Abstract
Pembrolizumab, a humanized monoclonal IgG4 antibody directed against programmed death-1, is an immune checkpoint inhibitor that has been introduced for the treatment of non-small-cell lung cancer. However, immune checkpoint inhibitors may cause severe immune-related adverse events. We herein present a case of lung cancer with complete atrioventricular block associated with acute myocarditis, which developed 16 days after the administration of pembrolizumab. The clinical course of this case suggested a strong need for close cardiac monitoring when pembrolizumab is administered on an outpatient basis.
Keywords: immune checkpoint inhibitors, pembrolizumab, immune-related adverse events, complete atrioventricular block, acute myocarditis
Introduction
Pembrolizumab, a humanized monoclonal IgG4 antibody directed against programmed death-1, is an immune checkpoint inhibitor that has been introduced for the treatment of non-small-cell lung cancer (NSCLC) (1). However, immune checkpoint inhibitors sometimes induce various immune-related adverse events (irAEs) that are uncommon with conventional cytotoxic anticancer agents. Pembrolizumab-associated irAEs most often affect the skin, colon, liver, lung, and endocrine glands (2). Although the incidence of myocarditis presenting as a cardiovascular irAE appears to be very low, it represents a potentially life-threatening development (3). We herein present a case of complete atrioventricular block associated with pembrolizumab-induced acute myocarditis, which necessitated permanent pacemaker implantation. This case raises an alarm regarding the administration of pembrolizumab at outpatient clinics because of the potential risk for pembrolizumab-induced acute myocarditis, which represents one of the most life-threatening irAEs.
Case Report
The patient was a 73-year-old man who was an ex-smoker (50 pack-years) with advanced NSCLC who was admitted to our hospital because of faintness and general fatigue; more than 95% of the patient's tumor cells were found to express programmed cell death ligand 1. At one year before this admission, he had been diagnosed with advanced NSCLC; bone and intrapulmonary metastases from NSCLC were detected; however, computed tomography and ultrasound cardiography (UCG) revealed no evidence of cardiac metastasis. He was initially treated with four cycles of cisplatin-based chemotherapy combined with pemetrexed and bevacizumab as a first-line treatment. A partial response was achieved. After induction therapy, he received pemetrexed and bevacizumab as maintenance therapy. However, he was considered to have progressive disease after seven maintenance cycles. Thus, 16 days before admission, pembrolizumab (200 mg) was administered as a second-line treatment. Although his chest X-ray revealed no widening of the cardiac silhouette and his electrocardiogram (ECG) revealed no conduction disturbance before the initiation of pembrolizumab treatment, at one day before admission he complained of faintness and general fatigue. He had no previous history of cardiovascular disease. His vital signs were as follows: body temperature, 36.5℃; blood pressure, 87/34 mmHg; heart rate, 30 bpm; and oxygen saturation, 98% on room air. ECG revealed a complete atrioventricular block with wide QRS complexes, which was not previously documented (Fig. 1). A laboratory analysis was positive for troponin T, and revealed elevated creatine kinase (CK) and transaminase levels (Table). UCG revealed a preserved left ventricular systolic function, an ejection fraction (EF) of 70%, and no myocardial edema that could indicate typical acute myocarditis (Fig. 2). A chest X-ray showed no signs of heart failure (Fig. 3). Coronary angiography did not reveal any significant stenosis (Fig. 4). Although hepatitis B/C virus markers and autoantibodies against collagen disease were not detected, a percutaneous liver biopsy sample obtained at admission showed interface hepatitis, hepatic necrosis and inflammation, and acidophilic bodies-findings which are consistent with hepatitis as an irAE (Fig. 5). He was diagnosed with acute hepatitis and a complete atrioventricular block associated with pembrolizumab-induced acute myocarditis, both of which were considered to represent irAEs. He was treated with high-dose glucocorticoids (intravenous methylprednisolone, 1,000 mg/day for 3 days) and temporary pacemaker implantation. The patient's symptomatic complete atrioventricular block persisted for 5 days. Thus, a permanent pacemaker was implanted (Fig. 6). After treatment with high-dose glucocorticoids, the patient's CK and transaminase levels normalized. The pacemaker check before discharge revealed the recovery of atrioventricular conduction. He was discharged on hospital day 21, at which time the dosage of oral glucocorticoids was being tapered.
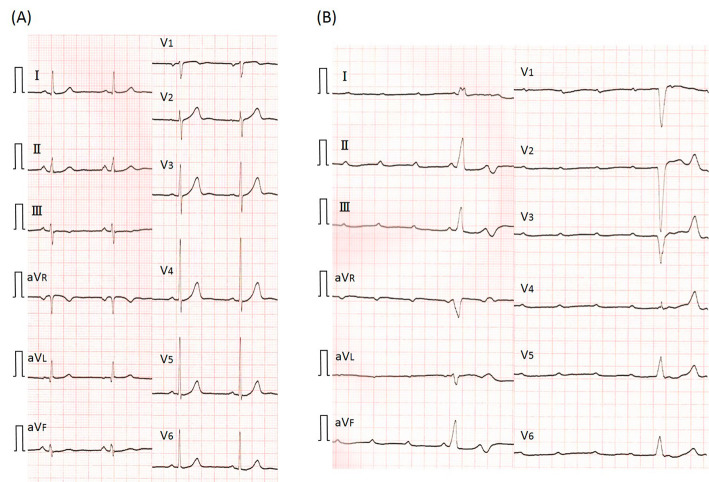
Figure 1.
Electrocardiogram (ECG) at 1 month before admission (A) revealed normal sinus rhythm. ECG on admission (B) showed a complete atrioventricular block with wide QRS complexes.
Table.
Laboratory Data on Admission.
| Variable | Reference range, Adults | On admission | ||
|---|---|---|---|---|
| WBC (/µL) | 3,000-9,500 | 4,800 | ||
| RBC (×106/µL) | 420-560 | 397 | ||
| Hb (g/dL) | 13-17 | 11.9 | ||
| Ht (%) | 40-51 | 35.0 | ||
| Plt (/µL) | 15-38 | 4.3 | ||
| TP (g/dL) | 6.3-8.3 | 6.5 | ||
| Alb (g/dL) | 3.8-5.3 | 3.7 | ||
| BUN (mg/dL) | 8-20 | 28.4 | ||
| Cr (mg/dL) | 0.4-1.1 | 0.77 | ||
| T-Bil (mg/dL) | 0.23-1.28 | 1.65 | ||
| AST (U/L) | 8-40 | 888 | ||
| ALT (U/L) | 4-42 | 1,685 | ||
| LDH (U/L) | 119-229 | 1,065 | ||
| ALP (U/L) | 105-340 | 642 | ||
| γ-GTP (U/L) | 0-78 | 442 | ||
| CK (U/L) | 40-220 | 455 | ||
| CK-MB (U/L) | 0-24 | 42 | ||
| Troponin-T | - | + | ||
| CRP (mg/dL) | 0-0.3 | 1.82 | ||
| IgG (mg/dL) | 870-1,700 | 1,155 | ||
| IgM (mg/dL) | 33-190 | 73 | ||
| IgA (mg/dL) | 110-410 | 161 |
Laboratory analysis results were positive for troponin T, and elevated creatine kinase and transaminase levels were observed.
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, TP: total protein, Alb: albumin, BUN: blood urea nitrogen, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, CK: creatine kinase, CK-MB: CK-MB isoenzyme, CRP: C-reactive protein, IgG: immunoglobulin G, IgM: immunoglobulin M, IgA: immunoglobulin A
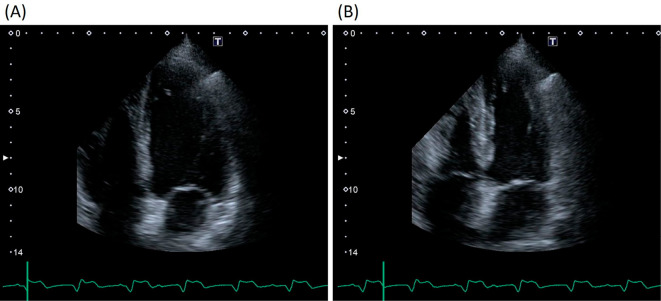
Figure 2.
Echocardiography on admission revealed a preserved left ventricular systolic function, with an ejection fraction of 70% and no myocardial edema. (A) End diastole and (B) End systole.
Figure 3.
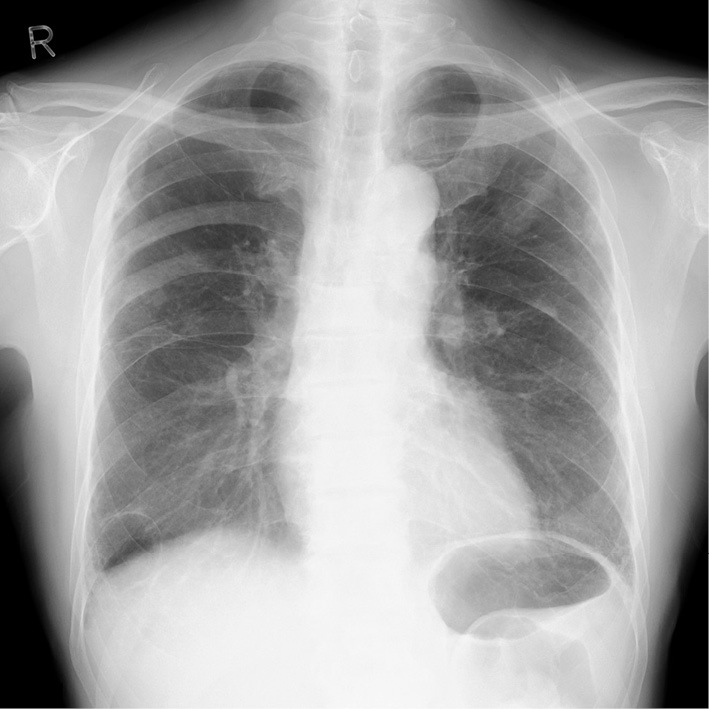
Chest X-ray on admission revealed no signs of heart failure.
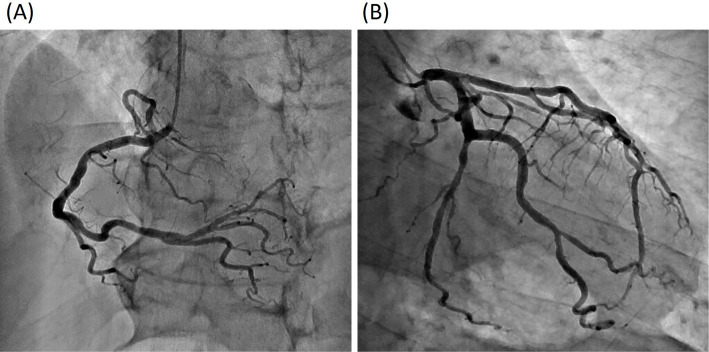
Figure 4.
Coronary angiography did not reveal any significant stenosis.
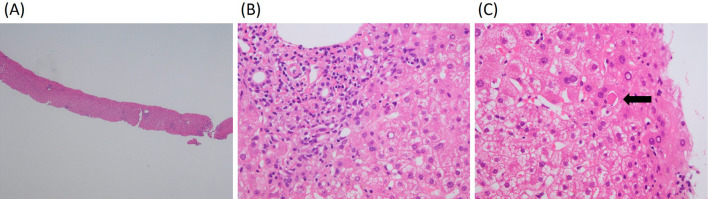
Figure 5.
The histological examination of a liver biopsy specimen (Hematoxylin and Eosin staining). (A, ×40) A very low-power field. (B, ×200) Lymphocytic/lymphoplasmacytic infiltration in the portal tracts extending into the lobule. (C, ×200) An apoptotic hepatocyte can be recognized as acidophilic body (arrow), induced by cytotoxic T-cells due to immune abnormalities.
Figure 6.
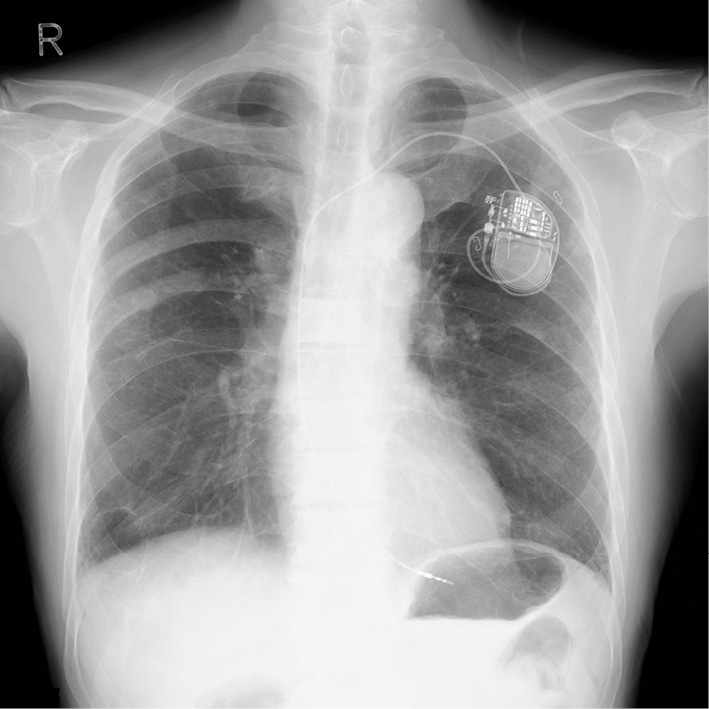
Chest X-ray after pacemaker implantation.
Discussion
To our knowledge, this is one of the first case reports in Japan to describe a case of complete atrioventricular block associated with pembrolizumab-induced myocarditis in a patient with advanced NSCLC. Our case is different from previous similar cases (4) because a complete atrioventricular block occurred with mild (but not fulminant) myocarditis, which presented with mildly elevated CK levels and a preserved EF, and because pembrolizumab-not nivolumab-caused acute myocarditis.
We found three important clinical issues. First, pembrolizumab-induced acute myocarditis could cause a complete atrioventricular block despite the patient's mildly elevated CK levels. Second, immune checkpoint inhibitors should be administered under close cardiac monitoring for the early detection of fatal cardiovascular irAEs. Finally, close cooperation between cardiologists and cancer specialists (i.e., a multidisciplinary team) is essential for correctly managing these irAEs.
This case is unique because a complete atrioventricular block occurred in a patient with pembrolizumab-induced acute myocarditis, despite the preservation of the EF and the mildly elevated CK level. Many previous reports have shown that a complete atrioventricular block is usually associated with immune checkpoint inhibitor-induced myocarditis with both a reduced EF and a considerably elevated CK level (4,5). The precise pathophysiology of irAEs remains unknown; however, several studies have shown that T-cells, antibodies, and the cytokine response may be associated with irAEs (6,7). Furthermore, there might be individual differences in the reaction to immune checkpoint inhibitors.
The second important point is that our case suggested that close cardiac examination before, during, and after the administration of receiving immune checkpoint inhibitors is of prime importance because death can be avoided if a complete atrioventricular block is detected at an early stage and subsequent pacemaker implantation is possible. Most chemotherapy treatments, including immune checkpoint inhibitor therapy, are currently administered on an outpatient basis. Close cardiac examinations before the use of immune checkpoint inhibitors, and cardiac monitoring during or after their administration are not conventionally performed at outpatient chemotherapy clinics. However, in our opinion, immune checkpoint inhibitors should be administered with more frequent outpatient clinic visits because of the potential risk of cardiovascular irAEs, such as a complete atrioventricular block and fulminant myocarditis. The median interval from the initial administration of ipilimumab and nivolumab (immune checkpoint inhibitors) to the development of myocarditis was reported to be 17 days (range, 13-64 days) (4). Similarly, myocarditis may most frequently occur at approximately 13-17 days after the final administration of pembrolizumab-based on the present case and the notification from the Japanese government (8). Considering that pembrolizumab is administered every 3 weeks (1), we strongly recommend close cardiac monitoring on an outpatient basis for at least 13-17 days after the final administration of pembrolizumab. A patient receiving pembrolizumab should probably undergo cardiac testing, including 12-lead ECG monitoring, and CK and troponin checks every other day from days 13 to 17. Cancer specialists need to pay special attention to pembrolizumab-induced myocarditis, as pembrolizumab may be more frequently used in the near future because it has been established as a first-line treatment for patients with advanced and programmed cell death ligand 1-positive NSCLC (1). At present, data regarding the incidence of cardiovascular irAEs remain scarce. We believe that the accumulation of an increased number of clinical cases would reveal the differences in the incidence of cardiovascular irAEs (including acute myocarditis) among immune checkpoint inhibitors.
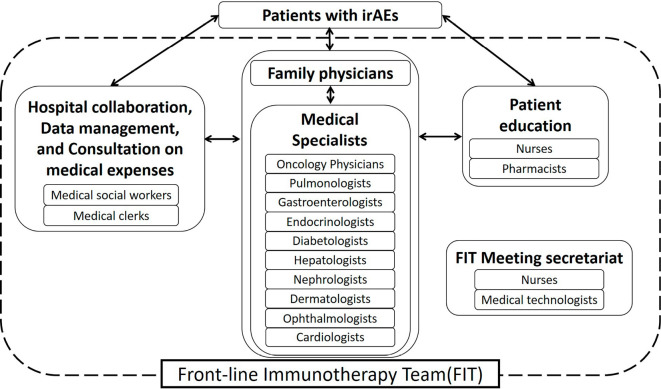
Finally, close cooperation is required between cancer specialists and cardiologists. Immune checkpoint inhibitors constitute a relatively novel therapy. Thus, the precise mechanism underlying the development of irAEs is not well established, irAEs are difficult to predict. Consequently, early detection is very important, particularly in cases involving a non-specific or atypical presentation of acute myocarditis. Acute myocarditis is typically associated with the presentation of chest pain and dyspnea. However, patients presenting with atypical symptoms, such as general fatigue, or symptoms of infectious disease are often observed (9). In addition, some patients complain of faintness, syncope, or palpitation due to lethal arrhythmias, such as a complete atrioventricular block or ventricular tachycardia, which are caused by acute myocarditis. Thus, cancer specialists and cardiologists should work in collaboration to monitor ECG, myocardial enzymes, and UCG when a patient who has received immune checkpoint inhibitors complains of any of the abovementioned symptoms. The close monitoring of patients by cancer specialists and cardiologists can prevent fatal outcomes in patients with cardiovascular irAEs. We recently established a multidisciplinary team, called the front-line immunotherapy team (FIT), for managing irAEs at our hospital. The FIT involves oncology physicians, pulmonologists, gastroenterologists, endocrinologists, diabetologists, hepatologists, nephrologists, dermatologists, ophthalmologists, and cardiologists, and also includes nurses, medical clerks, pharmacists, medical technologists, and social workers (Fig. 7). The FIT is expected to detect irAEs early and to provide adequate management.
Figure 7.
The organizational structure of the front-line immunotherapy team (FIT). The FIT members cooperated with each other to manage immune-related adverse events (irAEs).
The present case is associated with some limitations in our case. First, cardiac biopsy was not performed because we did not initially recognize the relationship between pembrolizumab and a myocarditis-associated complete atrioventricular block. Thus, the possibility of myocarditis presenting as a cardiovascular irAE should always be considered. Despite the lack of cardiac biopsy, the findings of the liver biopsy appeared to provide further evidence that irAE was involved in the pathogenesis of acute myocarditis in this case. Second, we did not evaluate the patient's viral antibody titers. However, viral myocarditis was considered to be unlikely in the present case because the patient's immunoglobulin M levels (which are usually elevated in viral infection), were within normal range both at admission and several days after admission.
In conclusion, extreme caution and close monitoring for the development of cardiovascular irAEs, including a myocarditis-associated complete atrioventricular block, are required after the initiation of pembrolizumab treatment on an outpatient basis. Establishing a multidisciplinary team that includes cardiologists and cancer specialists would contribute to the adequate management of cardiovascular irAEs.
The clinical description of this case report was approved by the Institutional Review Board of Sendai Kousei Hospital. Our patient gives his full permission for the publication, reproduction, broadcast and other use of photographs, recordings and other audio-visual material of himself and textual material in all editions of this product.
Author's disclosure of potential Conflicts of Interest (COI).
Shunichi Sugawara: Honoraria, MSD, Ono Pharmaceutical and Bristol-Myers Squibb.
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375: 1823-1833, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Garon EB, Rizvi NA, Hui R, et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018-2028, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 19: 21, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson DB, Balko JM, Compton ML, et al. . Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749-1755, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzerling L, Ott PA, Hodi FS, et al. . Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 4: 50, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 123: 2143-2153, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esfahani K, Miller WH Jr. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med 376: 1989-1991, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Notification issued by the Safety Division, Pharmaceutical and Medical Safety Bureau, Ministry of Health and Welfare, Japan, on April 20th, 2017 (in Japanese). [Internet]. [cited 2017 Aug. 14] Available from: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000163376.pdf
- 9.Kytö V, Saukko P, Lignitz E, et al. . Diagnosis and presentation of fatal myocarditis. Hum Pathol 36: 1003-1007, 2005. [DOI] [PubMed] [Google Scholar]