Abstract
Background
Partially hydrolyzed cow's milk protein-based formula (pHF) possesses low allergenicity. Here, we investigate the safety and efficacy of oral immunotherapy using pHF for children with cow's milk protein allergy (CMPA).
Objectives
A randomized, double-blind, controlled single-center trial was conducted to evaluate the efficacy and safety of pHF oral immunotherapy in children with CMPA.
Methods
Participants were randomized into double-blind pHF-pHF and extensively hydrolyzed cow's milk protein-based formula (eHF)-pHF groups. During this phase, the pHF-pHF group received pHF and the eHF-pHF group received eHF. During the open phase, all participants received pHF. The primary end point was a change in thresholds between baseline and the end of the first phase. Secondary end points were changes in thresholds between baseline and the end of the second phase, and casein-specific immunoglobulin (Ig)E, IgG4, and basophil activation.
Results
Twenty-five children, aged 1–9 years, were randomized into pHF-pHF and eHF-pHF groups. The threshold between baseline and the end of the first phase was significantly elevated in the pHF-pHF group (p = 0.048), but not in the eHF-pHF group. The threshold between other phases did not change significantly in either group. There were significant decreases in casein-specific IgE antibody levels between baseline and the second phase in the eHF-pHF group (p = 0.014). No participants suffered systemic allergic reactions requiring adrenaline or systemic corticosteroids after receiving the formulas.
Conclusions
The results of this trial suggest that, in children with CMPA, tolerance to cow's milk might be safely enhanced by intake of pHF, relative to that of eHF.
Keywords: Food allergy, Oral immunotherapy, Partially hydrolyzed formula, Cow's milk protein allergy
Introduction
The prevalence of cow's milk protein allergy (CMPA) ranges from 2 to 5% in children in Western countries [1]. There is no standard treatment for severe CMPA, except for strict avoidance of cow's milk protein. The risk of allergic reactions after accidental exposure diminishes the quality of life of the affected individual as well as the family [2].
The efficacy of oral immunotherapy (OIT) for CMPA was recently reported [3]; OIT, as investigated in this previous report, utilized unprocessed whole cow's milk. However, this type of therapy poses a risk of allergic reactions including severe anaphylaxis. The risk of an adverse reaction requiring epinephrine administration was found to be higher in children receiving milk OIT than in those receiving an elimination diet [4]. Therefore, the Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) guidelines do not recommend OIT with cow's milk for patients with CMPA [1].
Hydrolyzed formulas are available as 2 types: partially hydrolyzed cow's milk protein-based formula (pHF) and extensively hydrolyzed cow's milk protein-based formula (eHF). Both are prepared using enzymatic processes to convert native proteins into smaller fragments. There are no absolute criteria to define hydrolyzed cow's milk protein-based formulas; however, generally, pHF contains fewer peptides, with molecular weights of approximately < 5,000 Da. In contrast, eHF contains only peptides with molecular weights < 3,000 Da [5]. For infants with confirmed CMPA, recommendations suggest an elimination diet using a therapeutic formula. Currently, eHFs are recommended for infants and children with CMPA. At least 90% of infants and children with CMPA tolerate eHF [1]. Although partially hydrolyzed formula is not categorized as hypoallergenic, studies in mouse models have shown that pHF and eHF have low allergenicity [6, 7].
The safety and efficacy of hypoallergenic food products has been demonstrated [8, 9, 10]. Partially hydrolyzed whey and casein formula (E-akachan®) has been used in Japan for healthy infants with or without a family history of allergy. We hypothesized that OIT using pHF accelerates tolerance to cow's milk protein in a safe manner. Here, a randomized, double-blind, controlled trial was conducted to demonstrate that OIT using pHF improves the amount of cow's milk formula tolerated by children with CMPA.
Material and Methods
Trial Design
The trial was a randomized (1: 1), double-blind, controlled trial conducted at a single center (Fujita Health University) in Japan. Participants were randomized into 2 double-blind groups: a pHF-pHF and an eHF-pHF group.
The aim of the trial was to investigate whether OIT using pHF increases the amount of cow's milk tolerated by children with CMPA. Improvement was assessed by investigating the change in response to food challenge with regular cow's milk protein-based formula (rCMF), performed before, after 8 weeks, and after 16 weeks of OIT. Immunological changes were evaluated by assessing serum-specific immunoglobulin E (IgE), IgG4, and basophil activation, in response to casein, performed at the previously mentioned time points.
Participants
Inclusion criteria were as follows: an age of between 1 and 20 years at the start of the trial, and a known history of systemic symptoms induced by ingesting small amounts of milk allergens or high cow's milk-specific IgE values, with a 95% probability of CMPA (according to a previous trial [11]). Participants with a current diagnosis of severe persistent asthma were excluded from the trial. Pediatricians at the Fujita Health University explained the trial to the families of the participants, and informed consent was obtained from the families of individuals who met the criteria.
Participants were enrolled from April 2013 to January 2015. The trial (No. UMIN000013374) was registered with UMIN Clinical Trials Registry on November 8, 2012.
Formulas Used
We used 3 formulas in this trial: pHF (E-akachan®), eHF (MA-mi®), and rCMF (Hagukumi®), all from Morinaga Milk Industry Co., Ltd., Tokyo, Japan. The terms pHF and eHF denote cow's milk formulas prepared by enzymatic hydrolysis of milk protein, which decreases the antigenicity of casein and whey. The hydrolysate from eHF was treated with ultrafiltration. The protein content of pHF and rCMF is 1.64 g/100 mL and eHF contains 1.76 g/100 mL. The molecular weight profiles of the formulas are presented in Table 1.
Table 1.
Molecular weight profiles of the study formulas
| Molecular weight, Da | pHF E-akachan® | eHF MA-mi® | rCMF Hagukumi® |
|---|---|---|---|
| <500 | 54.1 | 65.2 | 46.7 |
| 500–1,000 | 21.0 | 22.6 | 14.6 |
| 1,000–1,200 | 7.5 | 6.7 | 4.9 |
| 1,200–2,000 | 9.2 | 4.4 | 7.4 |
| 2,000–3,500 | 5.4 | 1.1 | 5.5 |
| >3,500 | 2.9 | trace amountsa | 21.0 |
Defatted formula samples were applied to a high-performance liquid chromatography system (LC-20AD, Shimadzu, Tokyo, Japan) with a poly-hydroxyethyl aspartamide column (PolyLC, Columbia, MD, USA). pHF, partially hydrolyzed cow's milk formula; eHF, extensively hydrolyzed cow's milk formula; rCMF, regular cow's milk formula.
The hydrolysate for eHF was treated with ultrafiltration.
Food Challenge
Food Challenge and Definition of Thresholds at Baseline
Participants in both groups underwent food challenges 3 times using pHF, eHF, or rCMF. All food challenges at baseline were performed in a randomized, double-blind manner in a hospital ward, on 3 separate days, with a 1-week interval between the challenges. Parents of participants receiving antihistamines were requested to withhold the administration of these for 72 h before and during the challenge. A total volume of 20 mL of pHF, eHF, or rCMF was administered every 30 min in 5–7 instalments. The challenge was discontinued if objective allergic symptoms such as systemic urticaria, cough, and wheezing occurred, or if subjective allergic symptoms such as abdominal pain occurred; a pediatric allergist confirmed that symptoms were induced by the formula. Participants who presented allergic reactions remained under observation until the associated symptoms were resolved. Participants who consumed the formula without presenting symptoms were observed for 2 h after the last administration; in these participants, the food challenge results were confirmed to be negative by a pediatric allergist.
Food Challenge after Treatment
Open food challenge was performed using rCMF 8 and 16 weeks after treatment. Food challenges were performed in the same manner as the baseline food challenge.
Threshold Dose
The threshold dose was defined as the highest dose in the food challenge that did not elicit an adverse reaction. All participants were informed of the threshold for the 3 formulas after the baseline food challenges.
Randomization and Blinding
Participants were randomly assigned to either the pHF-pHF group or the eHF-pHF group in a 1: 1 ratio. Randomization was based on a permuted block of sizes of 2 and 4 participants, stratified by age (< 7 years or ≥7 years) and sex using a central computer. Randomization was performed by a technician who had no clinical association with this trial.
Oral Immunotherapy
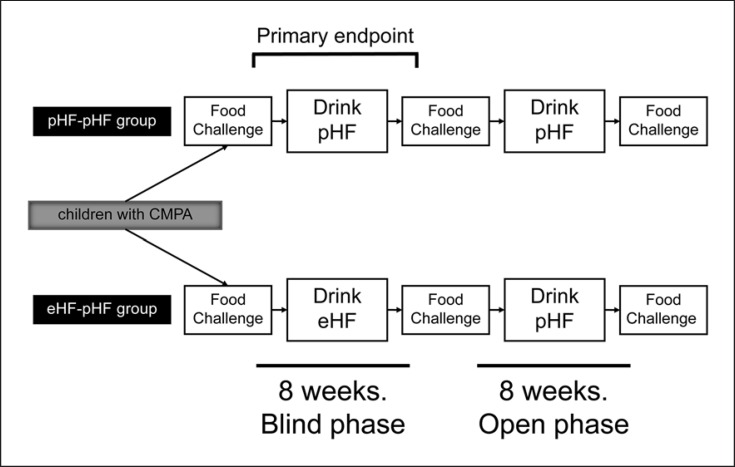
Participants who were unable to consume 20 mL of rCMF received OIT. The participants who were able to drink 20 mL rCMF without presenting symptoms were excluded. All participants consumed the assigned formula at amounts equal to the threshold for pHF in the baseline food challenge. Participants received unlabeled milk formula cans containing pHF or eHF during the blind phase. During the first 8 weeks of the trial (the double-blind phase), participants in the pHF-pHF group consumed ≤20 mL of pHF comprising the amount required for the pHF threshold once per day, and participants in the eHF-pHF group consumed ≤20 mL of eHF comprising the amount required for the pHF threshold, but not eHF thresholds. During the second 8 weeks of the trial (the open phase), all participants consumed ≤20 mL of pHF at the amount required for pHF thresholds, once per day. The protocol is shown in Figure 1.
Fig. 1.
Trial design. Participants with cow's milk protein allergy (CMPA) were randomized into 2 double-blind groups: a partially hydrolyzed cow's milk protein-based formula (pHF)-pHF group and an extensively hydrolyzed cow's milk protein-based formula (eHF)-pHF group. At baseline, all participants were administered 3 separate food challenges with ≤20 mL of pHF, eHF, and regular (r)CMF. The threshold was defined as the highest dose in the food challenge that did not elicit an adverse reaction (no observed adverse effect level [threshold]).
Blood Collection
Blood samples were collected from participants < 2 weeks before the food challenge at baseline, and < 1 week after each phase.
Assays for Serum Cow's Milk- or Casein-Specific IgE and IgG4
Participants' serum cow's milk-specific or casein-specific IgE and IgG4 antibody levels were determined using the Immulite 2000 3gAllergyTM system. The assay was performed by Siemens AG (Munich, Germany). The cutoff levels were 0.01 IUA/mL for specific IgE and 200 mg/L for specific IgG4.
Basophil Activation Test
Basophil activation was determined using an allergenicity kit (Beckman Coulter Inc., Fullerton, CA, USA), according to the manufacturer's instructions. All assays used fresh whole blood within 24 h of sampling. Briefly, heparin-anticoagulated peripheral blood samples were incubated at 37°C for 15 min with FITC-labeled anti-CRTH2, phycoerythrin (PE)-labeled anti-CD203c, and PE-cyanine 7-labeled anti-CD3 monoclonal antibodies from the kit in the presence of the allergen. PBS and anti-IgE antibodies (10 μg/mL) were used as negative and positive controls, respectively. After incubation, the stop solution from the kit was added to stop the reaction. Subsequently, samples were mixed with the lysing solution, and the mixture was incubated for 10 min at room temperature (18–25°C). The suspension was centrifuged for 5 min at 200 g at room temperature after lysing, washed with PBS, centrifuged for 5 min at 200 g at room temperature again, and suspended in 500 μL of PBS with 0.1% formaldehyde. Samples were analyzed using a FACSCaliburTM cell analyzer with CellQuestTM software (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). Basophils were identified based on their forward- and side-scatter properties, the absence of CD3 expression, and the presence of CRTH2 expression.
To assess allergen-specific basophil activation, 1 μg/mL casein (α-Casein, Sigma-Aldrich, St. Louis, MO, USA) was used. Data were acquired for 500 basophils, and samples with < 200 cells were excluded. Nonresponder status was defined as an anti-IgE-induced CD203c expression of < 10% (these samples were excluded).
Data Analysis
The primary end point was an improvement of the thresholds to the rCMF food challenge between baseline and the end of the first double-blind phase. The aim was to evaluate the effect of each hydrolyzed formula. The secondary end points were improvements of thresholds between baseline and the end of the second phase, and immunological changes. The aim was to evaluate the effect of the pHF intake duration. If a participant presented with an allergic reaction following the first intake, the threshold was considered 0.0 mL.
Immunological evaluation, including assessment of IgE, IgG4, and basophil activation levels in response to cow's milk, was performed before and after each phase.
These results, which were compared using Friedman's nonparametric analysis of variance (ANOVA) followed by post hoc Dunn's multiple comparison test, are presented as medians and interquartile ranges. p values < 0.05 were considered to represent statistical significance. Statistical analyses were performed using GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Participant Characteristics
Participants included 18 males and 7 females with a median age of 4.25 years (range 1–9 years). The median milk-specific IgE level was 76.5 IUA/mL (range 23.50–169.3 UA/mL).
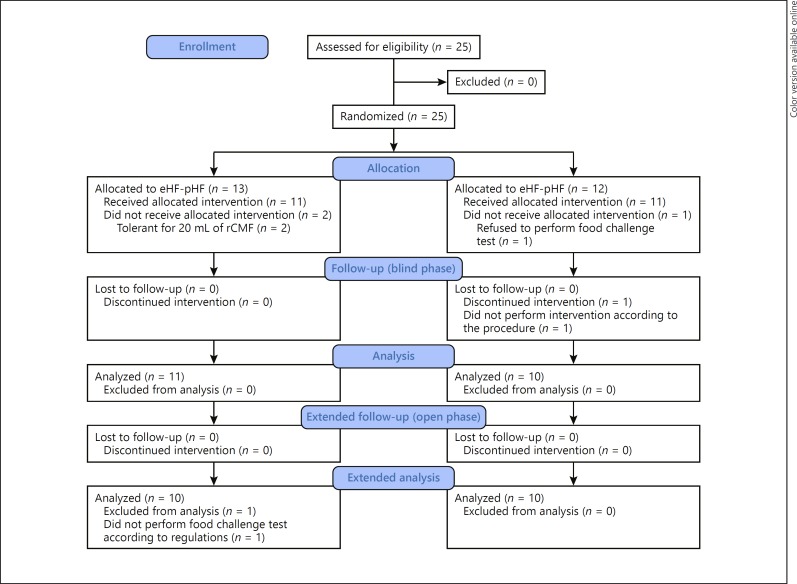
The trial flow diagram for the pHF-pHF group versus the eHF-pHF group is shown in Figure 2. The diagram includes detailed information on the excluded participants. In total, 25 participants were enrolled in this trial and randomly assigned to the pHF-pHF (n = 13) or eHF-pHF (n = 12) groups. There was no significant difference in age, threshold, and milk-specific IgE levels between the 2 groups (Table 2). All participants received the food challenge test using pHF, eHF, and rCMF, and had thresholds for each formula. The threshold for pHF ranged from 0.5 to 20 mL.
Fig. 2.
The number of participants assessed for eligibility for oral immunotherapy using partially hydrolyzed formula, who completed the trial. In total, 25 participants were enrolled and randomly assigned to 2 groups. At the baseline food challenge, 2 participants in the partially hydrolyzed cow's milk protein-based formula (pHF)-pHF group were tolerant to 20 mL of regular cow's milk formula (rCMF) and 1 in the extensively hydrolyzed cow's milk protein-based formula (eHF)-pHF group refused the food challenge test; these participants did not undergo the intervention. During the blind phase, 1 participant in the eHF-pHF group consumed his assigned formula mixed with other milk products during the first 2 weeks without our knowledge. When this became known, the participant was excluded from the trial. At the food challenge at the end of the open phase, 1 participant did not receive the food challenge formula according to the protocol; data for this participant were thus excluded from the analysis. The assignment of participants was blinded after all participants had undergone the intervention.
Table 2.
Clinical characteristics of 2 groups of participants with cow's milk protein allergy
| Patient No. | Age | Sex | Cow's milk-specific IgE, IUA/mL | First episode of cow's milk protein allergic response |
First episode of anaphylaxis induced by cow's milk protein |
|
|---|---|---|---|---|---|---|
| symptoms | age | age | ||||
| pHF-pHF group | ||||||
| 1 | 2 y 0 m | M | 275 | U | 0 y 8 m | - |
| 2 | 3 y 3 m | M | 205 | U | 0 y 8 m | - |
| 3 | 4 y 1 m | M | 71.8 | U, AB | 1 y 2 m | 2 y 4 m |
| 4 | 3 y 2 m | F | 32 | CO, U | 0 y 10 m | 0 y 10 m |
| 5 | 8 y 8 m | F | 14.4 | U (FC) | 3 y 10 m | - |
| 6 | 5 y 10 m | F | >500 | WH, UR, AB | 0 y 7 m | 0 y 7 m |
| 7 | 2 y 1 m | M | 40.2 | UR, AB, HT | 0 y 7 m | 0 y 7 m |
| 8 | 1 y 8 m | M | 3.13 | WH, UR | 1 y 5 m | 1 y 5 m |
| 9 | 9 y 7 m | M | 124 | U | 8 y 2 m | - |
| 10 | 3 y 10 m | M | 149 | WH | 2 y 0 m | 2 y 0 m |
| Median | 3 y 6 m | 97.9 | ||||
| eHF-pHF group | ||||||
| 11 | 5 y 5 m | M | 91.2 | UR, WH | 0 y 6 m | 0 y 6 m |
| 12 | 5 y 6 m | F | 34.9 | U | 0 y 5 m | 4 y 6 m |
| 13 | 4 y 3 m | M | >500 | CO, WH | 0 y 4 m | 0 y 4 m |
| 14 | 4 y 0 m | F | 23.1 | CO, U (FC) | 3 y 7 m | 3 y 7 m |
| 15 | 5 y 3 m | F | 197 | U | 0 y 7 m | 2 y 4 m |
| 16 | 8 y 8 m | F | 110 | U | 0 y 6 m | 6 y 3 m |
| 17 | 4 y 1 m | M | 81.2 | CO, WH, UR, HT | 1 y 3 m | 1 y 3 m |
| 18 | 3 y 0 m | M | 23.6 | CO, U (FC) | 2 y 9 m | 2 y 9 m |
| 19 | 7 y 11 m | M | 19.5 | U | 4 m | 3 y 0 m |
| 20 | 4 y 10 m | M | n.a. | U (FC) | 2 y 8 m | - |
| Median | 5 y 1 m | 58.05 | ||||
AB, abdominal pain; CO, cough; F, female; IgE, immunoglobulin E; M, male; n.a., not applicable; y, years; m, months; FC, symptom occurring during food challenge test; HT, hypotension; U, urticaria; WH, wheeze.
Assessment of Clinical Responses
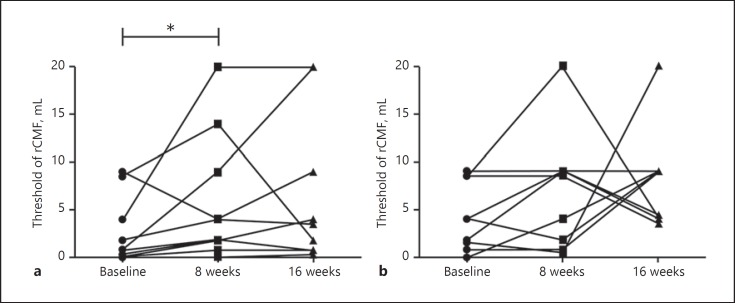
Twenty participants completed treatment. Of these, 10 were in the pHF-pHF group and 10 in the eHF-pHF group. Detailed data are shown in Table 3. The primary end point was a significant increase in the threshold in the pHF-pHF group (p = 0.048; Fig. 3a), but not in the eHF-pHF group (p = 0.23; Fig. 3b). In both groups, the improvement in thresholds between baseline and at the end of the second phase was not significant. Among the participants with severe allergy (patients 1, 3, 4, 5, 6, 7, and 8) whose baseline thresholds were < 4 mL, there was a significant change in thresholds between baseline and at the end of the trial in the pHF-pHF group (p = 0.023).
Table 3.
Clinical and immunological outcomes
| Patient No. | Baseline |
At 8 weeks |
At 16 weeks |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| threshold, mL | casein-specific IgE, IUA/mL | casein-specific IgG4, IUA/mL | casein BAL, % | threshold, mL | casein-specific IgE, IUA/mL | casein-specific IgG4, IUA/mL | casein BAL, % | threshold, mL | casein-specific IgE, IUA/mL | casein-specific IgG4, IUA/mL | casein BAL, % | |
| pHF-pHF group | ||||||||||||
| 1 | 0.35 | 260.0 | 15,763 | NR | 1.85 | 283.0 | 9,512 | NR | 0.80 | 266.0 | 14,286 | NR |
| 2 | 8.50 | 178.0 | 7,980 | 44.4 | 14.00 | 224.0 | 4,449 | 19.9 | 1.80 | 212.0 | 5,669 | 63.2 |
| 3 | 1.80 | 75.7 | 2,775 | NR | 4.00 | 110.0 | 2,965 | NR | 9.00 | 110.0 | 3,044 | NR |
| 4 | 0.00 | 32.5 | 558 | 15.9 | 0.80 | 30.9 | 282 | 13.2 | 0.80 | 31.6 | 259 | 19.4 |
| 5 | 0.00 | 13.0 | 1,621 | 25.7 | 1.80 | 11.3 | 1,263 | 63.2 | 4.00 | 12.0 | 1,360 | 52.0 |
| 6 | 0.80 | >500 | 2,723 | NR | 1.80 | >500 | 3,400 | NR | 0.80 | >500 | 3,628 | NR |
| 7 | 0.00 | 29.2 | <200 | 30.1 | 0.00 | 29.5 | <200 | 28.5 | 0.30 | 26.2 | <200 | 77.3 |
| 8 | 0.80 | 3.1 | 3,064 | 13.1 | 9.00 | 2.3 | 1,529 | 12.7 | 20.00 | 2.8 | 1,392 | 58.0 |
| 9 | 4.00 | 106.0 | 920 | 91.1 | 20.00 | 91.0 | 691 | 81.9 | 20.00 | 86.1 | 687 | 77.5 |
| 10 | 9.00 | 147.0 | 11,573 | NR | 4.00 | 137.0 | 12,197 | NR | 3.50 | 110.0 | 12,624 | NR |
| Median | 0.80 | 90.9 | 2,749 | 27.9 | 2.93 | 100.5 | 2,247 | 24.2 | 2.65 | 98.1 | 2,218 | 60.6 |
| eHF-pHF group | ||||||||||||
| 11 | 8.50 | 82.1 | 4,368 | 82.3 | 8.50 | 68.1 | 4,312 | 67.6 | 3.50 | 64.0 | 5,009 | 90.7 |
| 12 | 1.50 | 26.2 | 365 | NR | 0.50 | 20.9 | 390 | NR | 20.00 | 27.5 | 484 | NR |
| 13 | 4.00 | 425.0 | 14,254 | 67.6 | 1.80 | 464.0 | 18,853 | 94.0 | 9.00 | 347.0 | 17,104 | 7.6 |
| 14 | 4.00 | 17.8 | 1,146 | NR | 9.00 | 11.0 | 1,429 | NR | 4.00 | 10.8 | 2,329 | NR |
| 15 | 0.00 | 127.0 | 46,212 | 24.5 | 4.00 | 140.0 | 42,648 | 54.1 | 9.00 | 56.2 | 25,319 | 79.1 |
| 16 | 9.00 | 94.8 | 972 | 59.9 | 9.00 | 76.5 | 370 | 40.3 | 4.00 | 86.2 | 519 | 60.5 |
| 17 | 0.80 | 71.7 | <200 | 70.6 | 0.80 | 38.9 | <200 | 68.5 | 9.00 | 28.1 | <200 | 93.3 |
| 18 | 8.50 | 18.9 | 739 | 64.3 | 20.00 | 14.4 | 543 | 83.5 | 4.00 | 12.0 | 635 | 68.2 |
| 19 | 4.00 | 14.3 | 1,048 | 63.9 | 9.00 | 13.4 | 658 | 46.2 | 9.00 | 11.7 | 539 | 53.8 |
| 20 | 1.80 | n.a. | n.a. | n.a. | 9.00 | n.a. | n.a. | n.a. | 4.40 | n.a. | n.a. | n.a. |
| Median | 4.00 | 71.7 | 1,048 | 64.3 | 8.75 | 38.9 | 658 | 67.6 | 6.70 | 28.1 | 635 | 68.2 |
BAL, basophil activation level; n.a., not applicable; NR, nonresponder.
Fig. 3.
Clinical and immunological outcomes in each group. A significant change in threshold between baseline and at the end of the first phase was observed in the pHF-pHF group (p < 0.05), but not in the eHF-pHF group.
Immunological Correlations
The median casein-specific IgE antibody level at the end of the trial was lower than at baseline in the eHF-pHF group (p = 0.014), but not in the pHF-pHF group (p > 0.05). In both groups, milk-specific IgE and IgG4, casein-specific IgG4, and casein-specific basophil activation levels in the participants did not change between phases.
Adverse Events
No participants showed severe systemic allergic reactions requiring administration of adrenaline or systemic corticosteroids after consuming formulas in either phase. During the first 8 weeks of the trial, 1 participant presented anaphylaxis after accidentally consuming juice containing cow's milk protein. This event occurred 12 h after the consumption of pHF and was therefore not attributed to the assigned formula. Following this event, the participant continued to consume the same assigned amount of formula. Two participants in the pHF-pHF group reported itching in their mouths after ingesting the formula in the first week; these individuals were subsequently administered a decreased amount of pHF (i.e., from 1.8 to 1.0 mL) every day.
Discussion
To our knowledge, this study reports the first double-blind, controlled, randomized trial using pHF for OIT in children with CMPA. The findings indicate that OIT increased the amount of milk that could be tolerated without systemic allergic symptoms.
Numerous studies have reported the efficacy and safety of OIT using hypo- or low-allergenicity food. Unlike typical OIT, sublingual immunotherapy (SLIT) does not require the additional use of medication for allergic reactions induced by the OIT. However, the efficacy of SLIT is lower than that of typical OIT [12]. An open OIT trial conducted in children with CMPA using edible baked milk products revealed enhanced tolerance to unheated milk compared to that achieved through a strict avoidance diet [9]. Furthermore, an open OIT trial conducted in children with CMPA using low-dose cow's milk (3 mL) showed that participants exhibited improved tolerance to unheated cow's milk (3 and 25 mL) compared to that achieved through strict avoidance. Although some participants presented adverse allergic reactions induced by the OIT, most reactions were mild, and the rate of severe reactions was far lower (with only 1 incidence and it did not require adrenaline injection) than that observed following previous and typical OIT trials using a higher dose of cow's milk, which required some adrenaline injections [10, 13].
The pHF E-akachan® used in this trial is mainly fed to healthy infants, with or without a family history of allergy, in Japan. A previous animal study of this partially hydrolyzed formula product showed that intravenous challenge induced anaphylaxis in β-lactoglobulin-sensitized mice, but not in casein-sensitized mice [7]. Kido et al. [14] reported that 40 of 55 children (75%) with CMPA were able to ingest this pHF without any adverse reaction in an open food challenge test. OIT using this pHF therefore has the potential to decrease systemic reactions in CMPA. Various hydrolyzed cow's milk-based formulas are available, and differences in their hydrolyzed components might elicit different allergic reactions [15]. Prior oral exposure induction of specific β-lactoglobulin peptides in some partially hydrolyzed whey protein reduced the acute allergic skin response by whey protein [16]. Regarding the pHF used in this study, another group demonstrated that prior ingestion of the hydrolysates suppresses the sensitization to β-lactoglobulin and has submitted the results.
Previous studies of OIT involving the administration of low doses of cow's milk have comprised 2 phases: up-dosing and maintenance. During the up-dosing phase, the dose of milk ingested was gradually increased. After the maintenance dose was reached, this was administered daily. Most side effects induced by OIT occurred during the up-dosing phase [17]. According to our trial protocol, the amount of cow's milk allergen ingested did not change during the trial; participants received the same amount of pHF throughout the trial, which was guaranteed to be safe based on the baseline food challenge.
No improvement in threshold was observed between baseline and at the end of the second phase in the pHF-pHF group, or between the end of the first phase and the end of the second phase in the eHF-pHF group. These findings were attributed to several factors. First, the duration of OIT in this study was short, so the efficacy of the OIT might have been weak. A previous low-dose cow's milk intake OIT trial showed that a year of OIT induced tolerance [10]. A longer duration of the OIT in this study would possibly result in stronger efficacy for CMPA; additional study is needed to determine the optimal duration for each patient with CMPA. Second, at the end of the open phase, the thresholds of some participants were decreased relative to those at the end of blind phase. This might have been due to the placebo effect. Third, the eHF used in this trial contains trace amounts of milk peptides with a molecular weight > 2 kDa (Table 2). A previous trial suggested that although not significant, eHF might improve tolerance to cow's milk in children with CMPA compared with an amino acid-based or soy formula [18]. The trace amounts of milk peptides in the eHF milk might have affected the tolerance of participants in the eHF-pHF group, as indicated by the decrease in casein-specific IgE levels between baseline and at the end of the second phase in the eHF-pHF group.
The correlations in immunological changes following OIT were not consistent with those reported in previous studies [10, 14, 19, 20, 21]. However, some trials did not show the change in cow's milk-specific IgE [19] and other trials showed a decrease in cow's milk-specific IgE [10, 21]. One trial comparing SLIT with OIT for children with CMPA showed that cow's milk-specific IgE levels increased early and then decreased in the OIT group but were not significantly changed in the SLIT group [12]. Most trials show an increase in cow's milk-specific IgG4 during the trial.
As described, the casein-specific IgE levels decreased between baseline and at the end of the second phase in the eHF-pHF group. This finding was attributed to the effect of the stepwise introduction of cow's milk protein on the immune system of the participants in the eHF-pHF group. The Prevention of Egg Allergy with Tiny Amount InTake (PETIT) study demonstrated efficacy and safety using a similar stepwise approach [22]. Infants participating in the egg-consuming group consumed egg, starting at 50 mg/day for 3 months and increasing to 250 mg/day over 3 months. At the end of the trial, 92% of the egg-consuming infants passed the oral food challenge in contrast with only 62% of the egg-avoiding infants. In our study, the eHF-pHF group consumed eHF for the first 8 weeks; following this, pHF was consumed. eHF was considered to only weakly stimulate the immune system of the participants during the first phase; this was putatively followed by stronger stimulation of the immune system by pHF during the second phase. Stepwise milk protein intake may have affected such immunological changes.
Our trial had some limitations. First, the pHF amounts administered were small (20 mL); it is possible that some children are capable of ingesting much larger amounts of pHF. We enrolled children with severe cow's milk allergy; thus, food challenges with large amounts of formula posed a risk of inducing anaphylaxis and anaphylactic shock. Among the participants with severe CMPA whose baseline thresholds were < 4 mL, there was a significant change in thresholds between baseline and at the end of this trial in the pHF-pHF group. Considerably larger amounts of pHF could be effective for the participants with thresholds > 20 mL. Additional study is needed to determine the optimal dose of pHF for each patient with CMPA. Second, the participants were randomized prior to conducting the initial food challenge; therefore, the rCMF thresholds were not taken into account during randomization. The threshold between groups was not significantly different. Third, our sample size was small and young (median age 4.25 years). Compared to those in the eHF-pHF group, the rCMF thresholds in participants in the pHF-pHF group were lower, levels of cow's milk-specific IgE were higher, and ages were lower. Although these differences were not significant, these parameters might affect the results of this trial. A larger sample size would be required to reduce the differences. The differences in symptom scores and tolerance might be found by studying older cohorts. We plan to evaluate the effect of OIT using pHF in older cohorts. Furthermore, compared with pHF, typical eHF has an undesirable flavor. To mitigate the unpleasant bitter taste, the eHF in this study was prepared by reducing the degree of hydrolysis and with ultrafiltration technology. No participant in the eHF-pHF group refused to drink the assigned formula.
In conclusion, this trial suggests that OIT involving the intake of pHF improves tolerance to cow's milk in children with CMPA, relative to that with eHF intake, in a safe manner. Therefore, OIT with pHF represents a potential therapeutic strategy for children with CMPA.
Statement of Ethics
The procedures used in this trial and the possible risks were explained to all participants and their parents, and written informed consent was obtained. This trial conformed to the guidelines established by the Declaration of Helsinki. and the trial protocol was approved by the Research Ethics Committee of Fujita Health University, Aichi, Japan (No. 12–127).
Disclosure Statement
The authors had no conflicts of interest related to this work. Morinaga Milk Industry Co., Ltd. supported the trial, providing the formulas, and Siemens AG performed analysis of allergen components using IMMULITE® 2000 3gAllergyTM. Neither played any role in conducting the trial, analyzing the data, writing the manuscript, or in the decision to submit the manuscript for publication.
Funding Sources
This trial was supported by a Grant-in-Aid for Scientific Research © (23500973) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, which did not play any role in conducting the trial, analyzing the data, writing the manuscript, or the decision to submit the manuscript for publication.
Author Contributions
C.I. conceived the study, participated in its design and coordination, and prepared the first draft of the manuscript. K.T., S.S., Y.N., K.Y., I.T., A.U., and Y.K. conducted the food challenge test and helped to draft the manuscript. All authors read and approved the final version.
Acknowledgement
The authors thank Miyuki Teshigawara, Tomoaki Teshigawara, and Yoshiki Tsuboi for providing technical assistance. We would like to thank Editage (www.editage.com) for English language editing and publication support.
References
- 1.Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Special Committee on Food Allergy World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. Pediatr Allergy Immunol. 2010 Jul;21(Suppl 21):1–125. doi: 10.1111/j.1399-3038.2010.01068.x. [DOI] [PubMed] [Google Scholar]
- 2.Indinnimeo L, Baldini L, De Vittori V, Zicari AM, De Castro G, Tancredi G, et al. Duration of a cow-milk exclusion diet worsens parents' perception of quality of life in children with food allergies. BMC Pediatr. 2013 Dec;13((1)):203. doi: 10.1186/1471-2431-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung JP, Kloda LA, McDevitt J, Ben-Shoshan M, Alizadehfar R. Oral immunotherapy for milk allergy. Cochrane Database Syst Rev. 2012 Nov;11:CD009542. doi: 10.1002/14651858.CD009542.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brożek JL, Terracciano L, Hsu J, Kreis J, Compalati E, Santesso N, et al. Oral immunotherapy for IgE-mediated cow's milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012 Mar;42((3)):363–74. doi: 10.1111/j.1365-2222.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 5.Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on Nutrition American Academy of Pediatrics Section on Allergy and Immunology; Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008 Jan;121((1)):183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 6.van Esch BC, Knipping K, Jeurink P, van der Heide S, Dubois AE, Willemsen LE, et al. In vivo and in vitro evaluation of the residual allergenicity of partially hydrolysed infant formulas. Toxicol Lett. 2011 Mar;201((3)):264–9. doi: 10.1016/j.toxlet.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto H, Matsubara T, Nakazato Y, Namba K, Takeda Y. Evaluation of the antigenicity of hydrolyzed cow's milk protein formulas using the mouse basophil activation test. Toxicol Lett. 2016 Feb;242:53–9. doi: 10.1016/j.toxlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008 Aug;122((2)):342–7. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Nowak-Węgrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011 Jul;128((1)):125–131. doi: 10.1016/j.jaci.2011.04.036. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagida N, Sato S, Asaumi T, Okada Y, Ogura K, Ebisawa M. A single-center, case-control study of low-dose-induction oral immunotherapy with cow's milk. Int Arch Allergy Immunol. 2015;168((2)):131–7. doi: 10.1159/000442157. [DOI] [PubMed] [Google Scholar]
- 11.Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007 May;119((5)):1272–4. doi: 10.1016/j.jaci.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012 Feb;129((2)):448–55. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato S, Yanagida N, Ogura K, Asaumi T, Okada Y, Koike Y, et al. Immunotherapy in food allergy: towards new strategies. Asian Pac J Allergy Immunol. 2014 Sep;32((3)):195–202. [PubMed] [Google Scholar]
- 14.Kido J, Nishi N, Sakaguchi M, Matsumoto T. Most cases of cow's milk allergy are able to ingest a partially hydrolyzed formula. Ann Allergy Asthma Immunol. 2015 Oct;115((4)):330–331. doi: 10.1016/j.anai.2015.07.013. e2. [DOI] [PubMed] [Google Scholar]
- 15.Fritsché R. Animal models in food allergy: assessment of allergenicity and preventive activity of infant formulas. Toxicol Lett. 2003 Apr;140–141:303–9. doi: 10.1016/s0378-4274(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 16.Meulenbroek LA, van Esch BC, Hofman GA, den Hartog Jager CF, Nauta AJ, Willemsen LE, et al. Oral treatment with β-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow's milk allergy. Pediatr Allergy Immunol. 2013 Nov;24((7)):656–64. doi: 10.1111/pai.12120. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009 Aug;124((2)):286–91. doi: 10.1016/j.jaci.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S, Cosenza L, et al. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013 Sep;163((3)):771–7. doi: 10.1016/j.jpeds.2013.03.008. e1. [DOI] [PubMed] [Google Scholar]
- 19.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008 Dec;122((6)):1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nucera E, Pecora V, Buonomo A, Rizzi A, Aruanno A, Pascolini L, et al. Utility of Basophil Activation Test for monitoring the acquisition of clinical tolerance after oral desensitization to cow's milk: pilot study. United European Gastroenterol J. 2015 Jun;3((3)):272–6. doi: 10.1177/2050640615570694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perezábad L, Reche M, Valbuena T, López-Fandiño R, Molina E, López-Expósito I. Oral food desensitization in children with IgE-mediated cow's milk allergy: immunological changes underlying desensitization. Allergy Asthma Immunol Res. 2017 Jan;9((1)):35–42. doi: 10.4168/aair.2017.9.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, et al. PETIT Study Team Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Jan;389((10066)):276–86. doi: 10.1016/S0140-6736(16)31418-0. [DOI] [PubMed] [Google Scholar]