Abstract
A 78-year-old man had a fever and exhibited disordered consciousness, which led to his transportation to our hospital. On arrival, he exhibited discharge from the ear. Because extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli was detected in the ear discharge and cerebrospinal fluid specimens, it was inferred to be the causal bacteria. Pulsed-field gel electrophoresis indicated the same ESBL-producing E. coli pattern in the patient's ear discharge, external auditory canal granulation, cerebrospinal fluid, and stool, indicating their common molecular epidemiological origin. Although ESBL-producing E. coli is an extremely rare cause of bacterial meningitis, it should be considered as a potential causal bacteria for community-acquired meningitis.
Keywords: bacterial meningitis, ESBL-producing E. coli, otitis media with cholesteatoma
Introduction
Bacterial meningitis is a bacterial infection of the brain as well as the arachnoid and pia mater surrounding the spinal cord. Its main characteristics are a sudden onset, headache, and fever; it is indicated by the proliferation of cells in the cerebrospinal fluid with polymorphonuclear cell predominance. Because early treatment has a major impact on the patient's outcome, it is considered a neurological emergency.
Extended-spectrum beta-lactamase (ESBL) is an enzyme that breaks down most beta-lactamase antibiotics, including penicillin, cephem, and monobactam. Infection-causing ESBL-producing bacteria are associated with a poor patient outcome (1). Although ESBL-producing bacteria were previously considered responsible for mainly nosocomial infections, in recent years, community-acquired infections have also been reported, and this has led to concern that the frequency of such cases may increase in the future.
In this study, we report a case of an elderly man with community-acquired bacterial meningitis induced by the direct infiltration of otitis media with cholesteatoma caused by ESBL-producing Escherichia coli.
Case Report
The patient was a 78-year-old man with chief complaints of a fever and disordered consciousness. He had a medical history of atrial fibrillation and otitis media and no significant family history. He had been smoking 5-6 cigarettes/day for over 50 years, occasionally consumed alcoholic beverages, and was able to live independently.
One month prior to hospital admission, the patient noticed subjective symptoms of unsteadiness when standing and moving. After noticing difficulty in hearing from the right ear, the patient was diagnosed with right otitis media with cholesteatoma (7 days before admission) at the Department of Otorhinology of our hospital and scheduled for surgery at the Department of Otorhinology at another hospital. Antibiotic ear drops were prescribed, but not antibiotic internal medications. At approximately 1 AM on the day of hospitalization, he developed a fever of 39℃ and suffered urinary incontinence. Consequently, he was transported to the emergency department of our hospital because of a reduced responsiveness to questions. He had not complained of any symptoms of headache or earache.
The general characteristics of the patient noted at the time of admission were as follows: height, 165 cm; weight, 56 kg; blood pressure, 145/65 mmHg; heart rate, 111 bpm and irregular; and temperature, 37.5℃. Although the general findings included no thoracoabdominal abnormalities, discharge from the right ear was observed as well as a mass with scabbing on the upper posterior of the right external auditory canal. The neurological findings were a Glasgow Coma Scale (GCS) of E2V3M3, dull responses, and urinary incontinence. No neurological abnormalities were observed in the reflexes or sensory system. However, the cranial nervous system was difficult to evaluate in detail due to the patient's disturbance of consciousness. The meningeal irritation sign of stiff neck and Kernig's sign were also observed.
Blood tests indicated a white blood cell count of 11,930/μL, C-reactive protein level of 5.00 mg/dL, and creatinine level of 1.13 mg/dL as well as a slightly elevated inflammatory response and renal dysfunction. A cerebrospinal fluid examination indicated an opening pressure of 250 mmH2O, cell count of 656/μL (polymorphonuclear leukocytes, 64%), protein at 302 mg/dL, and cerebrospinal fluid glucose at 45 mg/dL (blood glucose, 145 mg/dL).
Electrocardiography indicated atrial fibrillation. Chest radiography findings were normal. Plain computed tomography (CT) of the chest, abdomen, and pelvis indicated no abnormal findings indicating the source of the fever.
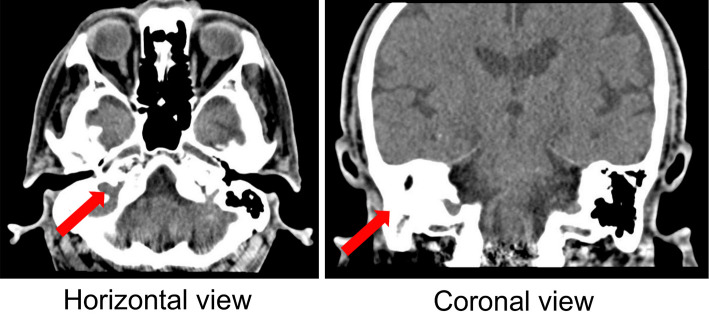
Although CT of the head showed no notable abnormalities, the right mastoid cells had poor pneumatization, and there was a shadow in the soft tissue from the right epitympanum to the middle ear (Fig. 1). Magnetic resonance imaging of the head indicated slight cerebral atrophy, but there were no clear intracranial structural disorders.
Figure 1.
Head CT performed on admission shows poor pneumatization of the right mastoid cells and a shadow in the soft tissue from the right epitympanum to the middle ear.
Owing to the presence of stiff neck and the cerebrospinal fluid findings, such as protein of 302 mg/dL, cerebrospinal fluid glucose of 45 mg/dL (blood glucose, 145 mg/dL), cell count of 656/μL (neutrophils, 64%), and proliferation of cells with polymorphonuclear cell dominance, we concluded that there was a high probability of bacterial meningitis. This patient was above the age of 50 and was not a compromised host, with no recent history of brain surgery.
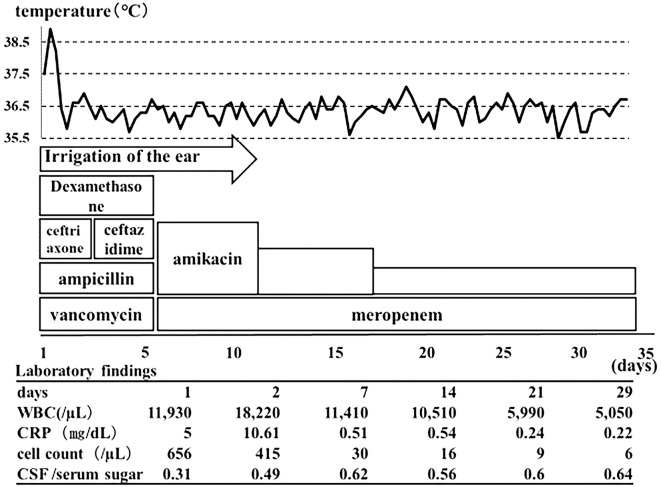
In accordance with the 2014 Japanese Guidelines for the Clinical Management of Bacterial Meningitis, we started the patient on initial treatment with a regimen of ceftriaxone sodium hydrate (CTRX) at 4 g/day while monitoring the renal function, ampicillin hydrate (ABPC) at 8 g/day, vancomycin hydrochloride (VCM) at 1.5 g/day, and dexamethasone sodium phosphate at 39.6 mg/day. In addition, irrigation of the ear was also simultaneously performed over several days by the Department of Otorhinology.
His fever subsided on day 2 of hospitalization and his consciousness also improved. However, on the same day, the results of ear discharge culture performed seven days prior to hospitalization indicated Pseudomonas aeruginosa; the patient's medication was therefore switched from CTRX to ceftazidime hydrate (CAZ) at 6 g/day. Dexamethasone sodium phosphate was discontinued after four days. On day 4 of hospitalization, ESBL-producing E. coli was identified in the ear discharge culture performed seven days prior to hospitalization as well as in all of the cultures performed at hospitalization for cerebrospinal fluid, ear discharge, external ear canal granulation, and stool, so the antibiotics were again switched to meropenem hydrate (MEPM) at 6 g/day and amikacin sulfate (AMK) at 800 mg/day. P. aeruginosa was not identified from the cerebrospinal fluid but was identified in the ear discharge culture performed at hospitalization and seven days prior to it.
Given that the blood culture performed prior to the antibiotic administration had been negative, we suspected that ESBL-producing E. coli had entered the ear from the stool, and the subsequent otitis media caused meningitis via direct infiltration. As the signs of ear infection began to subside on day 10 of hospitalization, ear irrigation was switched to once a week. The AMK dose was adjusted based on the renal function and blood drug concentration and was administered with MEPM until day 34 of hospitalization. Over the course of hospitalization, the patient's fever, disordered consciousness, and clinical symptoms improved. The final cerebrospinal fluid cell count was 6/μL, and as there were no notable sequelae, the patient was discharged on day 35 of hospitalization. After discharge, he was admitted to the Department of Otorhinology at another hospital for the previously scheduled surgery (Fig. 2).
Figure 2.
The patient’s clinical course.
To identify the infection route, the patient's cerebrospinal fluid, ear discharge, external ear canal granulation, and stool samples were assessed using pulsed-field gel electrophoresis. The results indicated that all four samples had a type A pulsed-field gel electrophoresis pattern, indicating that all four samples were from the same molecular epidemiological source (Fig. 3).
Figure 3.
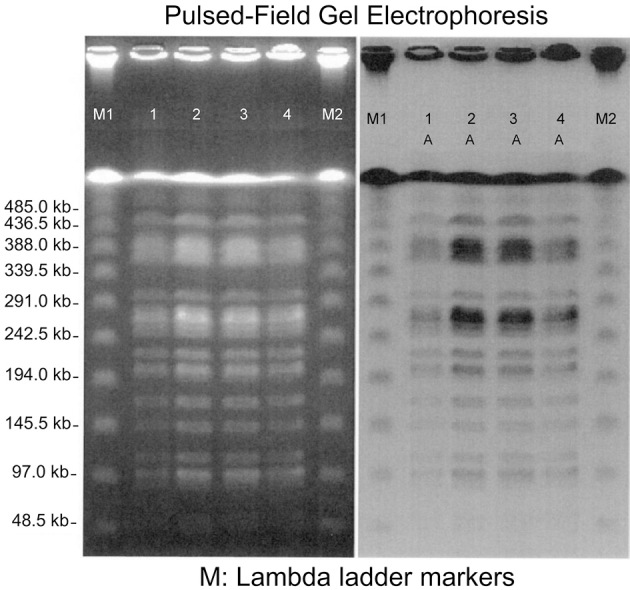
Pulsed-field gel electrophoresis (PFGE). 1: Cerebrospinal fluid, 2: Ear discharge, 3: External ear canal granulation, 4: Stool. All four specimens had the same type A PFGE pattern.
Discussion
In 1983, Knothe et al. reported that the Friedländer bacillus and other bacteria were resistant to cefotaxime (CTX) and other third-generation cephem antibiotics (2). This was the first report on ESBL-producing bacteria. ESBL is a generic name for a group of beta-lactamases with wide substrate specificity that arises from a spontaneous mutation in the class A beta-lactamase-producing gene; these beta-lactamases have the ability to hydrolyze CTX, ceftazidime, aztreonam, and other cephem antibiotics as well as monobactam antibiotics (3). Beta-lactamase genotypes include TEM, SHV, CMY, GES, OXA, and CTM-M, which are further categorized into a large number of subtypes. Because ESBL genes are located on plasmids, they may be horizontally transmitted to other Gram-negative bacilli strains aside from E. coli. In addition, because they are liable to become resistant to multiple drugs, they represent a major risk factor for infection (4). ESBL-producing bacteria seem to be the cause of multiple drug-resistant infections that can prolong hospitalization and increase the cost of treatment (1). In Europe, ESBL-producing bacteria are frequently detected, often in patients in the intensive-care unit (ICU), indicating that they are serious causal agents of nosocomial infections.
The risk factors for ESBL-producing bacteria include intubation and artificial respiration, a period of admission in the ICU, all types of catheter usage, frequent antibiotic usage, and a severe illness. However, in recent years, some studies have reported that ESBL-producing bacteria have also been identified among community-acquired infection-causing bacteria (3,5-10).
To our knowledge, there have been only 17 reported cases of meningitis caused by ESBL-producing bacteria, particularly ESBL-producing E. coli (Table) (7-22). Although 10 cases were reported in children (11,12,14-20), only 7 have been reported in adults (7-10,13,21,22), among which 4 were community-acquired cases, as in the present case (7-10). Genetic testing of ESBL was also conducted in eight cases (9-12,14,15,19).
Table.
Previous Reports of Meningitis Caused by Extended-spectrum Beta-lactamase (ESBL)-producing Escherichia Coli.
| Case (Ref.no.) |
Year | Country | Age | Infectious pattern | Background | ESBL-type | Antibiotic treatment | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (11) | 2006 | Algeria | Child | Nosocomial | No information | CTX-M-15 | No information | Cured | ||||||||
| (11) | 2006 | Algeria | Child | Community | No information | CTX-M-15 | No information | Cured | ||||||||
| (12) | 2008 | France | Child | Nosocomial | Very low birth weight infant | CTX-M-15 | Ceftazidime, vancomycin, netilmicin | Died | ||||||||
| (13) | 2008 | Japan | Adult | Nosocomial | Immunocompromised | Not determined | Meropenem | Cured | ||||||||
| (14) | 2010 | France | Child | Nosocomial | Very low birth weight infant | TEM-52 | Imipenem, gentamicin, ciprofloxacin | Cured | ||||||||
| (15) | 2010 | Brazil | Child | Undescribed | No information | CTX-M-2 | No information | Died | ||||||||
| (16) | 2011 | Thailand | Child | Nosocomial | Infected cephal hematoma | Not determined | Meropenem | Cured | ||||||||
| (7) | 2011 | Japan | Adult | Community | Diabetes mellitus | Not determined | Meropenem, cefotaxime, gentamicin, levofloxacin | Cured | ||||||||
| (8) | 2012 | France | Adult | Community | Alcoholism, aortic mycotic aneurisms | Not determined | Meropenem, ciprofloxacin | Meningitis cured, died during surgery | ||||||||
| (9) | 2012 | Turkey | Adult | Community | Chronic otitis media, cranialsurgery, cerebrospinal fluid fistula | CTX-M-15 | Meropenem, amikacin | Cured | ||||||||
| (17) | 2012 | Thailand | Child | Community | Multiple anomalies of ophthalmic dermoid tumor, cleft lip, cleftpalate, polydactyly, bifid vertebra, and right ear pinna anomalies | Not determined | Meropenem | Cured | ||||||||
| (18) | 2012 | Japan | Child | Nosocomial | Low birth weight infant | Not determined | Meropenem | Cured | ||||||||
| (19) | 2013 | Japan | Child | Nosocomial | Normal | CTX-M-1, TEM | Meropenem | Cured | ||||||||
| (20) | 2014 | Japan | Child | Nosocomial | Low birth weight infant | Not determined | Carbapenem series | Cured | ||||||||
| (21) | 2015 | Canada | Adult | Nosocomial | Ventriculitis after aneurysm clipping | Not determined | Meropenem, gentamicin | Cured | ||||||||
| (22) | 2016 | Japan | Adult | Nosocomial | Immunocompromised | Not determined | No information | Cured | ||||||||
| (10) | 2016 | Japan | Adult | Community | After myocardial infarction | CTX-M-9, TEM | Meropenem | Cured | ||||||||
| Present case | 2016 | Japan | Adult | Community | Serous otitis media | Not determined | Meropenem, amikacin | Cured |
The detection rate of ESBL-producing bacteria varies by region and institution. A multinational survey reported that 34.5% of Gram-negative bacilli were ESBL-producing bacteria (23). The major risk factors for community-acquired E. coli meningitis are alcoholism, cirrhosis of the liver, malignant tumor, diabetes, and the use of immunosuppressants. Nosocomial E. coli meningitis often occurs in patients following brain surgery and is resistant to multiple drugs in many cases (8). Rodríguez-Baño et al. reported that the risk factors for community-acquired infection due to ESBL-producing bacteria are diabetes, advanced age (≥60 years), female gender, repeated urinary tract infection, a history of invasive procedures performed in the urinary tract, outpatient care, and the use of antibiotics, such as aminopenicillin, cephalosporin, and fluoroquinolone (24). Yumuk et al. reported that independent risk factors for community-acquired ESBL-producing E. coli (particularly CTM-M type) were fluoroquinolone use, advanced age, and a severe underlying illness (25). However, the only risk factors applicable to the present case were advanced age and outpatient care. Although the present case suffered from atrial fibrillation, he was a healthy elderly individual otherwise. Several case studies of ESBL-producing E. coli meningitis have been reported in children, particularly low-birth-weight children (12,14,18,20). However, adults require the presence of certain background circumstances, such as being a compromised patient undergoing immunosuppressant therapy (13,22), alcoholism (8), diabetes (8), a history of brain surgery (21), or a history of middle ear surgery (9). Therefore, patients, such as the present case, who have few underlying illnesses and who contract community-acquired ESBL-producing E. coli meningitis are extremely rare.
Many recent studies have reported an increase in community-based carriers of ESBL-producing bacteria (26). Although the cause of this upsurge is unknown, it has been suggested that healthy individuals' intestines carry bacteria transmitted via food (27). These community-based carriers may bring ESBL-producing bacteria into medical facilities, which then cause hospital transmission and an increase in ESBL-producing bacteria. ESBL-producing E. coli carriers account for approximately 10% of all carriers (26-29). In particular, ESBL-producing E. coli is often detected in the stool of individuals ≥60 years of age (27). Therefore, in cases such as the present one wherein a patient with community-acquired meningitis had few underlying illnesses and was elderly, the possible involvement of ESBL-producing bacteria should be considered during examinations.
Bacteria usually reach the intraspinal region through one of the following routes: one in which the invasion of bacteremia takes place by the choroid plexus, or one in which the invasion occurs when bacteria cross the blood-brain barrier in other sites. It is important to consider the influence of neighboring organs when attempting to identify the origin of meningitis. Reportedly, 25% of patients with meningitis have a middle ear infection or paranasal sinusitis at the onset of meningitis (30). In the present case, although the bacteria was not identified using the blood culture, both cerebrospinal fluid and ear discharge cultures indicated ESBL-producing E. coli, and the pulsed-field gel electrophoresis patterns also indicated that all specimens had the same molecular epidemiological origin. We therefore inferred that the infection route of ESBL-producing E. coli was from the stool to the ear and then from a middle ear infection to meningitis via direct infiltration.
Based on experience, in cases of suspected bacterial meningitis, it is necessary to begin antibiotic administration at the point at which the disease is suspected without waiting for the results of bacteria culture procedures. In such cases, the initial antibiotic selection is made on the basis of the results of Gram staining and the patient's history of surgery and immunocompetence. van de Beek et al. (30) reported that the recommended initial antibiotics to be used in cases of community-acquired bacterial meningitis were the combined administration of vancomycin and third-generation cephem antibiotics in patients 16-50 years of age, with the additional use of ampicillin in patients >50 years of age. The treatment guidelines for bacterial meningitis released by the Infectious Diseases Society of America recommend the use of third-generation cephem antibiotics in cases suspected of infection with E. coli (31). The antibiotic selection criteria for community-acquired bacterial meningitis listed by this guideline indicate that antibiotics are ineffective in cases such as the present case in which ESBL-producing E. coli is present. However, based on the accumulation of reports on bacterial meningitis caused by ESBL-producing E. coli in Japan, the 2014 guideline states that carbapenem series are recommended when ESBL-producing bacteria are assumed to be involved.
In conclusion, meningitis caused by Gram-negative bacteria is often associated with poor patient outcomes, so the selection of antibiotics to be used in the initial treatment is an important factor that determines the patient's prognosis (3,24,32-34). In cases of community-acquired meningitis in adults and in patients with underlying factors, such as diabetes and exposure to resistant bacteria, the potential infection of ESBL-producing bacteria should be considered, particularly in cases of elderly patients with few underlying factors. In such cases, we believe that it is necessary to consider the use of carbapenem antibiotics as an initial therapy.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32: 1162-1171, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11: 315-317, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14: 933-951, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliebe C, Nies BA, Meyer JF, Tolxdorff-Neutzling RM, Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother 28: 302-307, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colodner R, Rock W, Chazan B, et al. . Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis 23: 163-167, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Pitout JD, Hanson ND, Church DL, Laupland KB. Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: importance of community isolates with blaCTX-M genes. Clin Infect Dis 38: 1736-1741, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Gon Y, Otsubo R, Murase S, Park K, Nakazawa K, Hara H. A case of an elderly individual with community-acquired meningitis caused by extended-spectrum beta-lactamase (ESBL)-producing E. coli. Clin Neurol 52: 12-18, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Weyrich P, Ettahar N, Legout L, Meybeck A, Leroy O, Senneville E. First initial community-acquired meningitis due to extended-spectrum beta-lactamase producing Escherichia coli complicated with multiple aortic mycotic aneurysms. Ann Clin Microbiol Antimicrob 11: 4, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elaldi N, Gozel MG, Kolayli F, Engin A, Celik C, Bakici MZ, Vahaboglu H. Community-acquired CTX-M-15-type ESBL-producing Escherichia coli meningitis: a case report and literature review. J Infect Dev Ctries 7: 424-431, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Yonezawa H, Kamada K, Tamai K, Tanimoto H. A case of bacterial meningitis caused by ESBL-producing Escherichia coli resulting from community-acquired urinary tract infection. J Jpn Soc Clin Microbiol 27: 340, 2016(in Japanese). [Google Scholar]
- 11.Ramdani-Bouguessa N, Mendonca N, Leitao J, Ferreira E, Tazir M, Canica M. CTX-M-3 and CTX-M-15 extended-spectrum beta-lactamases in isolates of Escherichia coli from a hospital in Algiers, Algeria. J Clin Microbiol 44: 4584-4586, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer-Mariotte S, Duboc P, Bonacorsi S, Lemeland JF, Bingen E, Pinquier D. CTX-M-15-producing Escherichia coli in fatal neonatal meningitis: failure of empirical chemotherapy. J Antimicrob Chemother 62: 1472-1474, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Otsubo M, Fujishima Y, Yokotani A, et al. . A case of acute renal failure caused by ESBL-producing E. coli meningitis. Proceedings of the 556th Kanto Regional meeting of the Japanese Society of Internal Medicine; 2008 Sep. Japanese Society of Internal Medicine, Tokyo, Japan, 2008: 23(in Japanese). [Google Scholar]
- 14.Moissenet D, Salauze B, Clermont O, et al. . Meningitis caused by Escherichia coli producing TEM-52 extended-spectrum beta-lactamase within an extensive outbreak in a neonatal ward: epidemiological investigation and characterization of the strain. J Clin Microbiol 48: 2459-2463, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade LN, Minarini LA, Pitondo-Silva A, et al. . Determinants of beta-lactam resistance in meningitis-causing Enterobacteriaceae in Brazil. Can J Microbiol 56: 399-407, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Nakwan N, Nakwan N, Wannaro J, Dissaneevate P, Kritsaneepaiboon S, Chokephaibulkit K. Septicemia, meningitis, and skull osteomyelitis complicating infected cephalhematoma caused by ESBL-producing Escherichia coli. Southeast Asian J Trop Med Public Health 42: 148-151, 2011. [PubMed] [Google Scholar]
- 17.Chaiyakulsil C, Prommalikit O. Successful medical treatment in a child with E. coli ESBL meningitis with acute communicating hydrocephalus and ventricular empyema: a case report. J Med Assoc Thai 95 (Suppl): S138-S141, 2012. [PubMed] [Google Scholar]
- 18.Hashimoto A, Oikawa K, Nakamura S, et al. . A case of a premature infant with anemic shock and meningitis caused by ESBL-producing E. coli. J Jpn Soc Premature Newborn Med 24: 699, 2012(in Japanese). [Google Scholar]
- 19.Takanashi M, Kenmochi M, Yamaguchi A, et al. . A case of severe neonatal bacterial meningitis caused by ESBL-producing E. coli. J Jpn Soc Perinatal Neonatal Med 51: 1253-1259, 2015(in Japanese). [Google Scholar]
- 20.Oikawa K, Koyamo K, Nakamura S, et al. . A case of a neonate with recurrent bacterial meningitis caused by ESBL-producing E. coli. J Jpn Pediatric Soc 118: 1122, 2014. [Google Scholar]
- 21.Zeiler FA, Silvaggio J. ESBL Escherichia coli ventriculitis after aneurysm clipping: a rare and dfficult therapeutic challenge. Case Rep Neurol Med 2015: 694807, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai K, Tashiro K, Kannae M, Morita T, Nakamura A, Takasu O. A case of rheumatic arthritis with bacterial meningitis caused by ESBL-producing E. coli during combined therapy with Adalimumab and Methotrexate. J Jpn Assoc Acute Med 27: 609, 2016. [Google Scholar]
- 23.Ben-Ami R, Rodriguez-Bano J, Arslan H, et al. . A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49: 682-690, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Baño J, Alcala JC, Cisneros JM, et al. . Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med 168: 1897-1902, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Yumuk Z, Afacan G, Nicolas-Chanoine MH, Sotto A, Lavigne JP. Turkey: a further country concerned by community-acquired Escherichia coli clone O25-ST131 producing CTX-M-15. J Antimicrob Chemother 62: 284-288, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Shimizu C, Inui S, et al. . The effectiveness of ESBL-producing E. coli screening in feces. J Jpn Soc Clin Microbiol 19: 230-235, 2009. [Google Scholar]
- 27.Yoshikawa K, Nagakawa T, Sonoda M, Shimatani Y, Takeda M, Kinoshita Y. Detection of ESBL- and MBL-producing E. coli bacteria in feces. J Jpn Soc Clin Microbiol 24: 9-16, 2014. [Google Scholar]
- 28.Kader AA, Kumar A, Kamath KA. Fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in patients and asymptomatic healthy individuals. Infect Control Hosp Epidemiol 28: 1114-1116, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Valverde A, Coque TM, Sanchez-Moreno MP, Rollan A, Baquero F, Canton R. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J Clin Microbiol 42: 4769-4775, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 351: 1849-1859, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Tunkel AR, Hartman BJ, Kaplan SL, et al. . Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39: 1267-1284, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Huang CR, Lu CH, Chang HW, Lee PY, Lin MW, Chang WN. Community-acquired spontaneous bacterial meningitis in adult diabetic patients: an analysis of clinical characteristics and prognostic factors. Infection 30: 346-350, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Dellinger RP, Carlet JM, Masur H, et al. . Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32: 858-873, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Skaf GS, Domloj NT, Fehlings MG, et al. . Pyogenic spondylodiscitis: an overview. J Infect Public Health 3: 5-16, 2010. [DOI] [PubMed] [Google Scholar]