Abstract
Background:
Recurrent pregnancy loss is a challenging reproductive problem, and chromosomal anomalies approximately affect 2%–8% of couples with recurrent pregnancy loss. The chromosomal abnormality, especially balanced translocation rearrangement in either parent, is the important cause of recurrent spontaneous abortion.
Aims:
The aim of this study was to investigate the role and prevalence of chromosomal anomalies in recurrent miscarriages. The results will be helpful for counseling and make the decision for alternative options and precaution for the affected couples and also support to make a national database.
Settings and Design:
The present retrospective study was carried out in 172 couples (344 individuals) having the history of three or more recurrent spontaneous abortion. The cytogenetic analysis was done in all 344 individuals using G-banding and karyotyping.
Results:
Out of 172 couples, 17 couples (9.88%) had different types of structural or numerical chromosomal abnormalities. The structural aberrations were observed in 15 (8.72%) couples, and numerical aberrations were seen in 2 (1.16%) couples. Out of 17 couples, 8 (47.05%) had balanced translocations, 2 (11.76%) had the Robertsonian translocation, 5 (29.41%) had the pericentric inversion of chromosome 8, 9, and Y, and only 2 (11.76%) women showed sex chromosome numerical aberrations.
Conclusions:
Cytogenetic analysis should be an important routine investigation in couples with repeated miscarriages. Cytogenetic analysis is essential and helpful for genetic counseling to take precaution and implementing proper reproductive alternatives. Studies on the genetic basis of pregnancy loss should be taken up to generate data on these issues from different regions.
KEYWORDS: Chromosomal abnormalities, genetic counseling, recurrent pregnancy loss
INTRODUCTION
The occurrence of three or more consecutive detectable pregnancy losses on or before 20 weeks of gestation or the loss of three consecutive fetuses is usually known as recurrent abortion.[1] Recurrent pregnancy loss (RPL) or miscarriages affects approximately 10%–15% couples of all clinically recognized pregnancies.[2] There are several etiological factors considered for recurrent abortion, including chromosomal anomalies, other genetic and nongenetic factors. Most of the miscarriages are spontaneous but nonrecurrent. Hence, it is estimated that more pregnancies are lost spontaneously than are actually carried to full term.[2]
Worldwide, the couples suffering from recurrent abortions due to chromosomal abnormalities have been found to be 2%–8%.[3] It has also been reported that 50%–60% spontaneously aborted product of conception have chromosomal anomalies.[3] Most of the chromosomal abnormalities in recurrent abortions have been noted due to abnormal chromosomal segregation during parental gametogenesis and parents carrying balanced reciprocal translocation.[2] Moreover, unbalanced translocations account for approximately 1% of spontaneous miscarriages. Most of the miscarriages are random and nonrecurrent and are caused by the segregational error in zygote formation.[4] In case of recurrent abortions, one of the parents is a carrier having balanced chromosomal rearrangement producing unbalanced gametes which increase the risk of recurrent abortion, malformed baby, or sterility in a couple.[5] Chromosomal aneuploidy in the embryo is another common cause of abortion. Trisomies of chromosome 13, 18, and 21 are the common chromosomal aneuploidies followed by X chromosome monosomy (45, X).[6] Translocation makes structural rearrangements of the chromosome of different composition, and the chromosomes are stable chromosome. Chromosomal abnormalities in the couple are considered to be strong etiologies for recurrent abortions. Thus, parental karyotyping is recommended to assess the genetic cause of recurrent pregnancy losses.[7] Some researchers advocated that prenatal genetic diagnosis would be a useful test, and the test may highlight some clues for couples with a history of recurrent abortions.[8] It has been noted that abnormal aneuploid gametes, fertilization, or nondisjunction of chromosomes during mitotic cell division in postzygotic stage cause abnormal fetuses and increase chance of abortion randomly.[6] Consanguineous marriages also significantly increase the incidence of inherited recessive disorders and cause some reproductive and developmental health problems. In addition to these, consanguineous marriages also promote recurrent pregnancy loss.[9] Heteromorphisms of chromosome 1, 9, 16, and Y has been studied by some researchers in relation to RPL, but still, it was difficult to significantly correlate it with recurrent abortion.[10]
Recurrent abortions make the couples feel guilty and mentally depressed. Until date, routine chromosomal analysis of couples or aborted materials has not become regular practice for proper management of recurrent abortion. In this retrospective study, we evaluate the frequencies of chromosomal abnormalities in couples suffering from recurrent abortions. This data will be helpful to the clinicians in the context of recurrent abortions and chromosomal abnormalities for proper genetic counseling to the reproductive failure or affected couples and also to make a national database in relations of chromosomal anomalies and recurrent abortions.
MATERIALS AND METHODS
The study was carried out among 172 couples for the last 18 years (2000–2017) with clinically diagnosed recurrent abortions. The couples with recurrent abortions visiting the Department of Obstetrics and Gynecology of our institute were referred to our cytogenetic laboratory for karyotyping. Most of the couples encountered with three abortions and four or five abortions were noted in a few couples. Complete case histories of both male and female partners were recorded. The case history covered family history, reproductive failure details, medication during pregnancy, laboratory investigation reports, and a pedigree chart to assess any history of hereditary disease and consanguinity.[11] The age of the female partners ranges from 19 to 41 years (mean and standard deviation [SD]: 25.6 ± 4.61), and age of the male partners ranges from 22 to 49 years (mean age and SD: 31.2 ± 5.71). For each, whole peripheral blood culture was set up using our standard lymphocytes culture and harvesting protocol.[12] Giemsa-trypsin G-banding preparation had been used to G-band karyotyping for chromosomal identification. A minimum number of 50 metaphases were scanned, and microphotography was done for routine karyotyping. Karyotype of metaphase chromosomes was done, and cytogenetic abnormalities were noted as per the guidelines provided by the international system for human cytogenetics nomenclature.[13]
RESULTS
A total of 172 couples with a history of three or more repeated miscarriages were included in this study. The mean age of the female partner was 25.6 years (19–41), and the male partners were 31.2 years (22–49). The mean number of miscarriages was 4.04 ranging from 3 to 7 in individuals having chromosomal abnormalities. In the present study, all females were nonsmoker and nonalcoholic. However, the percentage of tobacco chewing in males was high, and about 20% of men were alcoholic. It was observed that 41.27% of women had toxoplasmosis, rubella, cytomegalovirus, and herpes simplex infection (TORCH), while 8.13% of women showed endocrinal problems. Consanguineous marriages were found in 13.95% of couples among the study population. Among 172 couples (344 individuals), chromosome abnormalities were detected in 17 couples (9.88%). In other words, 4.94% (17/344) of individuals or 9.88% (17/172) of couples were affected. Details of chromosomal abnormalities of both partners are shown in Tables 1 and 2.
Table 1.
Abnormal cytogenetic findings in couples with recurrent abortions
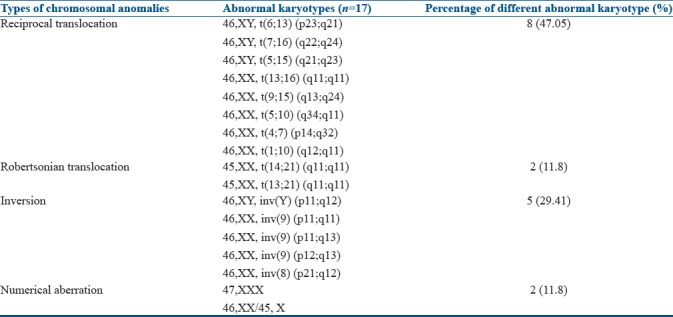
Table 2.
Frequency and types of chromosomal aberration in male and female partners

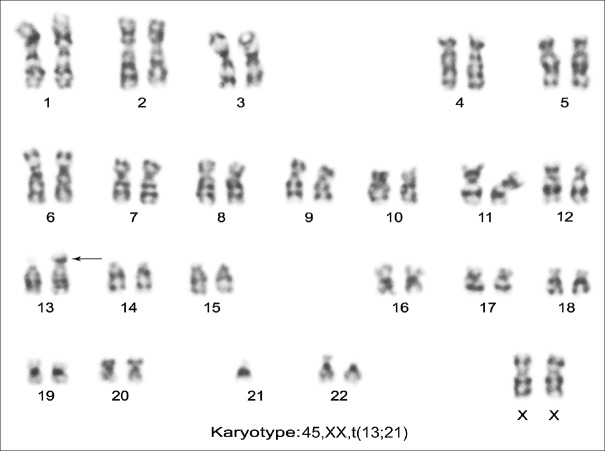
Among 17 couples, 15 (88.23%) showed structural aberration whereas 2 (11.76%) carried numerical abnormalities. Out of 17 cases, 8 (47.05%) had balanced translocation, 2 (11.76%) had the Robertsonian translocation, 5 (29.41%) had the inversion of chromosome 8, 9, or inversion Y, and two cases (11.76%) showed sex chromosome numerical aberration. Of the 172 couples, 17 individuals showed abnormal karyotypes, 13 (7.55%) were female, and 4 (2.32%) were male. Thus, there was an increased frequency of chromosomal aberration in females as compared to males [Table 2]. Among eight individuals with reciprocal translocation, five were seen in females [Figure 1], and the remaining three reciprocal translocations were observed in male partners. The only two Robertsonian translocations had been detected due to the translocation between D and G group of chromosome involving 14, 21 and 13; and 21 [Figure 2] chromosomes in female only. Among five cases of inversion, three were observed in chromosome 9, one was found in chromosome 8, and Y in a male partner. Two female cases were noted with numerical sex chromosome anomalies, one with karyotype (47, XXX) and another one with karyotype (46, XX/45, and X).
Figure 1.
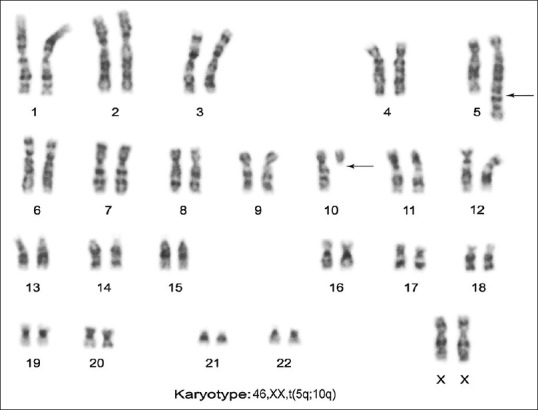
Female karyotype showing translocations 46, XX, and t(5q; 10q) (q34; q11)
Figure 2.
Female karyotype showing Robertsonian translocations 45, XX, and rob(13q; 21q)
DISCUSSION
Clinically, three or more consecutive pregnancy losses before 20 weeks of gestation are known as recurrent abortions. RPL is the most significant complication in the human reproductive life. Overall, RPL is about 10%–15% of all clinically recognized pregnancies.[2] A number of etiologies, including genetic factors, are responsible for recurrent pregnancy loss. Various causative factors that have been involved in spontaneous abortions are uterine abnormalities, hormonal imbalance, immunological and hematological disorders, stress, parental genetic composition, and environmental factors.[14,15] Idiopathic spontaneous abortion in the first trimester, possibly, is due to chromosomal abnormalities, genomic instability, single-gene mutation, epigenetic changes, X-chromosome inactivation, and sperm chromosomal rearrangement.[2,16] There is increased severity of the recurrent abortions in the first trimester of gestation and are caused by fetal chromosomal aneuploidy.[6] The genetic etiology for recurrent spontaneous abortion is due to fetal unbalanced chromosomal composition, which may be the result of one parent being a carrier for a balanced chromosomal translocation. Balanced translocations are responsible for 2%–5% of recurrent abortions. The carrier parent with a balanced translocation may face the problem of infertility and give birth to a malformed baby.[7]
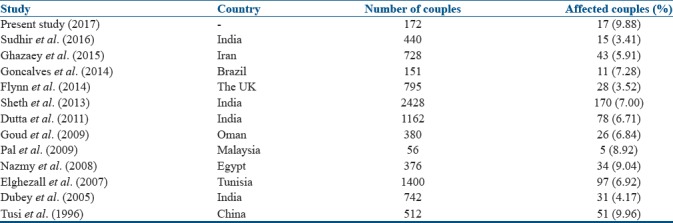
In the present study, 4.94% of individuals of the affected couples or 9.88% of couples were showing RPL due to chromosomal abnormalities. Our results showed an increasing number of chromosomal aberrations in females (7.5%) as compared to males (2.3%). Most of the studies have reported the predominance of chromosomal abnormalities in females,[17,18] and this is an agreement with our observation. All chromosomal aberrations observed in females have age <35 years, and in the present study, average maternal age was 29 years who presented with chromosomal abnormalities. In some studies, it has been reported that increased maternal age was associated with more number of miscarriages.[19] However, in this study, we could not find any such correlation with maternal age; however, increased numbers of abortions were seen in individuals with chromosomal abnormalities. Several studies reported different types of structural and numerical chromosomal anomalies in RPL, and the percentage of affected couples varies from 4% to 9%;[20,21,22,23,24] however, a few reports suggested low percentages of affected couples [Table 3].[25] Such variations may be due to differences in sample size, ethnicity, consanguinity, different geographical, cultural variation, perinatal infection, and variable criteria assigned for investigation of cases.[26]
Table 3.
Different studies on chromosomal abnormalities observed in couples with recurrent pregnancy loss
In this study, increased incidence of chromosomal aberrations in females as compared to males (2.5:1) was seen. Previous studies had also shown a nearly equal incidence of chromosome aberration ratio in male and female.[27] Carrier males with reciprocal translocation were supposed to have lower fertility rate because of poor motility of sperms with structural chromosomal aberrations, and those males showing abnormal semen profile with azoospermic or severe oligoasthenoteratozoospermia were associated with subfertility, infertility, and even sterility in males.[3,16] The structural abnormalities play an important role in the couple with recurrent abortions.[7] Our study result showed that reciprocal translocation was most common (50%) and frequently involved chromosomal abnormalities in pregnancy loss, and chromosomes 1, 4, 5, 6, 7, 10, 13, 15, and 16 were involved in different reciprocal translocations [Table 1]. Robertsonian translocation was less frequent (7.1%) and was found in only two female partners involving chromosomes 13, 21 and 14, and 21. It is estimated that the risk of miscarriages in couples with Robertsonian translocation is approximately 25% whereas it is increased in reciprocal translocation up to 25%–50%.[28] A carrier of Robertsonian translocation is phenotypically normal; however, there is a risk of unbalanced gametes which result in unbalanced offspring with recurrent abortion or down syndrome live baby.[29] In the present study, we encountered the occurrence of pericentric inversion of chromosome 8, 9, and chromosome Y. Pericentric inversion on chromosome 9, which is common in humans, is considered to be a normal variant rather than an abnormal karyotype.[30] In three cases, pericentric inversion of chromosome 9 occurred and in each one case of pericentric inversion of chromosome 8 and Y was detected [Table 2]. The risk of pregnancy loss with an inversion is not common as the inversion makes only structural changes but no loss or gain of genetic material. However, there are studies reporting an association of inversion 9 with subfertility, recurrent miscarriage, and abnormal phenotype.[31] All three cases with pericentric inversion 9 had a history of recurrent spontaneous abortion. In each one case with inversion 8 and Y also had spontaneous recurrent abortion. This indicates the possibility of inversion having a role in the etiology of spontaneous abortion; however, this needs molecular explanation and confirmation. An increased tendency to early spontaneous abortion in familial pericentric inversions has been well documented.[32]
Sex chromosome aneuploidy is usually encountered in the form of chromosomal aberrations in low frequency.[6] Numerical aberration involving sex chromosome was involved in two female individuals, i.e. 14.3% (2/14), one case with trisomy X (47, XXX) and another case with X-chromosome mosaicism (46, XX/45, and X). Mostly, such women with abnormal genotype are infertile or subfertile. However, occasionally, they are fertile with RPL, and they might have the chance for a normal healthy child also.[33] A number of studies advocated that heteromorphism of long arm (q) of chromosomes 1, 9, 16, and Y played a role in recurrent pregnancy loss.[10,34] Heteromorphism of the long arm (q) of chromosomes 1, 9 and 16, and Y were considered as factors associated with reproductive failure, and thus failures may be due to the fact that heterochromatin may contain hidden functional genes that regulate cellular roles in reproduction.[35] Consanguineous marriage occurs between biologically related individuals who share a common ancestor.[9] Researchers carried out some studies to evaluate the relationship between consanguineous marriages and recurrent pregnancy loss. Turki et al., in Saudi Arabia, depicted a significant correlation between consanguineous marriages and recurrent pregnancy loss.[36] In another study the frequency of recurrent spontaneous abortions was more in consanguineous than nonconsanguineous marriages.[37]
When both the partners confirmed normal karyotype, then some other factors, such as TORCH, uterine abnormalities, endocrine dysfunction, immunological factors or some others factors, are involved in recurrent pregnancy loss. Maternal infections with TORCH (virus) are considered as one of the risk factors for abnormal pregnancy outcome.[38] In the present study, 41.76% (71/170) of females who were cytogenetically normal had two or more recurrent abortions with TORCH infections. TORCH agents cause recurrent miscarriage since it can cross the placental barrier. Surpam et al. commented upon the significantly positive incidence of TORCH group of infection in patients of bad obstetric history when compared with normal healthy controls.[39] Recurrent miscarriages with normal karyotypes of both partners male and female, the conditions, such as uterine abnormalities or endocrine dysfunction or immunological disorders of the female partners, have been more likely to be associated with underlying recurrent pregnancy loss.
CONCLUSIONS
RPL is a challenging reproductive problem. The results on chromosomal abnormalities in RPL provide information regarding the role of chromosomal anomalies in RPL and also the usefulness of cytogenetic investigation to rule out the possible genetic cause of recurrent pregnancy loss. The genetic counseling and appropriate patients management can be made easily and accurately. The cytogenetic analysis has been an essential tool for couples for predicting the success of reproductive options. However, further molecular studies need to be done for the assessment of the recurrent risk of miscarriages due to genetic anomalies or some other factors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Mr. V. P. Kavinesan for technical support and the Indian Council of Medical Research, New Delhi, for financial assistance.
REFERENCES
- 1.Celep F, Karagüzel A, Ozeren M, Bozkaya H. The frequency of chromosomal abnormalities in patients with reproductive failure. Eur J Obstet Gynecol Reprod Biol. 2006;127:106–9. doi: 10.1016/j.ejogrb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 3.Hyde KJ, Schust DJ. Genetic considerations in recurrent pregnancy loss. Cold Spring Harb Perspect Med. 2015;5:a023119. doi: 10.1101/cshperspect.a023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. 2012;1822:1951–9. doi: 10.1016/j.bbadis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Flynn H, Yan J, Saravelos SH, Li TC. Comparison of reproductive outcome, including the pattern of loss, between couples with chromosomal abnormalities and those with unexplained repeated miscarriages. J Obstet Gynaecol Res. 2014;40:109–16. doi: 10.1111/jog.12133. [DOI] [PubMed] [Google Scholar]
- 6.Jia CW, Wang L, Lan YL, Song R, Zhou LY, Yu L, et al. Aneuploidy in early miscarriage and its related factors. Chin Med J (Engl) 2015;128:2772–6. doi: 10.4103/0366-6999.167352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozawa N, Maruyama T, Nagashima T, Ono M, Arase T, Ishimoto H, et al. Pregnancy outcomes of reciprocal translocation carriers who have a history of repeated pregnancy loss. Fertil Steril. 2008;90:1301–4. doi: 10.1016/j.fertnstert.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 8.Testart J, Gautier E, Brami C, Rolet F, Sedbon E, Thebault A. Intracytoplasmic sperm injection in infertile patients with structural chromosome abnormalities. Hum Reprod. 1996;11:2609–12. doi: 10.1093/oxfordjournals.humrep.a019179. [DOI] [PubMed] [Google Scholar]
- 9.Saad FA, Jauniaux E. Recurrent early pregnancy loss and consanguinity. Reprod Biomed Online. 2002;5:167–70. doi: 10.1016/s1472-6483(10)61620-3. [DOI] [PubMed] [Google Scholar]
- 10.Boronova I, Bernasovska J, Cakanova G, Ferenc P, Petrejcikova E, Szabadosova V. Heterochromatin variants in Slovak women with reproductive failure. Int J Hum Genet. 2015;15:1–5. [Google Scholar]
- 11.Royal College of Obstetricians and Gynaecologists. The Management of Recurrent Miscarriage. London, United Kingdom: Royal College of Obstetricians and Gynaecologists; 1998. [Google Scholar]
- 12.Ambulkar PS, Ghosh SK, Ingole IV, Pal AK. Genotoxic and cytotoxic effects of antibacterial drug, ciprofloxacin, on human lymphocytes in vitro. Nepal Med Coll J. 2009;11:147–51. [PubMed] [Google Scholar]
- 13.McGowan-Jordan J, Simons A, Schmid M, editors. An international system for human cytogenomic nomenclature (2016) Basel, Freiburg: S. Karger; 2016. ISCN 2016. [Google Scholar]
- 14.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24–9. [PubMed] [Google Scholar]
- 15.Homer HA, Li TC, Cooke ID. The septate uterus: A review of management and reproductive outcome. Fertil Steril. 2000;73:1–4. doi: 10.1016/s0015-0282(99)00480-x. [DOI] [PubMed] [Google Scholar]
- 16.Chandley AC. Infertility and recurrent abortions. In: Emery AE, Rimoin D, editors. Principles and Practice of Medical Genetics. London: Churchill Livingstone; 1999. pp. 313–9. [Google Scholar]
- 17.Sheth FJ, Liehr T, Kumari P, Akinde R, Sheth HJ, Sheth JJ. Chromosomal abnormalities in couples with repeated fetal loss: An Indian retrospective study. Indian J Hum Genet. 2013;19:415–22. doi: 10.4103/0971-6866.124369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves RO, Santos WV, Sarno M, Cerqueira BA, Gonçalves MS, Costa OL. Chromosomal abnormalities in couples with recurrent first trimester abortions. Rev Bras Ginecol Obstet. 2014;36:113–7. doi: 10.1590/s0100-72032014000300004. [DOI] [PubMed] [Google Scholar]
- 19.de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17:1649–56. doi: 10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- 20.Dubey S, Chowdary MR, Prahlad B, Kumar V, Mathur R, Hamilton S. Cytogenetic causes for recurrent spontaneous abortions – An experience of 742 couples (1484 cases) Ind J Hum Genet. 2005;11:94–8. [Google Scholar]
- 21.Dutta UR, Rajitha P, Pidugu VK, Dalal AB. Cytogenetic abnormalities in 1162 couples with recurrent miscarriages in Southern region of India: Report and review. J Assist Reprod Genet. 2011;28:145–9. doi: 10.1007/s10815-010-9492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goud TM, Mohammed Al Harassi S, Khalfan Al Salmani K, Mohammed Al Busaidy S, Rajab A. Cytogenetic studies in couples with recurrent miscarriage in the Sultanate of Oman. Reprod Biomed Online. 2009;18:424–9. doi: 10.1016/s1472-6483(10)60104-6. [DOI] [PubMed] [Google Scholar]
- 23.Elghezal H, Hidar S, Mougou S, Khairi H, Saad A. Prevalence of chromosomal abnormalities in couples with recurrent miscarriage. Fertil Steril. 2007;88:721–3. doi: 10.1016/j.fertnstert.2006.11.160. [DOI] [PubMed] [Google Scholar]
- 24.Nazmy NA. Cytogenetic studies of couples with reproductive failure in Alexandria, Egypt. J Egypt Public Health Assoc. 2008;83:255–71. [PubMed] [Google Scholar]
- 25.Sudhir N, Kaur T, Beri A, Kaur A. Cytogenetic analysis in couples with recurrent miscarriages: A retrospective study from Punjab, North India. J Genet. 2016;95:887–94. doi: 10.1007/s12041-016-0713-3. [DOI] [PubMed] [Google Scholar]
- 26.Al-Ghamdi AA, Makhashen SF. Etiology of recurrent pregnancy loss in Saudi females. Saudi J Med Med Sci. 2016;4:187–91. doi: 10.4103/1658-631X.188258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal S, Ma SO, Norhasimah M, Suhaida MA, Siti Mariam I, Ankathil R, et al. Chromosomal abnormalities and reproductive outcome in Malaysian couples with miscarriages. Singapore Med J. 2009;50:1008–12. [PubMed] [Google Scholar]
- 28.Ghazaey S, Keify F, Mirzaei F, Maleki M, Tootian S, Ahadian M, et al. Chromosomal analysis of couples with repeated spontaneous abortions in Northeastern Iran. Int J Fertil Steril. 2015;9:47–54. doi: 10.22074/ijfs.2015.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner M, Reh A, Grifo J, Perle MA. Characteristics of chromosomal abnormalities diagnosed after spontaneous abortions in an infertile population. J Assist Reprod Genet. 2012;29:817–20. doi: 10.1007/s10815-012-9781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao L, Murthy K, Babu A, Venkata P, Deenadayal M, Singh L. Chromosome inversions and a novel chromosome insertion associated with recurrent miscarriages in South India. Arch Gynecol Obstet. 2005;272:273–7. doi: 10.1007/s00404-005-0027-9. [DOI] [PubMed] [Google Scholar]
- 31.Purandare H, Fernandez NV, Deshmukh SV, Chavan S. Heterochromatic variations and pregnancy losses in humans. Int J Hum Genet. 2011;11:167–75. [Google Scholar]
- 32.El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: Current perspectives. Int J Womens Health. 2017;9:331–45. doi: 10.2147/IJWH.S100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsui KM, Yu WL, Lo FM, Lam TS. A cytogenetic study of 514 Chinese couples with recurrent spontaneous abortion. Chin Med J (Engl) 1996;109:635–8. [PubMed] [Google Scholar]
- 34.Madon PF, Athalye AS, Parikh FR. Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod Biomed Online. 2005;11:726–32. doi: 10.1016/s1472-6483(10)61691-4. [DOI] [PubMed] [Google Scholar]
- 35.Minocherhomji S, Athalye AS, Madon PF, Kulkarni D, Uttamchandani SA, Parikh FR, et al. Acase-control study identifying chromosomal polymorphic variations as forms of epigenetic alterations associated with the infertility phenotype. Fertil Steril. 2009;92:88–95. doi: 10.1016/j.fertnstert.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 36.Turki RF, Assidi M, Banni HA, Zahed HA, Karim S, Schulten HJ. Associations of recurrent miscarriages with chromosomal abnormalities, thrombophilia allelic polymorphisms and/or consanguinity in Saudi Arabia. BMC Med Genet. 2016;17:69. doi: 10.1186/s12881-016-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gowri V, Udayakumar AM, Bsiso W, Al Farsi Y, Rao K. Recurrent early pregnancy loss and consanguinity in Omani couples. Acta Obstet Gynecol Scand. 2011;90:1167–9. doi: 10.1111/j.1600-0412.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 38.Kavalier F. Investigation of recurrent miscarriages. BMJ. 2005;331:121–2. doi: 10.1136/bmj.331.7509.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surpam RB, Kamlakar UP, Khadse RK, Qazi MS, Jalgaonkar SV. Serological study for TORCH infections in women with bad obstetric history. J Obstet Gynecol India. 2006;56:41–3. [Google Scholar]