Abstract
Nivolumab is an anti-programmed cell death-1 (PD-1) antibody that is utilized as an immune checkpoint inhibitor (ICI) for cancer therapy. We herein present the case of a 57-year-old man who developed acute kidney injury during treatment with nivolumab for lung cancer. A renal biopsy revealed acute tubulointerstitial nephritis. Immunohistochemical staining demonstrated marked infiltration of macrophages and T cells together with mild B cell infiltration. Of note, strong CD163+ M2 macrophage infiltration was observed. The cessation of nivolumab and high-dose prednisolone therapy improved the renal function of the patient. Further, we review the pertinent literature on renal-infiltrating cells in ICI-induced tubulointerstitial nephritis.
Keywords: acute tubulointerstitial nephritis, anti-PD-1 antibody, immune checkpoint inhibitor, M2 macrophage
Introduction
Immune checkpoint inhibitors (ICIs) are attracting attention as novel cancer therapeutic agents. Two main groups are targeted by ICIs: cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), and programmed cell death-1 (PD-1) and its ligand PD-L1 (1,2). The anti-CTLA-4 monoclonal antibody ipilimumab was the first ICI to be approved by the United States Food and Drug Administration. This was followed by the approval of two anti-PD-1 antibodies (nivolumab and pembrolizumab) and two anti-PD-L1 antibodies (atezolizumab and durvalumab) (3). These agents are considered to reactivate cytotoxic T cells, leading to tumor cell lysis by blunting the braking mechanisms of the immune system (2,4).
The ICI-mediated damage observed in various organs is recognized under the umbrella term of immune-related adverse events (irAEs), since these events result from the disruption of immune tolerance (1,2). The spectrum of irAEs ranges from common manifestations such as dermatological, gastrointestinal and endocrine events, to rare organ damage involving the nervous, hematopoietic, and urinary systems (3). Specifically, in the urinary system, acute kidney injury (AKI) is clinically important; an increased number of reports on ICI-induced renal injuries has been observed in recent years (5-13).
In this report, we present a case of acute tubulointerstitial nephritis that developed during nivolumab treatment. To characterize the pathological characteristics of nivolumab-induced tubulointerstitial nephritis, we conducted a precise examination of the renal-infiltrating immune cells and reviewed the current literature on ICI-mediated renal damage.
Case Report
A 57-year-old man was admitted to our department with AKI. He had been diagnosed with stage IIA adenocarcinoma of the lung (T2aN1M0) four years previously. After the surgical resection of the left lower lobe of the lung, the patient received four courses of carboplatin and paclitaxel. However, two years after the initial diagnosis, the cancer progressed to stage IV (T0N3M1b) with multiple metastases to the lung, bone, and brain. Radiotherapy followed by repeated courses of multiple chemotherapies with cisplatin, pemetrexed, and bevacizumab was not effective. The patient was then started on biweekly treatments with nivolumab (170 mg, by intravenous drip infusion). After four courses of treatment, an acute increase was observed in the patient's serum creatinine level (from 0.80 mg/dL to 1.57 mg/dL). He was referred and admitted to our department. The patient, who had a history of appendicitis and stomach ulcers, was taking rabeprazole and magnesium oxide at the time of admission.
On admission, the patient was alert. His height was 176.7 cm and his weight was 54.2 kg. His blood pressure was 109/80 mmHg. There were no ophthalmologic findings suggesting uveitis. The results of the laboratory examination were as follows: hemoglobin, 11.7 g/dL; white blood cell count, 9,900/μL (neutrophils, 75.7% and eosinophils, 2.3%); platelet count, 49.0×104/μL; serum albumin, 3.0 g/dL; serum creatinine, 2.82 mg/dL; and C-reactive protein, 10.7 mg/dL. The patient was negative for antinuclear and antineutrophil cytoplasmic antibodies. A urinalysis showed 1+ protein and ± occult blood, and a microscopic examination revealed 281 white blood cells per high power field. May-Giemsa staining revealed that mononuclear cells-but not eosinophils-were dominant. The urinary protein creatinine ratio was 0.63 g/gCr. The N-acetyl-β-D-glucosaminidase activity and β-2 microglobulin levels were elevated at 24.1 U/L and 33,600 μg/L, respectively.
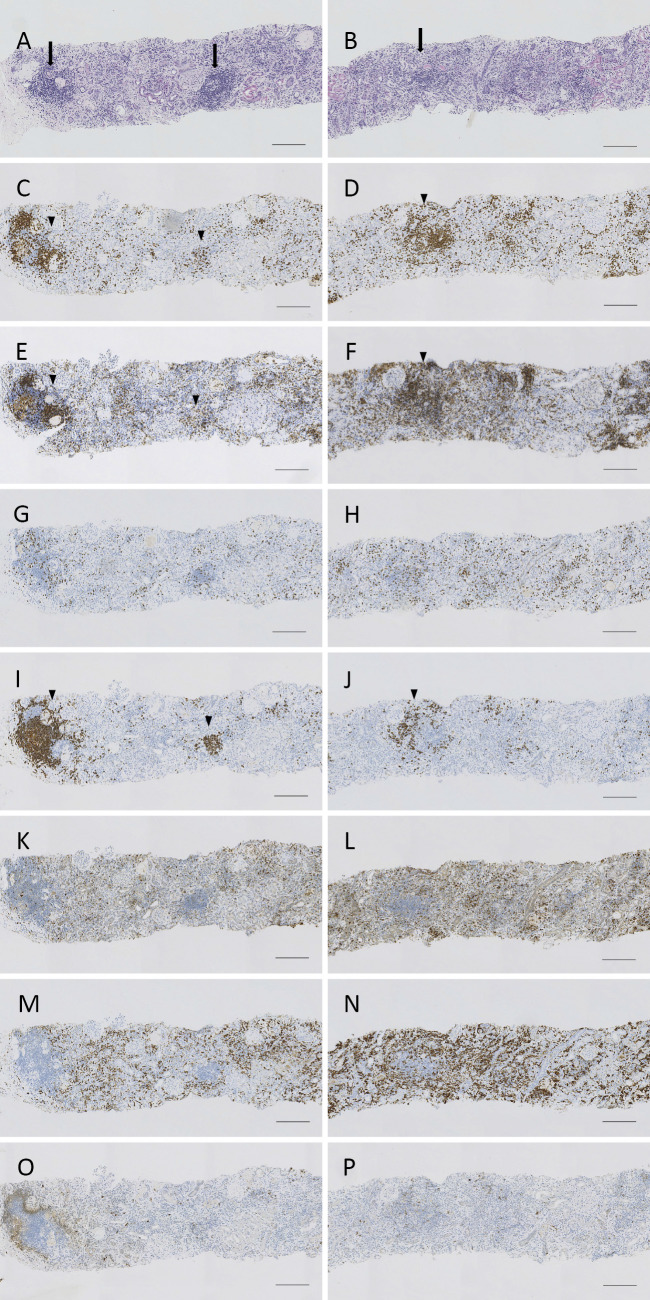
A renal biopsy was immediately performed. It revealed acute tubulointerstitial nephritis with marked mononuclear cell infiltration and some lymphoid follicles (Fig. 1A and B). Neither granulomatous nor vasculitic lesions were observed. Immunofluorescence did not reveal any significant deposits of immunoglobulins or complements in the kidney (data not shown). Immunohistochemical staining revealed the strong infiltration of CD3+ T cells, CD4+ T helper cells, CD68+ macrophages and CD163+ M2 macrophages, together with the mild infiltration of CD8+ cytotoxic T cells CD20+ B cells, and CD1c+ dendritic cells (Fig. 1C-P).
Figure 1.
The histological findings of the kidney. (A and B) Light microscopy findings. Periodic acid-Schiff staining. Marked mononuclear cell infiltration is observed in the tubulointerstitial area. (C-P) Immunohistochemical staining for CD3 (C and D), CD4 (E and F), CD8 (G and H), CD20 (I and J), CD68 (K and L), CD163 (M and N) and CD1c (O and P). Note some lymphoid follicles (arrows). The marked infiltration of CD3+, CD4+ and CD20+ cells is observed within the lymphoid follicles (arrowheads). However, the CD20+ infiltration is mild in the other tubulointerstitial areas. Scale bar indicates 200 μm.
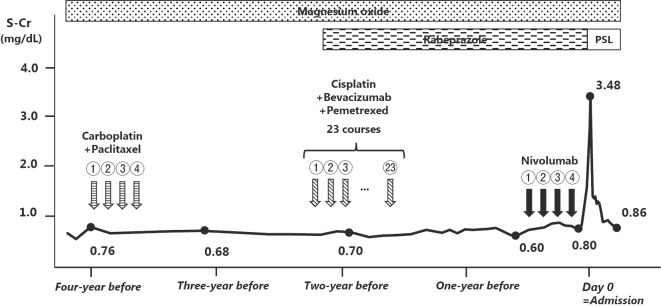
The patient was diagnosed with nivolumab-induced acute tubulointerstitial nephritis, and prednisolone (55 mg, daily) treatment was initiated. Nivolumab and rabeprazole were stopped based on a recent report suggesting a possible association between ICI-induced tubulointerstitial nephritis and proton pump inhibitors (7). The patient's serum creatinine level rapidly improved to 0.88 mg/dL at one month after the initiation of treatment. The patient's clinical course, including the anti-cancer treatments the patient received before nivolumab, are summarized in Fig. 2.
Figure 2.
The clinical course after the initiation of anti-cancer therapy. PSL: Prednisolone, Cr: serum creatinine
Discussion
The incidence of renal adverse events was reported to be relatively low (<1%) in randomized clinical trials of ICIs (ipilimumab, nivolumab, or pembrolizumab) (14-16). The risk of renal events was reported to increase by 6% when ipilimumab and nivolumab were used in combination (17). However, since 2014-when Izzedine et al. reported two cases of ipilimumab-induced acute granulomatous interstitial nephritis and identified four other similar cases by a literature review-the number of reported cases of ICI-induced kidney injury has been increasing (5-12). This trend might be partially due to the expanding indications of ICIs for malignancies ranging from melanoma to various cancers, including non-small cell lung cancer, renal cell carcinoma, and Hodgkin's lymphoma.
AKI with a histological feature of acute tubulointerstitial nephritis is a severe condition and represents the most common ICI-associated renal irAE (5-8,10-13). Shirali et al. reported six cases of AKI and acute tubulointerstitial nephritis, which occurred during treatment with one of the anti-PD-1 antibodies (nivolumab or pembrolizumab) (7). Cortazar et al. described 13 cases of ICI-induced AKI (8). In their series, 12 patients were found to have acute tubulointerstitial nephritis based on the examination of a renal biopsy specimen, and granulomatous lesions were found in three patients.
Table summarizes the previously reported cases in which infiltrating cells in the interstitium were analyzed by immunohistochemistry (8,10-13). CD3+ T cells were the major infiltrating cells in the kidney. Both CD4+ T cells and CD8+ T cells were detected in all previously reported cases. At present, the loss of peripheral tolerance of autoreactive T cells against tubular cells is hypothesized as an underlying mechanism of ICI-induced tubulointerstitial nephritis (7,8). An alternative hypothesis proposes that ICIs reduce tolerance to effector T cell-targeting drugs that are known to induce acute tubulointerstitial nephritis, such as proton pump inhibitors (7,8). The finding of substantial T cell infiltration into the tubulointerstitial area in this case is consistent with these hypotheses. Berney-Meyer et al. reported the histological features of omeprazole-induced tubulointerstitial nephritis using 25 biopsy-proven cases (18). They showed that the predominant infiltrating cells were CD4+ interleukin-17+ cells, suggesting a Th17-mediated inflammatory process. The role of Th17 cells in ICI-induced acute tubulointerstitial nephritis should be determined in future studies. Limited data are available for the other cell types that might be involved in ICI-induced acute tubulointerstitial nephritis. CD20+ B cells were detected at lower levels in Case 2 (Table) as well as the present case. Macrophage infiltration was observed in Cases 6, 7, 8 (Table), as well as in our case as well. In the present case, marked infiltration by both CD68+ and CD163+ macrophages was observed. CD68 is a pan-macrophage marker, and CD163 is recognized as a marker of activated M2 macrophages (19). M2 macrophages are considered to tune the inflammatory response, thereby promoting tissue remodeling or repair and sometimes driving the fibrotic response during tissue injury. In contrast, classical M1 macrophages produce proinflammatory cytokines and induce acute tissue damage (20,21).
Table.
Tubulointerstitial Infiltrating Cells in Immune Checkpoint Inhibitor-induced Acute Tubulointerstitial Nephritis.
| No. | Age | Sex | ICIs | PPI | Period between initiation of ICIs and AKI (months) | S-Cr (mg/dL) | Infiltrating cells in the kidney*1 | Treatment | Outcome | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| peak | CD3 | CD4 | CD8 | CD20 | CD68 | CD163 | Others | |||||||||
| 1 | 75 | Male | Ipi+Nivo | - | 1 | 7.31 | NA | ++ | ++ | NA | NA | NA | granzyme B+, perforin+, Foxp3+ | PSL / MMF | Dead | 10 |
| 2 | 75 | Male | Ipi+Nivo | - | 1.5 | 7.3 | ++ | ++ | ++ | + | NA | NA | PSL | PR | 8 | |
| 3 | 67 | Female | Nivo | + | 1.5 | 4.00 | ++ | ++ | ++ | NA | NA | NA | PSL | PR | 11 | |
| 4 | 52 | Female | Pem | + | 4 | 1.64 | ++ | ++ | + | NA | NA | NA | PSL | PR | ||
| 5 | 68 | Female | Ipi | + | 4 | 1.83 | + | + | + | NA | NA | NA | PSL | CR | ||
| 6 | 78 | Female | Nivo | + | 2 | 3.14*2 | ++ | ++ | ++ | NA | + | NA | PSL | CR | 12 | |
| 7 | 64 | Male | Pem | + | 4 | 4.3*2 | ++ | ++ | + | NA | ++ | NA | PSL | PR | ||
| 8 | 67 | Female | Nivo | + | 1 | 5.0 | ++ | ++ | ++ | + | ++ | NA | CD38++, CD1a+, Foxp3+ | PSL | PR | 13 |
| 9 | 57 | Male | Nivo | + | 2 | 3.48 | ++ | ++ | + | + | ++ | ++ | CD1c+ | PSL | CR | Our case |
ICI: immune checkpoint inhibitor, Ipi: Ipilimumab, PPI: proton pump inhibitor, NA: not available, AKI: acute kidney injury, Nivo: nivolumab, Pem: pembrolizumab, S-Cr: serum creatinine, PSL: prednisolone or prednisone, MMF: micophenolate mofetil, PR: patial recovery of serum creatinine, CR: normalization of serum creatinine
*1 The degree of infiltrating cells are expressed as follows, based upon the presented photos in the manuscript:++, strong; ’+mild.
*2 At presentation
The current information on CD163+ M2 macrophage infiltration in human nephritis is limited (18,22,23). Berney-Meyer et al. reported the presence of mild to moderate CD163+ macrophage infiltration in omeprazole-induced acute tubulointerstitial nephritis; however, CD4+ lymphocytes accounted for the majority of the infiltrating cells (18). Palmer et al. described that increased macrophage infiltration in patients with acute tubular injury was associated with minimal-change nephrotic syndrome. They reported that 75% of macrophages were CD163+ and considered that these cells might be engaged in the repair of injured tubular epithelial cells (22). Ikezumi et al. examined CD163+ M2 macrophage infiltration in IgA nephropathy. They reported that CD163+ M2 macrophages were detected not only in the glomeruli but also in the tubulointerstitial area; furthermore, they reported that the degree of CD163+ macrophage infiltration was well correlated with the degree of interstitial fibrosis (23). The exact role of the marked CD163+ M2 macrophage infiltration that was observed in our case is unclear. Considering that the renal biopsy specimen was obtained during the early phase of acute progressive kidney injury, CD163+ macrophages might have played a role in promoting tissue injury without contributing to tissue repair in the current case. Further studies are necessary to determine whether abundant CD163+ macrophage infiltration is ubiquitously observed in ICI-induced acute tubulointerstitial nephritis and to define the functional roles of CD163+ macrophage in tubulointerstitial injury, with a specific focus on the relationship between these macrophages and T cells.
In summary, we reported a case of nivolumab-induced acute tubulointerstitial nephritis and reviewed seven other cases in which immunohistochemical analyses were conducted. Most cases showed moderate to marked CD3+ T cell infiltration with CD4+ and CD8+ phenotypes. We also observed marked CD163+ M2 macrophage infiltration in our case. The further histological investigation of ICI-induced tubulointerstitial nephritis is necessary to elucidate the underlying mechanisms.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 6: 3479-3492, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michot JM, Bigenwald C, Champiat S, et al. . Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 54: 139-148, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8: 49, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kourie HR, Awada G, Awada AH. Learning from the “tsunami” of immune checkpoint inhibitors in 2015. Crit Rev Oncol Hematol 101: 213-220, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Izzedine H, Gueutin V, Gharbi C, et al. . Kidney injuries related to ipilimumab. Invest New Drugs 32: 769-773, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Thajudeen B, Madhrira M, Bracamonte E, Cranmer LD. Ipilimumab granulomatous interstitial nephritis. Am J Ther 22: e84-e87, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287-291, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Cortazar FB, Marrone KA, Troxell ML, et al. . Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638-647, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol 17: 188, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami N, Borges TJ, Yamashita M, Riella LV. Severe acute interstitial nephritis after combination immune-checkpoint inhibitor therapy for metastatic melanoma. Clin Kidney J 9: 411-417, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belliere J, Meyer N, Mazieres J, et al. . Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer 115: 1457-1461, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escandon J, Peacock S, Trabolsi A, Thomas DB, Layka A, Lutzky J. Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor. J Immunother Cancer 5: 3, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida A, Watanabe M, Nawata A, et al. . Tubulointerstitial nephritis as adverse effect of programmed cell death 1 inhibitor, nivolumab, showed distinct histological findings. CEN Case Rep 6: 169-174, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert C, Schachter J, Long GV, et al. . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372: 2521-2532, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer J, Reckamp KL, Baas P, et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garon EB, Rizvi NA, Hui R, et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018-2028, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Wolchok JD, Kluger H, Callahan MK, et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369: 122-133, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berney-Meyer L, Hung N, Slatter T, Schollum JB, Kitching AR, Walker RJ. Omeprazole-induced acute interstitial nephritis: a possible Th1-Th17-mediated injury? Nephrology (Carlton) 19: 359-365, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, David MZ, Hyjek E, Chang A, Meehan SM. M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol 10: 54-62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677-686, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Nikolic-Paterson DJ, Wang S, Lan HY. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl (2011) 4: 34-38, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer MB, Vichot AA, Cantley LG, Moeckel GW. Quantification and localization of M2 macrophages in human kidneys with acute tubular injury. Int J Nephrol Renovasc Dis 7: 415-419, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikezumi Y, Suzuki T, Karasawa T, et al. . Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology 58: 198-210, 2011. [DOI] [PubMed] [Google Scholar]