 15 000-50 000 residents as suburban stores (n = 12), and with >50 000 residents as urban stores (n = 14). Participating stores were spread across all 5 of Virginia’s health planning regions: 10 were located in the northwest region of Virginia, 6 in the eastern region, 6 in the southwest region, 5 in the central region, and 5 in the northwest region.22
Pharmacist Training
All pharmacists working in Walgreens stores where testing was offered completed in-person training, which covered administration of the HIV test, quality assurance procedures, and counseling approaches to the delivery of reactive test results (to supplement basic counseling skills already learned during pharmacy school). A 2-person team comprising 1 member from VDH’s implementation team and 1 member from VDH’s training contractor conducted the training. In addition to the in-person training, the pharmacists completed CDC’s Online Rapid Testing Training Course23 and reviewed Virginia’s quality assurance manual for HIV testing. Walgreens also requires all of its pharmacists to take a course on HIV-related stigma as part of their continuing education.
Testing Session Protocols
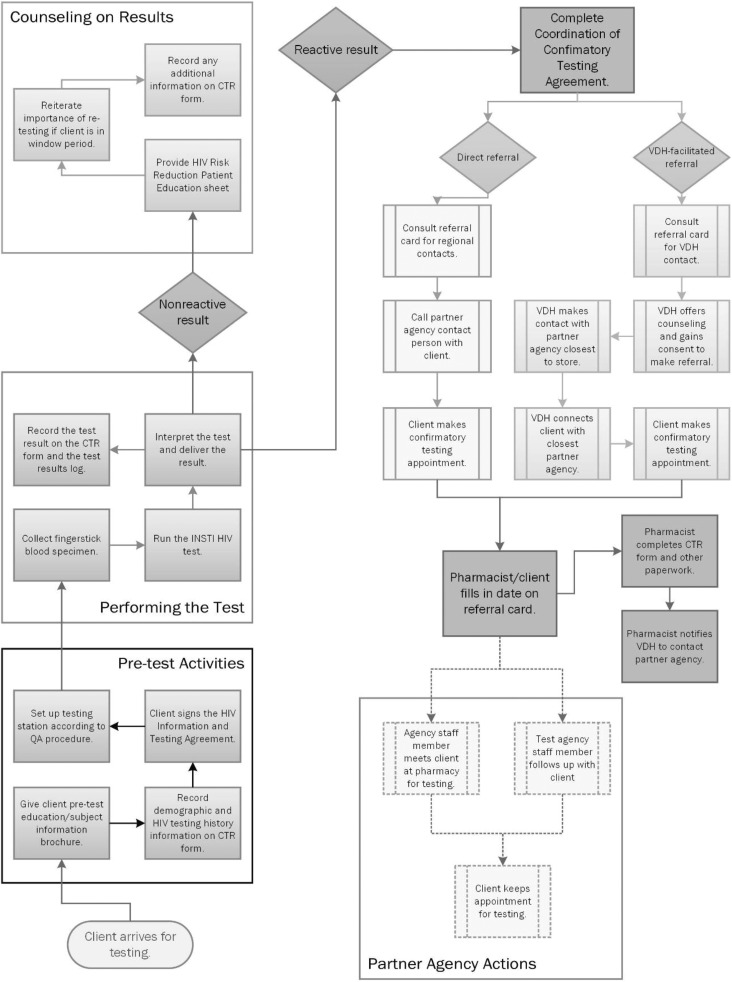
HIV testing was offered on a walk-in basis to anyone aged ≥18 during each pharmacy’s hours of operation (store hours varied; several pharmacies were open 24/7) (Figure). Clients who wished to receive an HIV test were required to complete a standard consent form while the pharmacist prepared the consultation room for the testing session. Consistent with CDC guidelines for testing programs in high-traffic health care settings (eg, emergency departments, community health centers), pharmacists did not perform risk assessments or conduct risk-reduction counseling.24 Pharmacists collected data on demographic characteristics, including sex (male, female, unknown, not asked); current gender (male, female, transgender, unknown, not asked); race (American Indian/Alaska Native, Asian, black/African American, Native Hawaiian/Pacific Islander, white, don’t know, declined to answer, not asked); ethnicity (Hispanic/Latino, non-Hispanic/non-Latino, don’t know, declined, not asked); year of birth; county of residence; and receipt of a previous HIV test (yes, no, don’t know, declined to answer, not asked). Pharmacists did not collect data on transmission risk. In the event of a reactive result, linkage staff members at partnering organizations collected data on transmission risk during the confirmatory testing process.
Figure.
Flow diagram for walk-in HIV testing at 32 Walgreens retail pharmacies participating in the Virginia Department of Health (VDH) pharmacy testing program, Virginia, June 1, 2014, through September 29, 2016. The testing program was part of the Care and Prevention in the United States Demonstration Project (2012-2016), which aimed to reduce HIV-related morbidity and mortality among racial/ethnic minority groups in 8 states.15 The diagram begins with activities conducted before administration of the INSTI HIV-1/HIV-2 rapid test, continues to the steps involved with documenting the test and interpreting the test result, and concludes with a walk-through of the 2 options for referral to confirmatory testing, the various steps involved, and the activities performed by the agency that conducted the confirmatory test. Abbreviations: CTR, counseling, testing, and referral; QA, quality assurance.
Pharmacists administered the INSTI test, which typically yielded results in 1 minute. If the result was nonreactive, the pharmacist delivered basic HIV prevention messages, asked about possible HIV exposure within the test’s window period (between potential infection and the earliest point that the test could detect HIV antibodies), and provided messages about routine HIV testing. Pharmacists provided educational materials and referrals to local CBOs for clients who sought complete risk-reduction counseling. Pharmacists referred clients whose test results were reactive for confirmatory testing and linkage to care by using the processes described in the next section.
Referrals and Linkage to Care
VDH established a multistep linkage model that began at the time of a reactive test result, with the pharmacist and client together calling either a regional partner agency (eg, the local health department or CBO, if available) or a 24-hour telephone line staffed by a member of the VDH implementation team (Figure). If the direct referral was used, the client made the confirmatory testing appointment with the partner agency during the initial telephone call. If the VDH-facilitated referral was used, a member of the VDH implementation team counseled clients by telephone about their result and the importance of follow-up confirmatory testing, and then walked clients through the process of connecting them to a test counselor at a CBO or to a disease intervention specialist. Clients were able to choose the style of referral. Clients were provided a referral card containing the partner agency name, as well as the date and time of their appointment, at the conclusion of the session. In many instances, VDH staff members remained on the line and were able to dial in the test counselor or disease intervention specialist as a third party. Sometimes, if the confirming agency was not available, VDH staff members gave clients contact numbers and instructions. Regardless, VDH staff members remained in contact with clients until they connected with a test counselor or disease intervention specialist, who then arranged and conducted the confirmatory testing. The confirmatory testing agency then linked those clients confirmed to be infected with HIV to medical care. We considered clients HIV positive if their HIV test result was positive at their confirmatory testing appointment, and we considered them linked to care when they had a documented CD4 or viral load test result reported to the Enhanced HIV/AIDS Reporting System.25
Costs of Intervention
We calculated the cost per person tested by dividing total expenditures for the program by the number of clients tested, and we calculated the cost per reactive result by dividing total expenditures by the number of clients with a reactive result. Total expenditures included Walgreens’ negotiated rate of reimbursement, which comprised its costs for the pharmacists’ time spent training, conducting testing, counseling clients, and participating in linkage-to-care activities (during the initial testing session), as well as for the supplies needed for testing (gloves, adhesive bandages, gauze pads), which Walgreens sourced from its internal stock; expenses associated with purchasing and shipping INSTI test kits and controls (eg, lancets, pipettes, and alcohol swabs); and costs of auxiliary testing supplies (eg, absorbent disposable underpads [Chux] and biohazard containers).
Results
From June 1, 2014, through September 29, 2016, Walgreens pharmacists at participating stores performed 3630 HIV tests. Testing attainment varied widely among pharmacies. The fewest tests conducted by a pharmacy was 4 and the most was 670 (mean, 113). The initial 13 stores averaged 220 tests per pharmacy, whereas the 19 stores added during the expansion phase averaged 40 tests per pharmacy.
In general, stores located in communities with larger populations performed more tests. Of the 3630 tests performed, urban stores accounted for 2013 (55.5%) tests, suburban stores for 1057 (29.1%) tests, and rural stores for 560 (15.4%) tests. Testing attainment varied among the 5 health planning regions in Virginia: 1339 (36.9%) were performed in the northern region, 1208 (33.3%) in the central region, 645 (17.8%) in the eastern region, 250 (6.9%) in the northwest region, and 188 (5.2%) in the southwest region. Also, among all tests, 1416 (39.0%) were administered during traditional business hours (9 am to 6 pm, Monday through Friday) and 2214 (61.0%) were administered outside of traditional business hours (6 pm to 9 am, Monday through Friday) or on weekends.
Of the 3630 clients tested, 1827 (50.3%) were non-Hispanic black, 1061 (29.2%) were non-Hispanic white, and 368 (10.1%) were Hispanic of any race. The median age of clients was 30 (range, 19-69). Of those tested, 2122 (58.5%) were male and 1668 (46.0%) had either never been tested or were unsure if they had been tested (Table 1).
Table 1.
Nonreactive and reactive results of HIV testing among 3630 pharmacy clients at 32 Walgreens retail pharmacies participating in a Virginia Department of Health pharmacy testing program, by demographic characteristics, Virginia, June 1, 2014, through September 29, 2016a
| Tested | Nonreactive | Reactive | ||||
|---|---|---|---|---|---|---|
| Characteristics | No. | No. | % of All Nonreactive | No. | % of All Reactive | % of Tested, by Row |
| Total | 3630 | 3600 | 100b | 30 | 100 | 0.8 |
| Race/ethnicity | ||||||
| American Indian/Alaska Native | 23 | 23 | 0.6 | 0 | 0 | 0 |
| Asian | 160 | 160 | 4.4 | 0 | 0 | 0 |
| Black/African American | 1827 | 1804 | 50.1 | 23 | 76.7 | 1.3 |
| Hispanic/Latino | 368 | 368 | 10.2 | 0 | 0 | 0 |
| >1 race | 38 | 38 | 1.1 | 0 | 0 | 0 |
| Native Hawaiian/Pacific Islander | 11 | 11 | 0.3 | 0 | 0 | 0 |
| White | 1061 | 1055 | 29.3 | 6 | 20.0 | 0.6 |
| Unknown | 142 | 141 | 3.9 | 1 | 3.3 | 0.7 |
| Previous HIV test | ||||||
| Don’t know | 286 | 282 | 7.8 | 4 | 13.3 | 1.4 |
| No | 1382 | 1369 | 38.0 | 13 | 43.3 | 0.9 |
| Not asked | 124 | 123 | 3.4 | 1 | 3.3 | 0.8 |
| Yes | 1819 | 1807 | 50.2 | 12 | 40.0 | 0.7 |
| Declined to answer | 19 | 19 | 0.5 | 0 | 0 | 0 |
| Age range, y | ||||||
| 13-19 | 107 | 106 | 2.9 | 1 | 3.3 | 0.9 |
| 20-29 | 1589 | 1575 | 43.8 | 14 | 46.7 | 0.9 |
| 30-39 | 1073 | 1062 | 29.5 | 11 | 36.7 | 1.0 |
| 40-49 | 421 | 418 | 11.6 | 3 | 10.0 | 0.7 |
| 50-59 | 223 | 223 | 6.2 | 0 | 0 | 0 |
| ≥60 | 82 | 81 | 2.3 | 1 | 3.3 | 1.2 |
| No age data | 135 | 135 | 3.8 | 0 | 0 | 0 |
| Gender | ||||||
| Female | 1473 | 1467 | 40.8 | 6 | 20.0 | 0.4 |
| Male | 2122 | 2098 | 58.3 | 24 | 80.0 | 1.1 |
| Transgender | 6 | 6 | 0.2 | 0 | 0 | 0 |
| Unknown | 29 | 29 | 0.8 | 0 | 0 | 0 |
a Testing was conducted in the following cities: Aldie, Alexandria, Centreville, Charlottesville, Chesterfield, Collinsville, Culpeper, Danville, Fairfax, Fredericksburg, Hampton, Hopewell, Louisa, Lynchburg, Manassas, Martinsville, Newport News, Norfolk, Petersburg, Reston, Richmond, Roanoke, South Boston, Suffolk, Virginia Beach, Williamsburg, Winchester, and Woodbridge. The testing program was part of the Care and Prevention in the United States Demonstration Project (2012-2016), which aimed to reduce HIV-related morbidity and mortality among racial/ethnic minority groups in 8 states.15
b Some percentages do not total to 100.0 because of rounding.
Of the 3630 clients tested, 30 (0.8%) were reactive for HIV antibodies (Table 1). Of the 30 clients who had reactive test results, 23 (76.7%) were non-Hispanic black, the median age was 30 (range, 19-69), and 17 (56.7%) had either never been tested or were unsure if they had been tested. Also, of the 30 clients with a reactive test, 26 (86.7%) had positive confirmatory testing and 4 (13.3%) were lost to follow-up. Of the 26 clients who had a confirmed HIV diagnosis, 8 (30.8%) were heterosexual, 1 (3.8%) was an intravenous drug user, 15 (57.7%) were men who have sex with men (MSM), and 1 (3.8%) had unknown risk factors. Among the 26 clients who were confirmed to have infection, 22 were linked to care: 14 were linked to care within 30 days, 4 were linked to care within 60 days, 1 was linked to care within 90 days, and 3 were linked to care after 90 days.
VDH spent $151 710 on the pharmacy testing program (Table 2), which included $108 421 on reimbursements to Walgreens, $39 727 on INSTI test kits and controls, and $3562 on auxiliary testing supplies. The mean cost per person tested was $41.79, and the mean cost per reactive result was $5057.
Table 2.
HIV testing attainment, expenditures, and costs of HIV testing among 3630 pharmacy clients at 32 Walgreens retail pharmacies participating in a Virginia Department of Health (VDH) pharmacy testing program,a Virginia, June 1, 2014, through September 29, 2016, compared with results of a CDC pharmacy testing pilot project at 21 sites in 6 cities, United States, August 2011 to July 2013
| Variable | VDH Pharmacy Testing Program | CDC Pharmacy Testing Pilot Project14,22 |
|---|---|---|
| Attainment, no. | ||
| Clients tested | 3630 | 939 |
| Clients with reactive result | 30 | 17 |
| Total expenditures, $ | 151 710b | 44 328c |
| Reimbursements for service | 108 421 | NA |
| Test kits and controls | 39 727 | NA |
| Auxiliary supplies | 3562 | NA |
| Cost, $ | ||
| Per person tested | 41.79 | 47.21 |
| Per reactive result | 5057 | 2608 |
Abbreviations: CDC, Centers for Disease Control and Prevention; NA, not applicable.
a Testing sites were located in the following cities: Aldie, Alexandria, Centreville, Charlottesville, Chesterfield, Collinsville, Culpeper, Danville, Fairfax, Fredericksburg, Hampton, Hopewell, Louisa, Lynchburg, Manassas, Martinsville, Newport News, Norfolk, Petersburg, Reston, Richmond, Roanoke, South Boston, Suffolk, Virginia Beach, Williamsburg, Winchester, and Woodbridge. The testing program was part of the Care and Prevention in the United States Demonstration Project (2012-2016), which aimed to reduce HIV-related morbidity and mortality among racial/ethnic minority groups in 8 states.15
b Total expenditures for VDH program included: Walgreens’ negotiated rate of reimbursement, composed of its costs for the pharmacists’ time spent training and conducting testing and for the supplies needed for testing (gloves, adhesive bandages, and gauze pads); expenses associated with purchasing and shipping INSTI HIV-1/HIV-2 test kits and controls (eg, lancets, pipettes, and alcohol swabs); and costs of auxiliary testing supplies (eg, absorbent disposable underpads [Chux] and biohazard containers).
c Total expenditures for the CDC project were not itemized but included costs of test kits, control kits, shipping, test supplies, training, reporting, program administration, and advertising. The documented time pharmacists of various earning levels spent providing pretest counseling and posttest counseling was also included in expenditures.
Discussion
Selecting Walgreens stores using data on community composition at the census-tract level appeared to successfully expand access to HIV testing, reaching large proportions of racial/ethnic minority groups, people who had never been tested, and people with undiagnosed HIV infection. Testing attainment varied across the state, and the cost of the intervention was tolerable.
Impact of Site Selection Strategy
We selected Walgreens stores located in census tracts with large racial/ethnic minority populations, which is one predictor of increased HIV prevalence, to increase the likelihood that average customers would belong to a group with high HIV prevalence. Our results suggest that this site selection strategy was effective because nearly two-thirds of clients who were tested were people of color. Furthermore, the 0.8% HIV reactive rate for this VDH pharmacy testing program was higher than both the VDH CBO (ie, CBOs providing outreach-based testing) targeted HIV testing program (0.6%) and clinical (ie, general public at local health departments, emergency departments; 0.4%) HIV testing reactive rates during the same period (VDH, unpublished data, 2017).
Our results also suggest that this site selection strategy may have improved the likelihood of reaching clients who were unaware of their HIV status. During the study period, 46% of clients reported that they had either never previously had an HIV test or were unsure if they had been tested. In contrast, during the same period, 33% of clients tested statewide (via publicly funded CBOs, local health departments, and other clinical sites) had never been tested or were unsure if they had been tested (VDH, unpublished data, 2017). This discrepancy was even more pronounced among people who had a reactive HIV test result—57% of those in our program compared with 22% of clients tested statewide had never been tested or were unsure if they had been tested (VDH, unpublished data, 2017).
Testing Attainment
Although eastern Virginia is the state’s most populous region and has the highest HIV incidence and prevalence rates in the state,26 fewer HIV tests were conducted there than in either central Virginia or northern Virginia. This regional variation in testing attainment may be attributable to several factors. First, as reported by others, the perceived community need for the service among Walgreens’ district supervisors or store managers may have differed.27 For example, unlike most stores in Virginia, 2 stores in central Virginia advertised the availability of HIV testing, and these 2 stores accounted for nearly one-quarter of all tests performed in Virginia during the evaluation period. Second, regional variation in attainment may also have been affected by the numbers and types of stores we chose for the pilot and expansion phases of the program. For example, during the pilot phase, only 1 of the first 13 pharmacies to implement testing was located in the northwest region, and the southwest region had no urban or suburban stores. Although during the expansion phase we attempted to even out the number and type of stores in each region, none of the 19 stores that we added reached the same number of clients as the stores selected during the pilot phase. These findings suggest that, in addition to the demographic and socioeconomic compositions of regions, decisions made by Walgreens’ leaders and VDH may have influenced the regional variation in testing attainment that we observed and may have affected our results.
Intervention Cost
Our data appear to validate some results from the CDC pharmacy-based HIV testing pilot project in 6 US cities, which demonstrated a cost of $47.21 per person tested.28 However, unlike the CDC cost analysis, which relied on reimbursing pharmacists of various earning levels for their documented time spent providing pretest counseling and posttest counseling,14,28 we reimbursed Walgreens on a fee-for-service basis, using an estimate of the average length of a testing session to negotiate a rate of reimbursement per test. Because this reimbursement rate was fixed and supply costs in our study were based on actual expenditures, our $41.79 cost per person tested may be a more useful gauge of what this service might cost if it were to be replicated in other jurisdictions or brought to scale under conditions similar to those in Virginia.
Positive Client Outcomes
Research into the perceptions of pharmacists about HIV testing has suggested that although pharmacists, in keeping with their professional oath,29 have the desire to ensure that clients are linked to care, some pharmacists are unfamiliar with the process of linkage to care, and some are unaware of the wide range of barriers that can arise during the process and impede linkage.30,31 Before implementation of this HIV testing program, we were aware that the most challenging aspect of the program would be the referral and linkage to care of clients who had a reactive test result. Although pharmacists may be willing and able to help clients process a reactive result, competing priorities within the pharmacy (eg, managing pharmacy staff members, answering the telephone, filling prescriptions, consulting with clients) may prevent them from providing clients with the individual attention needed to realistically address the barriers to linkage to care that clients face.30 These same competing priorities also may be what prevents pharmacists from providing on-site confirmatory HIV testing. To address this linkage challenge, we established a 24-hour telephone line for the purpose of bridging the gap between the time of clients’ reactive HIV test result and their linkage to the disease intervention specialist or CBO conducting confirmatory testing.
This method provided a reasonably functional pathway for linking clients with a confirmed HIV diagnosis to medical care. Although just shy of the 90% linkage goal for new diagnoses outlined in the National HIV/AIDS Strategy,1 85% of the clients who received a confirmed HIV diagnosis through our pharmacy testing program were linked to medical care, with most linked to care within 30 days. This linkage-to-care rate was comparable to the rates for CBO-targeted HIV testing programs and clinical HIV testing in Virginia (VDH, unpublished data, 2017). However, it should be noted that our approach relied on having VDH staff members operate a 24/7 hotline for our pharmacy testing clients and on the willingness of CBO staff members to respond to confirmatory testing needs in the middle of the night, as happened on several occasions. It may not be feasible to include these features for all pharmacies statewide.
Limitations
The model we used had several limitations. Because this program was implemented as part of CAPUS and was meant to expand access to testing to black and Latino populations, and was not intended as formal research, the primary goal of the work was to establish a functioning pharmacy HIV testing program that met those objectives. As such, we did not implement HIV testing in communities with large affluent or small racial/ethnic minority populations to serve as control sites, and we did not survey clients or pharmacists about their attitudes relating to HIV testing or their perceptions about the services offered. We also did not control for the length of time that the HIV testing service was offered at various pharmacies. In addition, some stores conducted few tests despite being located in areas where we anticipated high levels of HIV testing uptake, and we were unable to systematically analyze why this occurred.
Furthermore, because only a pharmacist could conduct testing in this program, the operational protocols for the program were purposely designed to prevent disruption to the pharmacy, in part by minimizing the length of an average pharmacist–client session. Consistent with what is done in other nontargeted clinical HIV testing programs,24 Walgreens pharmacists neither collected data on risk from clients nor offered risk-reduction counseling to clients. As a result, although our relatively high HIV reactive rate suggests that we reached high-risk clients, we were unable to identify the risk categories of most clients or whether their perceptions of their own risk influenced their decision to test at a Walgreens. Because methodologies now exist to estimate the number of resident MSM at state and county levels, by using data from both of the sources we used to select our priority locations,32,33 future pharmacy-based HIV testing programs may benefit from estimating the presence of this population when selecting service locations.
In addition, local pharmacy conditions may have influenced our results. First, our program was implemented in a chain pharmacy that offered other point-of-care services (eg, blood glucose testing, vaccinations, blood pressure measurements). Offering these services may have made it easier for clients to approach the pharmacy counter because doing so would not be a de facto indicator to onlookers that the client was requesting an HIV test. Second, although VDH promoted the HIV testing program through multiple forms of media, testing attainment was highest at the 2 Walgreens stores that performed their own additional marketing. Because VDH did not collect information related to Walgreens staff member perceptions of the program or any quantitative data related to additional marketing by Walgreens staff members, we were unable to assess whether internal and external marketing may have influenced our results. Further research may help determine to what extent local retail pharmacy conditions played a role in client willingness to request an HIV test.
Finally, because we did not include control groups in our evaluation and all clients received the same services, we were unable to say categorically that our approach contributed to our success in linking clients to care. However, to our knowledge, previous pharmacy-based HIV testing research focused solely on client linkage to confirmatory testing, and no one has described successful methods for linking clients to medical care, even though this remains one of the main barriers to implementing pharmacy testing on a larger scale.9,10,34,35 Consequently, the methods for linkage to care used in our program may provide a basis for future research in this area.
Conclusions
The results of this pharmacy-based HIV testing program demonstrate that the retail pharmacy is a setting in which clients who have not previously been tested for HIV are willing to do so and that locating HIV testing in pharmacies can lead to the identification of new HIV infections. However, the success of retail pharmacy–based testing programs may be influenced by various factors, including community demographic characteristics, site selections, costs, pharmacist perceptions, competing priorities within the pharmacy, and external marketing.
This pilot program showed the value of taking a “no wrong door” approach to HIV testing, which seeks to normalize HIV testing by increasing the number of routes by which a client can access the service. To achieve the goals of the National HIV/AIDS Strategy, it is important that access to HIV testing is expanded through private–public partnerships. Our program suggests that normalizing HIV testing through programs such as pharmacy-based testing may help expand access to the service and provide new opportunities for public health departments, private corporations, and nonprofit organizations to improve the health of their communities.
Acknowledgments
The authors acknowledge the hard work and support from the members of the Virginia site team of the US Department of Health and Human Services Secretary’s Minority AIDS Initiative Fund for Care and Prevention in the United States (CAPUS) Demonstration Project federal partnership, which helped foster this project: Drs Kirk Henny, Mesfin Mulatu, and Kim Williams, as well as Emilio German, Tamika Hoyte, Benjamin Laffoon, and Pilgrim Spikes (Centers for Disease Control and Prevention [CDC]); Kim Brown (Health Resources and Services Administration); Ilze Ruditis (Substance Abuse and Mental Health Services Administration); and Todd Harvey (formerly of the National Alliance of State and Territorial AIDS Directors). The authors also acknowledge the following collaborating partners: Ambrose Delpino, Rusty Maney, Glen Pietrandoni, Alain Porte, and Ashley Samoila (Walgreens); Jennifer Brown and Leahjane Lavin (bioLytical Laboratories); Joanna McKee (Virginia Commonwealth University); Leigh Guarinello (Inova Health Systems); Tanya Kearney (Eastern Virginia Medical School); and Zakia McKensey (Nationz Foundation). Finally, the authors are grateful to the Walgreens pharmacists who participated in this project.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The CAPUS project was supported by the Secretary’s Minority AIDS Initiative Fund and led by CDC (grant #5U62PS003949).
References
- 1. Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. 2010. https://obamawhitehouse.archives.gov/administration/eop/onap/nhas. Accessed September 3, 2018.
- 2. Anderson TJ, Atkins D, Baker-Cirac C, et al. Revised guidelines for HIV counseling, testing, and referral—technical expert panel review of CDC HIV counseling, testing, and referral guidelines. MMWR Morb Mortal Wkly Rep. 2001;50(RR19):1–58.11215787 [Google Scholar]
- 3. NIH-funded study finds community-based efforts increase HIV testing, prompt behavior change [press release]. Bethesda, MD: National Institute of Mental Health; 2013. https://www.nimh.nih.gov/archive/news/2013/nih-funded-study-finds-community-based-efforts-increase-hiv-testing-prompt-behavior-change.shtml. Accessed September 3, 2018. [Google Scholar]
- 4. Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. [DOI] [PubMed] [Google Scholar]
- 5. Virginia Department of Health, Office of Epidemiology. Virginia HIV epidemiologic profile 2016. 2017. http://www.vdh.virginia.gov/content/uploads/sites/10/2016/01/HIV_Epi_Profile_2016.pdf. Accessed September 3, 2018.
- 6. Reif S, Golin CE, Smith SR. Barriers to accessing HIV/AIDS care in North Carolina: rural and urban differences. AIDS Care. 2005;17(5):558–565. [DOI] [PubMed] [Google Scholar]
- 7. Institute of Medicine. No Time to Lose: Getting More From HIV Prevention. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Establishing a Holistic Framework to Reduce Inequities in HIV, Viral Hepatitis, STDs, and Tuberculosis in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 9. Dugdale C, Zaller N, Bratberg J, Berk W, Flanigan T. Missed opportunities for HIV screening in pharmacies and retail clinics. J Manag Care Spec Pharm. 2014;20(4):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gubbins PO, Klepser ME, Dering-Anderson AM, et al. Point-of-care testing for infectious diseases: opportunities, barriers, and considerations in community pharmacy. J Am Pharm Assoc (2003). 2014;54(2):163–171. [DOI] [PubMed] [Google Scholar]
- 11. Darin KM, Klepser ME, Klepser DE, et al. Pharmacist-provided rapid HIV testing in two community pharmacies. J Am Pharm Assoc (2003). 2015;55(1):81–88. [DOI] [PubMed] [Google Scholar]
- 12. Fuller CM, Galea S, Caceres W, Blaney S, Sisco S, Vlahov D. Multilevel community-based intervention to increase access to sterile syringes among injection drug users through pharmacy sales in New York City. Am J Public Health. 2007;97(1):117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calderon Y, Cowan E, Rhee JY, Brusalis C, Leider J. Counselor-based rapid HIV testing in community pharmacies. AIDS Patient Care STDS. 2013;27(8):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weidle PJ, Lecher S, Botts LW, et al. HIV testing in community pharmacies and retail clinics: a model to expand access to screening for HIV infection. J Am Pharm Assoc (2003). 2014;54(5):486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. The Care and Prevention in the United States (CAPUS) Demonstration Project. 2016. https://www.cdc.gov/hiv/research/demonstration/capus/index.html. Accessed September 3, 2018.
- 16. Walgreens HIV. Centers of Excellence pharmacies now top 500 nationwide in areas most impacted by HIV/AIDS [press release]. Deerfield, IL: Walgreens; 2011. http://news.walgreens.com/press-releases/community-news/walgreens-hiv-centers-of-excellence-pharmacies-now-top-500-nationwide-in-areas-most-impacted-by-hivaids.htm. Accessed September 3, 2018. [Google Scholar]
- 17. Galli RA, Green KF, La Marca A, et al. Evaluation of the accuracy and ease of use of a rapid HIV-1 antibody test performed by untrained operators at the point of care. J Clin Virol. 2013;58(suppl 1):e65–e69. [DOI] [PubMed] [Google Scholar]
- 18. US Census Bureau. 2010. US Census. https://www.census.gov/2010census. Accessed September 3, 2018.
- 19. US Census Bureau. American Community Survey 2007-2011. https://www.census.gov/programs-surveys/acs. Accessed September 3, 2018.
- 20. Denning P, DeNenno E. Communities in crisis: is there a generalized HIV epidemic in impoverished urban areas of the United States? 2017. https://www.cdc.gov/hiv/group/poverty.html. Accessed September 3, 2018.
- 21. Levy ME, Wilton L, Phillips G, II, et al. Understanding structural barriers to accessing HIV testing and prevention services among black men who have sex with men (BMSM) in the United States. AIDS Behav. 2014;18(5):972–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virginia Department of Health. Health planning regions in Virginia. 2007. http://www.vdh.virginia.gov/content/uploads/sites/3/2016/03/Maps_2007Bsmall.pdf. Accessed August 24, 2018.
- 23. Centers for Disease Control and Prevention. High impact prevention: HIV testing. August 2017 https://www.cdc.gov/hiv/policies/hip/hip.html. Accessed September 3, 2018.
- 24. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep. 2006;55(RR14):1–17. [PubMed] [Google Scholar]
- 25. Cohen SM, Gray KM, Ocfemia MCB, et al. The status of the national HIV surveillance system, United States, 2013. Public Health Rep. 2014;129(4):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virginia Department of Health. Virginia HIV surveillance annual report 2016. 2017. http://www.vdh.virginia.gov/content/uploads/sites/10/2017/08/Annual_Report_2016_july.pdf. Accessed September 3, 2018.
- 27. Amesty S, Blaney S, Crawford ND, Rivera AV, Fuller C. Pharmacy staff characteristics associated with support for pharmacy-based HIV testing in pharmacies participating in the New York State expanded access syringe exchange program. J Am Pharm Assoc (2003). 2012;52(4):472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lecher SL, Shrestha RK, Botts LW, et al. Cost analysis of a novel HIV testing strategy in community pharmacies and retail clinics. J Am Pharm Assoc (2003). 2015;55(5):488–492. [DOI] [PubMed] [Google Scholar]
- 29. American Association of Colleges of Pharmacy. Oath of a pharmacist. 2016. https://www.aacp.org/sites/default/files/2017-11/OATHOFAPHARMACIST2008-09.pdf. Accessed September 3, 2018.
- 30. Ryder PT, Meyerson BE, Coy KC, von Hippel CD. Pharmacists’ perspectives on HIV testing in community pharmacies. J Am Pharm Assoc (2003). 2013;53(6):595–600. [DOI] [PubMed] [Google Scholar]
- 31. Meyerson BE, Ryder PT, van Hippel C, Coy K. We can do more than just sell the test: pharmacist perspectives about over-the-counter rapid HIV tests. AIDS Behav. 2013;17(6):2019–2113. [DOI] [PubMed] [Google Scholar]
- 32. Lieb S, Thompson DR, Misra S, et al. Estimating populations of men who have sex with men in the southern United States. J Urban Health. 2009;86(6):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grey JA, Bernstein KT, Sullivan PS, et al. Estimating the population sizes of men who have sex with men in US states and counties using data from the American Community Survey. JMIR Public Health Surveill. 2016;2(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Avert. HIV and AIDS in the United States of America (USA). Updated April 2018. https://www.avert.org/professionals/hiv-around-world/western-central-europe-north-america/usa. Accessed September 3, 2018.
- 35. Centers for Disease Control and Prevention. Today’s HIV/AIDS epidemic. August 2016. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/todaysepidemic-508.pdf. Accessed September 3, 2018.